Astragalus Complanatus Ethanol Attenuates Septic Shock by Exerting Anti-Inflammatory Effects on Macrophages
Abstract
:1. Introduction
2. Results
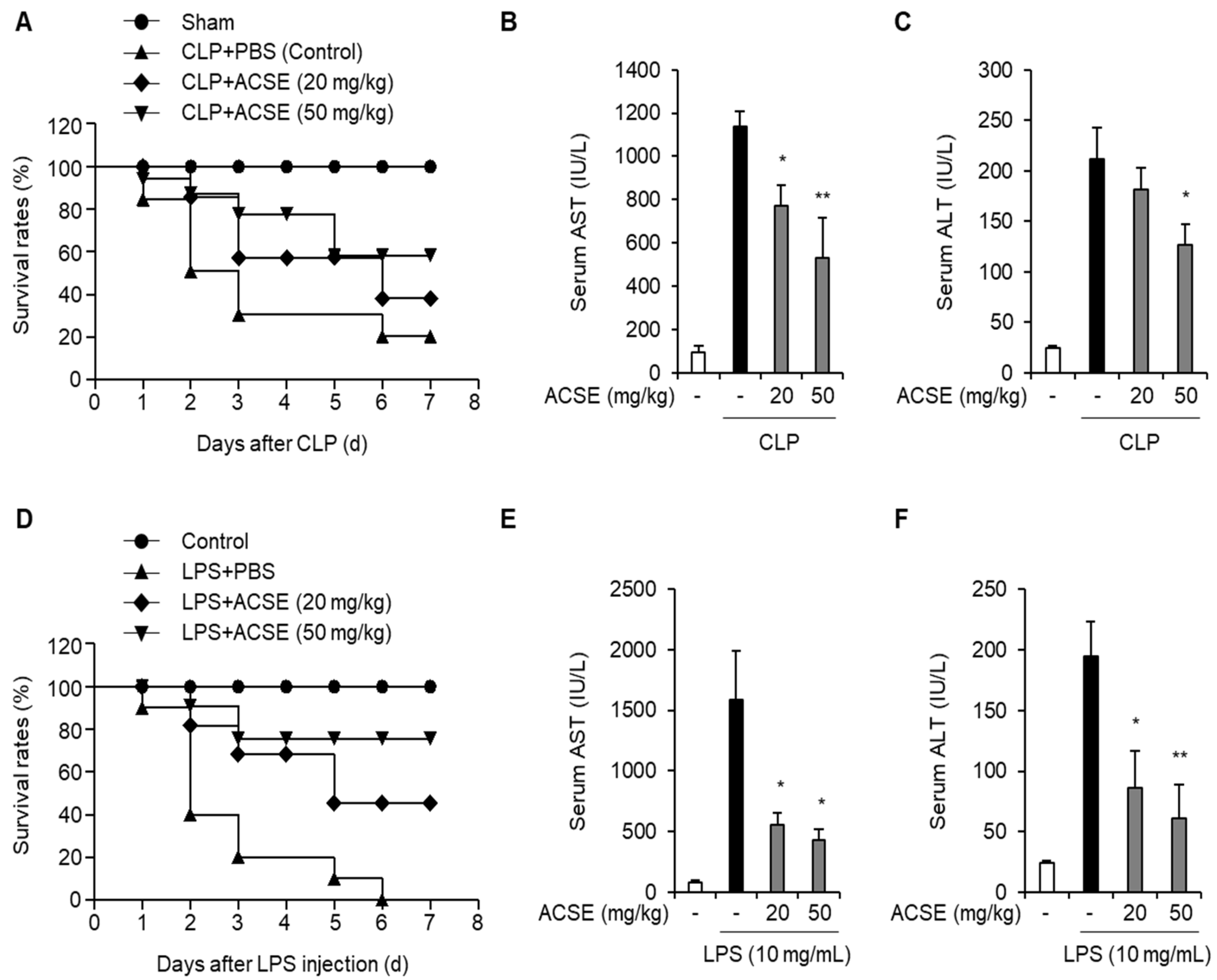
2.1. ACSE Increases Survival Outcomes and Alleviates Liver Injury in Sepsis Mouse Models
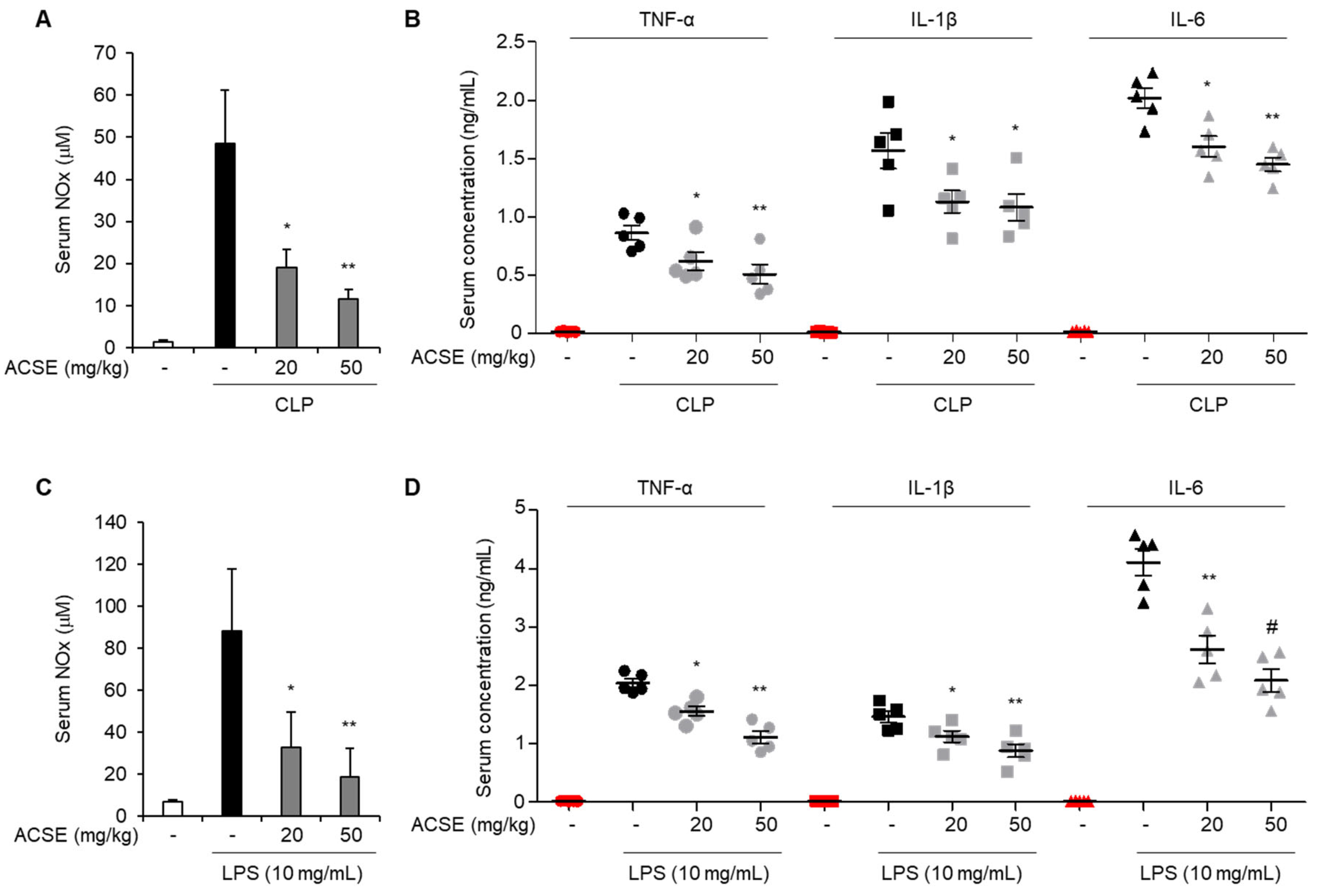
2.2. ACSE Attenuates Inflammatory Responses in Septic Mice
2.3. ACSE Suppresses the Expression of Inflammatory Mediators Induced by LPS in Macrophages
2.4. ACSE Attenuates NF-κB Activation by LPS Stimulation in J774 Cells
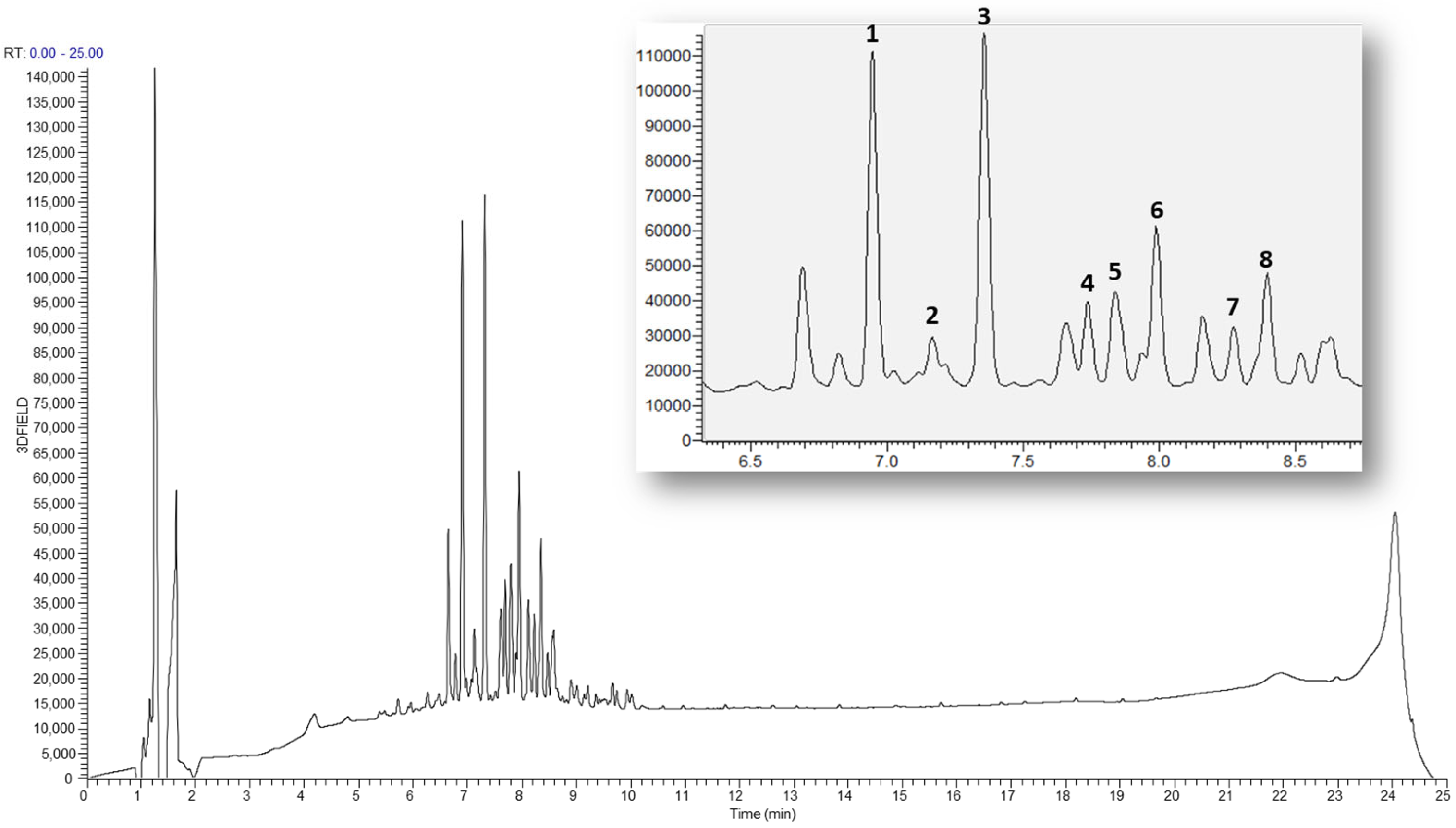
2.5. LC-MS Base Ion Peak Chromatogram of Astragali Complanate Semen
3. Discussion
4. Materials and Methods
4.1. Preparation of ACSEs
4.2. Cell Culture
4.3. Animal Studies
4.4. Blood Sample Collection
4.5. Cell Viability
4.6. NO Analytical Measurements
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Immunoblotting
4.9. RT-PCR and Quantitative Real-Time PCR Analysis
4.10. LC-MS Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Astragali Complanati Semen |
| ACSE | Astragali Complanati Semen extract |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| CLP | Cecal ligation and puncture |
| COX-2 | Cyclooxygenase-2 |
| CRP | C-reactive protein |
| DAMP | Damage-associated molecular pattern |
| IKK | IκB kinase |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| LC-MS | Liquid chromatography-mass spectrometry |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MODS | Multiple organ dysfunction syndrome |
| NF-κB | Nuclear factor-κB |
| NO | Nitric oxide |
| PAMP | Pathogen-associated molecular pattern |
| PRR | Pattern recognition receptor |
| STAT | Signal transducer and activator of transcription |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
References
