Level of Secretion and the Role of the Nerve Growth Factor in Patients with Keratoconus before and after Collagen Fibre Cross-Linking Surgery
Abstract
:1. Introduction
2. Results
2.1. NGF-β Concentration
2.2. Correlations of NGF-β with Corneal Topographic Parameters
2.3. Corneal Sensitivity
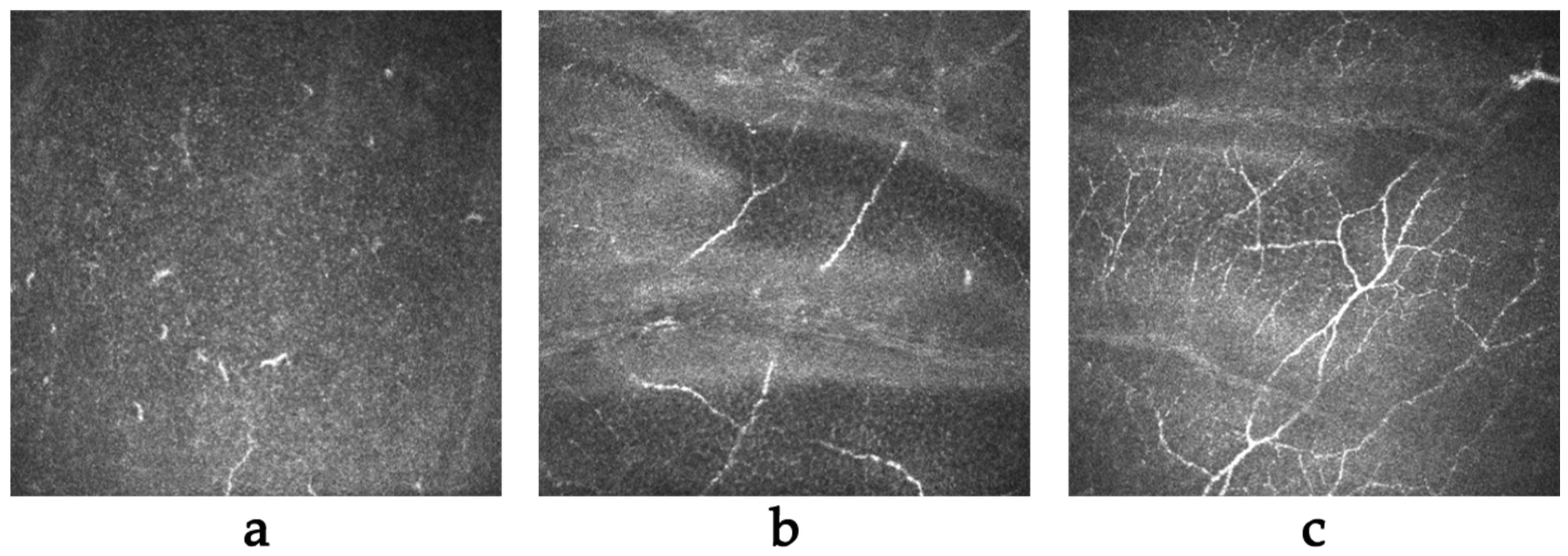
2.4. In Vivo Confocal Microscopy (IVCM)
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Cross-Linking Treatment
4.3. Measurements
4.4. Tear Collection and Analysis
4.5. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated re-view. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Heydarian, S.; Hooshmand, E.; Saatchi, M.; Yekta, A.; Aghamirsalim, M.; Valadkhan, M.; Mortazavi, M.; Hashemi, A.; Khabazkhoob, M. The Prevalence and Risk Factors for Keratoconus. A Systematic Review and Meta-Analysis. Cornea 2020, 39, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, P.; Dadachanji, Z.; Shetty, R.; Nagarajan, S.A.; Khamar, P.; Sethu, S.; D’Souza, S. Relevance of IgE, allergy and eye rubbing in the pathogenesis and management of Keratoconus. Indian J. Ophthalmol. 2020, 68, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Loh, I.P.; Sherwin, T. Is Keratoconus an Inflammatory Disease? The Implication of Inflammatory Pathways. Ocul. Immunol. Inflamm. 2022, 30, 246–255. [Google Scholar] [CrossRef]
- Lema, I.; Sobrino, T.; Durán, J.A.; Brea, D.; Díez-Feijoo, E. Subclinical keratoconus and inflammatory molecules from tears. Br. J. Ophthalmol. 2009, 93, 820–824. [Google Scholar] [CrossRef]
- Jun, A.S.; Cope, L.; Speck, C.; Feng, X.; Lee, S.; Meng, H.; Hamad, A.; Chakravarti, S. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS ONE 2011, 27, e16437. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Mohan, S.; Pye, D.C.; Willcox, M.D. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012, 90, 303–309. [Google Scholar] [CrossRef]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Taheri, N.; Ahoor, M.H. Tear film inflammatory mediators in patients with keratoconus. Int. Ophthalmol. 2015, 35, 467–472. [Google Scholar] [CrossRef]
