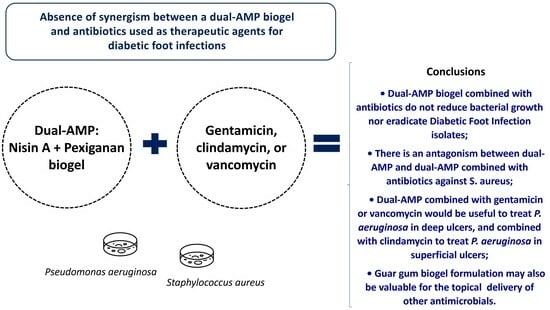
Absence of Synergism between a Dual-AMP Biogel and Antibiotics Used as Therapeutic Agents for Diabetic Foot Infections
Abstract
:1. Introduction
2. Results
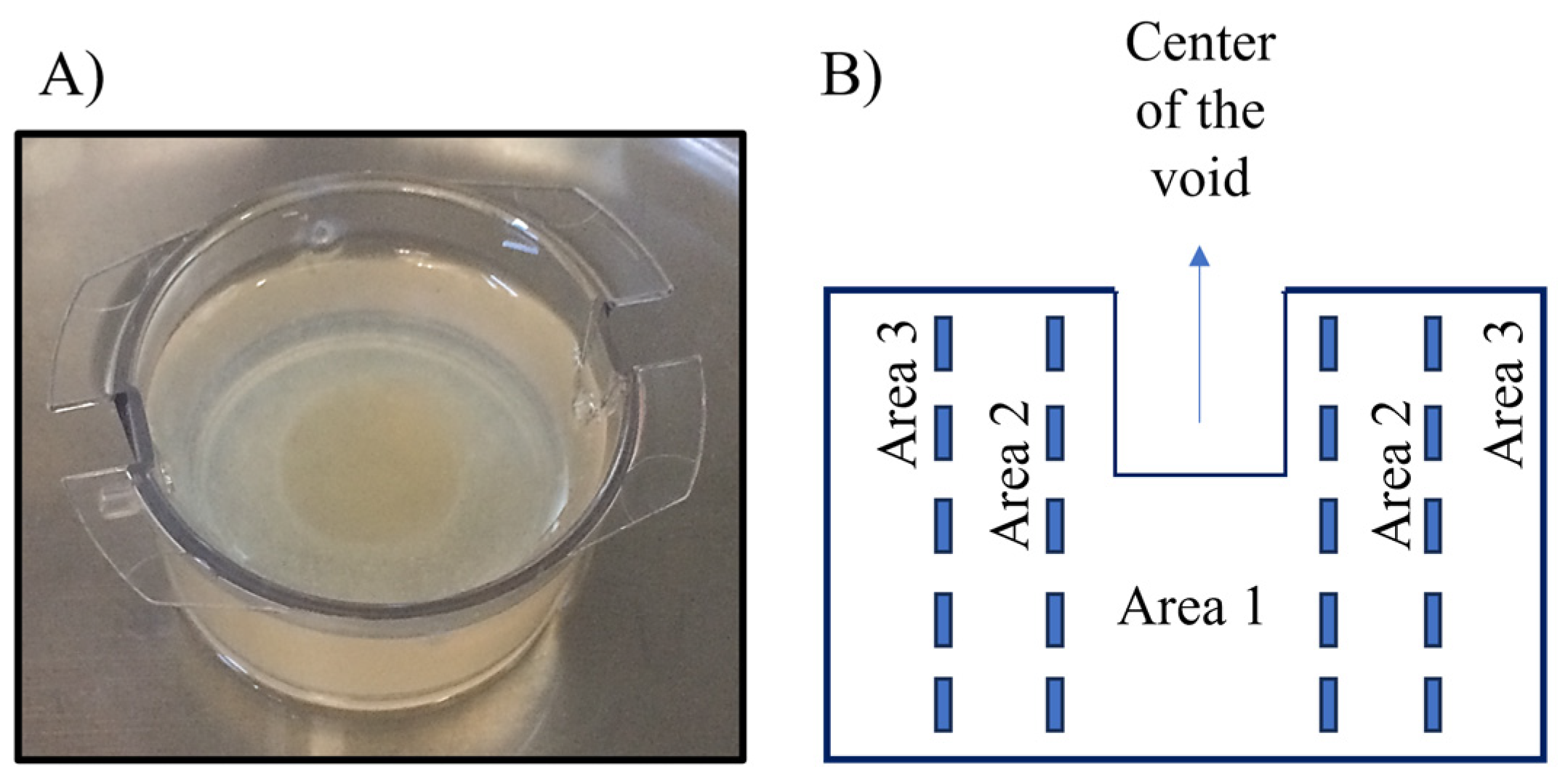
2.1. Antimicrobial and Bacterial Diffusion in a DFI Collagen 3D Model
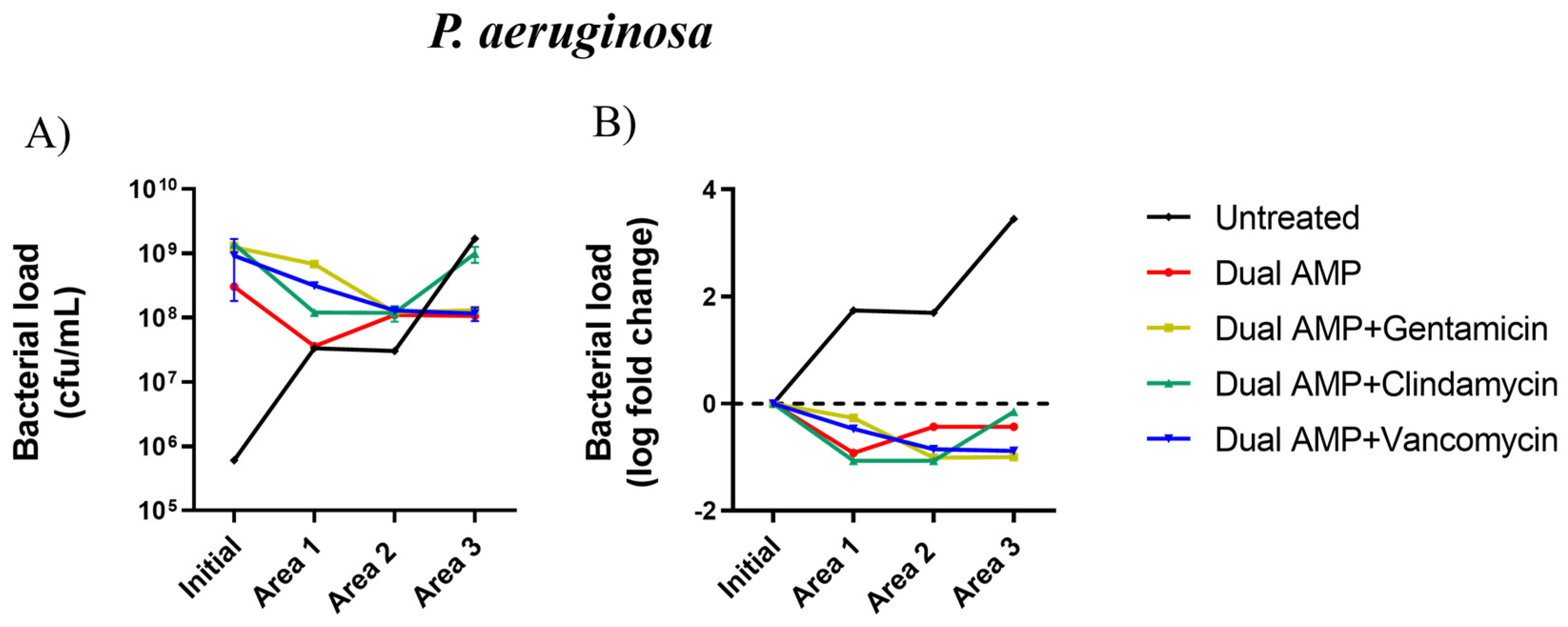
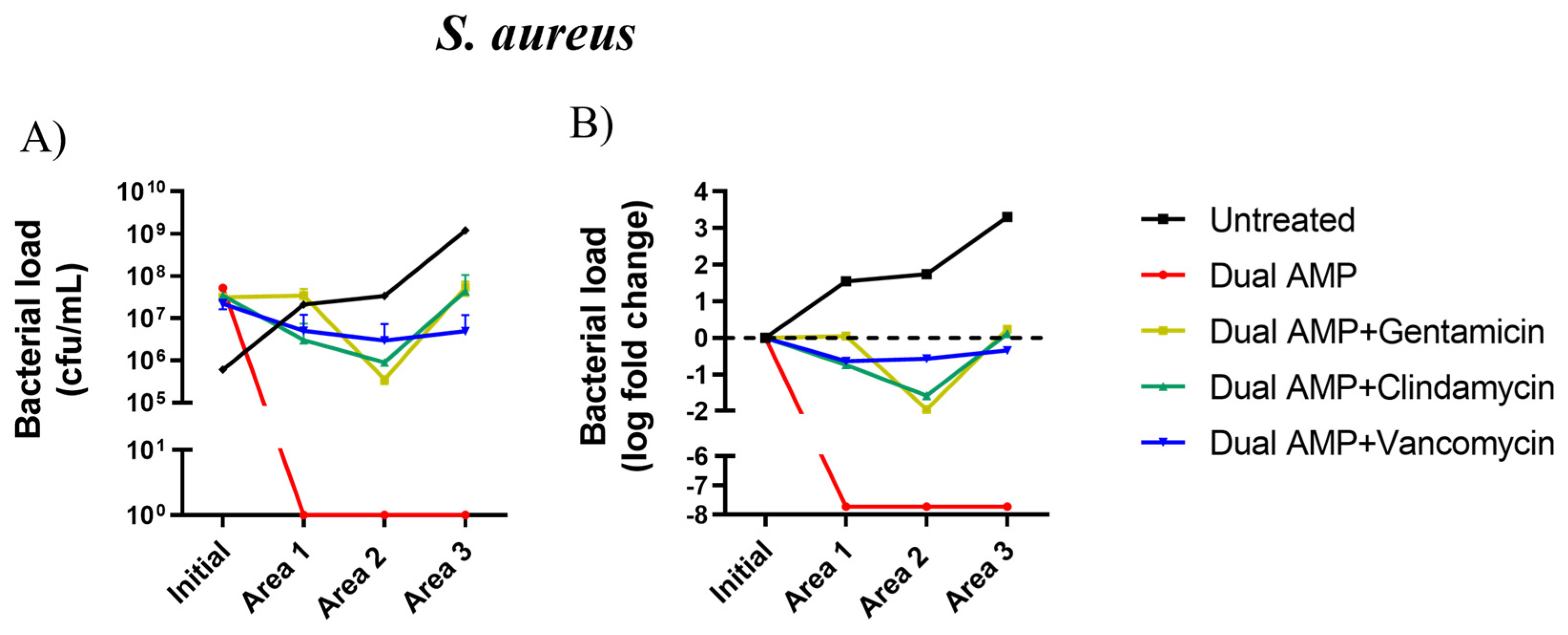
2.2. Inhibitory Activity of Pexiganan-Nisin Dual AMP Biogel Combined with Antibiotics
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Antibiotics and Antimicrobial Peptides
4.3. Collagen Model
4.4. Antimicrobials and Bacterial Diffusion Using the Collagen Model
4.5. Assessment of the Antimicrobial Activity of the Gel
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A. Diabetic foot infections: Current treatment and delaying the ‘post-antibiotic era’. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 246–253. [Google Scholar] [CrossRef] [PubMed]
- Raspovic, K.M.; Wukich, D.K. Self-reported quality of life and diabetic foot infections. J. Foot Ankle Surg. 2014, 53, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.J.; Marques-Costa, A.; Vilela, C.; Neves, J.; Candeias, N.; Cavaco-Silva, P.; Melo-Cristino, J. Clinical and bacteriological survey of diabetic foot infections in Lisbon. Diabetes Res. Clin. Pract. 2012, 95, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Saltoglu, N.; Ergonul, O.; Tulek, N.; Yemisen, M.; Kadanali, A.; Karagoz, G.; Batirel, A.; Ak, O.; Sonmezer, C.; Eraksoy, H.; et al. Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int. J. Infect. Dis. 2018, 70, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Tascini, C. Resistant Infections in the Diabetic Foot: A Frightening Scenario. In The Diabetic Foot Syndrome; Piaggesi, A., Apelqvist, J., Eds.; S. Karger AG: Basel, Switzerland, 2017; Volume 26, pp. 161–166. [Google Scholar]
- Roque-Borda, C.A.; da Silva, P.B.; Rodrigues, M.C.; Azevedo, R.B.; Di Filippo, L.; Duarte, J.L.; Chorilli, M.; Festozo Vicente, E.; Pavan, F.R. Challenge in the Discovery of New Drugs: Antimicrobial Peptides against WHO-List of Critical and High-Priority Bacteria. Pharmaceutics 2021, 13, 773. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Mansour, S.C.; de la Fuente-Núñez, C.; Hancock, R.E. Peptide IDR-1018: Modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Sci. 2015, 21, 323–329. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Gottler, L.M.; Ramamoorthy, A. Structure, membrane orientation, mechanism, and function of pexiganan—A highly potent antimicrobial peptide designed from magainin. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2009, 1788, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, A.; Masso-Silva, J.; Haddaoui, I.; Shymaa, E. Exploring the arsenal of antimicrobial peptides: Mechanisms, diversity, and applications. Biochimie 2023, 214, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Holroyd, K.J.; Zasloff, M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: A randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin. Infect. Dis. 2008, 47, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Lamb, H.M.; Wiseman, L.R. Pexiganan acetate. Drugs 1998, 56, 1047–1052, discussion 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Greber, K.E.; Dawgul, M. Antimicrobial Peptides Under Clinical Trials. Curr. Top. Med. Chem. 2017, 17, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Flamm, R.K.; Rhomberg, P.R.; Simpson, K.M.; Farrell, D.J.; Sader, H.S.; Jones, R.N. In vitro spectrum of pexiganan activity when tested against pathogens from diabetic foot infections and with selected resistance mechanisms. Antimicrob. Agents Chemother. 2015, 59, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- Christ, K.; Wiedemann, I.; Bakowsky, U.; Sahl, H.-G.; Bendas, G. The role of lipid II in membrane binding of and pore formation by nisin analyzed by two combined biosensor techniques. Biochim. Biophys. Acta (BBA)—Biomembr. 2007, 1768, 694–704. [Google Scholar] [CrossRef]
- Dosler, S.; Gerceker, A.A. In vitro activities of nisin alone or in combination with vancomycin and ciprofloxacin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. Chemotherapy 2011, 57, 511–516. [Google Scholar] [CrossRef]
- Okuda, K.; Zendo, T.; Sugimoto, S.; Iwase, T.; Tajima, A.; Yamada, S.; Sonomoto, K.; Mizunoe, Y. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2013, 57, 5572–5579. [Google Scholar] [CrossRef]
