Spontaneous Attenuation of Alcoholic Fermentation via the Dysfunction of Cyc8p in Saccharomyces cerevisiae
Abstract
1. Introduction
2. Results
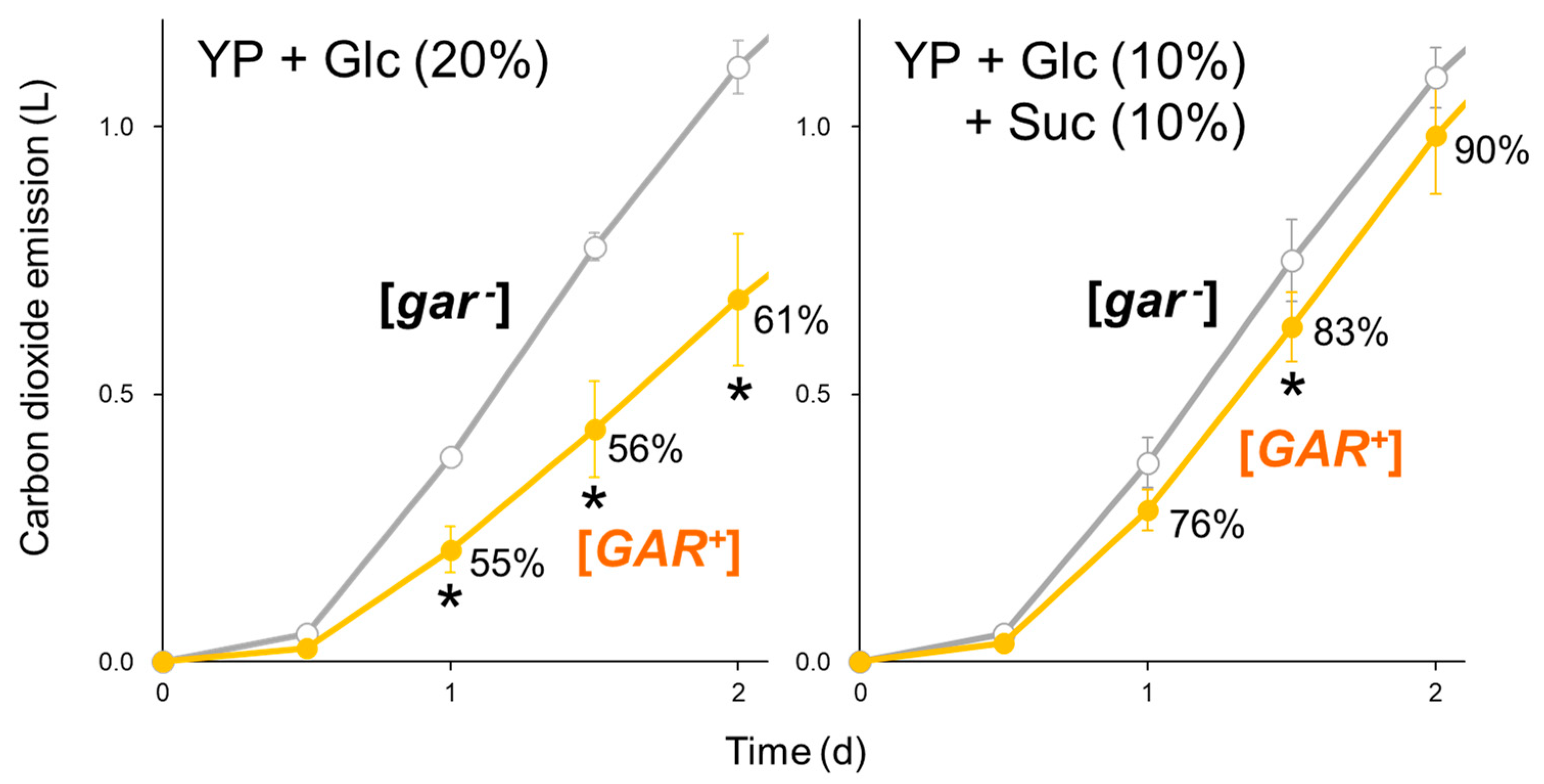
2.1. Spontaneous [GAR+] Retards Alcoholic Fermentation from Glucose
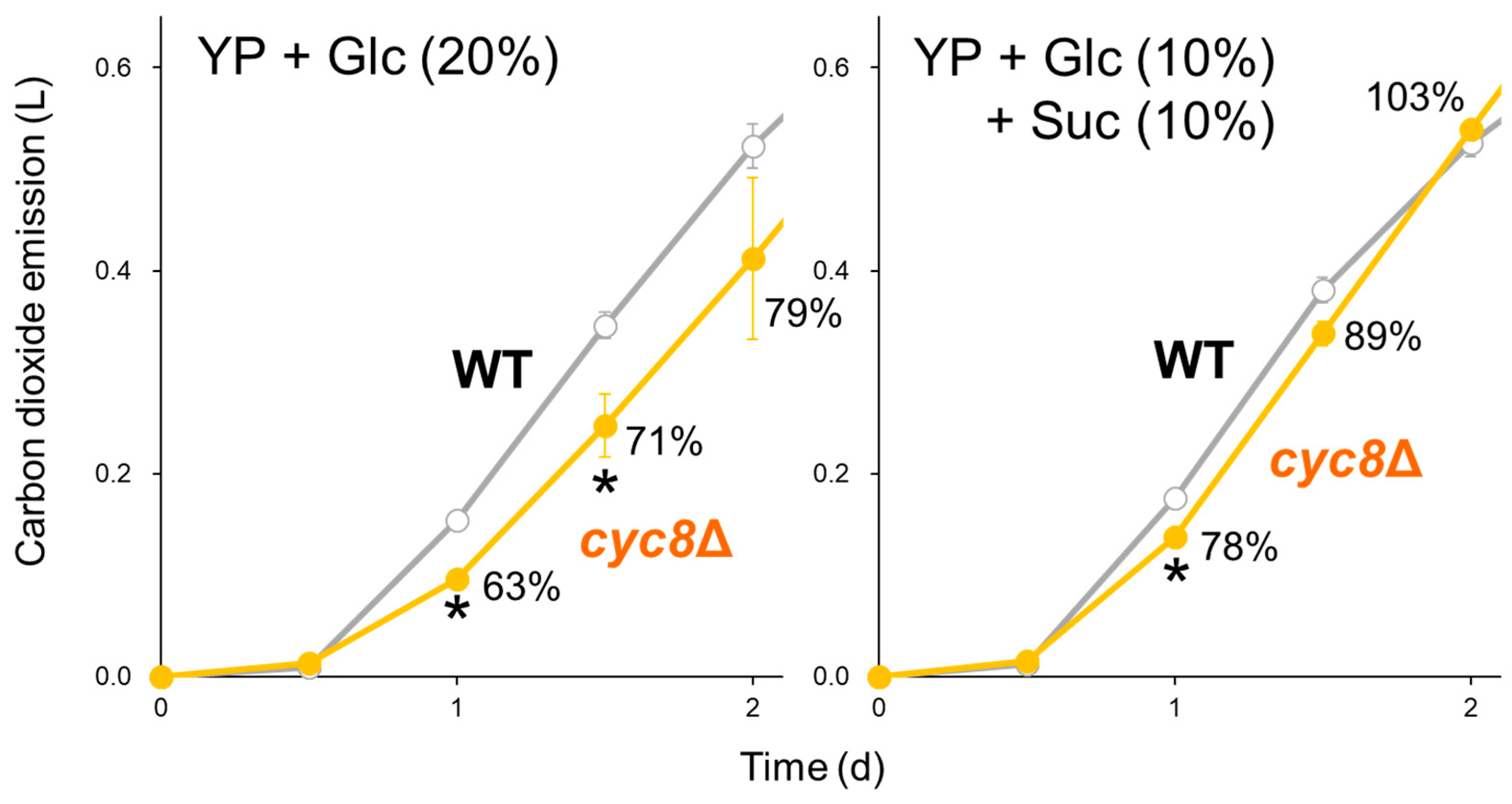
2.2. [GAR+] Alters Glucose Response via the Cyc8p–Tup1p Complex
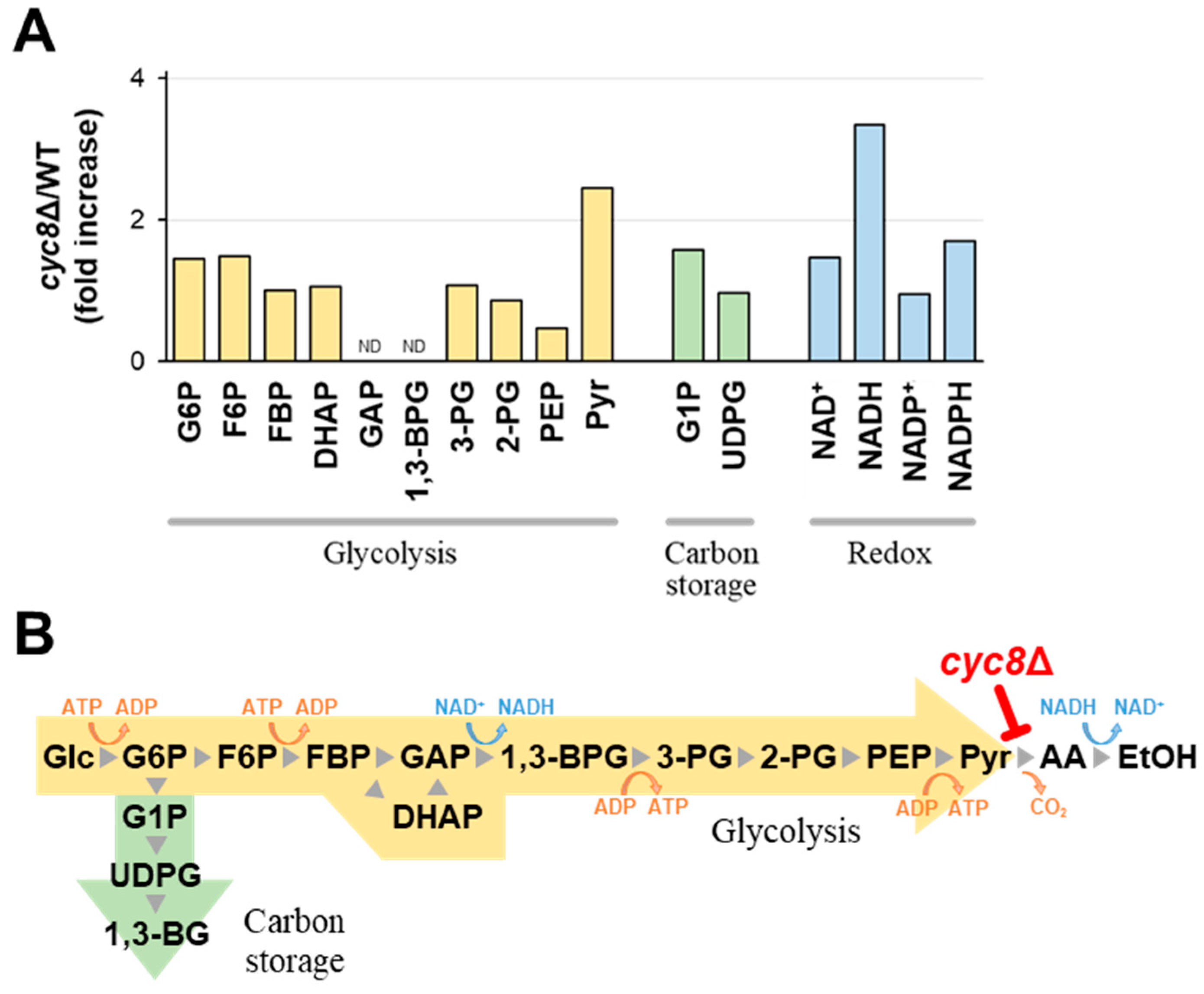
2.3. Dysfunction of Cyc8p Leads to Inactivation of Pyruvate Decarboxylase
3. Discussion
4. Materials and Methods
4.1. Yeast Strains Used in This Study
4.2. Fermentation Test
4.3. RNA-Seq Analysis
4.4. Analysis of Intracellular Metabolite Profiles
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, S.F. Cellular heterogeneity: Benefits besides bet-hedging. Curr. Biol. 2016, 26, R355–R357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wloch-Salamon, D.M.; Fisher, R.M.; Regenberg, B. Division of labour in the yeast: Saccharomyces cerevisiae. Yeast 2017, 34, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Harvey, Z.H.; Chen, Y.; Jarosz, D.F. Protein-based inheritance: Epigenetics beyond the chromosome. Mol. Cell 2018, 69, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Shaban, K.; Sauty, S.M.; Yankulov, K. Variation, variegation and heritable gene repression in S. cerevisiae. Front. Genet. 2021, 12, 630506. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.; Pandey, S.; Pelet, S. Cellular heterogeneity: Yeast-side story. Fungal Biol. Rev. 2022, 39, 34–45. [Google Scholar] [CrossRef]
- Čáp, M.; Štěpánek, L.; Harant, K.; Váchová, L.; Palková, Z. Cell differentiation within a yeast colony: Metabolic and regulatory parallels with a tumor-affected organism. Mol. Cell 2012, 46, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Bowen, C.H.; Liu, D.; Zhang, F. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis. Nat. Chem. Biol. 2016, 12, 339–344. [Google Scholar] [CrossRef]
