Serpin-4 Negatively Regulates Prophenoloxidase Activation and Antimicrobial Peptide Synthesis in the Silkworm, Bombyx mori
Abstract
:1. Introduction
2. Results
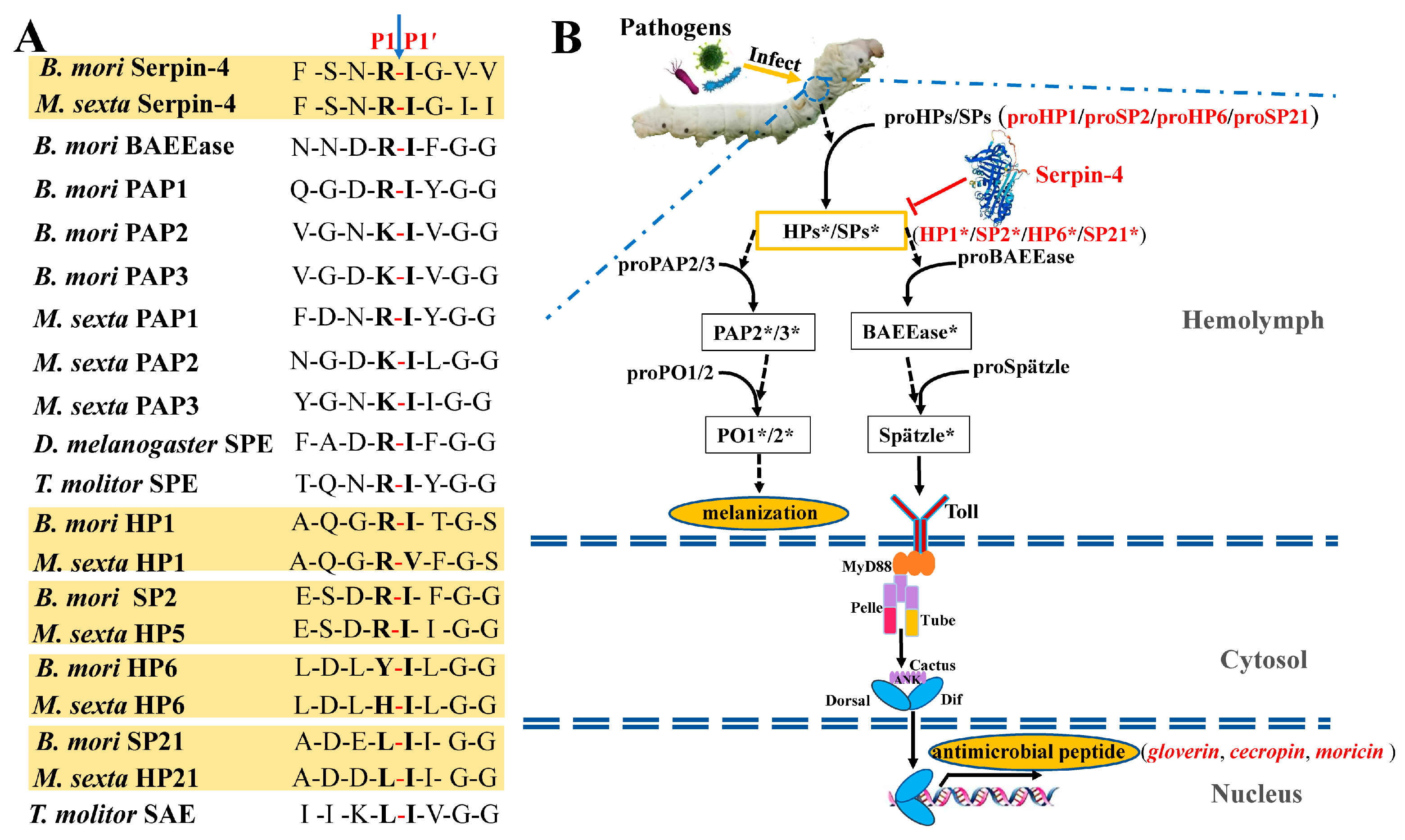
2.1. Sequence, Domain Identification, Three-Dimensional Structure, and Phylogenetic Assays of BmSerpin-4
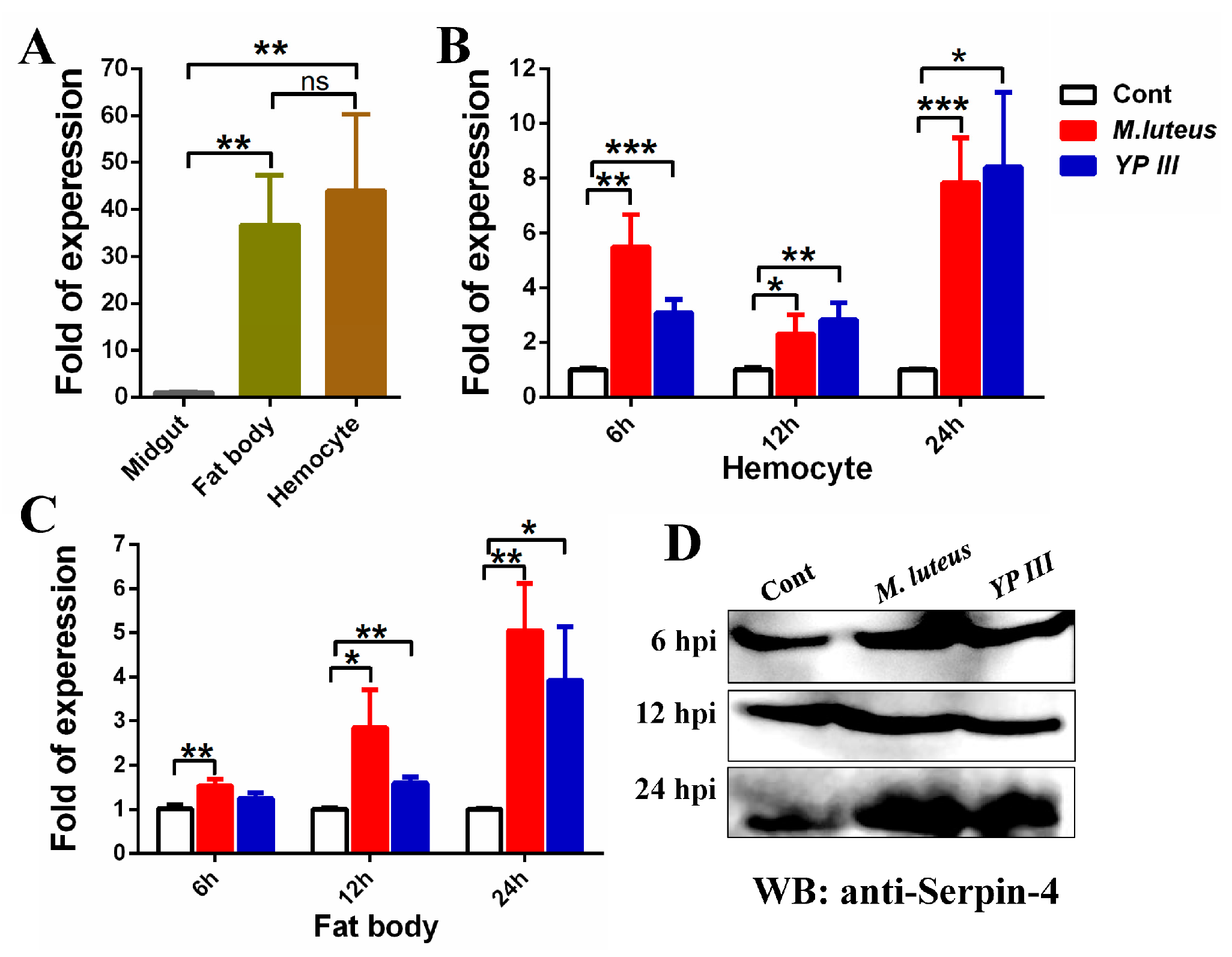
2.2. Bacterial Infection Induces BmSerpin-4 Expression in the Fat Body and Hemocytes of Larval Silkworm
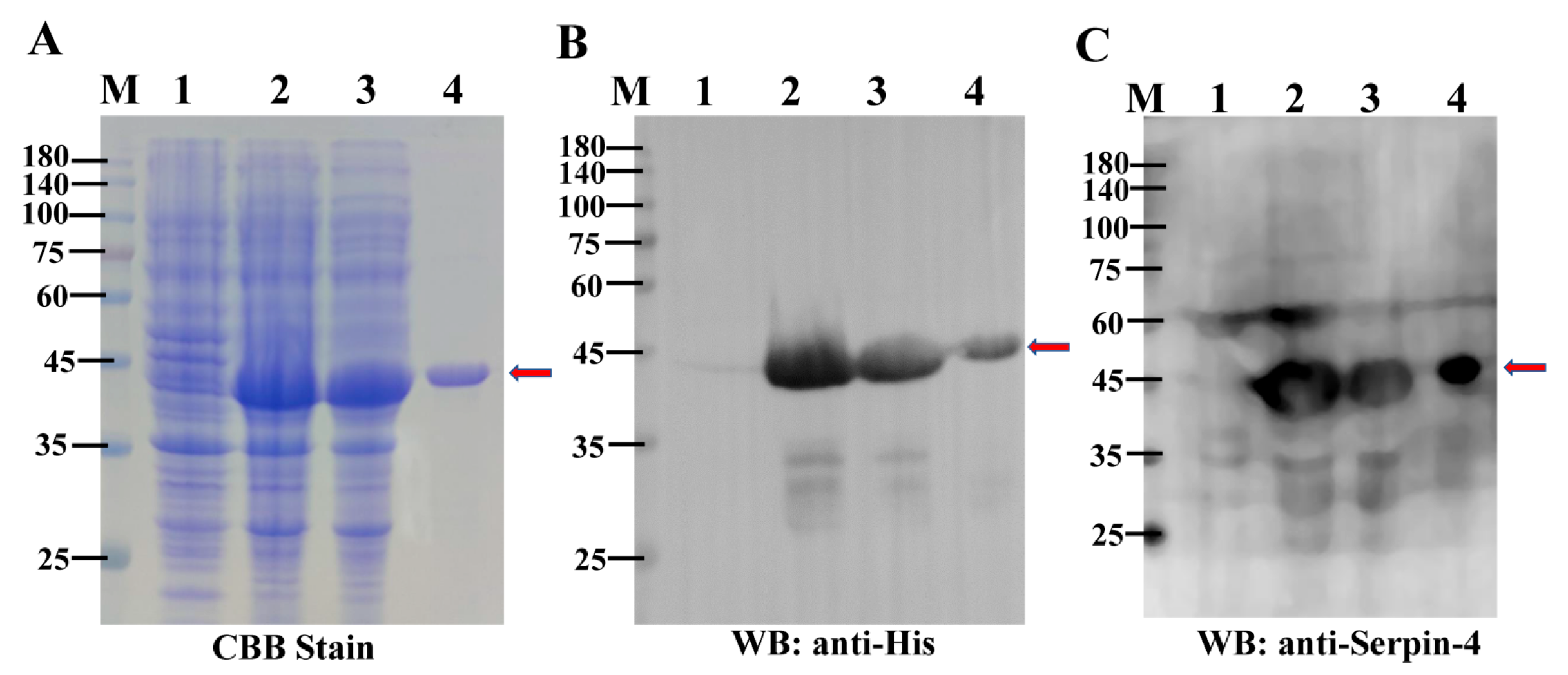
2.3. Purification of the Recombinant BmSerpin-4
2.4. BmSerpin-4 Inhibits Systemic Melanization in the Larval Silkworm
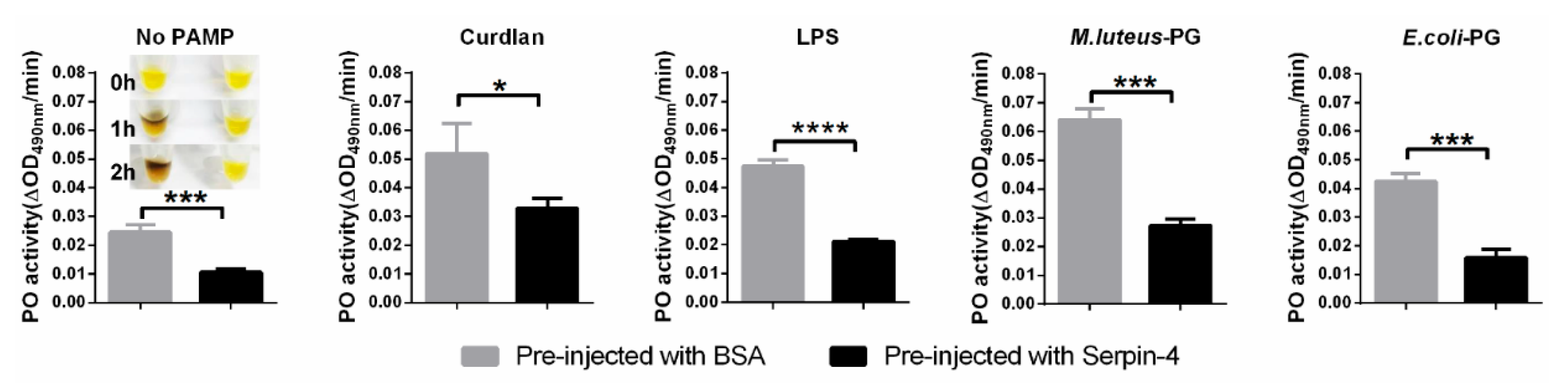
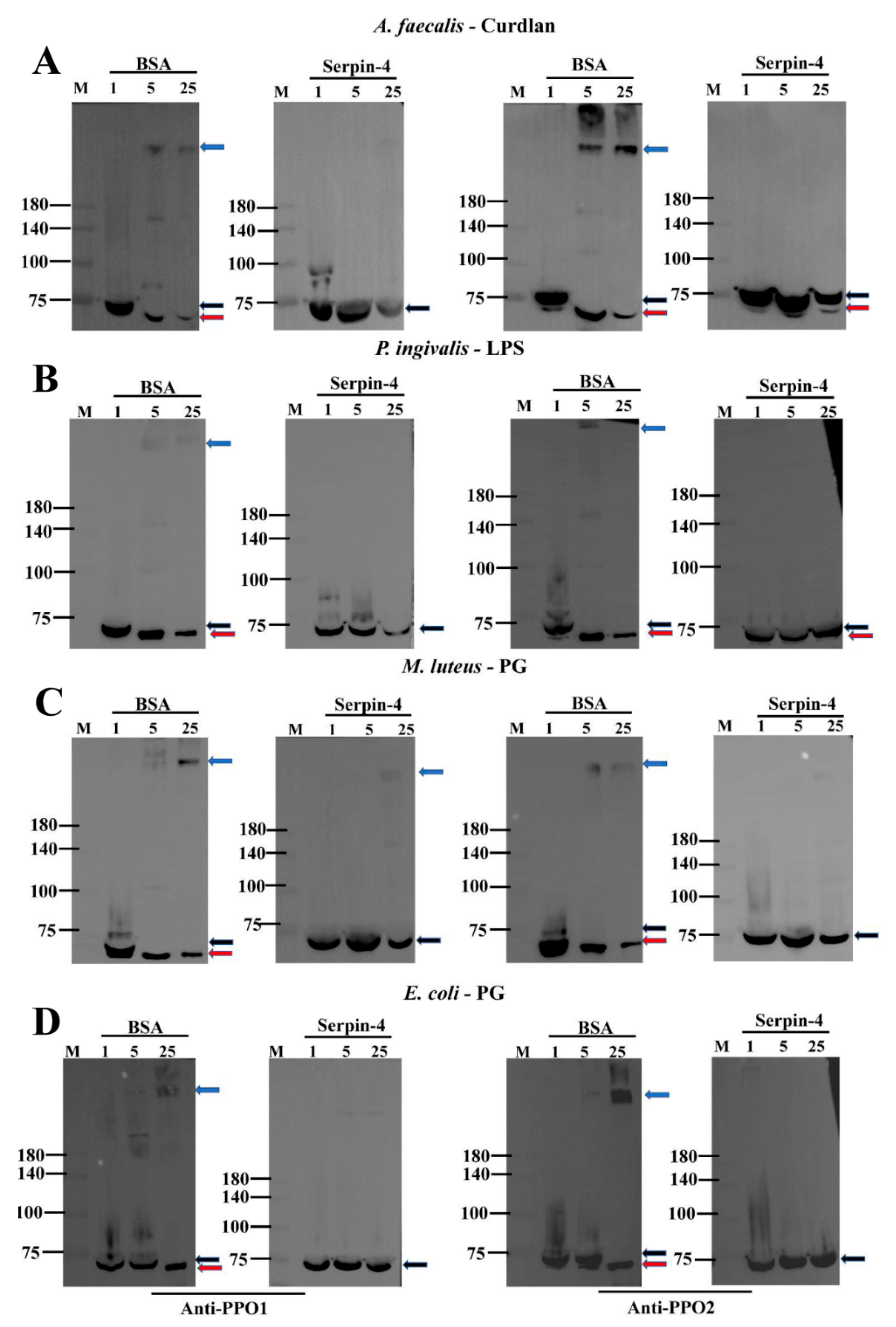
2.5. BmSerpin-4 Inhibits the Activation of PPOs
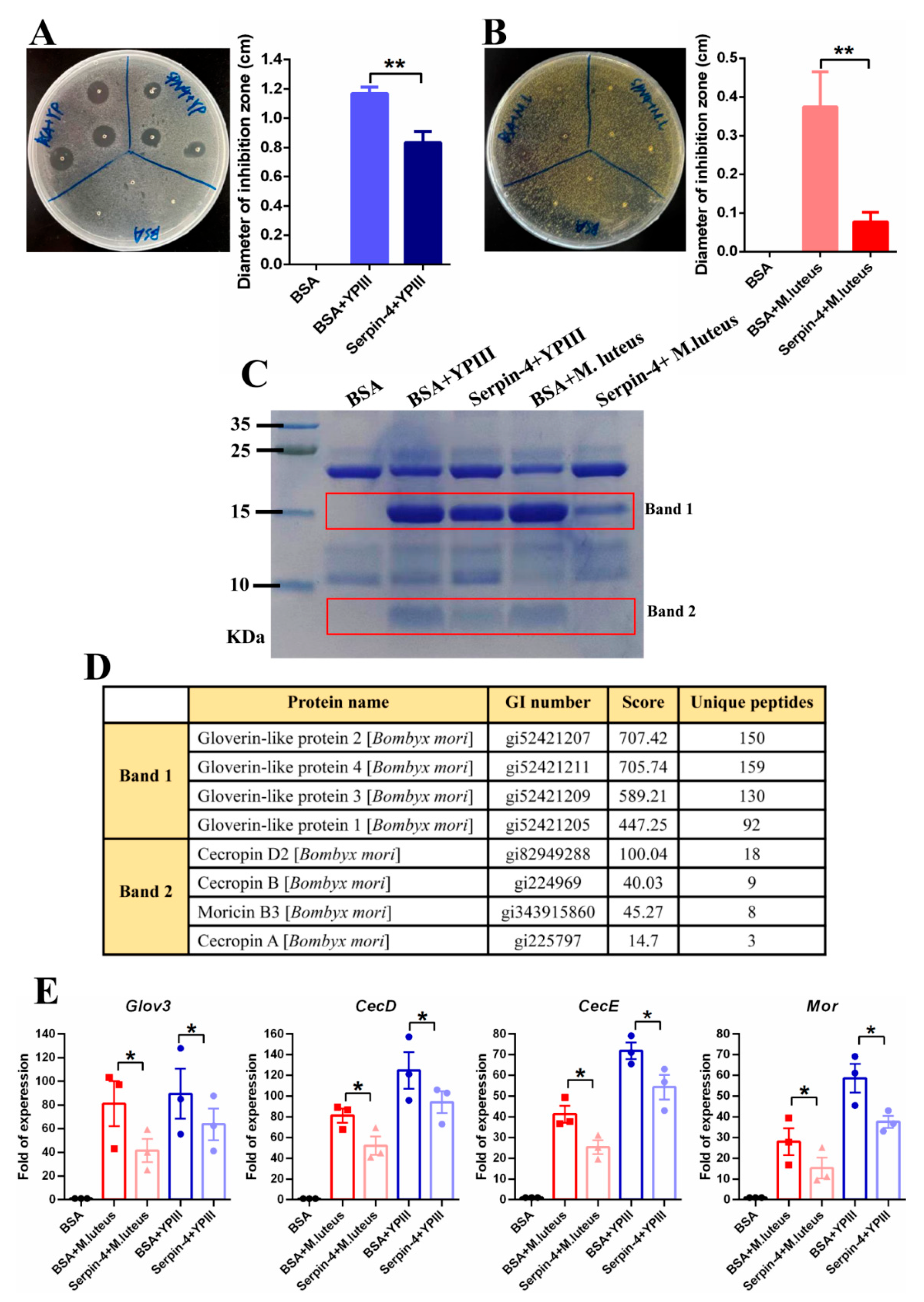
2.6. BmSerpin-4 Inhibits AMP Generation Induced by Bacteria
3. Discussion
4. Materials and Methods
4.1. Rearing of Silkworms and Bacterial Infection
4.2. Protein Sequence, Domain Identification, Three-Dimensional Structure, and Phylogenetic Assays
4.3. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (q-PCR)
4.4. Expression and Purification of Recombinant BmSerpin-4
4.5. The Preparation of BmSerpin-4 Polyclonal Antiserum
4.6. Phenoloxidase Activity and Prophenoloxidase Activation Assays
4.7. Hemolymph Antibacterial Activity Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed]