- Marques, J.C.; Ladislau de Carvalho, K.I.; Xavier, R.; Nosé, W.; Rizzo, L.V. Inflammatory profile of keratoconic corneal epithelium. BMC Ophthalmol. 2023, 23, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, X.; Liu, Y.; Wang, P.; Li, X. Tear Levels of Inflammatory Cytokines in Keratoconus: A Meta-Analysis of Case-Control and Cross-Sectional Studies. BioMed Res. Int. 2021, 30, 6628923. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, I.C.; Corbu, C.G.; Tanase, C.; Ionita, G.; Nicula, C.; Coviltir, V.; Potop, V.; Constantin, M.; Codrici, E.; Mihai, S.; et al. Overexpression of Tear Inflammatory Cytokines as Addi-tional Finding in Keratoconus Patients and Their First Degree Family Members. Mediat. Inflamm. 2018, 2, 2018. [Google Scholar] [CrossRef]
- Nichani, P.A.H.; Solomon, B.; Trinh, T.; Mimouni, M.; Rootman, D.; Singal, N.; Chan, C.C. Investigating the role of inflammation in keratoconus: A retrospective analysis of 551 eyes. Eur. J. Ophthalmol. 2023, 33, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.; Corbu, C.G.; Tanase, C.; Jonescu-Cuypers, C.; Nicula, C.; Dascalescu, D.; Cristea, M.; Voinea, L.M. Inflammatory Bi-omarkers Profile as Microenvironmental Expression in Keratoconus. Dis. Markers 2016, 2016, 1243819. [Google Scholar] [CrossRef] [PubMed]
- Galvis, V.; Sherwin, T.; Tello, A.; Merayo, J.; Barrera, R.; Acera, A. Keratoconus: An inflammatory disorder? Eye 2015, 29, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, H.; Huang, S. Role of NGF and its receptors in wound healing (Review). Exp. Ther. Med. 2021, 21, 599. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Liu, G.; Yin, S.; Ma, J.; Liu, J.; Zhang, M.; Wang, Y. p75 neurotrophin receptor regulates NGF-induced myofibroblast differentiation and collagen synthesis through MRTF-A. Exp. Cell Res. 2019, 383, 111504. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Lambiase, A.; Merlo, D.; Mollinari, C.; Bonini, P.; Rinaldi, A.M.; D’Amato, M.; Micera, A.; Coassin, M.; Rama, P.; Bonini, S.; et al. Molecular basis for keratoconus: Lack of TrkA expression and its transcriptional repression by Sp3. Proc. Natl. Acad. Sci. USA 2005, 102, 16795–16800. [Google Scholar] [CrossRef]