- Kamrad, S.; Correia-Melo, C.; Szyrwiel, L.; Aulakh, S.K.; Bähler, J.; Demichev, V.; Mülleder, M.; Ralser, M. Metabolic heterogeneity and cross-feeding within isogenic yeast populations captured by DILAC. Nat. Microbiol. 2023, 8, 441–454. [Google Scholar] [CrossRef]
- Fernandes, R.L.; Nierychlo, M.; Lundin, L.; Pedersen, A.E.; Puentes Tellez, P.E.; Dutta, A.; Carlquist, M.; Bolic, A.; Schäpper, D.; Brunetti, A.C.; et al. Experimental methods and modeling techniques for description of cell population heterogeneity. Biotechnol. Adv. 2011, 29, 575–599. [Google Scholar] [CrossRef]
- Rugbjerg, P.; Sommer, M.O.A. Overcoming genetic heterogeneity in industrial fermentations. Nat. Biotechnol. 2019, 37, 869–876. [Google Scholar] [CrossRef]
- Brown, J.C.S.; Lindquist, S. A heritable switch in carbon source utilization driven by an unusual yeast prion. Genes Dev. 2009, 23, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, D.F.; Lancaster, A.K.; Brown, J.C.S.; Lindquist, S. An evolutionarily conserved prion-like element converts wild fungi from metabolic specialists to generalists. Cell 2014, 158, 1072–1082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jarosz, D.F.; Brown, J.C.S.; Walker, G.A.; Datta, M.S.; Ung, W.L.; Lancaster, A.K.; Rotem, A.; Chang, A.; Newby, G.A.; Weitz, D.A.; et al. Cross-kingdom chemical communication drives a heritable, mutually beneficial prion-based transformation of metabolism. Cell 2014, 158, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Kumano, M.; Sugimoto, Y.; Ito, M.; Ohashi, M.; Sunada, K.; Takahashi, T.; Yamada, T.; Takagi, H. Metabolic switching of sake yeast by kimoto lactic acid bacteria through the [GAR+] non-genetic element. J. Biosci. Bioeng. 