Involvement of PAR-2 in the Induction of Cell-Specific Matrix Metalloproteinase-2 by Activated Protein C in Cutaneous Wound Healing
Abstract
:1. Introduction
2. Results
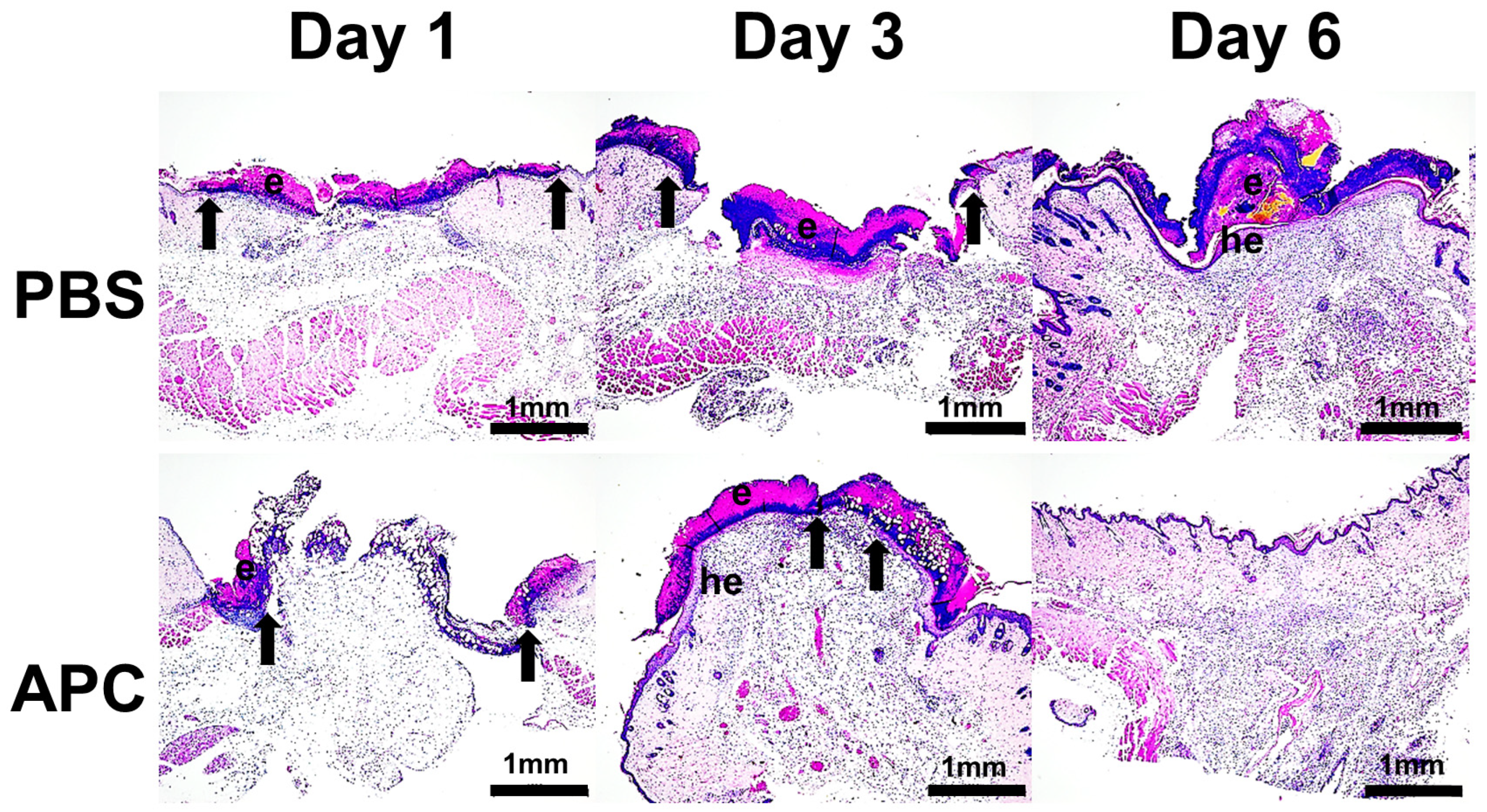
2.1. Activated Protein C Accelerates Murine Cutaneous Wound Healing
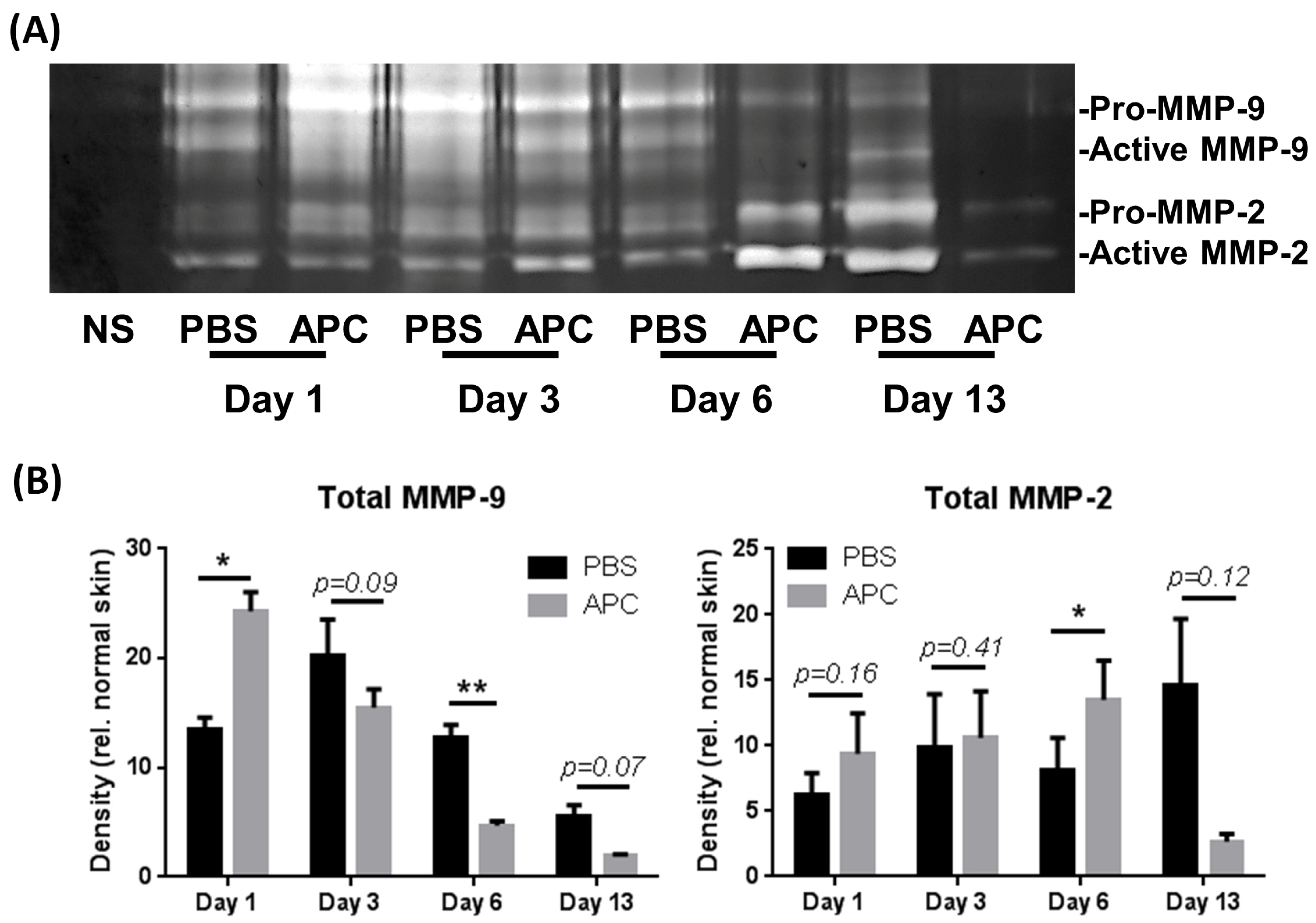
2.2. APC-Treated Wounds Induced Activity of MMP-2 and MMP-9 Earlier Than Control Wounds
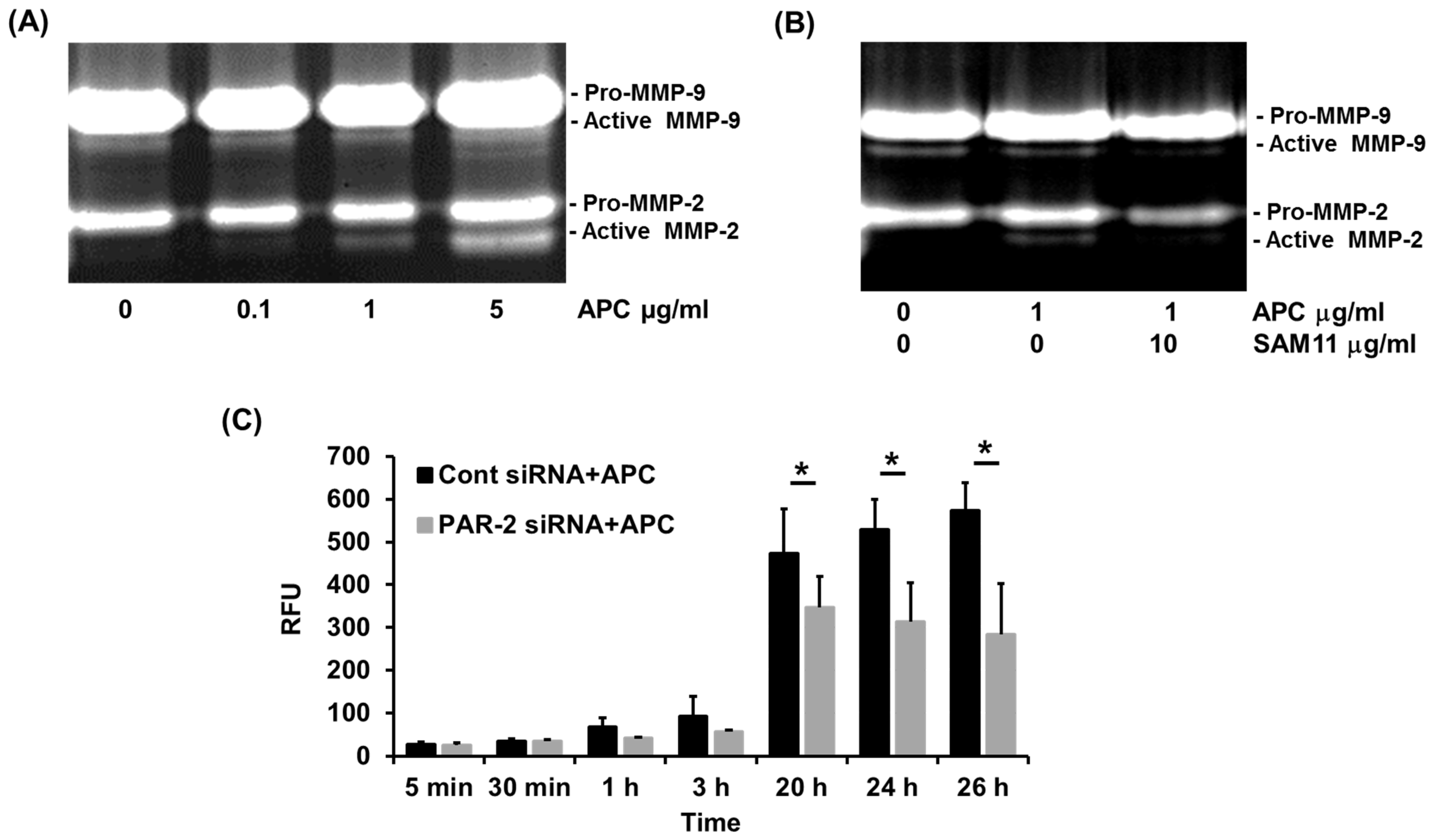
2.3. APC-Induced MMP-2 by Human Primary Keratinocytes Requires PAR-2
2.4. PAR-2 Deficiency Reduces MMP-2 Activation by Murine Dermal Fibroblasts
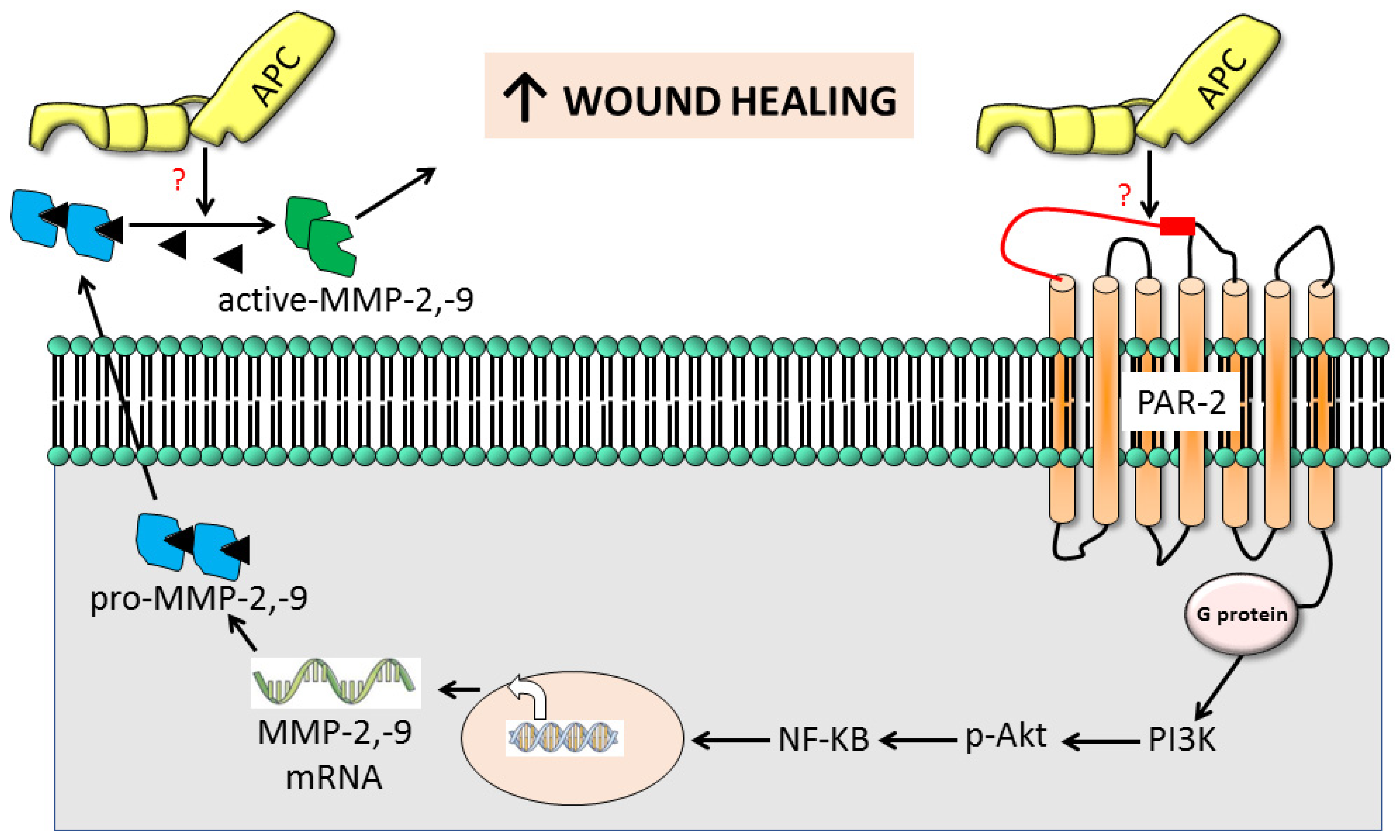
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Wound Healing Model
4.3. Histology and Immunohistochemistry
4.4. Cell Culture and Reagents
4.5. Knockdown of PAR-2 through siRNA
4.6. Zymography
4.7. Fluorometric Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Dekoninck, S.; Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 2019, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Lin, H.; Bereza-Malcolm, L.; Clarke, E.; Jackson, C.J.; Xue, M. Activated Protein C in Cutaneous Wound Healing: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 903. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Lechapt-Zalcman, E.; Prulière-Escabasse, V.; Advenier, D.; Galiacy, S.; Charrière-Bertrand, C.; Coste, A.; Harf, A.; d’Ortho, M.P.; Escudier, E. Transforming growth factor-beta1 increases airway wound repair via MMP-2 upregulation: A new pathway for epithelial wound repair? Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L1277–L1282. [Google Scholar] [CrossRef] [PubMed]
