Antimicrobial Activity of N,N-Diethyldithiocarbamate against Ureaplasma parvum and Ureaplasma urealyticum
Abstract
:1. Introduction
2. Results
2.1. The Bacteriostatic and Bactericidal Effect of DDC on Ureaplasma Cultures
2.1.1. The Bacteriostatic Effect of DDC on Ureaplasma Cultures
2.1.2. The Bactericidal Effect of DDC on Ureaplasma Cultures
2.2. The Percentage of U. parvum (Up) and U. urealyticum (Uu) Strains for Which Bacteriostatic and Bactericidal Effects of DDC Were Demonstrated
2.2.1. The Percentage of U. parvum (Up) Strain for Which Bacteriostatic (Cell Division) and Bactericidal Effects of DDC Were Demonstrated
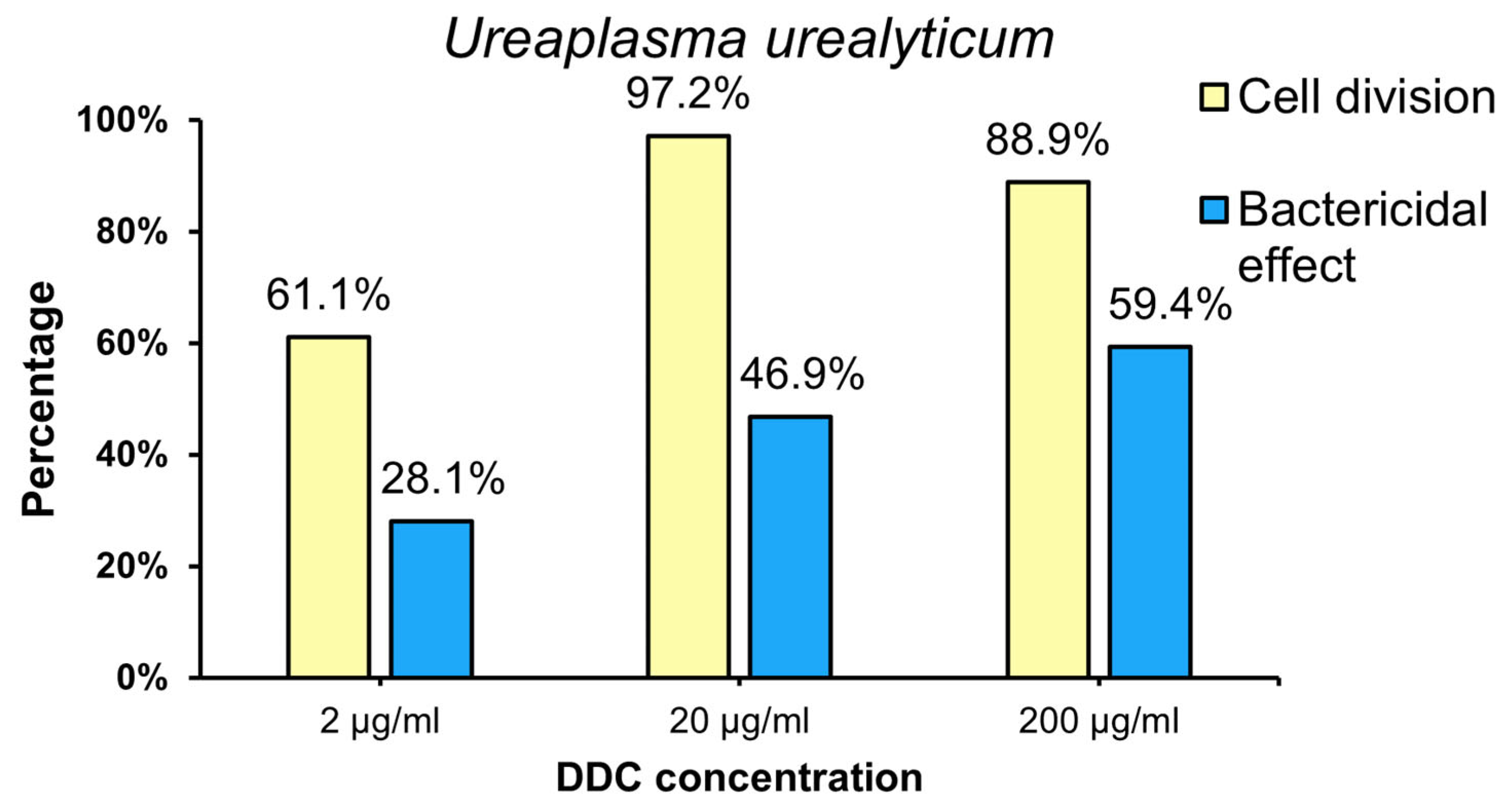
2.2.2. The Percentage of U. urealyticum (Uu) Strain for Which Bacteriostatic (Cell Division) and Bactericidal Effects of DDC Were Showed