- Govind, S. Innate immunity in Drosophila: Pathogens and pathways. Insect Sci. 2008, 15, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster—From microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell. Mol. Life. Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Luo, F.; Xu, Y.; Zhang, Y.; Jin, L.H. Drosophila innate immunity involves multiple signaling pathways and coordinated communication between different tissues. Front. Immunol. 2022, 13, 905370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Y.; Kanost, M.R. Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: A bacteria-inducible protein similar to Drosophila easter. Proc. Natl. Acad. Sci. USA 1998, 95, 12220–12225. [Google Scholar] [CrossRef] [PubMed]
- Binggeli, O.; Neyen, C.; Poidevin, M.; Lemaitre, B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog. 2014, 10, e1004067. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, Q.; Zhang, J.; Yang, B.; Wu, K.; Xie, W.; Luan, Y.X.; Ling, E. Insect prophenoloxidase: The view beyond immunity. Front. Physiol. 2014, 5, 252. [Google Scholar] [CrossRef]
- Cerenius, L.; Lee, B.L.; Soderhall, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef]
- Kanost, M.R.; Gorman, M.J. Phenoloxidases in insect immunity. In Insect Immunology; Beckage, N., Ed.; Academic Press: San Diego, CA, USA; Elsevier: Amsterdam, The Netherlands, 2008; pp. 69–96. [Google Scholar]
- Kanost, M.R. Serine proteinase inhibitors in arthropod immunity. Dev. Comp. Immunol. 1999, 23, 291–301. [Google Scholar] [CrossRef]