- Kolozsvári, B.L.; Petrovski, G.; Gogolák, P.; Rajnavölgyi, É.; Tóth, F.; Berta, A.; Fodor, M. Association between mediators in the tear fluid and the severity of keratoconus. Ophthalmic Res. 2014, 51, 46–51. [Google Scholar] [CrossRef]
- Fodor, M.; Vitályos, G.; Losonczy, G.; Hassan, Z.; Pásztor, D.; Gogolák, P.; Kolozsvári, B.L. Tear Mediators NGF along with IL-13 Predict Keratoconus Progression. Ocul. Immunol. Inflamm. 2021, 29, 1090–1101. [Google Scholar] [CrossRef]
- Kolozsvári, B.L.; Berta, A.; Petrovski, G.; Miháltz, K.; Gogolák, P.; Rajnavölgyi, E.; Hassan, Z.; Széles, P.; Fodor, M. Alterations of tear mediators in patients with keratoconus after corneal crosslinking associate with corneal changes. PLoS ONE 2013, 8, e76333. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Liu, L.; Wang, Y.; Ding, H.; Li, L.; Zhong, X. Early changes in ocular surface and tear inflammatory mediators after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis. PLoS ONE 2014, 9, e107370. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, H.; He, M.; Liu, L.; Liu, L.; Li, G.; Niu, B.; Zhong, X. Comparison of Early Changes in Ocular Surface and Inflammatory Mediators between Femtosecond Lenticule Extraction and Small-Incision Lenticule Extraction. PLoS ONE 2016, 11, e0149503. [Google Scholar] [CrossRef]
- Zwingelberg, S.B.; Bachmann, B.O.; Cursiefen, C. Real Life Data on Efficacy and Safety of Topical NGF Eye Drops (Cenegermin). Klinische Monatsblätter für Augenheilkunde 2020, 237, 1455–1461. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Inferrera, L.; Aragona, E.; Gargano, R.; Postorino, E.I.; Aragona, P. Clinical and instrumental assessment of the corneal healing in moderate and severe neurotrophic keratopathy treated with rh-NGF (Cenegermin). Eur. J. Ophthalmol. 2022, 32, 3402–3410. [Google Scholar] [CrossRef]
- Moura, G.S.; Santos, A.; Cenedeze, M.A.; Hiyane, M.I.; Camara, N.O.S.; Barbosa de Sousa, L.; Augusto de Oliveira, L. Increased lacrimal inflammatory mediators in patients with keratoconus. Mol. Vis. 2021, 27, 656–665. [Google Scholar]
