The Role of the Gut Microbiota in Anorexia Nervosa in Children and Adults—Systematic Review
Abstract
:1. Introduction
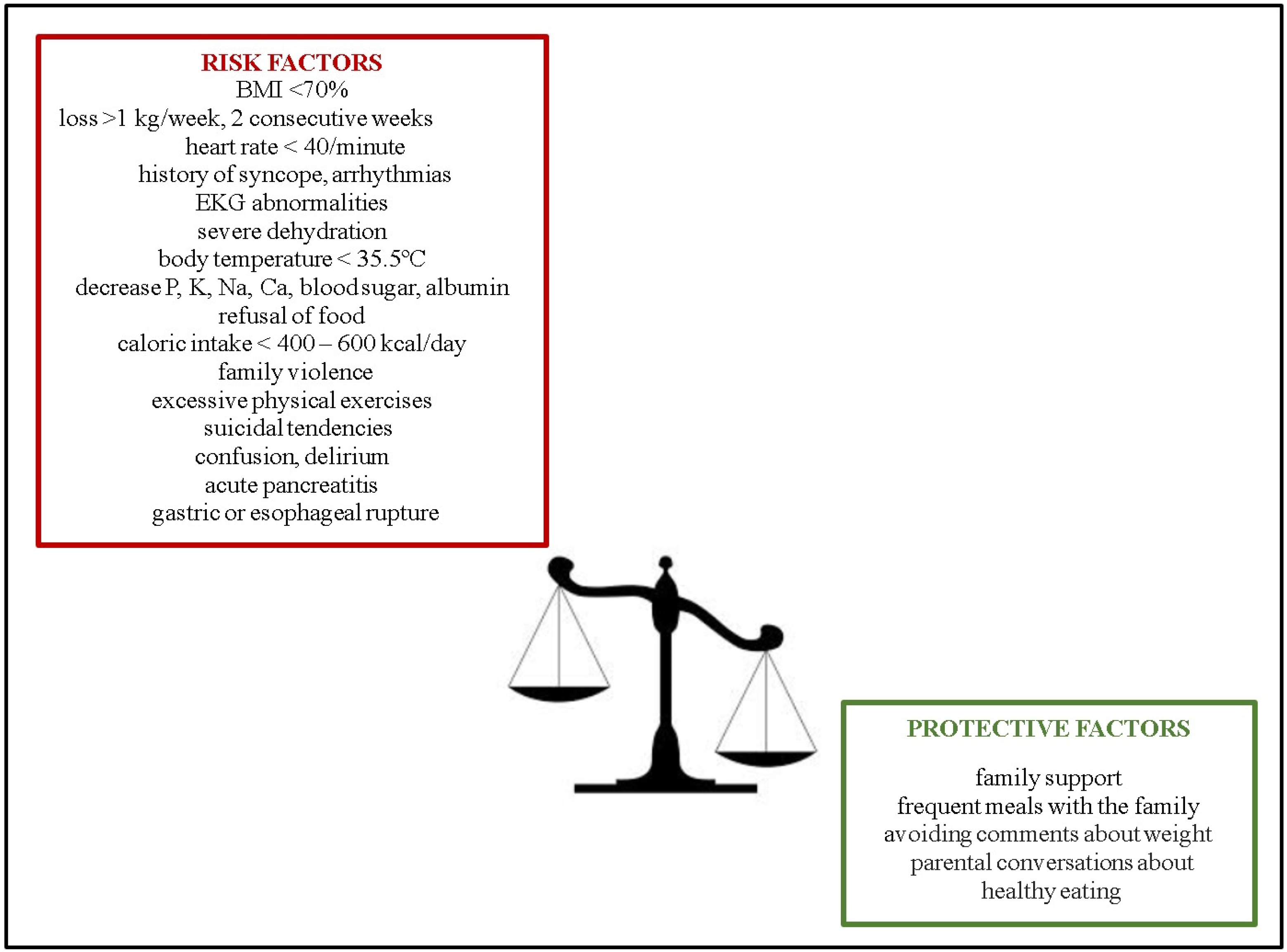
1.1. Epidemiology
1.2. Motivation
1.3. Objectives of the Study
2. Materials and Methods
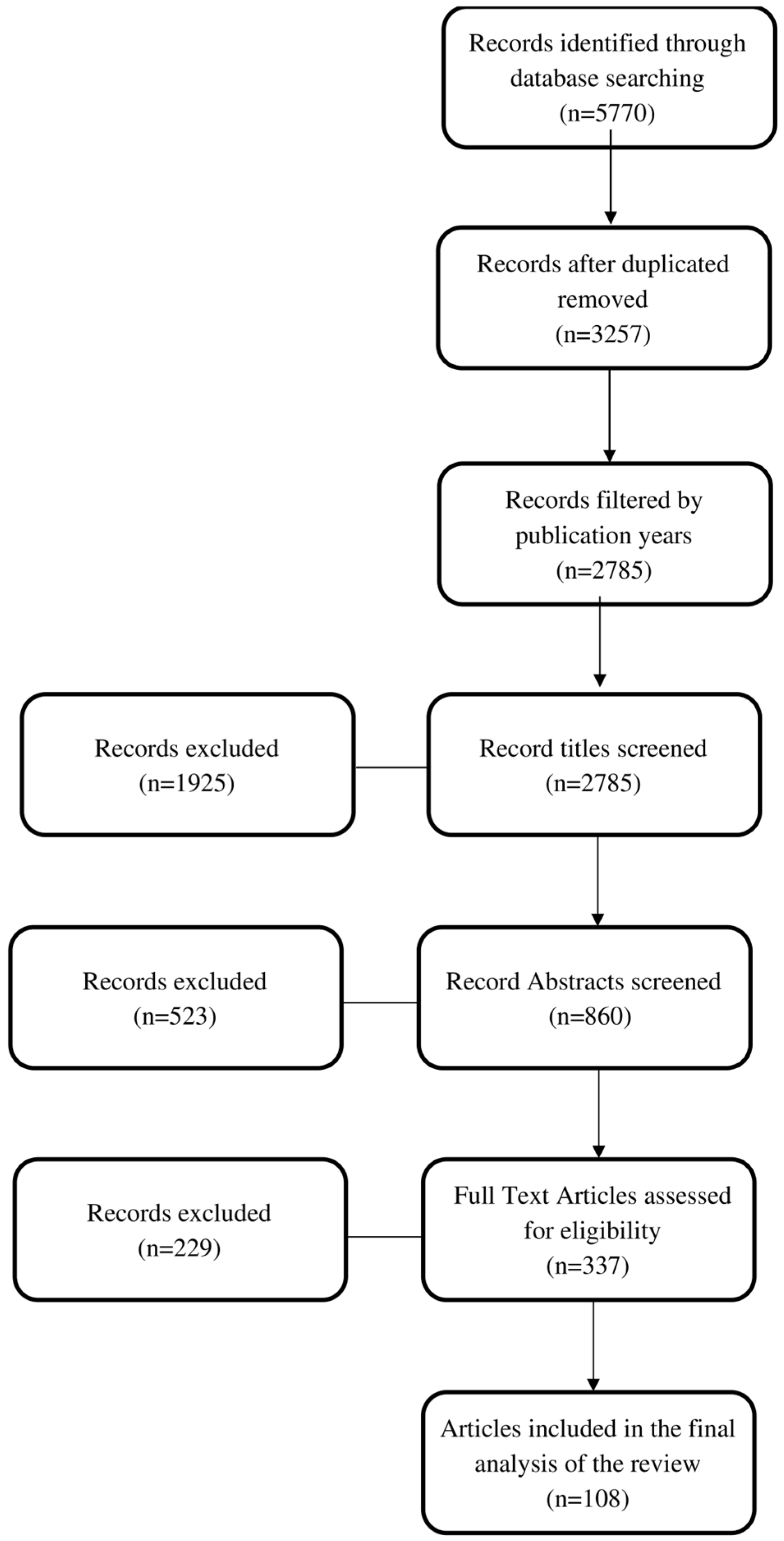
2.1. Search Strategy
2.2. Study Selection
3. Results
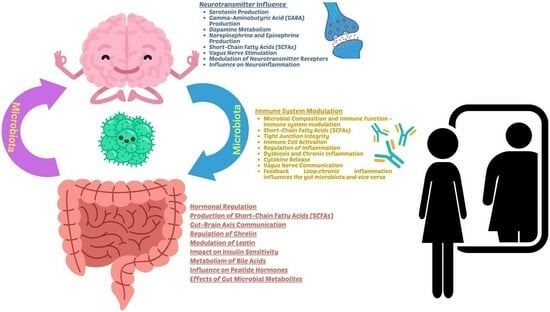
3.1. Influences of Gut Microbiota
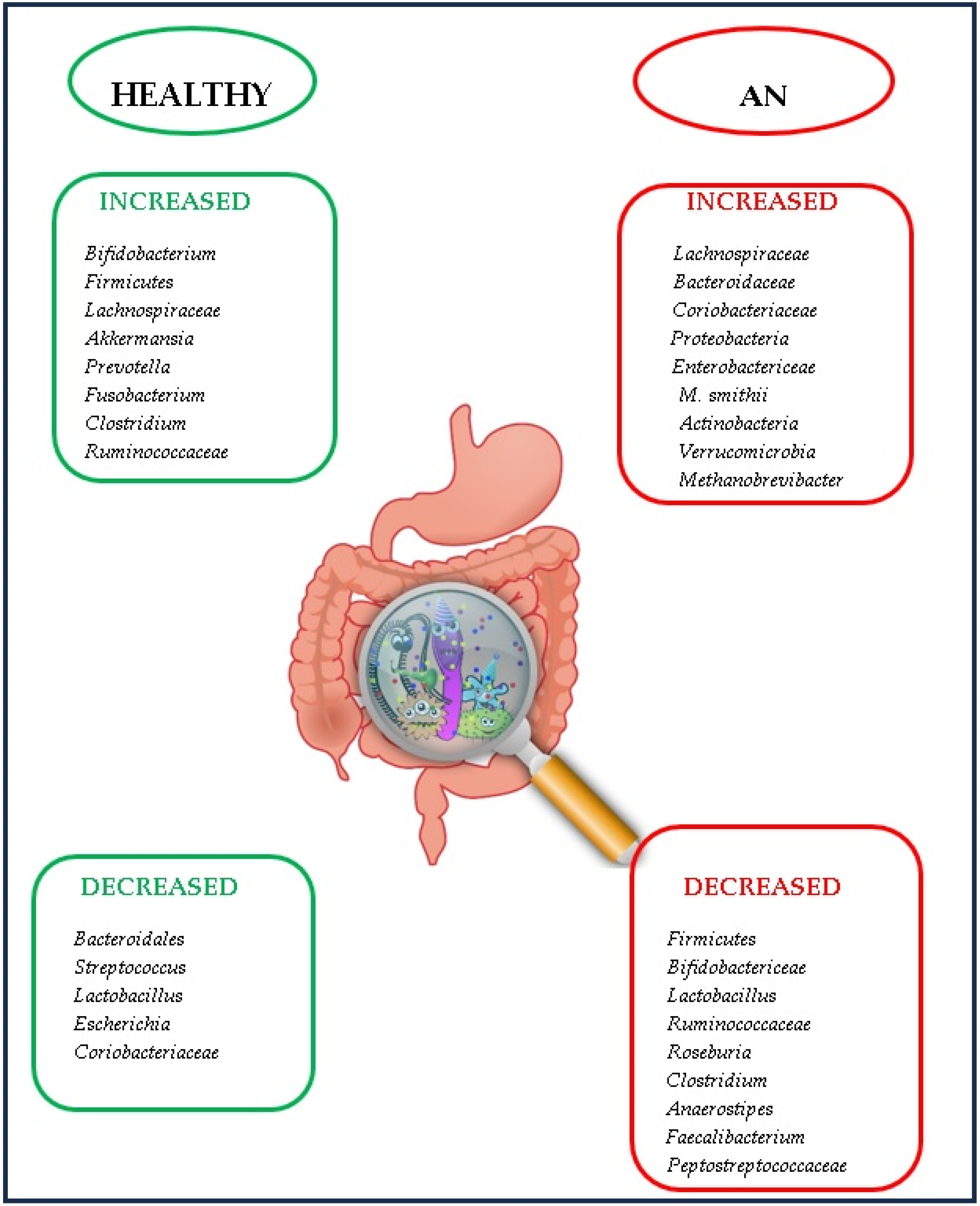
3.2. Gut Microbiota in AN
- The existence of changes in the microbiota induces and/or contributes to food restriction;