- McCawley, L.J.; O’Brien, P.; Hudson, L.G. Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)- mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J. Cell. Physiol. 1998, 176, 255–265. [Google Scholar] [CrossRef]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Frøssing, S.; Rønø, B.; Hald, A.; Rømer, J.; Lund, L.R. Skin wound healing in MMP2-deficient and MMP2/Plasminogen double-deficient mice. Exp. Dermatol. 2010, 19, e234–e240. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Wulsin, D.; Skokos, E.A.; Fleckman, P.; Pirrone, A.; Shipley, J.M.; Senior, R.M.; Bornstein, P. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol. 2009, 28, 65–73. [Google Scholar] [CrossRef]
- Hingorani, D.V.; Lippert, C.N.; Crisp, J.L.; Savariar, E.N.; Hasselmann, J.P.C.; Kuo, C.; Nguyen, Q.T.; Tsien, R.Y.; Whitney, M.A.; Ellies, L.G. Impact of MMP-2 and MMP-9 enzyme activity on wound healing, tumor growth and RACPP cleavage. PLoS ONE 2018, 13, e0198464. [Google Scholar] [CrossRef]
- Jackson, C.J.; Xue, M.; Thompson, P.; Davey, R.A.; Whitmont, K.; Smith, S.; Buisson-Legendre, N.; Sztynda, T.; Furphy, L.J.; Cooper, A.; et al. Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair. Regen. 2005, 13, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Thompson, P.; Kelso, I.; Jackson, C. Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp. Cell Res. 2004, 299, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Riewald, M.; Petrovan, R.J.; Donner, A.; Mueller, B.M.; Ruf, W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 2002, 296, 1880–1882. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The cytoprotective protein C pathway. Blood 2007, 109, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Isermann, B.; Vinnikov, I.A.; Madhusudhan, T.; Herzog, S.; Kashif, M.; Blautzik, J.; Corat, M.A.; Zeier, M.; Blessing, E.; Oh, J.; et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 2007, 13, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, D.; Gelbard, H.; Cheng, T.; Insalaco, R.; Fernández, J.A.; Griffin, J.H.; Zlokovic, B.V. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron 2004, 19, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Molinar-Inglis, O.; Birch, C.A.; Nicholas, D.; Orduña-Castillo, L.; Cisneros-Aguirre, M.; Patwardhan, A.; Chen, B.; Grimsey, N.J.; Coronel, L.J.; Lin, H.; et al. aPC/PAR1 confers endothelial anti-apoptotic activity via a discrete, β-arrestin-2-mediated SphK1-S1PR1-Akt signaling axis. Proc. Natl. Acad. Sci. USA 2021, 118, e2106623118. [Google Scholar] [CrossRef]
- Cheng, T.; Liu, D.; Griffin, J.H.; Fernández, J.A.; Castellino, F.; Rosen, E.D.; Fukudome, K.; Zlokovic, B.V. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 2003, 9, 338–342. [Google Scholar] [CrossRef]
- Nazir, S.; Gadi, I.; Al-Dabet, M.M.; Elwakiel, A.; Kohli, S.; Ghosh, S.; Manoharan, J.; Ranjan, S.; Bock, F.; Braun-Dullaeus, R.C.; et al. Cytoprotective activated protein C averts Nlrp3 inflammasome-induced ischemia-reperfusion injury via mTORC1 inhibition. Blood 2017, 130, 2664–2677. [Google Scholar] [CrossRef]
- Peach, C.J.; Edgington-Mitchell, L.E.; Bunnett, N.W.; Schmidt, B.L. Protease-activated receptors in health and disease. Physiol. Rev. 2023, 103, 717–785. [Google Scholar] [CrossRef]
- Vergnolle, N.; Wallace, J.L.; Bunnett, N.W.; Hollenberg, M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001, 22, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Julovi, S.M.; Xue, M.; Dervish, S.; Sambrook, P.N.; March, L.; Jackson, C.J. Protease activated receptor-2 mediates activated protein c–induced cutaneous wound healing via inhibition of p38. Am. J. Pathol. 2011, 179, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.; Seo, J.; Kim, H.K.; Han, Y.P.; Park, E.J.; Park, J.O.; Yang, C.S.; Kim, J.W. Fibroblast-targeting polymeric nanovehicles to enhance topical wound healing through promotion of PAR-2 receptor-mediated endocytosis. Biomater. Sci. 2023, 11, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.S.; Knight, D.A.; Stewart, G.A.; Henry, P.J. Role of PGE2 in protease-activated receptor-1, −2 and −4 mediated relaxation in the mouse isolated trachea. Br. J. Pharmacol. 