- An, C.; Kanost, M.R. Manduca sexta serpin-5 regulates prophenoloxidase activation and the Toll signaling pathway by inhibiting hemolymph proteinase HP6. Insect. Biochem. Mol. Biol. 2010, 40, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kanost, M.R.; Jiang, H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect. Sci. 2015, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Veillard, F.; Troxler, L.; Reichhart, J.M. Drosophila melanogaster clip-domain serine proteases: Structure, function and regulation. Biochimie 2016, 122, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Marieshwari, B.N.; Bhuvaragavan, S.; Sruthi, K.; Mullainadhan, P.; Janarthanan, S. Insect phenoloxidase and its diverse roles: Melanogenesis and beyond. J. Comp. Physiol. B 2023, 193, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gubb, D.; Sanz-Parra, A.; Barcena, L.; Troxler, L.; Fullaondo, A. Protease inhibitors and proteolytic signalling cascades in insects. Biochimie 2010, 92, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.; Lin, Z.; Zou, Z.; Lu, Z. Serpin-5 regulates prophenoloxidase activation and antimicrobial peptide pathways in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2016, 73, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Gettins, P.G. Serpin structure, mechanism, and function. Chem. Rev. 2002, 102, 4751–4804. [Google Scholar] [CrossRef] [PubMed]
- Reichhart, J.M. Tip of another iceberg: Drosophila serpins. Trends Cell. Biol. 2005, 15, 659–665. [Google Scholar] [CrossRef]
