BmRRS1 Protein Inhibits the Proliferation of Baculovirus Autographa californica Nucleopolyhedrovirus in Silkworm, Bombyx mori
Abstract
:1. Introduction
2. Results
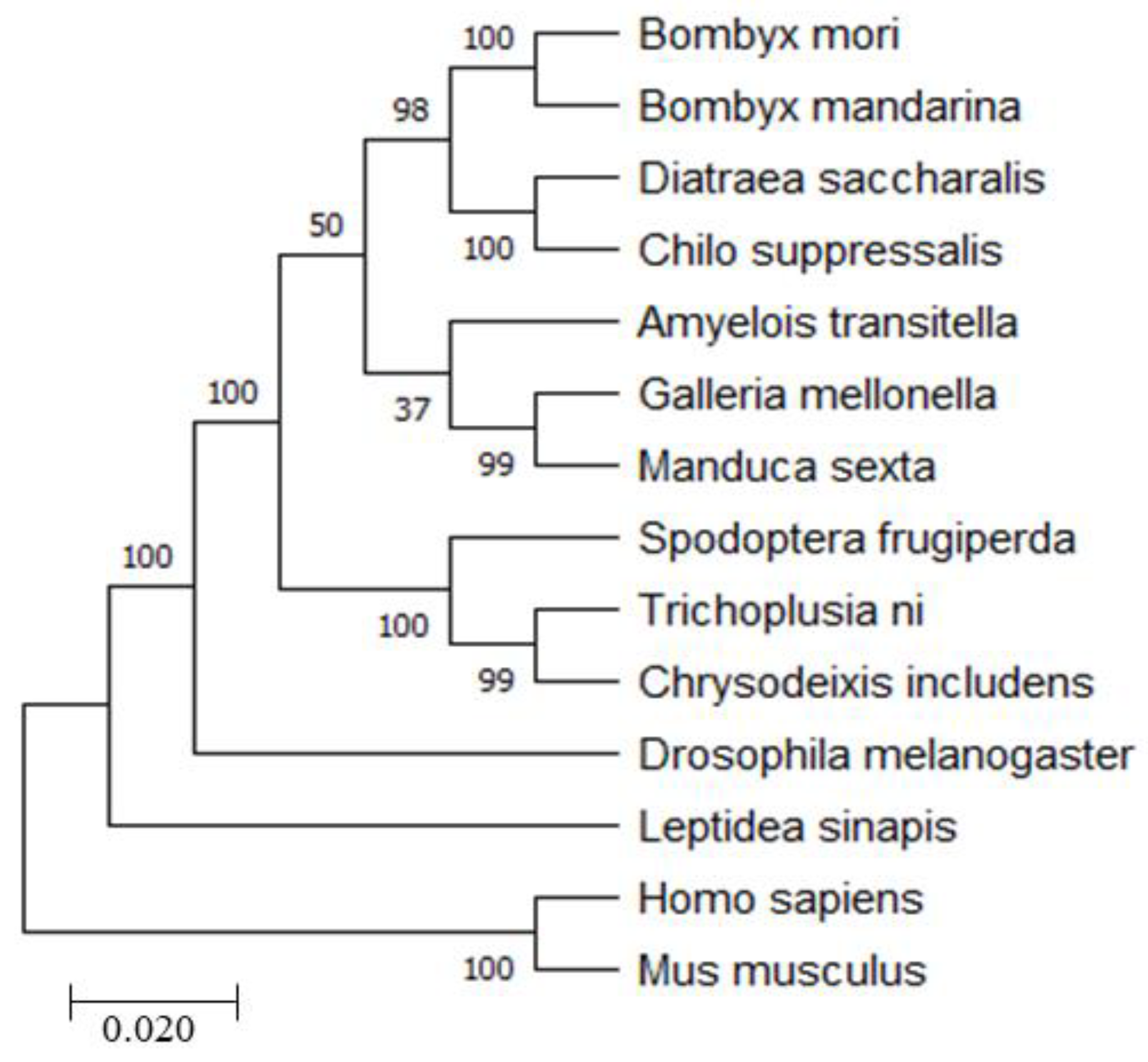
2.1. Bioinformatics Analysis of BmRRS1
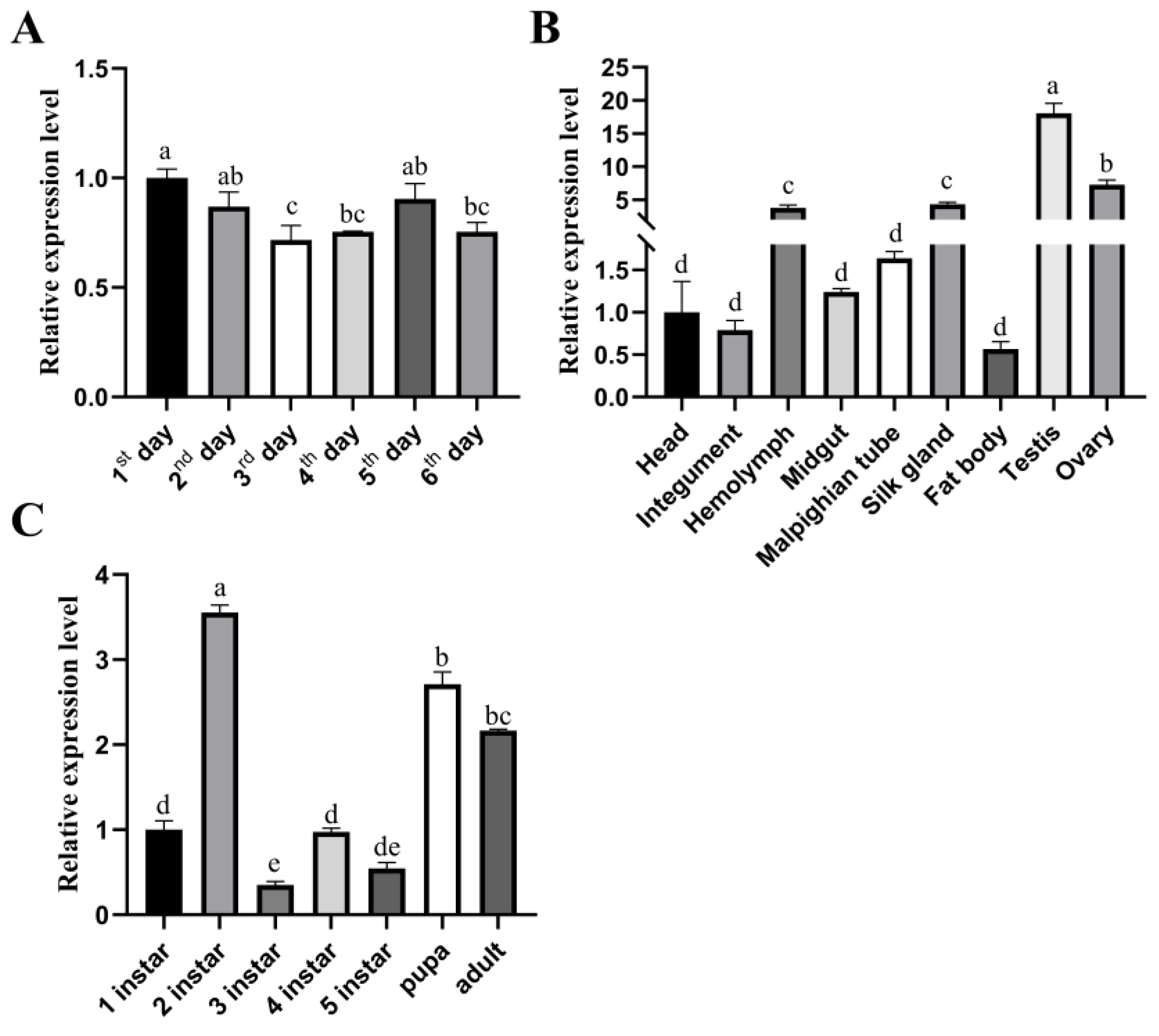
2.2. Spatiotemporal Expression Profile of BmRRS1
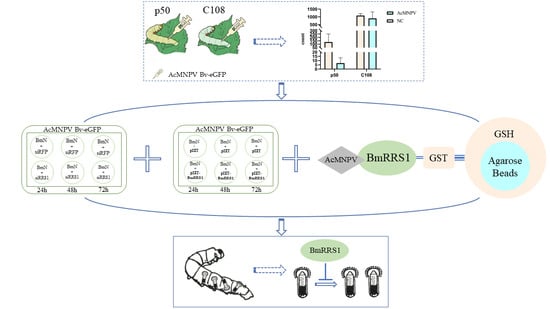
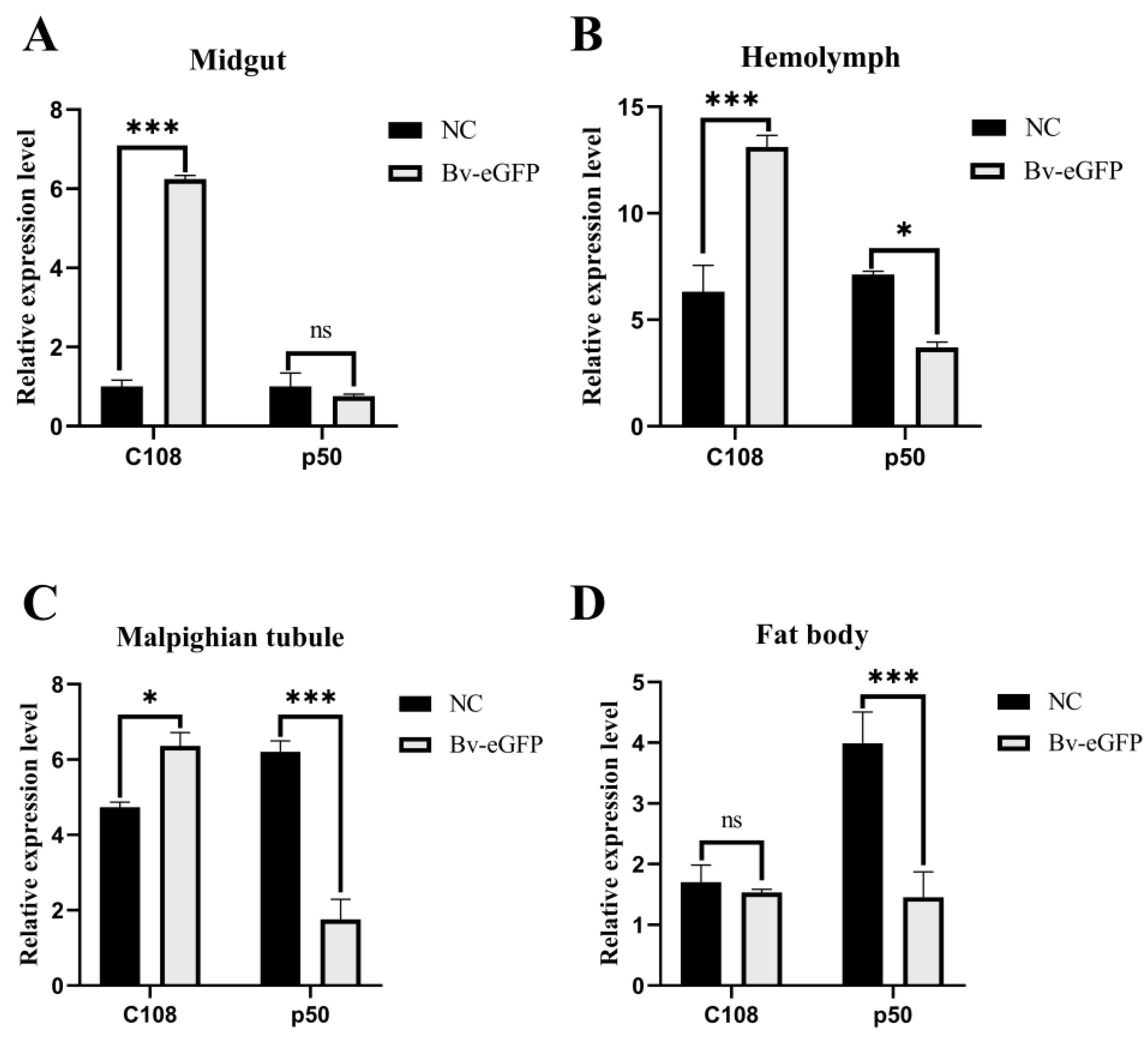
2.3. Expression of BmRRS1 in Immune System-Related Tissues of Different Resistant Silkworm Strains
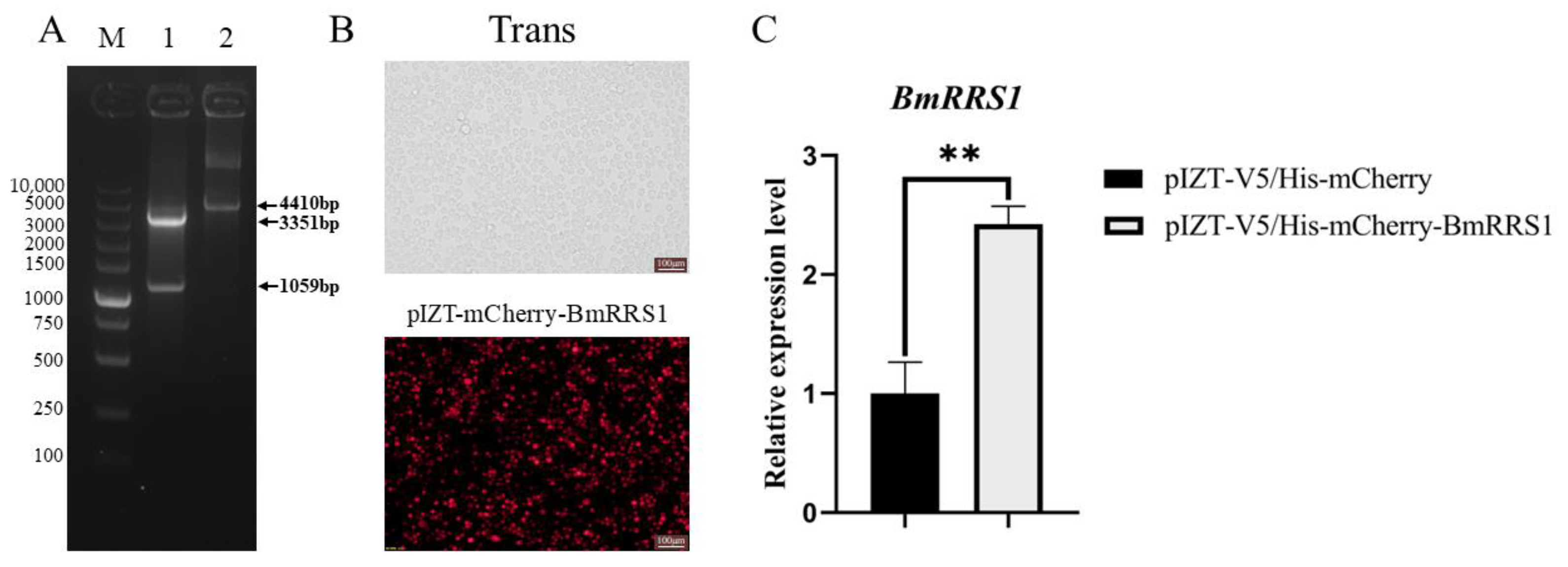
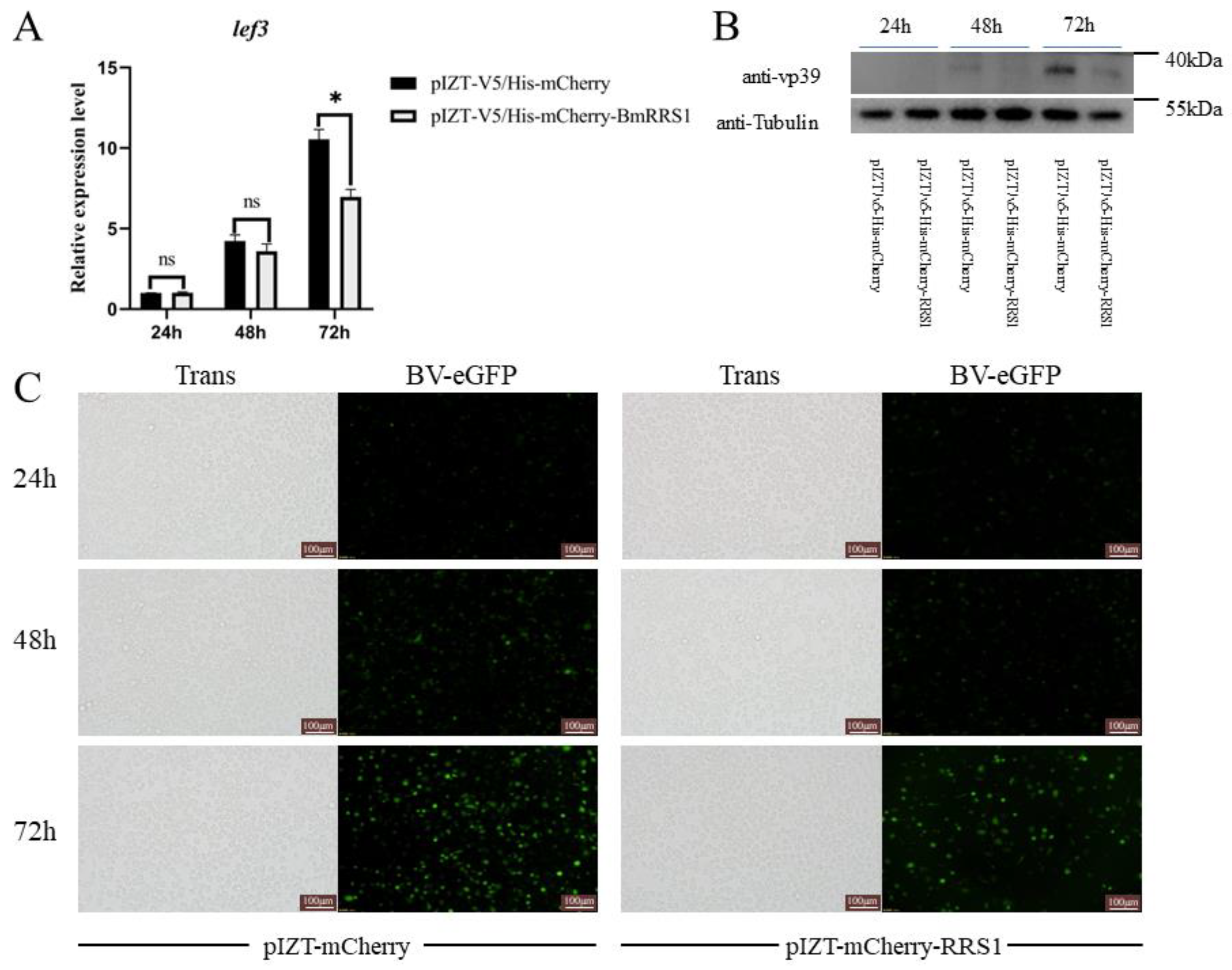
2.4. Overexpression of BmRRS1 Inhibited AcMNPV Proliferation in BmN Cells
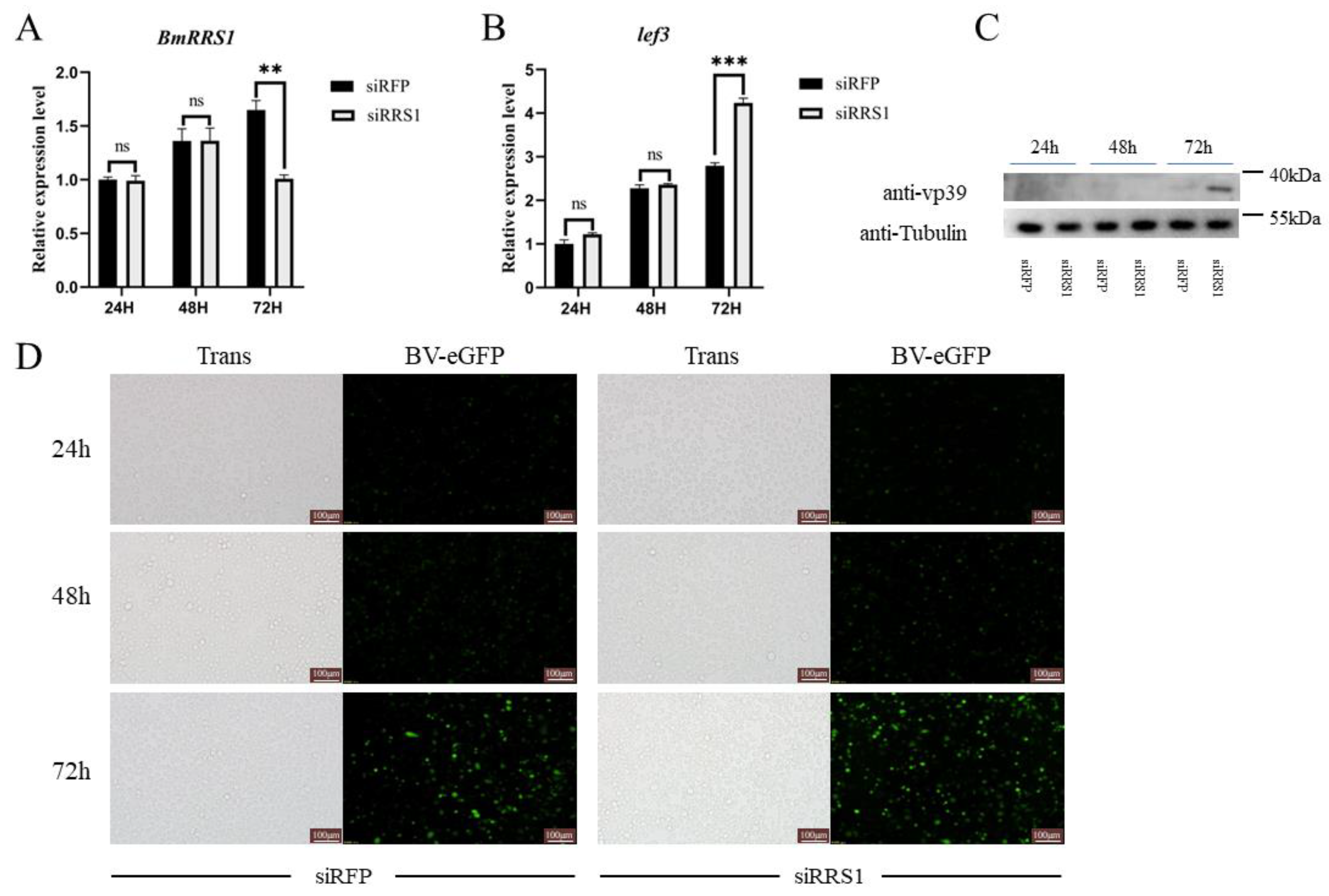
2.5. Knockdown of BmRRS1 Promoted AcMNPV Proliferation in BmN Cells
2.6. BmRRS1 Interacts with BV-eGFP to Affect Its Infection
2.7. Knockdown of SfRRS1 Decreases the Survival Rate of Spodoptera frugiperda to AcMNPV
3. Discussion
4. Materials and Methods
4.1. Silkworms and AcMNPV
4.2. Sample Preparation
4.3. RNA Extraction and cDNA Synthesis
4.4. Bioinformatic Analyses
4.5. Cell Culture and Transfection
4.6. Construction of pIZT-mCherry-BmRRS1 Overexpression Vector
4.7. Synthesis of siRNA
4.8. Construction of pGEX-4T-1-BmRRS1 Overexpression Vector
4.9. Real-Time Quantitative PCR (RT-qPCR)
4.10. Western Blot
4.11. GST-Tag Pull-Down
4.12. Bioassays for Spodoptera frugiperda to AcMNPV
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, L.; Chen, J.; Guo, Z.M.; Lv, W. Insecticidal activity and biological control effect of AcMNPV on Spodoptera frugiperda larvae. J. Huazhong Agric. Univ. 2021, 40, 46–53. [Google Scholar] [CrossRef]