3. Discussion
4. Materials and Methods
4.1. General
4.2. Detection and Identification of Ureaplasma spp.
4.3. Research Method Used
4.4. Bacteriostatic Effect of DDC on Ureaplasma Cultures
4.5. Bactericidal Activity of DDC
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deetjen, P.; Maurer, C.; Rank, A.; Berlis, A.; Schubert, S.; Hoffmann, R. Brain abscess caused by Ureaplasma urealyticum in an adult patient. J. Clin. Microbiol. 2014, 52, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Vittecoq, O.; Schaeverbeke, T.; Favre, S.; Daragon, A.; Biga, N.; Cambon-Michot, C.; Bébéar, C.; Le Loët, X. Molecular diagnosis of Ureaplasma urealyticum in an immunocompetent patient with destructive reactive polyarthritis. Arthritis Rheum. 1997, 40, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.S.; Skevaki, C.L.; Kafetzis, D.A. The significance of Ureaplasma urealyticum as a pathogenic agent in the paediatric population. Curr. Opin. Infect. Dis. 2006, 19, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Tantengco, O.A.G.; de Castro Silva, M.; Velayo, C.L. The role of genital mycoplasma infection in female infertility: A systematic review and meta-analysis. Am. J. Reprod. Immunol. 2021, 85, e13390. [Google Scholar] [CrossRef] [PubMed]
- Abele-Horn, M.; Wolff, C.; Dressel, P.; Pfaff, F.; Zimmermann, A. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J. Clin. Microbiol. 1997, 35, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Khosropour, C.M.; Manhart, L.E.; Gillespie, C.W.; Lowens, M.S.; Golden, M.R.; Jensen, N.L.; Kenny, G.E.; Totten, P.A. Efficacy of standard therapies against Ureaplasma species and persistence among men with non-gonococcal urethritis enrolled in a randomised controlled trial. Sex. Transm. Infect. 2015, 91, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Biernat-Sudolska, M.; Rojek-Zakrzewska, D.; Drzewiecki, A.; Lauterbach, R. Antimicrobial susceptibility of Ureaplasma urealyticum and Ureaplasma parvum isolated from premature infants with respiratory disorders. Prz. Epidemiol. 2007, 61, 371–376. (In Polish) [Google Scholar]
- Yang, T.; Pan, L.; Wu, N.; Wang, L.; Liu, Z.; Kong, Y.; Ruan, Z.; Xie, X.; Zhang, J. Antimicrobial resistance in clinical Ureaplasma spp. and mycoplasma hominis and structural mechanisms underlying quinolone resistance. Antimicrob. Agents Chemother. 2020, 64, e02560-19. [Google Scholar] [CrossRef]
- Lajarin-Reinares, M.; Pena-Rodríguez, E.; Cañellas-Santos, M.; Rosell-Vives, E.; Cortés, P.; Casas, M.L.; Calvo, M.À.; Fernandez-Campos, F. Repurposing Disulfiram as an Antimicrobial Agent in Topical Infections. Antibiotics 2022, 11, 1752. [Google Scholar] [CrossRef]
- Agarwal, R.P.; McPherson, R.A.; Phillips, M. Rapid degradation of disulfiram by serum albumin. Res. Commun. Chem. Pathol. Pharmacol. 1983, 42, 293–310. [Google Scholar]
- Cobby, J.; Mayersohn, M.; Selliah, S. The rapid reduction of disulfiram in blood and plasma. J. Pharmacol. Exp. Ther. 1977, 202, 724–731. [Google Scholar] [PubMed]
- Cvek, B. The Promiscuity of Disulfiram in Medicinal Research. ACS Med. Chem. Lett. 2023, 14, 1610–1614. [Google Scholar] [CrossRef]
- Kaul, L.; Abdo, A.I.; Coenye, T.; Krom, B.P.; Hoogenkamp, M.A.; Zannettino, A.C.W.; Süss, R.; Richter, K. The combination of diethyldithiocarbamate and copper ions is active against Staphylococcus aureus and Staphylococcus epidermidis biofilms in vitro and in vivo. Front. Microbiol. 2022, 13, 999893. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.J.; Harris, A.M.; Horne, J.E. The bacteriological and clinical assessment of a new preparation for the treatment of otitis extema in dogs and cats. J. Small Anim. Pr. Pract. 1974, 15, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, L.W.; Adler, A.; Trager, W. Tetraethylthiuram disulfide (Antabuse) inhibits the human malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1979, 76, 5303–5307. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.H.; Walker, E.M., Jr.; Bartelt, M.; Day, S.; Pappas, A.A. In-vitro antimicrobial activity of diethyldithiocarbamate and dimethyldithiocarbamate against methicillin-resistant staphylococcus. Ann. Clin. Lab. Sci. 1987, 17, 171–177. [Google Scholar] [PubMed]
- Kobatake, T.; Ogino, K.; Sakae, H.; Gotoh, K.; Watanabe, A.; Matsushita, O.; Okada, H.; Yokota, K. Antibacterial Effects of Disulfiram in Helicobacter pylori. Infect. Drug Resist. 2021, 14, 1757–1764. [Google Scholar] [CrossRef]