- Li, M.; Christen, J.M.; Dittmer, N.T.; Cao, X.; Zhang, X.; Jiang, H.; Kanost, M.R. The Manduca sexta serpinome: Analysis of serpin genes and proteins in the tobacco hornworm. Insect. Biochem. Mol. Biol. 2018, 102, 21–30. [Google Scholar] [CrossRef]
- Zou, Z.; Picheng, Z.; Weng, H.; Mita, K.; Jiang, H. A comparative analysis of serpin genes in the silkworm genome. Genomics 2009, 93, 367–375. [Google Scholar] [CrossRef]
- Suwanchaichinda, C.; Kanost, M.R. The serpin gene family in Anopheles gambiae. Gene 2009, 442, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gulley, M.M.; Zhang, X.; Michel, K. The roles of serpins in mosquito immunology and physiology. J. Insect. Physiol. 2013, 59, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.R.; Shimada, T.; Abe, H. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 2005, 50, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, L.; Yang, L.; Qian, C.; Wei, G.; Dai, L.; Li, J.; Zhu, B.; Liu, C. Serpin-15 from Bombyx mori inhibits prophenoloxidase activation and expression of antimicrobial peptides. Dev. Comp. Immunol. 2015, 51, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.C.; Dong, Z.; Xiao, L.; Li, T.; Zhang, Y.; He, H.; Xia, Q.; Zhao, P. Silk gland-specific proteinase inhibitor serpin16 from the Bombyx mori shows cysteine proteinase inhibitory activity. Biochem. Biophys. Res. Commun. 2015, 457, 31–36. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, L.; Dai, J.; Yuan, G.; Wang, L.; Qian, C.; Zhu, B.; Liu, C.; Wei, G. Characterization and functional analysis of serpin-28 gene from silkworm, Bombyx mori. J. Invertebr. Pathol. 2018, 159, 18–27. [Google Scholar] [CrossRef]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in lepidopteran insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar]
- Irving, J.A.; Pike, R.N.; Lesk, A.M.; Whisstock, J.C. Phylogeny of the serpin superfamily: Implications of patterns of amino acid conservation for structure and function. Genome Res. 2000, 10, 1845–1864. [Google Scholar] [CrossRef]
- Roh, K.B.; Kim, C.H.; Lee, H.; Kwon, H.M.; Park, J.W. Proteolytic Cascade for the Activation of the Insect Toll Pathway Induced by the Fungal Cell Wall Component. J. Biol. Chem. 2009, 284, 19474–19481. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Cao, X.; Zou, Z.; Lu, Z.; Kanost, M.R.; Jiang, H. Hemolymph protease-5 links the melanization and Toll immune pathways in the tobacco hornworm, Manduca sexta. Proc. Natl. Acad. Sci. USA 2020, 117, 23581–23587. [Google Scholar] [CrossRef]
- Tong, Y.; Jiang, H.; Kanost, M.R. Identification of plasma proteases inhibited by Manduca sexta serpin-4 and serpin-5 and their association with components of the prophenoloxidase activation pathway. J. Biol. Chem. 2005, 280, 14932–14942. [Google Scholar] [CrossRef] [PubMed]
