Chronic Stress Blocks the Endometriosis Immune Response by Metabolic Reprogramming
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
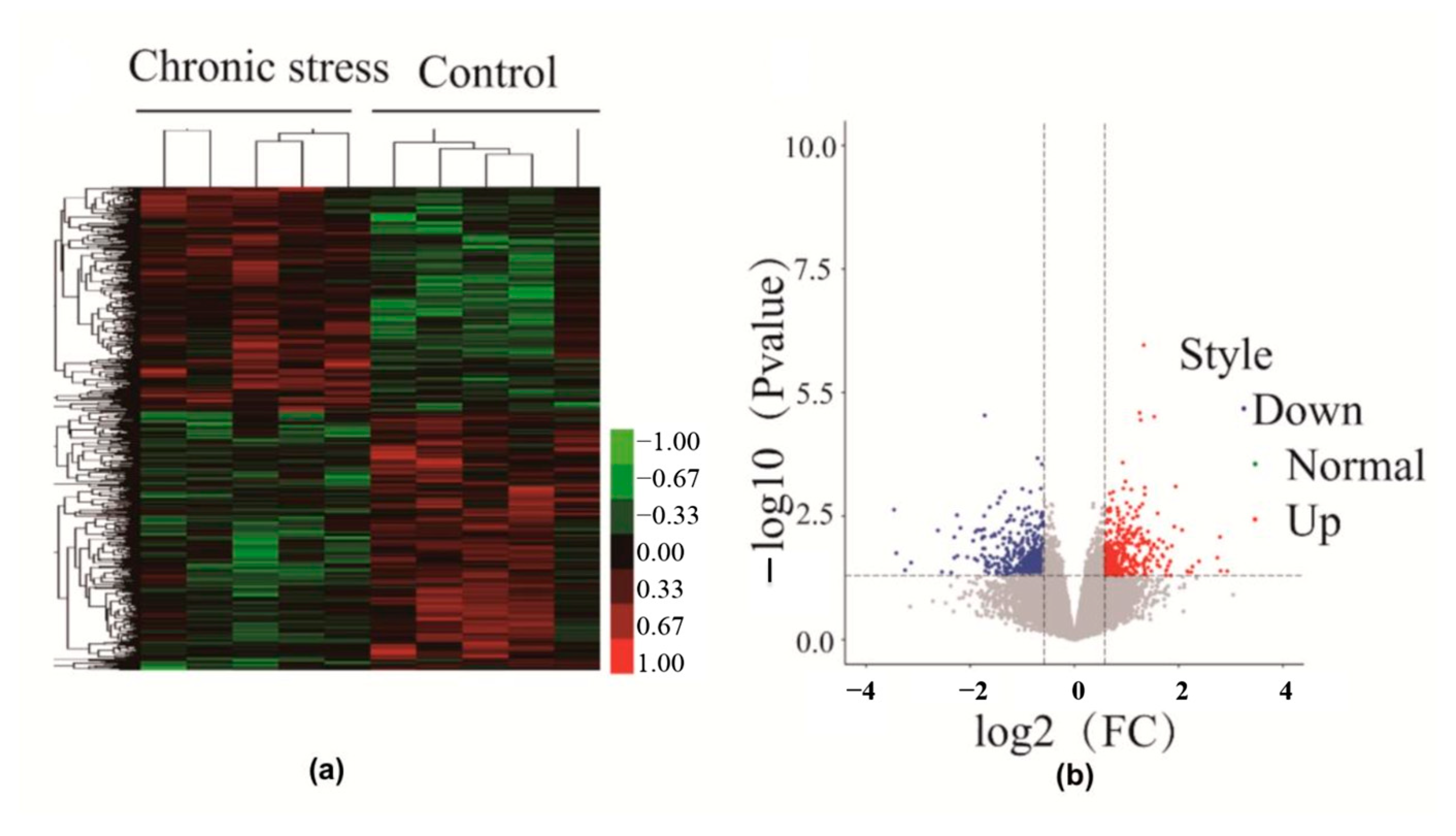
2.2. Differential Gene Expression in Endometriosis Patients with or without Chronic Stress
2.3. Gene Ontology (GO) Enrichment Analysis of Differentially Expressed Genes
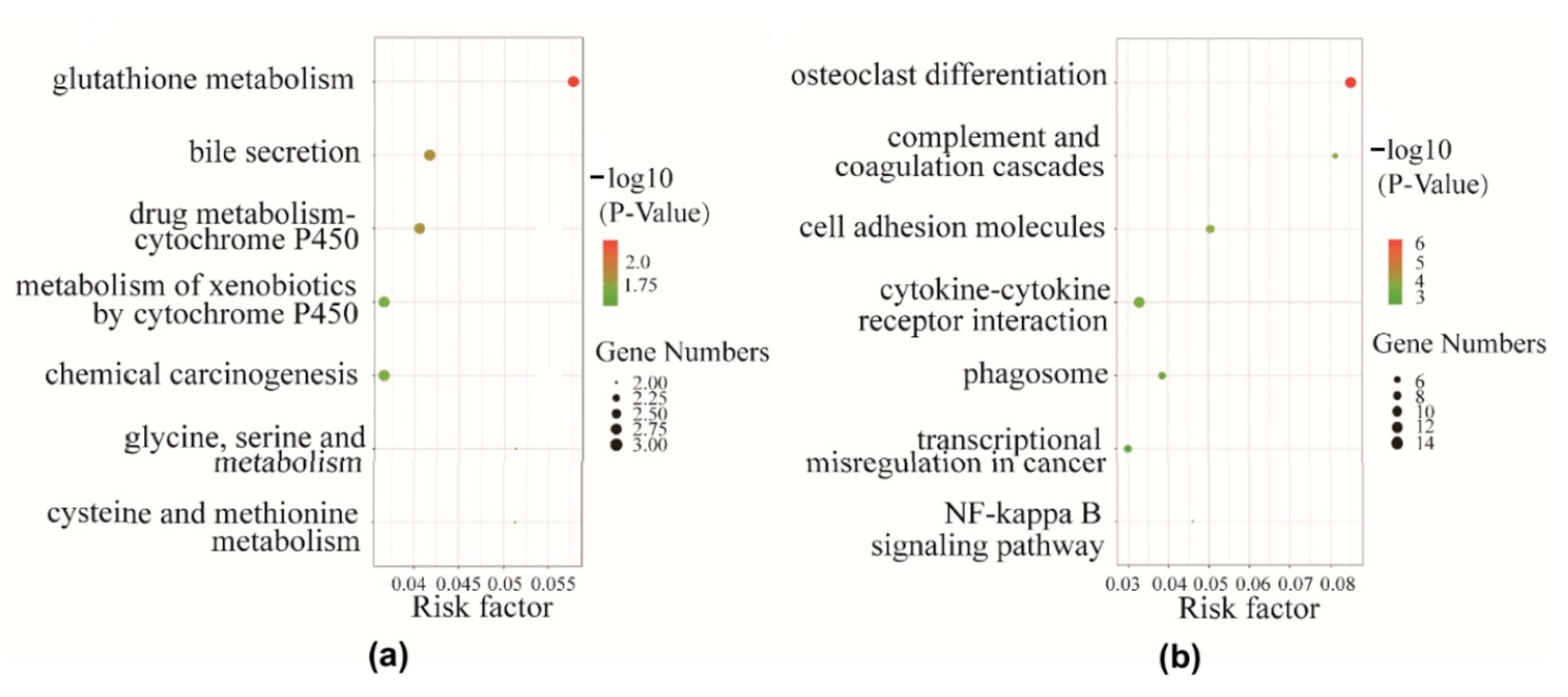
2.4. Pathway Analysis of Differentially Expressed Genes
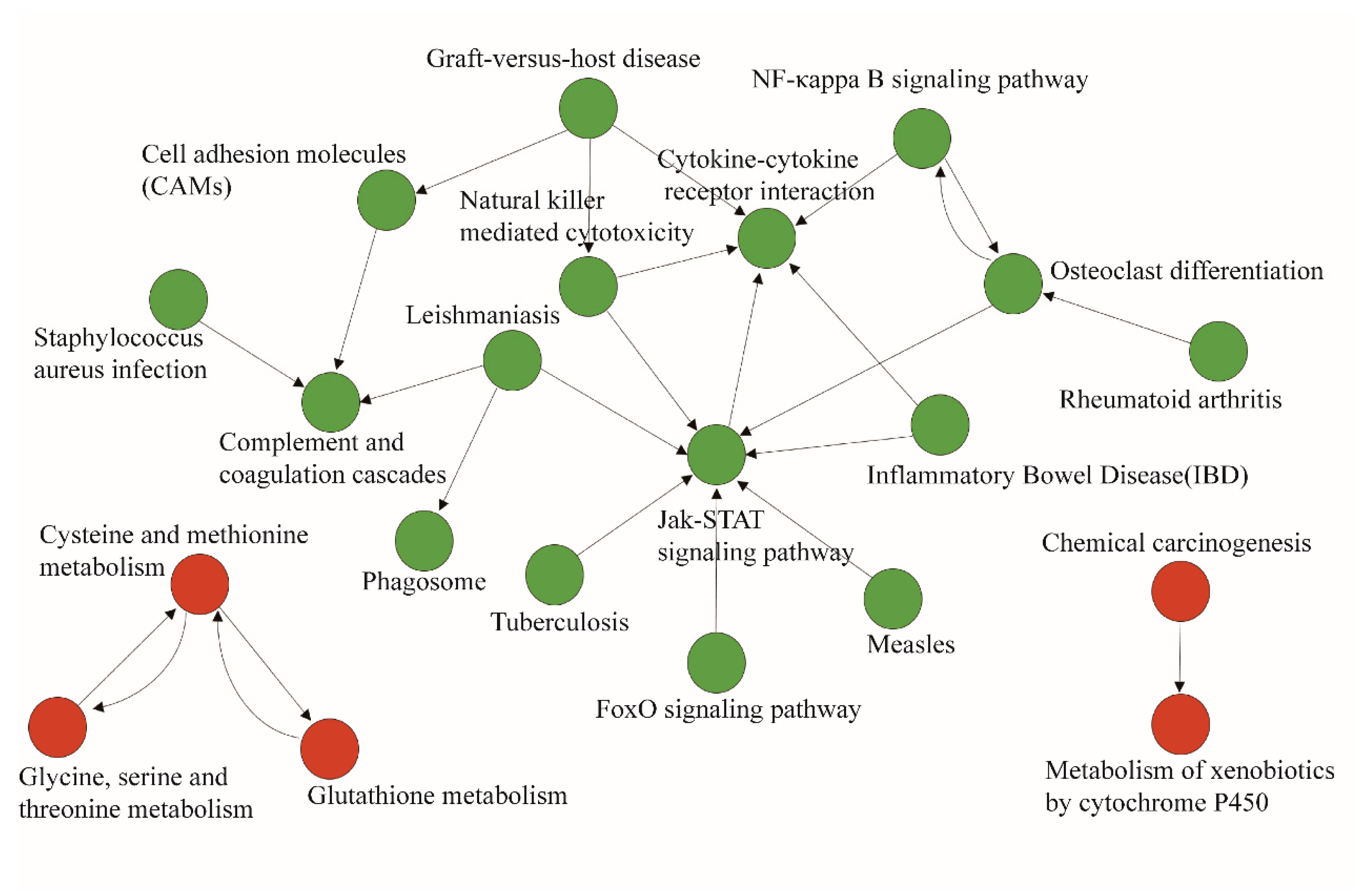
2.5. Pathway–Act Network Analysis
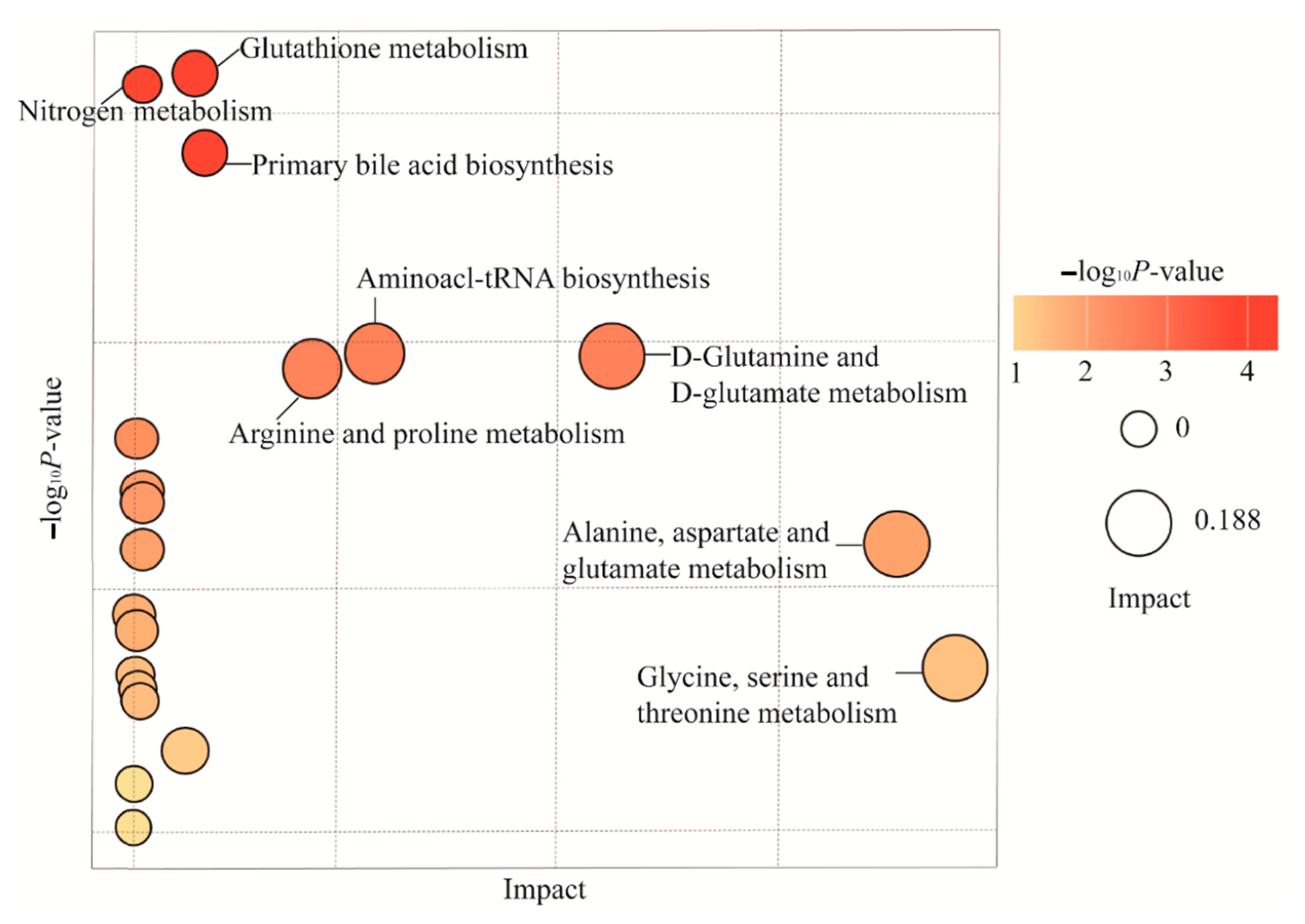
2.6. Differences in Metabolic Profiles and Pathways in Endometriosis Patients with or without Chronic Stress
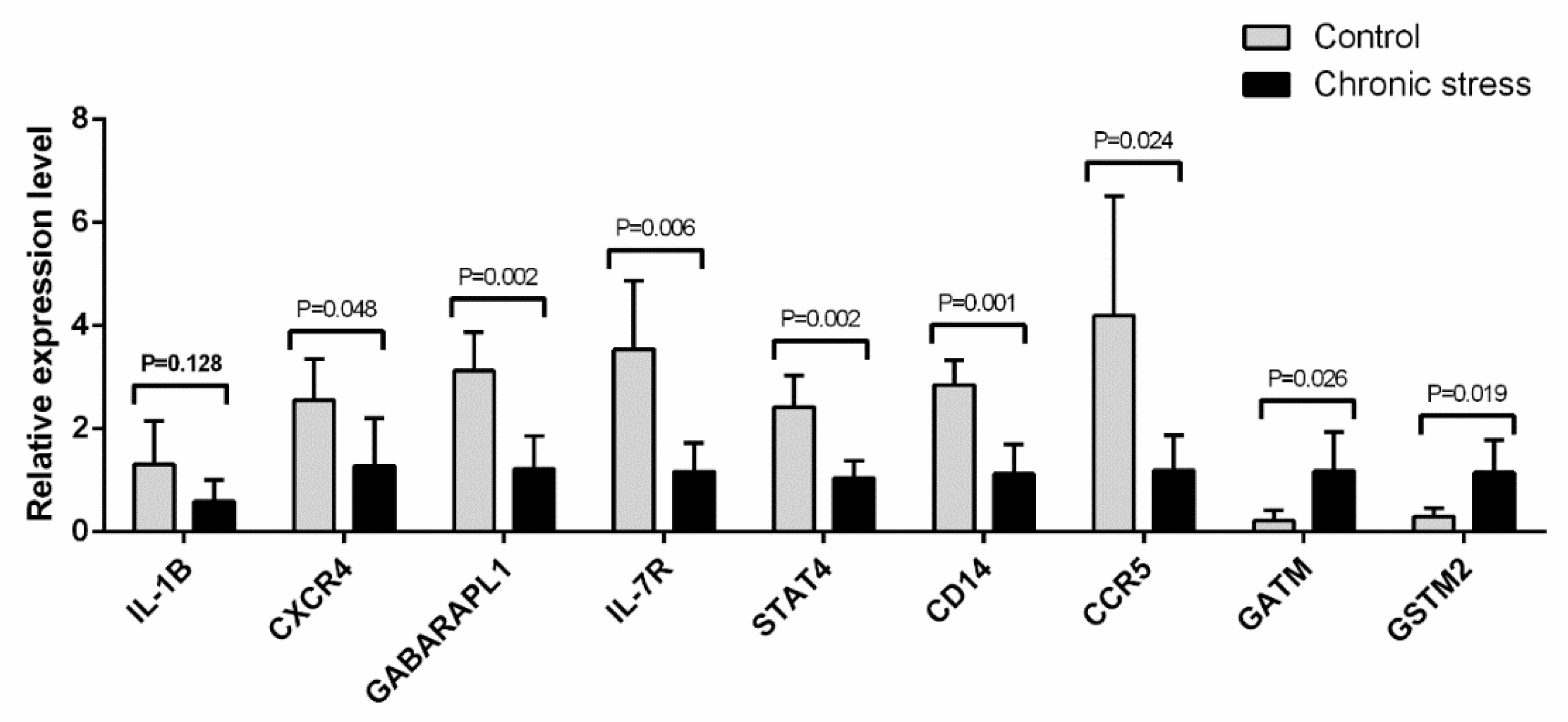
2.7. Quantitative Real-Time PCR Validation of Gene Expression Related to the Inflammatory Pathway and the Metabolic Pathway
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Gene Expression Profiling: RNA Extraction, Library Preparation, RNA-Sequencing, and Analyses of Differentially Expressed Genes
4.3. Metabolome Analyses: Tissue Metabolite Extraction and Metabolite Profiling Analysis
4.4. Quantitative RT–PCR
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, L.; Whitaker, L.H.R.; Mawson, R.L.; Hickey, M. Endometriosis. BMJ 2022, 379, e068950. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Parker, M.; Sneddon, A.; Lopez, V.; Ellwood, D. Impact of endometriosis on women’s lives: A qualitative study. BMC Womens Health 2014, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Genesis, genes and epigenetics of endometriosis-associated infertility. Nat. Rev. Endocrinol. 