- Lazzaro, A.; De Girolamo, G.; Filippi, V.; Innocenti, G.P.; Santinelli, L.; Ceccarelli, G.; Trecarichi, E.M.; Torti, C.; Mastroianni, C.M.; d’Ettorre, G.; et al. The Interplay between Host Defense, Infection, and Clinical Status in Septic Patients: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 803. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Legoabe, L.J.; Dall’Acqua, S.; Zengin, G. Plants’ bioactive secondary metabolites in the management of sepsis: Recent findings on their mechanism of action. Front. Pharmacol. 2022, 13, 1046523. [Google Scholar] [CrossRef]
- Thompson, K.; Venkatesh, B.; Finfer, S. Sepsis and septic shock: Current approaches to management. Intern. Med. J. 2019, 49, 160–170. [Google Scholar] [CrossRef]
- Herrero, R.; Sanchez, G.; Lorente, J.A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Transl. Med. 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Khan, M.A.; Singh, S.K. Constitutive Inflammatory Cytokine Storm: A Major Threat to Human Health. J. Interferon Cytokine Res. 2020, 40, 19–23. [Google Scholar] [CrossRef]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Khosrojerdi, A.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Implications for the role of lipopolysaccharide in the development of atherosclerosis. Trends Cardiovasc. Med. 2022, 32, 525–533. [Google Scholar] [CrossRef]
- Chen, F.; Zou, L.; Williams, B.; Chao, W. Targeting Toll-Like Receptors in Sepsis: From Bench to Clinical Trials. Antioxid. Redox Signal. 2021, 35, 1324–1339. [Google Scholar] [CrossRef]
- Platanitis, E.; Decker, T. Regulatory Networks Involving STATs, IRFs, and NFκB in Inflammation. Front. Immunol. 2018, 9, 2542. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Adib-Conquy, M. Monocytes/macrophages and sepsis. Crit. Care Med. 2005, 33, S506–S509. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S. Bench to bedside review: Therapeutic modulation of nitric oxide in sepsis-an update. Intensive Care Med. Exp. 2019, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Q.; Li, M.; Lao, J.; Tang, H.; Ming, S.; Wu, M.; Gong, S.; Li, L.; Liu, L.; et al. SLAMF7 regulates the inflammatory response in macrophages during polymicrobial sepsis. J. Clin. Investig. 2023, 133, e150224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Scherer, P.E. Immunologic and endocrine functions of adipose tissue: Implications for kidney disease. Nat. Rev. Nephrol. 2018, 14, 105–120. [Google Scholar] [CrossRef]
- Ng, Y.F.; Tang, P.C.; Sham, T.T.; Lam, W.S.; Mok, D.K.; Chan, S.W. Semen Astragali Complanati: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 155, 39–53. [Google Scholar] [CrossRef]
- Kuo, J.C.; Zhang, L.J.; Huang, H.T.; Liaw, C.C.; Lin, Z.H.; Liu, M.; Kuo, Y.H. Bioactive Flavonoid Glycosides and HPLC and UPLC Quantification of Commercial Astragali Complanati Semen. Molecules 2020, 25, 4762. [Google Scholar] [CrossRef]
- Sham, T.T.; Zhang, H.; Mok, D.K.W.; Chan, S.W.; Wu, J.; Tang, S.; Chan, C.O. Chemical Analysis of Astragali Complanati Semen and Its Hypocholesterolemic Effect Using Serum Metabolomics Based on Gas Chromatography-Mass Spectrometry. Antioxidants 2017, 6, 57. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.; Li, X.; Peng, L. Total flavonoids from Astragalus complanatus attenuates lung injury following paraquat poisoning in rats through inhibiting excessive endoplasmic reticulum stress and c-Jun N-terminal kinase pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014, 26, 383–387. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Fan, X.H.; Zhang, Z.Q.; Li, T.; Zhu, C.P.; Zhang, X.R.; Song, W. Extraction, antioxidant capacity and identification of Semen Astragali Complanati (Astragalus complanatus R. Br.) phenolics. Food Chem. 2013, 141, 1295–1300. [Google Scholar] [CrossRef]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2005, 4, 31–36. [Google Scholar] [CrossRef]