- Acar Eser, N.; Dikmetas, O.; Kocabeyoglu, S.; Tan, C.; Irkec, M. Evaluation of Keratoconus Disease with Tear Cytokine and Chemokine Levels Before and After Corneal Cross-Linking Treatment. Ocul. Immunol. Inflamm. 2023, 6, 1–7. [Google Scholar] [CrossRef]
- Levi-Montalcini, R. The nerve growth factor thirty-five years later. Vitr. Cell. Dev. Biol. 1987, 23, 227–238. [Google Scholar] [CrossRef]
- Aloe, L.; Chaldakov, G.N. Homage to Rita Levi-Montalcini, the queen of modern neuroscience. Cell Biol. Int. 2013, 37, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, G.; Minnone, G.; Strippoli, R.; De Pasquale, L.; Petrini, S.; Caiello, I.; Manni, L.; De Benedetti, F.; Bracci-Laudiero, L. Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J. Immunol. 2014, 192, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.G.; Davanger, M. The effect of steroids on the healing of the corneal endothelium. An in vivo and in vitro study in rabbits. Acta Ophthalmol. 1984, 62, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, S.; He, Y.; Zhang, Y. The research progress on the molecular mechanism of corneal cross-linking in keratoconus treatment. Contact Lens Anterior Eye 2023, 46, 101795. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; van Dijk, K.; Melles, G.R. Treatment options for advanced keratoconus: A review. Surv. Ophthalmol. 2015, 60, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Jain, P.; Goyal, J.L.; Gupta, D. Comparative Analysis of Refractive and Topographic Changes in Early and Advanced Keratoconic Eyes Undergoing Corneal Collagen Crosslinking. Cornea 2013, 32, 1359–1364. [Google Scholar] [CrossRef]
- Koller, T.; Mrochen, M.; Seiler, T. Complication and failure rates after corneal crosslinking. J. Cataract Refract. Surg. 2009, 35, 1358–1362. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, K.S.; Kim, H.C.; Lee, S.H.; Kim, E.K. Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am. J. Ophthalmol. 2005, 139, 965–971. [Google Scholar] [CrossRef]
- Cheung, I.M.; McGhee, C.N.; Sherwin, T. Deficient repair regulatory response to injury in keratoconic stromal cells. Clin. Exp. Optom. 2014, 97, 234–239. [Google Scholar] [CrossRef]
- Teo, A.W.J.; Mansoor, H.; Sim, N.; Lin, M.T.; Liu, Y.C. In Vivo Confocal Microscopy Evaluation in Patients with Keratoconus. J. Clin. Med. 2022, 11, 393. [Google Scholar] [CrossRef]