2018, 126, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; Henderson, C.M.; Luong, P.; Block, D.E.; Bisson, L.F. Downshifting yeast dominance: Cell physiology and phospholipid composition are altered with establishment of the [GAR+] prion in Saccharomyces cerevisiae. Front. Microbiol. 2020, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Maeda, N.; Okumura, H.; Shima, J. Emergence of [GAR+] cells in yeast from sake brewing affects the fermentation properties. Yeast 2023, 40, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gancedo, J.M. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 1998, 62, 334–361. [Google Scholar] [CrossRef]
- Kayikci, Ö.; Nielsen, J. Glucose repression in Saccharomyces cerevisiae. FEMS Yeast Res. 2015, 15, fov068. [Google Scholar] [CrossRef]
- Schüller, H.J. Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 139–160. [Google Scholar] [CrossRef]
- Malavé, T.M.; Dent, S.Y. Transcriptional repression by Tup1-Ssn6. Biochem. Cell Biol. 2006, 84, 437–443. [Google Scholar] [CrossRef]
- Payankaulam, S.; Li, L.M.; Arnosti, D.N. Transcriptional repression: Conserved and evolved features. Curr. Biol. 2010, 20, R764–R771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Roy, A.; Jouandot, D., 2nd; Cho, K.H. The glucose signaling network in yeast. Biochim. Biophys. Acta 2013, 1830, 5204–5210. [Google Scholar] [CrossRef]
- Whitmarsh, A.J. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta 2007, 1773, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Bruce, C.; Hart, C.; Leigh-Bell, J.; Gelperin, D.; Umansky, L.; Gerstein, M.B.; Snyder, M. Dynamic and complex transcription factor binding during an inducible response in yeast. Genes Dev. 2009, 23, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Carlson, M. Molecular analysis of SSN6, a gene functionally related to the SNF1 protein kinase of Saccharomyces cerevisiae. Mol. Cell. Biol. 1987, 7, 3637–3645. [Google Scholar] [PubMed]