2001, 132, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cocks, T.M.; Moffatt, J.D. Protease-activated Receptor-2 (PAR2) in the Airways. Pulm. Pharmacol. Ther. 2001, 14, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Whitmont, K.; McKelvey, K.J.; Fulcher, G.; Reid, I.; March, L.; Xue, M.; Cooper, A.; Jackson, C.J. Treatment of chronic diabetic lower leg ulcers with activated protein C: A randomised placebo-controlled, double-blind pilot clinical trial. Int. Wound J. 2013, 12, 422–427. [Google Scholar] [CrossRef]
- Babiarz-Magee, L.; Chen, N.; Seiberg, M.; Lin, C.B. Combination of activated protein c and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int. J. Low. Extrem. Wounds 2011, 10, 146–151. [Google Scholar]
- Babiarz-Magee, L.; Chen, N.; Seiberg, M.; Lin, C.B. The Expression and Activation of Protease-Activated Receptor-2 Correlate with Skin Color. Pigment. Cell Res. 2004, 17, 241–251. [Google Scholar] [CrossRef]
- Rattenholl, A.; Steinhoff, M. Proteinase-activated receptor-2 in the skin: Receptor expression, activation and function during health and disease. Drug News Perspect. 2008, 21, 369–381. [Google Scholar] [CrossRef]
- Moormann, C.; Artuc, M.; Pohl, E.; Varga, G.; Buddenkotte, J.; Vergnolle, N.; Brehler, R.; Henz, B.M.; Schneider, S.W.; Luger, T.A.; et al. Functional Characterization and Expression Analysis of the Proteinase-Activated Receptor-2 in Human Cutaneous Mast Cells. J. Investig. Dermatol. 2006, 126, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Howells, G.L.; Macey, M.G.; Chinni, C.; Hou, L.; Fox, M.T.; Harriott, P.; Stone, S.R. Proteinase-activated receptor-2: Expression by human neutrophils. J. Cell Sci. 1997, 110, 881–887. [Google Scholar] [CrossRef]