- Mazzotta, C.; Hafezi, F.; Kymionis, G.; Caragiuli, S.; Jacob, S.; Traversi, C.; Barabino, S.; Randleman, J.B. In Vivo Confocal Microscopy after Corneal Collagen Crosslinking. Ocul. Surf. 2015, 13, 298–314. [Google Scholar] [CrossRef]
- Yavuz Saricay, L.; Gonzalez Monroy, J.E.; Fulton, A.B. Can Nerve Growth Factor (NGF) Be a Treatment Option for Pediatric Eye Diseases? Semin. Ophthalmol. 2023, 38, 427–432. [Google Scholar] [CrossRef]
- Elhusseiny, A.M.; Traish, A.S.; Saeed, H.N.; Mantagos, I.S. Topical cenegermin 0.002% for pediatric neurotrophic keratopathy. Eur. J. Ophthalmol. 2022, 32, 3420–3424. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, S.; Jiang, L.; Lin, M.; Xu, Z.; Yu, Y.; Wang, Q.; Lu, F.; Hu, L. The effect of nerve growth factor on corneal nerve regeneration and dry eye after LASIK. Exp. Eye Res. 2021, 203, 108428. [Google Scholar] [CrossRef]
- Dawidowicz, M.; Kula, A.; Mielcarska, S.; Kiczmer, P.; Skiba, H.; Krygier, M.; Chrabańska, M.; Piecuch, J.; Szrot, M.; Robotycka, J.; et al. B7H4 Expression Is More Frequent in MSS Status Colorectal Cancer and Is Negatively Associated with Tumour Infiltrating Lymphocytes. Cells 2023, 12, 861. [Google Scholar] [CrossRef]
- Bronikowska, J.; Kłósek, M.; Janeczko, T.; Kostrzewa-Susłow, E.; Czuba, Z.P. The modulating effect of methoxy-derivatives of 2′-hydroxychalcones on the release of IL-8, MIF, VCAM-1 and ICAM-1 by colon cancer cells. Biomed. Pharmacother. 2022, 145, 112428. [Google Scholar] [CrossRef]
- Gothwal, V.K.; Gujar, R.; Sharma, S.; Begum, N.; Pesudovs, K. Factors affecting quality of life in keratoconus. Ophthalmic Physiol. Opt. 2022, 42, 986–997. [Google Scholar] [CrossRef]
| Parameter | Group | N | Moderate | SD | Median | Min | Max | Q1 | Q3 | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NGF-β | ALL | Studied group | 48 | 8.11 | 5.86 | 6.42 | 3.1 | 28.87 | 4.17 | 9.4 | p < 0.001 * |
| Control group | 20 | 16.73 | 7.71 | 14.36 | 7.21 | 32.91 | 10.07 | 22.28 | |||
| AGE | |||||||||||
| up to 19 years | Studied group | 15 | 8.86 | 5.9 | 7.89 | 3.94 | 28.87 | 6.42 | 9.45 | p < 0.001 * | |