- Dowell, R.D.; Ryan, O.; Jansen, A.; Cheung, D.; Agarwala, S.; Danford, T.; Bernstein, D.A.; Rolfe, P.A.; Heisler, L.E.; Chin, B.; et al. Genotype to phenotype: A complex problem. Science 2010, 328, 469. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Church, M.; Hokamp, K.; Alhussain, M.M.; Bamagoos, A.A.; Fleming, A.B. Systematic analysis of tup1 and cyc8 mutants reveals distinct roles for TUP1 and CYC8 and offers new insight into the regulation of gene transcription by the yeast Tup1-Cyc8 complex. PLoS Genet. 2023, 19, e1010876. [Google Scholar] [CrossRef]
- Watanabe, D.; Zhou, Y.; Hirata, A.; Sugimoto, Y.; Takagi, K.; Akao, T.; Ohya, Y.; Takagi, H.; Shimoi, H. Inhibitory role of Greatwall-like protein kinase Rim15p in alcoholic fermentation via upregulating the UDP-glucose synthesis pathway in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2016, 82, 340–351. [Google Scholar] [CrossRef]
- Watanabe, D.; Kawashima, M.; Yoshioka, N.; Sugimoto, Y.; Takagi, H. Rational design of alcoholic fermentation targeting extracellular carbon. NPJ Sci. Food 2023, 7, 37. [Google Scholar] [CrossRef]
- Patel, B.K.; Gavin-Smyth, J.; Liebman, S.W. The yeast global transcriptional co-repressor protein Cyc8 can propagate as a prion. Nat. Cell Biol. 2009, 11, 344–349. [Google Scholar] [CrossRef]
- Takao, Y.; Takahashi, T.; Yamada, T.; Goshima, T.; Isogai, A.; Sueno, K.; Fujii, T.; Akao, T. Characteristic features of the unique house sake yeast strain Saccharomyces cerevisiae Km67 used for industrial sake brewing. J. Biosci. Bioeng. 2018, 126, 617–623. [Google Scholar] [CrossRef]