- Guo, H.; Han, Z.; Fang, J.; Liu, B. Synergistie effect of tebufenozide combined with Autographa californica nuclear polyhedrosis virus against Spodoptera exigua. Acta Phytophylacica Sin. 2005, 32, 411–415. [Google Scholar] [CrossRef]
- Zhang, J.; Zafar, J.; Kong, J.; Wang, F.; Shao, X.; Zhang, R.; Pang, R.; Xu, H.; Xu, X.; Jin, F. MicroRNA-Mediated Host Immune Genes Manipulation Benefits AcMNPV Proliferation in Spodoptera frugiperda. J. Agric. Food Chem. 2023, 71, 17175–17187. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.R.; Yario, T.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′UTR as in the 3′UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Yang, K.; Lin, T.H.; Gong, Y.X.; Pan, L.J. Identification of the Apoptosis Inhibitor Gene p49 of Spodoptera litura Multicapsid Nucleopolyhedrovirus. Virus Genes 2005, 31, 145–151. [Google Scholar] [CrossRef]
- Thiem, S.M.; Chejanovsky, N. The role of baculovirus apoptotic suppressors in AcMNPV-mediated translation arrest in Ld652Y cells. Virology 2004, 319, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.J.; Legesse-Miller, A.; Coller, H.A. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc. Natl. Acad. Sci. USA 2008, 105, 14879–14884. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.Y.; Zhao, P.; Yuan, Y.; LV, Y.X.; Cui, Z.X.; Huang, Y.Z.; Zhu, X.W. Research progress of silkworm molecular marker-assisted breeding related genes. North Seric. 2021, 42, 8. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, Q.; Zhou, X.