| Control group | 20 | 16.73 | 7.71 | 14.36 | 7.21 | 32.91 | 10.07 | 22.28 | |||
| 20–26 years | Studied group | 16 | 8.76 | 6.61 | 7.39 | 3.73 | 28.87 | 4.09 | 10.11 | p = 0.001 * | |
| Control group | 20 | 16.73 | 7.71 | 14.36 | 7.21 | 32.91 | 10.07 | 22.28 | |||
| over 26 years | Studied group | 13 | 6.03 | 4.85 | 4.63 | 3.1 | 21.57 | 3.94 | 5.53 | p < 0.001 * | |
| Control group | 20 | 16.73 | 7.71 | 14.36 | 7.21 | 32.91 | 10.07 | 22.28 | |||
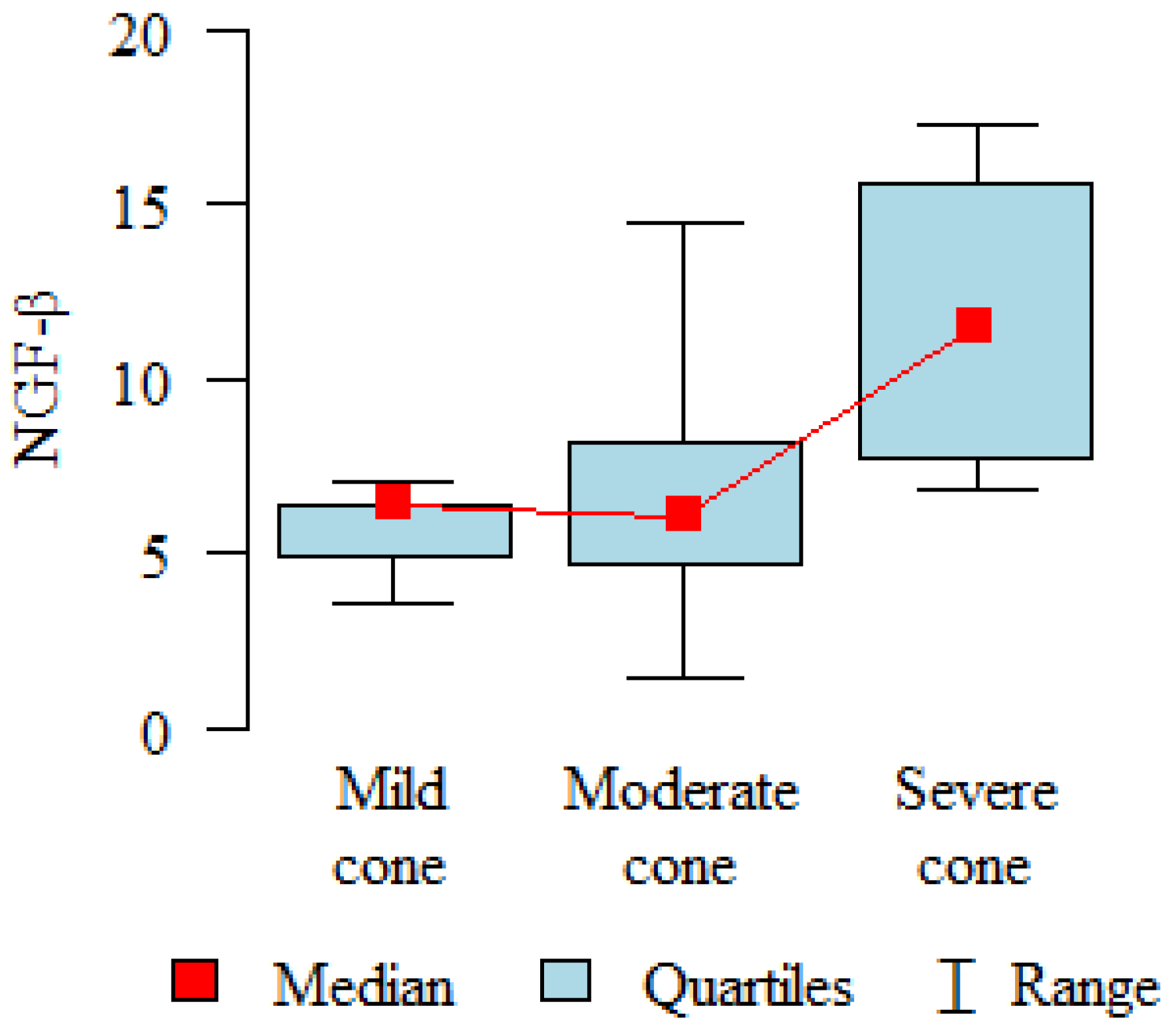
| SEVERITY | |||||||||||
| Mild | Studied group | 15 | 5.94 | 2.68 | 4.77 | 3.51 | 12.93 | 3.94 | 7.15 | p < 0.001 * | |
| Control group | 20 | 16.73 | 7.71 | 14.36 | 7.21 | 32.91 | 10.07 | 22.28 | |||
| Medium | Studied group | 25 | 9.3 | 7.29 | 7.23 | 3.1 | 28.87 | 4.17 | 9.93 | p < 0.001 * | |
| Control group | 20 | 16.73 | 7.71 | 14.36 | 7.21 | 32.91 | 10.07 | 22.28 | |||
| Severe | Studied group | 6 | 7.61 | 2.01 | 7.84 | 4.18 | 10.36 | 7.3 | 8.2 | p = 0.004 * | |
| Control group | 20 | 16.73 | 7.71 | 14.36 | 7.21 | 32.91 | 10.07 | 22.28 | |||
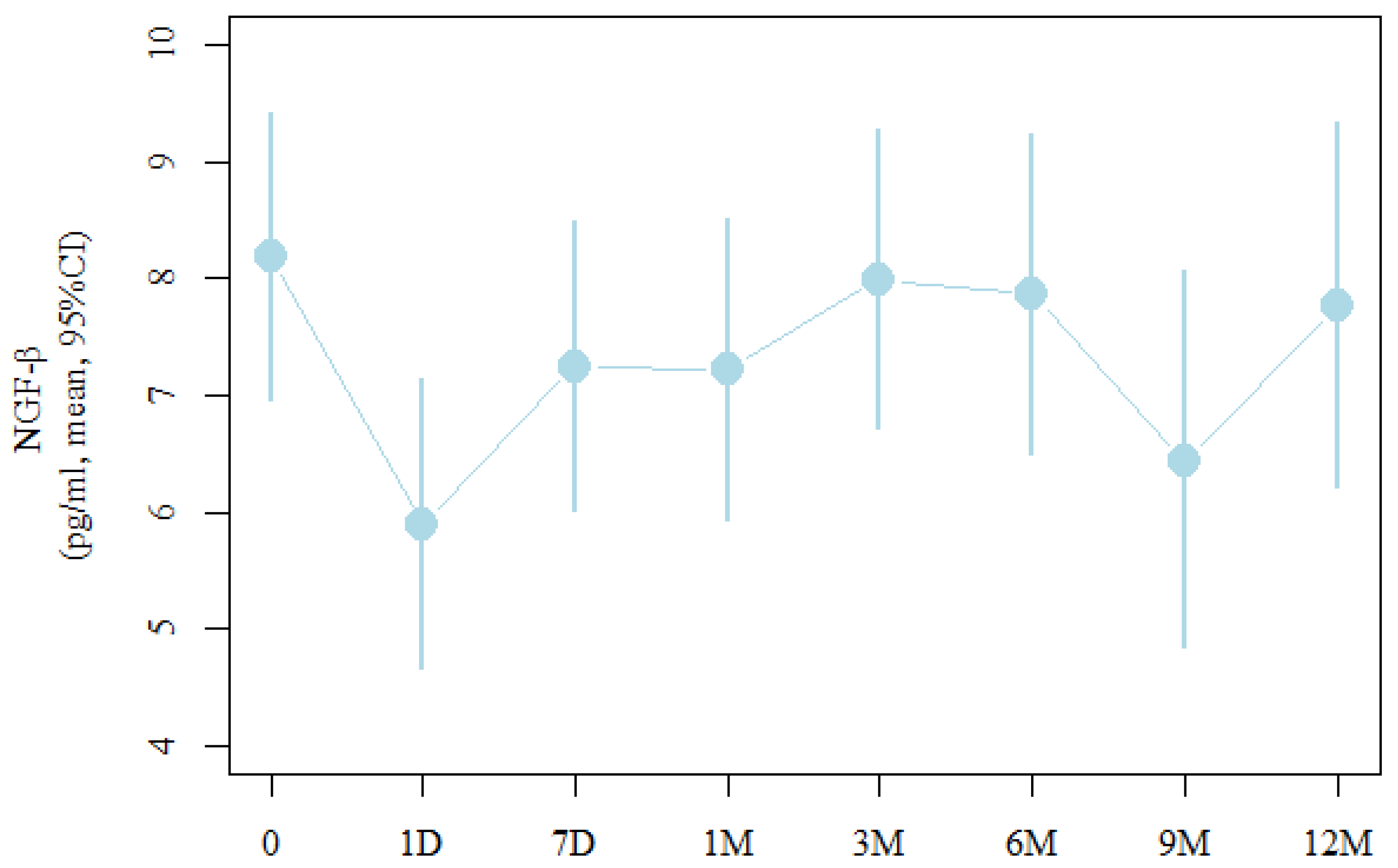
| Measure | Moderate | 95% | CI |
|---|---|---|---|
| Before treatment (0) | 8.2 | 7 | 9.4 |
| Day after the procedure (1 D) | 5.9 (p = 0.037 *) | 4.7 | 7.1 |
| After seven days (7 D) | 7.3 | 6 | 8.5 |
| After one month (1 M) | 7.2 | 5.9 | 8.5 |
| After three months (3 M) | 8 | 6.7 | 9.3 |
| After six months (6 M) | 7.9 | 6.5 | 9.2 |
| After nine months (9 M) | 6.4 | 4.8 | 8.1 |
| After 12 months (12 M) | 7.8 | 6.2 | 9.3 |
| Ks | Kf | CYL | PI—Apex | PI—Thinnest | |
|---|---|---|---|---|---|
| Measurement before treatment | r = 0.265, p = 0.075 | r = 0.315, p = 0.033 * | r = 0.08, p = 0.597 | r = −0.172, p = 0.254 | r = −0.222, p = 0.138 |
| Measurement after seven days | r = 0.297, p = 0.094 | r = 0.335, p = 0.057 | r = −0.01, p = 0.956 | r = 0.006, p = 0.972 | r = 0.06, p = 0.742 |
| Measurement after one month | r = 0.148, p = 0.368 | r = 0.32, p = 0.047 * | r = −0.092, p = 0.579 | r = −0.067, p = 0.687 | r = −0.058, p = 0.724 |
| Measurement after three months | r = 0.011, p = 0.95 | r = 0.021, p = 0.899 | r = 0.044, p = 0.791 | r = 0.123, p = 0.463 | r = 0.025, p = 0.883 |