- Gong, W.; Wen, H. Sepsis Induced by Cecal Ligation and Puncture. In Mouse Models of Innate Immunity; Methods in Molecular Biology Series; Humana Press: New York, NY, USA, 2019; Volume 1960, pp. 249–255. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Ceresa, F.; Campo, S.; Barbera, G.; Caruso, D.; Palazzo, E.; Patanè, F.; Monardo, P. Acute Kidney Injury and Sepsis after Cardiac Surgery: The roles of Tissue Inhibitor Metalloproteinase-2, Insulin-like Growth Factor Binding Protein-7, and Mid-Regional Pro-Adrenomedullin. J. Clin. Med. 2023, 12, 5193. [Google Scholar] [CrossRef] [PubMed]
- Bloos, F.; Trips, E.; Nierhaus, A. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients with Severe Sepsis or Septic Shock: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 1266–1276. [Google Scholar] [CrossRef]
- Fang, M.; Zou, T.; Yang, X.; Zhang, Z.; Cao, P.; Han, J.; Duan, Y.; Ruan, B.-F.; Li, Q.-S. Discovery of Novel Pterostilbene Derivatives that Might Treat Sepsis by Attenuating Oxidative Stress and Inflammation through Modulation of MAPKs/NF-κB Signaling Pathways. Antioxidants 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Rocca, C.; De Bartolo, A.; Grande, F.; Rizzuti, B.; Pasqua, T.; Giordano, F.; Granieri, M.C.; Occhiuzzi, M.A.; Garofalo, A.; Amodio, N.; et al. Cateslytin abrogates lipopolysaccharide-induced cardiomyocyte injury by reducing inflammation and oxidative stress through toll like receptor 4 interaction. Int. Immunopharmacol. 2021, 94, 107487. [Google Scholar] [CrossRef] [PubMed]
- Pradipta, I.S.; Sodik, D.C.; Lestari, K.; Parwati, I.; Halimah, E.; Diantini, A.; Abdulah, R. Antibiotic Resistance in Sepsis Patients: Evaluation and Recommendation of Antibiotic Use. N. Am. J. Med. Sci. 2013, 5, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Usmani, J.; Khan, T.; Ahmad, R.; Sharma, M. Potential role of herbal medicines as a novel approach in sepsis treatment. Biomed. Pharmacother. 