- Jiang, I.H.; Chosa, N.; Kim, S.H.; Nam, H.J.; Lemaitre, B. A Spätzle-Processing Enzyme Required for Toll Signaling Activation in Drosophila Innate Immunity. Dev. Cell. 2006, 10, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, S.J.; Kan, H.; Kwon, H.M.; Roh, K.B. A Three-step Proteolytic Cascade Mediates the Activation of the Peptidoglycan-induced Toll Pathway in an Insect. J. Biol. Chem. 2008, 283, 7599–7607. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Ishibashi, J.; Ragan, E.J.; Jiang, H.; Kanost, M.R. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J. Biol. Chem. 2009, 284, 19716–19726. [Google Scholar] [CrossRef]
- An, C.; Ragan, E.J.; Kanost, M.R. Serpin-1 splicing isoform J inhibits the proSpätzle-activating proteinase HP8 to regulate expression of antimicrobial hemolymph proteins in Manduca sexta. Dev. Comp. Immunol. 2011, 35, 135–141. [Google Scholar] [CrossRef]
- Gorman, M.J.; Wang, Y.; Jiang, H.B.; Kanost, M.R. Manduca sexta hemolymph proteinase 21 activates prophenoloxidase-activating proteinase 3 in an insect innate immune response proteinase cascade. J. Biol. Chem. 2007, 282, 11742–11749. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H. Reconstitution of a branch of the Manduca sexta prophenoloxidase activation cascade in vitro: Snake-like hemolymph proteinase 21 (HP21) cleaved by HP14 activates prophenol oxidase- activating proteinase-2 precursor. Insect Biochem. Mol. Biol. 2007, 37, 1015–1025. [Google Scholar] [CrossRef]