| Measurement after six months | r = 0.059, p = 0.765 | r = 0.049, p = 0.803 | r = 0.072, p = 0.714 | r = −0.138, p = 0.483 | r = 0.035, p = 0.86 |
| Measurement after nine months | r = 0.359, p = 0.144 | r = 0.066, p = 0.794 | r = 0.53, p = 0.024 * | r = 0, p = 1 | r = −0.038, p = 0.882 |
| Measurement after twelve months | r = 0.493, p = 0.012 * | r = 0.525, p = 0.007 * | r = 0.143, p = 0.495 | r = −0.201, p = 0.335 | r = −0.086, p = 0.681 |
| Group | Moderate | SD |
|---|---|---|
| 0 | 6 | 0 |
| 7 D | 0.06 | 0.24 |
| 1 M | 1.73 | 0.99 |
| 3 M | 3.55 | 1.38 |
| 6 M | 4.93 | 1.05 |
| 9 M | 5 | 1.22 |
| 12 M | 5.54 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krok, M.; Wróblewska-Czajka, E.; Łach-Wojnarowicz, O.; Bronikowska, J.; Czuba, Z.P.; Wylęgała, E.; Dobrowolski, D. Level of Secretion and the Role of the Nerve Growth Factor in Patients with Keratoconus before and after Collagen Fibre Cross-Linking Surgery. Int. J. Mol. Sci. 2024, 25, 366. https://doi.org/10.3390/ijms25010366
Krok M, Wróblewska-Czajka E, Łach-Wojnarowicz O, Bronikowska J, Czuba ZP, Wylęgała E, Dobrowolski D. Level of Secretion and the Role of the Nerve Growth Factor in Patients with Keratoconus before and after Collagen Fibre Cross-Linking Surgery. International Journal of Molecular Sciences. 2024; 25(1):366. https://doi.org/10.3390/ijms25010366
Chicago/Turabian StyleKrok, Magdalena, Ewa Wróblewska-Czajka, Olga Łach-Wojnarowicz, Joanna Bronikowska, Zenon P. Czuba, Edward Wylęgała, and Dariusz Dobrowolski. 2024. "Level of Secretion and the Role of the Nerve Growth Factor in Patients with Keratoconus before and after Collagen Fibre Cross-Linking Surgery" International Journal of Molecular Sciences 25, no. 1: 366. https://doi.org/10.3390/ijms25010366
APA StyleKrok, M., Wróblewska-Czajka, E., Łach-Wojnarowicz, O., Bronikowska, J., Czuba, Z. P., Wylęgała, E., & Dobrowolski, D. (2024). Level of Secretion and the Role of the Nerve Growth Factor in Patients with Keratoconus before and after Collagen Fibre Cross-Linking Surgery. International Journal of Molecular Sciences, 25(1), 366. https://doi.org/10.3390/ijms25010366