2021, 144, 112337. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.; Hoff, J.; Sommerfeld, O.; Zipprich, A.; Gassler, N.; Press, A.T. The liver in sepsis: Molecular mechanism of liver failure and their potential for clinical translation. Mol. Med. 2022, 28, 84. [Google Scholar] [CrossRef]
- Yip, T.C.; Lui, G.C.; Wong, V.W.; Chow, V.C.; Ho, T.H.; Li, T.C.; Tse, Y.K.; Hui, D.S.; Chan, H.L.; Wong, G.L. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut 2021, 70, 733–742. [Google Scholar] [CrossRef]
- Karakike, E.; Giamarellos-Bourboulis, E.J. Macrophage Activation-Like Syndrome: A Distinct Entity Leading to Early Death in Sepsis. Front. Immunol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Cai, X.; Chen, Y.; Xie, X.; Yao, D.; Ding, C.; Chen, M. Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-κB. Am. J. Transl. Res. 2019, 11, 1884–1894. [Google Scholar]
- Feng, D.; Guo, R.; Liao, W.; Li, J.; Cao, S. Plantamajoside alleviates acute sepsis-induced organ dysfunction through inhibiting the TRAF6/NF-κB axis. Pharm. Biol. 2023, 61, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Millar, M.W.; Fazal, F.; Rahman, A. Therapeutic Targeting of NF-κB in Acute Lung Injury: A Double-Edged Sword. Cells 2022, 11, 3317. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-κB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Chen, J.; Kieswich, J.E.; Chiazza, F.; Moyes, A.J.; Gobbetti, T.; Purvis, G.S.; Salvatori, D.C.; Patel, N.S.; Perretti, M.; Hobbs, A.J.; et al. IκB Kinase Inhibitor Attenuates Sepsis-Induced Cardiac Dysfunction in CKD. J. Am. Soc. Nephrol. 2017, 28, 94–105. [Google Scholar] [CrossRef]