- Watanabe, D.; Araki, Y.; Zhou, Y.; Maeya, N.; Akao, T.; Shimoi, H. A loss-of-function mutation in the PAS kinase Rim15p is related to defective quiescence entry and high fermentation rates of Saccharomyces cerevisiae sake yeast strains. Appl. Environ. Microbiol. 2012, 78, 4008–4016. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Kajihara, T.; Sugimoto, Y.; Takagi, K.; Mizuno, M.; Zhou, Y.; Chen, J.; Takeda, K.; Tatebe, H.; Shiozaki, K.; et al. Nutrient signaling via the TORC1-Greatwall-PP2AB55δ pathway is responsible for the high initial rates of alcoholic fermentation in sake yeast strains of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2019, 85, e02083-18. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Morley, A. An evolutionary perspective on the Crabtree effect. Front. Mol. Biosci. 2014, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Dashko, S.; Zhou, N.; Compagno, C.; Piškur, J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014, 14, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Hagman, A.; Piškur, J. A study on the fundamental mechanism and the evolutionary driving forces behind aerobic fermentation in yeast. PLoS ONE 2015, 10, e0116942. [Google Scholar] [CrossRef] [PubMed]
- Pronk, J.T.; Yde Steensma, H.; Van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- Candy, J.M.; Duggleby, R.G. Structure and properties of pyruvate decarboxylase and site-directed mutagenesis of the Zymomonas mobilis enzyme. Biochim. Biophys. Acta 1998, 1385, 323–338. [Google Scholar] [CrossRef]
- Hohmann, S. Characterisation of PDC2, a gene necessary for high level expression of pyruvate decarboxylase structural genes in Saccharomyces cerevisiae. Mol. Gen. Genet. 1993, 241, 657–666. [Google Scholar] [CrossRef]