- Hou, L.; Kapas, S.; Cruchley, A.T.; Macey, M.G.; Harriott, P.; Chinni, C.; Stone, S.R.; Howells, G.L. Immunolocalization of protease-activated receptor-2 in skin: Receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology 1998, 94, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Johansson, U.; Lawson, C.; Dabare, M.; Syndercombe-Court, D.; Newland, A.C.; Howells, G.L.; Macey, M.G. Human peripheral blood monocytes express protease receptor-2 and respond to receptor activation by production of IL-6, IL-8, and IL-1β. J. Leukoc. Biol. 2005, 78, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Prasad, R.; Ansari, S.A.; Roy, A.; Mukherjee, A.; Sen, P. Matrix metalloproteinase-2: A key regulator in coagulation proteases mediated human breast cancer progression through autocrine signaling. Biomed. Pharmacother. 2018, 105, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Falconer, A.M.D.; Chan, C.M.; Gray, J.; Nagashima, I.; Holland, R.A.; Shimizu, H.; Pickford, A.R.; Rowan, A.D.; Wilkinson, D.J. Collagenolytic matrix metalloproteinases antagonize proteinase-activated receptor-2 activation, providing insights into extracellular matrix turnover. J. Biol. Chem. 2019, 294, 10266–10277. [Google Scholar] [CrossRef]
- Wilson, S.R.; Gallagher, S.; Warpeha, K.; Hawthorne, S.J. Amplification of MMP-2 and MMP-9 production by prostate cancer cell lines via activation of protease-activated receptors. Prostate 2004, 60, 168–174. [Google Scholar] [CrossRef]
- Kawao, N.; Nagataki, M.; Nagasawa, K.; Kubo, S.; Cushing, K.; Wada, T.; Sekiguchi, F.; Ichida, S.; Hollenberg, M.D.; MacNaughton, W.K.; et al. Signal transduction for proteinase-activated receptor-2-triggered prostaglandin E2 formation in human lung epithelial cells. J. Pharmacol. Exp. Ther. 2005, 315, 576–589. [Google Scholar] [CrossRef]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef]