- Ding, M.; Lian, D.; Zhang, L.; Jiang, T.; Wang, W. IκB Kinase Inhibitor VII Modulates Sepsis-Induced Excessive Inflammation and Cardiac Dysfunction in 5/6 Nephrectomized Mice. Mediat. Inflamm. 2020, 2020, 4251682. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Jang, J.P.; Park, S.H.; Kim, A.; Jang, J.H.; Yoon, H.R.; Yoon, S.R.; Park, J.H.; Cho, H.J.; Lee, H.G. Ponciri Fructus Immaturus ethanol extract attenuates septic shock through inhibition of the STAT1 signaling pathway. Front. Nutr. 2022, 9, 988309. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, X.; Jiang, X.; Wang, Y.; Miao, Z.; He, W.; Yang, G.; Lv, Z.; Yu, Y.; Zheng, Y. Micheliolide inhibits LPS-induced inflammatory response and protects mice from LPS challenge. Sci. Rep. 2016, 6, 23240. [Google Scholar] [CrossRef]
- Grigoryan, R.; Costas-Rodriguez, M.; Van Wonterghem, E.; Vandenbroucke, R.E.; Vanhaecke, F. Effect of Endotoxemia Induced by Intraperitoneal Injection of Lipopolysaccharide on the Mg isotopic Composition of Biofluids and Tissues in Mice. Front. Med. 2021, 8, 664666. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.S.; Lim, J.; Yoon, H.R.; Park, S.-H.; Kim, A.; Jang, J.-P.; Cho, H.J.; Lee, H.G. Astragalus Complanatus Ethanol Attenuates Septic Shock by Exerting Anti-Inflammatory Effects on Macrophages. Int. J. Mol. Sci. 2024, 25, 384. https://doi.org/10.3390/ijms25010384
Hwang YS, Lim J, Yoon HR, Park S-H, Kim A, Jang J-P, Cho HJ, Lee HG. Astragalus Complanatus Ethanol Attenuates Septic Shock by Exerting Anti-Inflammatory Effects on Macrophages. International Journal of Molecular Sciences. 2024; 25(1):384. https://doi.org/10.3390/ijms25010384
Chicago/Turabian StyleHwang, Yo Sep, Jeewon Lim, Hyang Ran Yoon, Seong-Hoon Park, Aeyung Kim, Jun-Pil Jang, Hee Jun Cho, and Hee Gu Lee. 2024. "Astragalus Complanatus Ethanol Attenuates Septic Shock by Exerting Anti-Inflammatory Effects on Macrophages" International Journal of Molecular Sciences 25, no. 1: 384. https://doi.org/10.3390/ijms25010384