-L.; Zhu, Y.; Dong, Z.-Q.; Chen, P.; Pan, M.-H.; Lu, C. Bombyx mori Nuclear Polyhedrosis Virus (BmNPV) Induces Host Cell Autophagy to Benefit Infection. Viruses 2017, 10, 14. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhu, L.B.; Guo, Z.X.; Zhu, H.D.; Huang, Z.H.; Cao, H.H.; Yu, H.Z.; Liu, S.H.; Xu, J.P. Bombyx mori ferritin heavy-chain homolog facilitates BmNPV proliferation by inhibiting reactive oxygen species–mediated apoptosis. Int. J. Biol. Macromol. 2022, 217, 842–852. [Google Scholar] [CrossRef]
- Wang, X.; Lv, J.L.; Cheng, S.; Su, Z.H.; Qin, S.; Sun, X.; Tang, X.D.; Liu, Q.N.; Li, M.W.; Wang, X.Y. Bombyx mori transcription factor, E74A, beneficially affects BmNPV infection through direct interaction. Pest Manag. Sci. 2022, 78, 5302–5312. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Q.; He, F.C.; Zhou, G.Q. Function of Regulator of Ribosome Synthesis 1 and its Relationship with Diseases. Lett. Biotechol. 2014, 25, 871–874. [Google Scholar] [CrossRef]
- Tsuno, A.; Miyoshi, K.; Tsujii, R.; Miyakawa, T.; Mizuta, K. RRS1, a Conserved Essential Gene, Encodes a Novel Regulatory Protein Required for Ribosome Biogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 2000, 20, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhu, Z.; Wu, J.; She, H.; Han, R.; Xu, H.; Qin, Z.H. DRAM1 regulates autophagy and cell proliferation via inhibition of the phosphoinositide 3-kinase-Akt-mTOR-ribosomal protein S6 pathway. Cell Commun. Signal. 