- Corbin, A.; Pitts, B.; Parker, A.; Stewart, P.S. Antimicrobial penetration and efficacy in an in vitro oral biofilm model. Antimicrob. Agents Chemother. 2011, 55, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Dosler, S.; Mataraci, E. In vitro pharmacokinetics of antimicrobial cationic peptides alone and in combination with antibiotics against methicillin resistant Staphylococcus aureus biofilms. Peptides 2013, 49, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jia, P.; Kang, H.; Zhang, H.; Liu, Y.; Yang, P.; Yan, Y.; Zuo, G.; Guo, L.; Jiang, M.; et al. Upregulating Hif-1α by Hydrogel Nanofibrous Scaffolds for Rapidly Recruiting Angiogenesis Relative Cells in Diabetic Wound. Adv. Healthc. Mater. 2016, 5, 907–918. [Google Scholar] [CrossRef]
- Santos, R.; Gomes, D.; Macedo, H.; Barros, D.; Tibério, C.; Veiga, A.S.; Tavares, L.; Castanho, M.; Oliveira, M. Guar gum as a new antimicrobial peptide delivery system against diabetic foot ulcers Staphylococcus aureus isolates. J. Med. Microbiol. 2016, 65, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Ruza, D.; Cunha, E.; Tavares, L.; Oliveira, M. Diabetic foot infections: Application of a nisin-biogel to complement the activity of conventional antibiotics and antiseptics against Staphylococcus aureus biofilms. PLoS ONE 2019, 14, e0220000. [Google Scholar] [CrossRef]
- Pletzer, D.; Hancock, R.E. Antibiofilm Peptides: Potential as Broad-Spectrum Agents. J. Bacteriol. 2016, 198, 2572–2578. [Google Scholar] [CrossRef]
- Gomes, D.; Santos, R.; Soares, R.S.; Reis, S.; Carvalho, S.; Rego, P.; C. Peleteiro, M.; Tavares, L.; Oliveira, M. Pexiganan in Combination with Nisin to Control Polymicrobial Diabetic Foot Infections. Antibiotics 2020, 9, 128. [Google Scholar] [CrossRef]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of Persister Cells of Pseudomonas aeruginosa and Staphylococcus aureus by Chemical Treatment and Evaluation of Their Susceptibility to Membrane-Targeting Agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Yu, G.; Baeder, D.Y.; Regoes, R.R.; Rolff, J. Combination Effects of Antimicrobial Peptides. Antimicrob. Agents Chemother. 2016, 60, 1717–1724. [Google Scholar] [CrossRef]