- Mendes, O.; Kim, H.T.; Lungu, G.; Stoica, G. MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin. Exp. Metastasis 2007, 24, 341–351. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- van Hinsbergh, V.W.; Engelse, M.A.; Quax, P.H. Pericellular proteases in angiogenesis and vasculogenesis. Arter. Thromb. Vasc. Biol. 2006, 26, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, S.; Ploplis, V.A.; Sala, E.; Sandoval-Cooper, M.; Donahue, D.L.; Correale, C.; Arena, V.; Spinelli, A.; Repici, A.; Malesci, A.; et al. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc. Natl. Acad. Sci. USA 2011, 108, 19830–19835. [Google Scholar] [CrossRef] [PubMed]
- Hardy, E.; Fernandez-Patron, C. Destroy to Rebuild: The Connection Between Bone Tissue Remodeling and Matrix Metalloproteinases. Front. Physiol. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Reiss, M.J.; Han, Y.-P.; Garcia, E.; Goldberg, M.; Yu, H.; Garner, W.L. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery 2010, 147, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Van Den Steen, P.E.; Wuyts, A.; Husson, S.J.; Proost, P.; Van Damme, J.; Opdenakker, G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur. J. Biochem. 2003, 270, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.T.; Moradi, B.; Smith, M.M.; Jackson, C.J.; Little, C.B. Activation of Matrix Metalloproteinases 2, 9, and 13 by Activated Protein C in Human Osteoarthritic Cartilage Chondrocytes. Arthritis Rheumatol. 2014, 66, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- McQuibban, G.A.; Gong, J.-H.; Wong, J.P.; Wallace, J.L.; Clark-Lewis, I.; Overall, C.M. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 2002, 100, 1160–1167. [Google Scholar] [CrossRef]