2019, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Song, H.M.; Li, Z.W.; Jiang, P.; Zhou, S.H.; Li, H.R.; Liu, X.W. Effect of interaction between N Protein and Ribosomal Protein S20 of porcien Reproductive and Respiratory Syndrome Virus on Virue Replication. Acta Vet. Zootech. Sin. 2023, 54, 293–303. [Google Scholar] [CrossRef]
- Lv, H.; Dong, W.; Qian, G.; Wang, J.; Li, X.; Cao, Z.; Lv, Q.; Wang, C.; Guo, K.; Zhang, Y. uS10, a novel Npro-interacting protein, inhibits classical swine fever virus replication. J. Gen. Virol. 2017, 98, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Mohr, I. Ribosome biogenesis restricts innate immune responses to virus infection and DNA. Elife 2019, 8, e49551. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.B.; Yang, A.Q.; Li, P.Y.; Xia, X.; Han, Y.Q.; Zhou, G.M. Genomic gain of RRS1 promotes hepatocellular carcinoma through reducing the RPL11-MDM2-p53 signaling. Sci. Adv. 2021, 7, eabf4304. [Google Scholar] [CrossRef]
- Li, T.; Qin, S.; Sun, X.; Zhang, K.-X.; Ding, X.-Y.; Wang, X.-Y.; Li, M.-W. Transcriptome analysis reveals distinct innate immunity and ribosomal response at early stage of AcMNPV infection in haemocyte of silkworm resistant and susceptible strains. J. Asia-Pac. Entomol. 2022, 25, 101938. [Google Scholar] [CrossRef]
- Ding, X.Y.; Wang, X.Y.; Kong, Y.H.; Zhao, C.X.; Qin, S.; Sun, X.; Li, M.W. Comparative Transcriptome Analysis of Bombyx mori (Lepidoptera) Larval Hemolymph in Response to Autographa californica Nucleopolyhedrovirus in Differentially Resistant Strains. Processes 2021, 9, 1401. [Google Scholar] [CrossRef]