- Field, D.; O’ Connor, R.; Cotter, P.D.; Ross, R.P.; Hill, C. In Vitro Activities of Nisin and Nisin Derivatives Alone and In Combination with Antibiotics against Staphylococcus Biofilms. Front. Microbiol. 2016, 7, 508. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Scalise, G. In-vitro activity and killing effect of polycationic peptides on methicillin-resistant Staphylococcus aureus and interactions with clinically used antibiotics. Diagn. Microbiol. Infect. Dis. 2000, 38, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Silvestri, C.; Ghiselli, R.; Kamysz, W.; Minardi, D.; Castelli, P.; Orlando, F.; Kamysz, E.; Provinciali, M.; Muzzonigro, G.; et al. In vitro and in vivo effects of sub-MICs of pexiganan and imipenem on Pseudomonas aeruginosa adhesion and biofilm development. Infez. Med. 2013, 21, 287–295. [Google Scholar]
- Cirioni, O.; Simonetti, O.; Morroni, G.; Brescini, L.; Kamysz, W.; Kamysz, E.; Orlando, F.; Pierpaoli, E.; Caffarini, M.; Orciani, M.; et al. Efficacy of Pexiganan Combination with Tigecycline in a Mouse Model of Pseudomonas aeruginosa Sepsis. Curr. Top. Med. Chem. 2018, 18, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K.; Kamysz, W.; Piechowicz, L. Activity of antimicrobial peptides, alone or combined with conventional antibiotics, against Staphylococcus aureus isolated from the airways of cystic fibrosis patients. Virulence 2017, 8, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; MacDonald, D.L.; Holroyd, K.J.; Thornsberry, C.; Wexler, H.; Zasloff, M. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 1999, 43, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Mataraci, E.; Dosler, S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2012, 56, 6366–6371. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Seisling, N.; Cotter, P.D.; Ross, R.P.; Hill, C. Synergistic Nisin-Polymyxin Combinations for the Control of Pseudomonas Biofilm Formation. Front. Microbiol. 2016, 7, 1713. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef]
- Costa-Almeida, C.E. Collagen implant with gentamicin sulphate as an option to treat a neuroischaemic diabetic foot ulcer: Case report. Int. J. Surg. Case Rep. 2016, 21, 48–51. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Kuss, M.; Edmonds, M.; Reyzelman, A.; Sigal, F. Topical application of a gentamicin-collagen sponge combined with systemic antibiotic therapy for the treatment of diabetic foot infections of moderate severity: A randomized, controlled, multicenter clinical trial. J. Am. Podiatr. Med. Assoc. 