- Tokura, A.; Fu, G.S.; Sakamoto, M.; Endo, H.; Tanaka, S. Factors functioning in nodule melanization of insects and their mechanisms of accumulation in nodules. J. Insect Physiol. 2014, 60, 40–49. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhao, P.; Rayaprolu, S.; Wang, X.; Cao, X.; Jiang, H. Serpin-9 and -13 regulate hemolymph proteases during immune responses of Manduca sexta. Insect Biochem. Mol. Biol. 2017, 90, 71–81. [Google Scholar] [CrossRef]
- Liu, H.; Heng, J.; Wang, L.; Tong, X.; Guo, P. Identification, characterization, and expression analysis of clip-domain serine protease genes in the silkworm, Bombyx mori. Dev. Comp. Immunol. 2020, 105, 103584. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Chen, X.; Cao, P.; Beerntsen, B.T.; Ling, E. BmToll9, an Arthropod conservative Toll, is likely involved in the local gut immune response in the silkworm, Bombyx mori. Dev. Comp. Immunol. 2010, 34, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Y.; Yu, X.Q.; Kanost, M.R. Prophenoloxidase-activating proteinase-2 from hemolymph of Manduca sexta. A bacteria-inducible serine proteinase containing two clip domains. J. Biol. Chem. 2003, 278, 3552–3561. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Hultmark, D.; Boman, H.G. Insect immunity: Galleria mellonella and other lepidoptera have cecropia-P9-like factors active against gram negative bacteria. Insect Biochem. 1981, 11, 537–548. [Google Scholar] [CrossRef]
- Schagger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]


| Primer | Sequence (5′–3′) |
|---|---|
| Quantitative real-time PCR | |
| Q-Serpin-4 F | GCCATGGGTATCGAGGATTT |