- Piubelli, G.C.; Moscardi, F.; Hoffmann-Campo, C.B. Interactions among insect-resistant soybean genotypes extracts with populations of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) susceptible and resistant to its nucleopolyhedrovirus. An. Acad. Bras. Ciências 2009, 81, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Wennmann, J.T.; Radtke, P.; Eberle, K.E.; Gueli Alletti, G.; Jehle, J.A. Deciphering Single Nucleotide Polymorphisms and Evolutionary Trends in Isolates of the Cydia pomonella granulovirus. Viruses 2017, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, X.; Hu, Z.; Li, M.; O’Reilly, D.R.; Zuidema, D.; Vlak, J.M. Genetic engineering of Helicoverpa armigera single-nucleocapsid nucleopolyhedrovirus as an improved pesticide. J. Invertebr. Pathol. 2000, 76, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.J.; Cao, J.B.; Xu, C.G.; Fu, Y.J.; Zhang, Z.Y.; Liang, A.H. Study on Virulence to Larvae and Mammiferous Security of Recombinant Baculovirus (AcMNPV-BmKIT-Chi). Acta Agric. Boreali.-Sin. 2007, 22, 161–164. [Google Scholar] [CrossRef]
- Fatica, A.; Tollervey, D. Making ribosomes. Curr. Opin. Cell Biol. 2002, 14, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Fromont Racine, M.; Senger, B.; Saveanu, C.; Fasiolo, F. Ribosome assembly in eukaryotes. Gene 2003, 313, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Granneman, S.; Baserga, S.J. Ribosome biogenesis: Of knobs and RNA processing. Exp. Cell Res. 2004, 296, 43–50. [Google Scholar] [CrossRef]