2012, 102, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Bistas, K.; Le, J. Clindamycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Patel, S.; Preuss, C.; Bernice, F. Vancomycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chaves, B.; Tadi, P. Gentamycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Senneville, E. Antibacterial Treatment in Diabetic Foot Infections. In The Diabetic Foot Syndrome; Piaggesi, A., Apelqvist, J., Eds.; S. Karger AG: Basel, Switzerland, 2017; Volume 26, pp. 167–183. [Google Scholar]
- Kim, P.J.; Steinberg, J.S. Wound care: Biofilm and its impact on the latest treatment modalities for ulcerations of the diabetic foot. Semin. Vasc. Surg. 2012, 25, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Senneville, É.; Albalawi, Z.; van Asten, S.A.; Abbas, Z.G.; Allison, G.; Aragón-Sánchez, J.; Embil, J.M.; Lavery, L.A.; Alhasan, M.; Oz, O.; et al. IWGDF/IDSA Guidelines on the Diagnosis and Treatment of Diabetes-related Foot Infections (IWGDF/IDSA 2023). Clin. Infect. Dis. 2023, 1, e3687. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Duong, L.; Gross, S.P.; Siryaporn, A. Developing Antimicrobial Synergy With AMPs. Front. Med. Technol. 2021, 3, 640981. [Google Scholar] [CrossRef]
- Park, S.C.; Park, Y.; Hahm, K.S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef]
- He, J.; Starr, C.G.; Wimley, W.C. A lack of synergy between membrane-permeabilizing cationic antimicrobial peptides and conventional antibiotics. Biochim. Biophys. Acta 2015, 1848, 8–15. [Google Scholar] [CrossRef]
- Brennan-Krohn, T.; Kirby, J.E. When One Drug Is Not Enough: Context, Methodology, and Future Prospects in Antibacterial Synergy Testing. Clin. Lab. Med. 2019, 39, 345–358. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Oshiro, T.; Nagasawa, Z.; Hanaki, H.; Ikeda-Dantsuji, Y.; Nagayama, A. The antagonistic effects of a combination of vancomycin and minocycline in Staphylococcus aureus with heterogeneous resistance to vancomycin. J. Infect. Chemother. 2008, 14, 15–22. [Google Scholar] [CrossRef]