| Q-Serpin-4 R | TTTCTGCTTTGTGTACCACTTTC |
| Q-Glov3 F | GACACGAGAATGGGAGGAG |
| Q-Glov3 R | AAGACCCTGGTGCCGTAA |
| Q-CecD F | CTCCCGGCAACTTCTTCA |
| Q-CecD R | CGAACCCTCTGACCCATT |
| Q-CecE F | ACTGTTCGACATCGCCTCT |
| Q-CecE R | CGAATGTTCTGACCCACC |
| Q-Mor F | CTGAAGAAGGGTGGGAAAGTTA |
| Q-Mor R | ATGAAGTCTATAGCCACGTGC |
| Q-IF4A F | TCTGGCATCATACCTTCTACAA |
| Q-IF4A R | TCTGTGTCATCTTTTCCCTGTT |
| Gene cloning | |
| Serpin-4 F | TGCGGATCCCAAAATATTCCTAAGGCGACT |
| Serpin-4 R | TGCCTCGAGTTAGTAAAGTGATGGCTCCGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qie, X.; Yan, X.; Wang, W.; Liu, Y.; Zhang, L.; Hao, C.; Lu, Z.; Ma, L. Serpin-4 Negatively Regulates Prophenoloxidase Activation and Antimicrobial Peptide Synthesis in the Silkworm, Bombyx mori. Int. J. Mol. Sci. 2024, 25, 313. https://doi.org/10.3390/ijms25010313
Qie X, Yan X, Wang W, Liu Y, Zhang L, Hao C, Lu Z, Ma L. Serpin-4 Negatively Regulates Prophenoloxidase Activation and Antimicrobial Peptide Synthesis in the Silkworm, Bombyx mori. International Journal of Molecular Sciences. 2024; 25(1):313. https://doi.org/10.3390/ijms25010313
Chicago/Turabian StyleQie, Xingtao, Xizhong Yan, Wentao Wang, Yaya Liu, Lijun Zhang, Chi Hao, Zhiqiang Lu, and Li Ma. 2024. "Serpin-4 Negatively Regulates Prophenoloxidase Activation and Antimicrobial Peptide Synthesis in the Silkworm, Bombyx mori" International Journal of Molecular Sciences 25, no. 1: 313. https://doi.org/10.3390/ijms25010313