- Zhou, X.L.; Wei, Y.; Chen, X.Y.; Chen, P.; Tang, X.F.; Zhang, Q.; Dong, Z.Q.; Pan, M.H.; Lu, C. BmGeminin2 interacts with BmRRS1 and regulates Bombyx mori cell proliferation. Cell Cycle 2019, 18, 1498–1512. [Google Scholar] [CrossRef]
- Qian, W.; Yang, Y.; Li, Z.; Wu, Y.; He, X.; Li, H.; Cheng, D. Enhanced Myc Expression in Silkworm Silk Gland Promotes DNA Replication and Silk Production. Insects 2021, 12, 361. [Google Scholar] [CrossRef]
- Chung, H.C. Ultrastructure of the posterior silk gland cells in the silkworm, Bombyx mori. Acta Entomol. Sin. 1980, 23, 242–248. [Google Scholar] [CrossRef]
- Feng, L.; Shen, W. (Eds.) Silk gland. In Anatomical Physiology of Silkworm, 1st ed.; Higher Education Press: Beijing, China, 2015; Volume 1, p. 153. [Google Scholar]
- Guo, H.Y.; Zhu, J.; Liao, Q.H.; Tang, A.X.; Qi, R.B.; Tang, J.Y.; Yang, H.Z.; Liu, Q.G. Advances in Ribosomal Protein Regulation of Viral Life Cycle. Chin. J. Anim. Infect. Dis. 2022, 30, 173–180. [Google Scholar] [CrossRef]
- Li, Y.; Dong, W.; Shi, Y.; Deng, F.; Chen, X.; Wan, C.; Zhou, M.; Zhao, L.; Fu, Z.F.; Peng, G. Rabies virus phosphoprotein interacts with ribosomal protein L9 and affects rabies virus replication. Virology 2016, 488, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Blissard, G.W.; Theilmann, D.A. Baculovirus Entry and Egress from Insect Cells. Annu. Rev. Virol. 2018, 5, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.Q.; Wang, J.Y.; Guo, X.Y.; Wang, S.P.; Lu, C.D. Transient in vivo gene delivery to the silkworm Bombyx mori by EGT-null recombinant AcNPV using EGFP as a reporter. Arch. Virol. 2005, 150, 93–105. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Qin, S.; Sun, X.; Wang, S.; Li, M. The hemolymph melanization response is related to defence against the AcMNPV infection in Bombyx mori. Arch. Insect Biochem. Physiol. 2021, 108, e21764. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, Y.P.; Huang, S.H.; Liu, W.L.; Su, X.L.; Pan, Z.P. A study on artificial rearing technique of Spodoptera frugiperda in the laboratory. J. Environ. Entomol. 2019, 41, 6. [Google Scholar] [CrossRef]



| Primer Names | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| BmRRS1-1 | GGGGTACCATGGATATTGTAAATGAGATATTAGA | GCTCTAGATCTCCTTTTCCGTCCAGTA |
| BmRRS1-2 | CGGGATCCATGGATATTGTAAATGAGATATTAGAAAGGG | CCCTCGAGCTATCTCCTTTTCCGTCCAG |
| BmRRS1-3 | AACGCTGAAGACAAACAAAGATTCA | CGAACTACTATGGCCTCTTCGAT |
| BmGAPDH | CCGCGTCCCTGTTGCTAAT | CTGCCTCCTTGACCTTTTGC |
| Lef3 | CAAACGCGTTGCTTCGTACA | TGCTCGAGTCGGAAGAGGTA |
| SfRRS1-1 | AGCTTGATATTGGCACATTACTAGCTT | CTCTGTAGGAAGCTCCCATATCTTGTTT |
| SfGAPDH | AGAAGACTGTTGACGGACC | AGGAATGACTTTGCCGAC |
| Primer Names | Sequences (5′-3′) |
|---|---|
| BmRRS1-1 Olig-1 | GATCACTAATACGACTCACTATAGGGCCCGTGACAATGCTCAGCTACTACTTT |
| BmRRS1-1 Olig-2 | AAAGTAGTAGCTGAGCATTGTCACGGGCCCTATAGTGAGTCGTATTAGTGATC |
| BmRRS1-1 Olig-3 | AACCCGTGACAATGCTCAGCTACTACTCCCTATAGTGAGTCGTATTAGTGATC |
| BmRRS1-1 Olig-4 | GATCACTAATACGACTCACTATAGGGAGTAGTAGCTGAGCATTGTCACGGGTT |
| BmRRS1-2 Olig-1 | GATCACTAATACGACTCACTATAGGGTAAAGTATGGGAGCTTCCCACTGAATT |
| BmRRS1-2 Olig-2 | AATTCAGTGGGAAGCTCCCATACTTTACCCTATAGTGAGTCGTATTAGTGATC |
| BmRRS1-2 Olig-3 | AATAAAGTATGGGAGCTTCCCACTGAACCCTATAGTGAGTCGTATTAGTGATC |