- Lalouckova, K.; Skřivanová, E.; Rondevaldova, J.; Frankova, A.; Soukup, J.; Kokoska, L. In vitro antagonistic inhibitory effects of palm seed crude oils and their main constituent, lauric acid, with oxacillin in Staphylococcus aureus. Sci. Rep. 2021, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Brochado, A.R.; Telzerow, A.; Bobonis, J.; Banzhaf, M.; Mateus, A.; Selkrig, J.; Huth, E.; Bassler, S.; Zamarreño-Beas, J.; Zietek, M.; et al. Species-specific activity of antibacterial drug combinations. Nature 2018, 559, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sahoo, N.; Bhunia, A. Antimicrobial Peptides and their Pore/Ion Channel Properties in Neutralization of Pathogenic Microbes. Curr. Top. Med. Chem. 2016, 16, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra-Maillard, A.P.V.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta potential beyond materials science: Applications to bacterial systems and to the development of novel antimicrobials. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. Antibiotic Essentials; Physicians’ Press: Royal Oak, MI, USA, 2003; 406p. [Google Scholar]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Mottola, C.; Semedo-Lemsaddek, T.; Mendes, J.J.; Melo-Cristino, J.; Tavares, L.; Cavaco-Silva, P.; Oliveira, M. Molecular typing, virulence traits and antimicrobial resistance of diabetic foot staphylococci. J. Biomed. Sci. 2016, 23, 33. [Google Scholar] [CrossRef]
- Mottola, C.; Matias, C.S.; Mendes, J.J.; Melo-Cristino, J.; Tavares, L.; Cavaco-Silva, P.; Oliveira, M. Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections. BMC Microbiol. 2016, 16, 119. [Google Scholar] [CrossRef]
- Werthén, M.; Henriksson, L.; Jensen, P.; Sternberg, C.; Givskov, M.; Bjarnsholt, T. An in vitro model of bacterial infections in wounds and other soft tissues. Apmis 2010, 118, 156–164. [Google Scholar] [CrossRef]
- Price, B.L.; Lovering, A.M.; Bowling, F.L.; Dobson, C.B. Development of a Novel Collagen Wound Model to Simulate the Activity and Distribution of Antimicrobials in Soft Tissue during Diabetic Foot Infection. Antimicrob. Agents Chemother. 2016, 60, 6880–6889. [Google Scholar] [CrossRef] [PubMed]
- Oyibo, S.O.; Jude, E.B.; Tarawneh, I.; Nguyen, H.C.; Harkless, L.B.; Boulton, A.J. A comparison of two diabetic foot ulcer classification systems: The Wagner and the University of Texas wound classification systems. Diabetes Care 2001, 24, 84–88. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Area 1 | Area 2 | Area 3 | |||