2019, 15, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The social and psychological impact of endometriosis on women’s lives: A critical narrative review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [PubMed]
- De Graaff, A.A.; Van Lankveld, J.; Smits, L.J.; Van Beek, J.J.; Dunselman, G.A. Dyspareunia and depressive symptoms are associated with impaired sexual functioning in women with endometriosis, whereas sexual functioning in their male partners is not affected. Hum. Reprod. 2016, 31, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.; Urrutia, R.; Torres-Reveron, A.; Vincent, K.; Flores, I. Anxiety, coping skills and hypothalamus-pituitary-adrenal (HPA) axis in patients with endometriosis. J. Reprod. Biol. Health 2015, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.J.; Sharma, V.; Sharma, S.; Mazmanian, D. A Systematic Review of the Association Between Psychiatric Disturbances and Endometriosis. J. Obstet. Gynaecol. Can. 2015, 37, 1006–1015. [Google Scholar] [CrossRef]
- Lazzeri, L.; Orlandini, C.; Vannuccini, S.; Pinzauti, S.; Tosti, C.; Zupi, E.; Nappi, R.E.; Petraglia, F. Endometriosis and perceived stress: Impact of surgical and medical treatment. Gynecol. Obstet. Investig. 2015, 79, 229–233. [Google Scholar] [CrossRef]
- Van Stein, K.; Schubert, K.; Ditzen, B.; Weise, C. Understanding Psychological Symptoms of Endometriosis from a Research Domain Criteria Perspective. J. Clin. Med. 2023, 12, 4056. [Google Scholar] [CrossRef]