- Madhusudhan, T.; Kerlin, B.A.; Isermann, B. The emerging role of coagulation proteases in kidney disease. Nat. Rev. Nephrol. 2016, 12, 94–109. [Google Scholar] [CrossRef]
- Julovi, S.M.; Shen, K.; Mckelvey, K.; Minhas, N.; March, L.; Jackson, C.J. Activated Protein C Inhibits Proliferation and Tumor Necrosis Factor α–Stimulated Activation of p38, c-Jun NH2-Terminal Kinase (JNK) and Akt in Rheumatoid Synovial Fibroblasts. Mol. Med. 2013, 19, 324–331. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julovi, S.M.; McKelvey, K.; Minhas, N.; Chan, Y.-K.A.; Xue, M.; Jackson, C.J. Involvement of PAR-2 in the Induction of Cell-Specific Matrix Metalloproteinase-2 by Activated Protein C in Cutaneous Wound Healing. Int. J. Mol. Sci. 2024, 25, 370. https://doi.org/10.3390/ijms25010370
Julovi SM, McKelvey K, Minhas N, Chan Y-KA, Xue M, Jackson CJ. Involvement of PAR-2 in the Induction of Cell-Specific Matrix Metalloproteinase-2 by Activated Protein C in Cutaneous Wound Healing. International Journal of Molecular Sciences. 2024; 25(1):370. https://doi.org/10.3390/ijms25010370
Chicago/Turabian StyleJulovi, Sohel M., Kelly McKelvey, Nikita Minhas, Yee-Ka Agnes Chan, Meilang Xue, and Christopher J. Jackson. 2024. "Involvement of PAR-2 in the Induction of Cell-Specific Matrix Metalloproteinase-2 by Activated Protein C in Cutaneous Wound Healing" International Journal of Molecular Sciences 25, no. 1: 370. https://doi.org/10.3390/ijms25010370
APA StyleJulovi, S. M., McKelvey, K., Minhas, N., Chan, Y.-K. A., Xue, M., & Jackson, C. J. (2024). Involvement of PAR-2 in the Induction of Cell-Specific Matrix Metalloproteinase-2 by Activated Protein C in Cutaneous Wound Healing. International Journal of Molecular Sciences, 25(1), 370. https://doi.org/10.3390/ijms25010370