- Reimand, J.; Vaquerizas, J.M.; Todd, A.E.; Vilo, J.; Luscombe, N.M. Comprehensive reanalysis of transcription factor knockout expression data in Saccharomyces cerevisiae reveals many new targets. Nucleic Acids Res. 2010, 38, 4768–4777. [Google Scholar] [CrossRef]
- Winston, F.; Dollard, C.; Ricupero-Hovasse, S.L. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 1995, 11, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, R.K.; Johnston, J.R. Genealogy of principal strains of the yeast genetic stock center. Genetics 1986, 113, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Ota, T.; Nitta, F.; Akao, T.; Shimoi, H. Automatic measurement of sake fermentation kinetics using a multi-channel gas monitor system. J. Biosci. Bioeng. 2011, 112, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, D.; Kumano, M.; Sugimoto, Y.; Takagi, H. Spontaneous Attenuation of Alcoholic Fermentation via the Dysfunction of Cyc8p in Saccharomyces cerevisiae. Int. J. Mol. Sci. 2024, 25, 304. https://doi.org/10.3390/ijms25010304
Watanabe D, Kumano M, Sugimoto Y, Takagi H. Spontaneous Attenuation of Alcoholic Fermentation via the Dysfunction of Cyc8p in Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2024; 25(1):304. https://doi.org/10.3390/ijms25010304
Chicago/Turabian StyleWatanabe, Daisuke, Maika Kumano, Yukiko Sugimoto, and Hiroshi Takagi. 2024. "Spontaneous Attenuation of Alcoholic Fermentation via the Dysfunction of Cyc8p in Saccharomyces cerevisiae" International Journal of Molecular Sciences 25, no. 1: 304. https://doi.org/10.3390/ijms25010304
APA StyleWatanabe, D., Kumano, M., Sugimoto, Y., & Takagi, H. (2024). Spontaneous Attenuation of Alcoholic Fermentation via the Dysfunction of Cyc8p in Saccharomyces cerevisiae. International Journal of Molecular Sciences, 25(1), 304. https://doi.org/10.3390/ijms25010304