| BmRRS1-2 Olig-4 | GATCACTAATACGACTCACTATAGGGTTCAGTGGGAAGCTCCCATACTTTATT |
| RFP-Olig-1 | GATCACTAATACGACTCACTATAGGGGCACCCAGACCATGAGAATTT |
| RFP-Olig-2 | AAATTCTCATGGTCTGGGTGCCCCTATAGTGAGTCGTATTAGTGATC |
| RFP-Olig-3 | AAGCACCCAGACCATGAGAATCCCTATAGTGAGTCGTATTAGTGATC |
| RFP-Olig-4 | GA TCACTAATACGACTCACTATAGGGATTCTCATGGTCTGGGTGCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Ding, X.; Wang, Z.; Zhou, S.; Qin, S.; Sun, X.; Wang, X.; Li, M. BmRRS1 Protein Inhibits the Proliferation of Baculovirus Autographa californica Nucleopolyhedrovirus in Silkworm, Bombyx mori. Int. J. Mol. Sci. 2024, 25, 306. https://doi.org/10.3390/ijms25010306
Zhou L, Ding X, Wang Z, Zhou S, Qin S, Sun X, Wang X, Li M. BmRRS1 Protein Inhibits the Proliferation of Baculovirus Autographa californica Nucleopolyhedrovirus in Silkworm, Bombyx mori. International Journal of Molecular Sciences. 2024; 25(1):306. https://doi.org/10.3390/ijms25010306
Chicago/Turabian StyleZhou, Liqin, Xinyi Ding, Zhisheng Wang, Si Zhou, Sheng Qin, Xia Sun, Xueyang Wang, and Muwang Li. 2024. "BmRRS1 Protein Inhibits the Proliferation of Baculovirus Autographa californica Nucleopolyhedrovirus in Silkworm, Bombyx mori" International Journal of Molecular Sciences 25, no. 1: 306. https://doi.org/10.3390/ijms25010306
APA StyleZhou, L., Ding, X., Wang, Z., Zhou, S., Qin, S., Sun, X., Wang, X., & Li, M. (2024). BmRRS1 Protein Inhibits the Proliferation of Baculovirus Autographa californica Nucleopolyhedrovirus in Silkworm, Bombyx mori. International Journal of Molecular Sciences, 25(1), 306. https://doi.org/10.3390/ijms25010306