- Guo, S.W.; Zhang, Q.; Liu, X. Social psychogenic stress promotes the development of endometriosis in mouse. Reprod. Biomed. Online 2017, 34, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Kalfas, M.; Chisari, C.; Windgassen, S. Psychosocial factors associated with pain and health-related quality of life in Endometriosis: A systematic review. Eur. J. Pain 2022, 26, 1827–1848. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Lutgendorf, S.K.; Sood, A.K. The neuroendocrine impact of chronic stress on cancer. Cell Cycle 2007, 6, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Sood, A.K. Neuroendocrine influences on cancer biology. Semin. Cancer Biol. 2008, 18, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Imperato, A.; Angelucci, L.; Casolini, P.; Zocchi, A.; Puglisi-Allegra, S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992, 577, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Newton, R. Molecular mechanisms of glucocorticoid action: What is important? Thorax 2000, 55, 603–613. [Google Scholar] [CrossRef]
- Appleyard, C.B.; Cruz, M.L.; Hernandez, S.; Thompson, K.J.; Bayona, M.; Flores, I. Stress management affects outcomes in the pathophysiology of an endometriosis model. Reprod. Sci. 2015, 22, 431–441. [Google Scholar] [CrossRef]
- Cuevas, M.; Flores, I.; Thompson, K.J.; Ramos-Ortolaza, D.L.; Torres-Reveron, A.; Appleyard, C.B. Stress exacerbates endometriosis manifestations and inflammatory parameters in an animal model. Reprod. Sci. 2012, 19, 851–862. [Google Scholar] [CrossRef]