|---|---|---|---|---|---|---|
| Log Load Reduction | Log Load Reduction of the Dual AMP Alone | Log Load Reduction | Log Load Reduction of the Dual AMP Alone | Log Load Reduction | Log Load Reduction of the Dual AMP Alone | |
| Dual-AMP | −0.92 | −0.44 | −0.44 | |||
| Dual-AMP + gentamicin | −0.27 | −1.28 | −1.01 | −0.04 | −0.98 | −0.08 |
| Dual-AMP + clindamycin | −1.07 | −0.52 | −1.07 | −0.04 | −0.15 | −0.96 |
| Dual-AMP + vancomycin | −0.47 | −0.94 | −0.85 | −0.07 | −0.90 | −0.02 |
| Compounds | Area 1 | Area 2 | Area 3 | |||
|---|---|---|---|---|---|---|
| Log Load Reduction | Log Load Reduction of the Dual AMP Alone | Log Load Reduction | Log Load Reduction of the Dual AMP Alone | Log Load Reduction | Log Load Reduction of the Dual AMP Alone | |
| Dual-AMP | −7.72 | −7.72 | −7.72 | |||
| Dual-AMP + gentamicin | 0.04 | −7.54 * | −1.95 | −5.54 * | 0.23 | −7.73 * |
| Dual-AMP + clindamycin | −0.75 | −6.79 * | −1.58 | −5.95 * | 0.10 | −7.64 * |
| Dual-AMP + vancomycin | −0.64 | −6.70 * | −0.56 | −6.78 * | −0.35 | −7.00 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, R.S.; Gomes, D.; Serrano, I.; Cunha, E.; Tavares, L.; Oliveira, M. Absence of Synergism between a Dual-AMP Biogel and Antibiotics Used as Therapeutic Agents for Diabetic Foot Infections. Int. J. Mol. Sci. 2024, 25, 407. https://doi.org/10.3390/ijms25010407
Soares RS, Gomes D, Serrano I, Cunha E, Tavares L, Oliveira M. Absence of Synergism between a Dual-AMP Biogel and Antibiotics Used as Therapeutic Agents for Diabetic Foot Infections. International Journal of Molecular Sciences. 2024; 25(1):407. https://doi.org/10.3390/ijms25010407
Chicago/Turabian StyleSoares, Rui Silva, Diana Gomes, Isa Serrano, Eva Cunha, Luís Tavares, and Manuela Oliveira. 2024. "Absence of Synergism between a Dual-AMP Biogel and Antibiotics Used as Therapeutic Agents for Diabetic Foot Infections" International Journal of Molecular Sciences 25, no. 1: 407. https://doi.org/10.3390/ijms25010407
APA StyleSoares, R. S., Gomes, D., Serrano, I., Cunha, E., Tavares, L., & Oliveira, M. (2024). Absence of Synergism between a Dual-AMP Biogel and Antibiotics Used as Therapeutic Agents for Diabetic Foot Infections. International Journal of Molecular Sciences, 25(1), 407. https://doi.org/10.3390/ijms25010407