- Totten, A.H.; Crawford, C.L.; Dalecki, A.G.; Xiao, L.; Wolschendorf, F.; Atkinson, T.P. Differential Susceptibility of Mycoplasma and Ureaplasma Species to Compound-Enhanced Copper Toxicity. Front. Microbiol. 2019, 10, 1720. [Google Scholar] [CrossRef]
- Dégrange, S.; Renaudin, H.; Charron, A.; Bébéar, C.; Bébéar, C.M. Tetracycline resistance in Ureaplasma spp. and Mycoplasma hominis: Prevalence in Bordeaux, France, from 1999 to 2002 and description of two tet(M)-positive isolates of M. hominis susceptible to tetracyclines. Antimicrob. Agents Chemother. 2008, 52, 742–744. [Google Scholar] [CrossRef]
- Hillier, S.; Roberts, Z.; Dunstan, F.; Butler, C.; Howard, A.; Palmer, S. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: A case-control study. J. Antimicrob. Chemother. 2007, 60, 92–99. [Google Scholar] [CrossRef]
- Deguchi, T.; Yoshida, T.; Miyazawa, T.; Yasuda, M.; Tamaki, M.; Ishiko, H.; Maeda, S. Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex. Transm. Dis. 2004, 31, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, G.; Romero, R.; Shim, S.S.; Kim, E.C.; Yoon, B.H. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: Distribution, intrauterine inflammatory response and pregnancy outcomes. J. Perinat. Med. 2003, 31, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Luki, N.; Lebel, P.; Boucher, M.; Doray, B.; Turgeon, J.; Brousseau, R. Comparison of polymerase chain reaction assay with culture for detection of genital mycoplasmas in perinatal infections. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, K.A.; Flick-Smith, H.; Barnes, K.B.; Pereira-Leal, J.B.; Surkont, J.; Hampson, R.; Atkins, H.S.; Harding, S.V. Disulfiram, an alcohol dependence therapy, can inhibit the in vitro growth of Francisella tularensis. Int. J. Antimicrob. Agents 2019, 54, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Velasco-García, R.; Zaldívar-Machorro, V.J.; Mújica-Jiménez, C.; González-Segura, L.; Muñoz-Clares, R.A. Disulfiram irreversibly aggregates betaine aldehyde dehydrogenase—A potential target for antimicrobial agents against Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2006, 341, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Kaul, L.; Süss, R.; Zannettino, A.; Richter, K. The revival of dithiocarbamates: From pesticides to innovative medical treatments. iScience 2021, 24, 102092. [Google Scholar] [CrossRef]
- Glass, J.I.; Lefkowitz, E.J.; Glass, J.S.; Heiner, C.R.; Chen, E.Y.; Cassell, G.H. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature 2000, 407, 757–762. [Google Scholar] [CrossRef]
- Robertson, J.A.; Stemke, G.W.; Davis, J.W.; Harasawa, R.; Thirkell, D.; Kong, F.; Shepard, M.C.; Ford, D.K. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int. J. Syst. Evol. Microbiol. 2002, 52, 587–597. [Google Scholar] [CrossRef]
- Paralanov, V.; Lu, J.; Duffy, L.B.; Crabb, D.M.; Shrivastava, S.; Methé, B.A.; Inman, J.; Yooseph, S.; Xiao, L.; Cassell, G.H.; et al. Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol. 2012, 12, 88. [Google Scholar] [CrossRef]
- Shepard, M.C.; Lunceford, C.D. Differential agar medium (A7) for identification of Ureaplasma urealyticum (human T mycoplasmas) in primary cultures of clinical material. J. Clin. Microbiol. 1976, 3, 613–625. [Google Scholar] [CrossRef]
- Biernat-Sudolska, M.; Rojek-Zakrzewska, D.; Lauterbach, R. Assesment of various diagnostic methods of ureaplasma respiratory tract infections in newborns. Acta Biochim. Pol. 2006, 53, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.B.; Nicholas, R. Serological identification of mycoplasmas by growth and metabolic inhibition tests. Methods Mol. Biol. 1998, 104, 105–111. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biernat-Sudolska, M.; Rojek-Zakrzewska, D.; Drożdż, K.; Bilska-Wilkosz, A. Antimicrobial Activity of N,N-Diethyldithiocarbamate against Ureaplasma parvum and Ureaplasma urealyticum. Int. J. Mol. Sci. 2024, 25, 40. https://doi.org/10.3390/ijms25010040
Biernat-Sudolska M, Rojek-Zakrzewska D, Drożdż K, Bilska-Wilkosz A. Antimicrobial Activity of N,N-Diethyldithiocarbamate against Ureaplasma parvum and Ureaplasma urealyticum. International Journal of Molecular Sciences. 2024; 25(1):40. https://doi.org/10.3390/ijms25010040
Chicago/Turabian StyleBiernat-Sudolska, Małgorzata, Danuta Rojek-Zakrzewska, Kamil Drożdż, and Anna Bilska-Wilkosz. 2024. "Antimicrobial Activity of N,N-Diethyldithiocarbamate against Ureaplasma parvum and Ureaplasma urealyticum" International Journal of Molecular Sciences 25, no. 1: 40. https://doi.org/10.3390/ijms25010040