- Long, Q.; Liu, X.; Qi, Q.; Guo, S.W. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor β2. Hum. Reprod. 2016, 31, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef] [PubMed]
- Nassiri Kigloo, H.; Itani, R.; Montreuil, T.; Feferkorn, I.; Raina, J.; Tulandi, T.; Mansour, F.; Krishnamurthy, S.; Suarthana, E. Endometriosis, chronic pain, anxiety, and depression: A retrospective study among 12 million women. J. Affect. Disord. 2023, 346, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Jorge, C. Endometriosis and Depression: A Psychoimmune Vicious Cycle. J. Minim. Invasive Gynecol. 2015, 22, S165–S166. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, D.; Bray, J.D.; Su, E.; Pavone, M.E.; Dyson, M.T.; Navarro, A.; Kakinuma, T.; Bulun, S.E. Activated glucocorticoid and eicosanoid pathways in endometriosis. Fertil. Steril. 2012, 98, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fang, M.; Davies, H.; Hu, Z. Mifepristone: A potential clinical agent based on its anti-progesterone and anti-glucocorticoid properties. Gynecol. Endocrinol. 2014, 30, 169–173. [Google Scholar] [CrossRef]
- Kang, Y.; Nagaraja, A.S.; Armaiz-Pena, G.N.; Dorniak, P.L.; Hu, W.; Rupaimoole, R.; Liu, T.; Gharpure, K.M.; Previs, R.A.; Hansen, J.M.; et al. Adrenergic Stimulation of DUSP1 Impairs Chemotherapy Response in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 1713–1724. [Google Scholar] [CrossRef]
- Partecke, L.I.; Speerforck, S.; Käding, A.; Seubert, F.; Kühn, S.; Lorenz, E.; Schwandke, S.; Sendler, M.; Keßler, W.; Trung, D.N.; et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology 2016, 16, 423–433. [Google Scholar] [CrossRef]
- Yamauchi, N.; Harada, T.; Taniguchi, F.; Yoshida, S.; Iwabe, T.; Terakawa, N. Tumor necrosis factor-alpha induced the release of interleukin-6 from endometriotic stromal cells by the nuclear factor-kappaB and mitogen-activated protein kinase pathways. Fertil. Steril. 2004, 82 (Suppl. S3), 1023–1028. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, T.; Tong, D.; Li, S.; Yu, X.; Liu, B.; Jiang, L.; Liu, K. Research advances in endometriosis-related signaling pathways: A review. Biomed. Pharmacother. 2023, 164, 114909. [Google Scholar] [CrossRef] [PubMed]
- Wickiewicz, D.; Chrobak, A.; Gmyrek, G.B.; Halbersztadt, A.; Gabryś, M.S.; Goluda, M.; Chełmońska-Soyta, A. Diagnostic accuracy of interleukin-6 levels in peritoneal fluid for detection of endometriosis. Arch. Gynecol. Obstet. 2013, 288, 805–814. [Google Scholar] [CrossRef]
- Li, B.; Chen, M.; Liu, X.; Guo, S.W. Constitutive and tumor necrosis factor-alpha-induced activation of nuclear factor-kappaB in adenomyosis and its inhibition by andrographolide. Fertil. Steril. 2013, 100, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Du, W.; Zhang, Y.J.; Hong, J.; Su, W.Y.; Tang, J.T.; Wang, Y.C.; Lu, R.; Fang, J.Y. Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol. Carcinog. 2012, 51, 174–184. [Google Scholar] [CrossRef]
- George, A.F.; Jang, K.S.; Nyegaard, M.; Neidleman, J.; Spitzer, T.L.; Xie, G.; Chen, J.C.; Herzig, E.; Laustsen, A.; Marques de Menezes, E.G.; et al. Seminal plasma promotes decidualization of endometrial stromal fibroblasts in vitro from women with and without inflammatory disorders in a manner dependent on interleukin-11 signaling. Hum. Reprod. 2020, 35, 617–640. [Google Scholar] [CrossRef]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef]
- Okamoto, M.; Nasu, K.; Abe, W.; Aoyagi, Y.; Kawano, Y.; Kai, K.; Moriyama, M.; Narahara, H. Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum. Reprod. 2015, 30, 632–641. [Google Scholar] [CrossRef]
- Hernandez, S.; Cruz, M.L.; Torres-Reveron, A.; Appleyard, C.B. Impact of physical activity on pain perception in an animal model of endometriosis. J. Endometr. Pelvic Pain Disord. 2015, 7, 89–114. [Google Scholar] [CrossRef]
- Toth, B. Stress, inflammation and endometriosis: Are patients stuck between a rock and a hard place? J. Mol. Med. 2010, 88, 223–225. [Google Scholar] [CrossRef]
- Furuya, M.; Tanaka, R.; Miyagi, E.; Kami, D.; Nagahama, K.; Miyagi, Y.; Nagashima, Y.; Hirahara, F.; Inayama, Y.; Aoki, I. Impaired CXCL4 expression in tumor-associated macrophages (TAMs) of ovarian cancers arising in endometriosis. Cancer Biol. Ther. 2012, 13, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Izumiya, C.; Taniguchi, K.; Matsushima, S.; Fukaya, T. Role of NK cells and HLA-G in endometriosis. Front. Biosci. 2012, 4, 1568–1581. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.A.; Joesting, J.J.; Freund, G.G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 2013, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Szymanowski, K.; Niepsuj-Biniaś, J.; Dera-Szymanowska, A.; Wołuń-Cholewa, M.; Yantczenko, A.; Florek, E.; Opala, T.; Murawski, M.; Wiktorowicz, K. An influence of immunomodulation on Th1 and Th2 immune response in endometriosis in an animal model. BioMed Res. Int. 2013, 2013, 849492. [Google Scholar] [CrossRef] [PubMed]
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Stefanek, M.; Sood, A.K. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat. Rev. Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Brown, J.K.; Maybin, J.; Saunders, P.T.; Duncan, W.C.; Horne, A.W. Transforming growth factor-beta induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, 3450–3459. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Imanaka, S. Understanding the molecular mechanisms of macrophage polarization and metabolic reprogramming in endometriosis: A narrative review. Reprod. Med. Biol. 2022, 21, e12488. [Google Scholar] [CrossRef]
- Sun, L.; Song, L.; Wan, Q.; Wu, G.; Li, X.; Wang, Y.; Wang, J.; Liu, Z.; Zhong, X.; He, X.; et al. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015, 25, 429–444. [Google Scholar] [CrossRef]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.H.; Jones, R.G. Fueling immunity: Insights into metabolism and lymphocyte function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Goetze, K.; Walenta, S.; Ksiazkiewicz, M.; Kunz-Schughart, L.A.; Mueller-Klieser, W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol. 2011, 39, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, E.; Kunz-Schughart, L.A.; Ebner, S.; Mueller-Klieser, W.; Hoves, S.; Andreesen, R.; Mackensen, A.; Kreutz, M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006, 107, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Phillips, M.M.; Sheaff, M.T.; Szlosarek, P.W. Targeting arginine-dependent cancers with arginine-degrading enzymes: Opportunities and challenges. Cancer Res. Treat. 2013, 45, 251–262. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Xu, J.; Li, K.; Wang, J.; Dai, Y.; Chen, Y.; Chai, R.; Xu, C.; Kang, Y. Chronic Stress Blocks the Endometriosis Immune Response by Metabolic Reprogramming. Int. J. Mol. Sci. 2024, 25, 29. https://doi.org/10.3390/ijms25010029
Lu C, Xu J, Li K, Wang J, Dai Y, Chen Y, Chai R, Xu C, Kang Y. Chronic Stress Blocks the Endometriosis Immune Response by Metabolic Reprogramming. International Journal of Molecular Sciences. 2024; 25(1):29. https://doi.org/10.3390/ijms25010029
Chicago/Turabian StyleLu, Chong, Jing Xu, Ke Li, Jing Wang, Yilin Dai, Yiqing Chen, Ranran Chai, Congjian Xu, and Yu Kang. 2024. "Chronic Stress Blocks the Endometriosis Immune Response by Metabolic Reprogramming" International Journal of Molecular Sciences 25, no. 1: 29. https://doi.org/10.3390/ijms25010029
APA StyleLu, C., Xu, J., Li, K., Wang, J., Dai, Y., Chen, Y., Chai, R., Xu, C., & Kang, Y. (2024). Chronic Stress Blocks the Endometriosis Immune Response by Metabolic Reprogramming. International Journal of Molecular Sciences, 25(1), 29. https://doi.org/10.3390/ijms25010029