- For each patient, the intestinal microbiota determines the type of malnutrition (marasmus or kwashiorkor) [22].
- -
- -
- A reduction in Bacteroidetes [50].
- -
- A decrease in Bacteroidetes, Faecalibacterium, Agathobacter, Balutia, and Lachnospira, an increase in Ruminococcaceae, Alistipes, and Clostridiales and no difference regarding fungi [51].
- -
- An increase in Enterobacteriaceae, but without significant differences compared to the control group [52].
- -
- An increased level of Enterobacteriaceae and Alistipes and a decreased level of Faecalibacterium [53].
3.3. The Operational Mechanisms through Which the Gut Microbiota Functions in AN
3.4. Depression Linked to AN through Gut Microbiota
- -
- The growth of Actinobacteria [90].
- -
- A decrease in Actinobacteria, Dialistea, and Coprococcus and an increase in Proteobacteria, and Alistipes [91].
- -
- Variable modifications of Bacteroidetes, a reduction in Faecalibacterium and Coprococcus, and an increase in Eggertehella [92].
- -
- -
- Low Faecalibacterium in AN patients with major depression [92].
- -
3.5. Gut Microbiota and BMI
3.6. Gut Microbiota after Weight Gain
- -
- An increase in Firmicutes, Ruminococcaceae, Faecalibacterium, Fusicatenibacter, and Lachnospiraceae.
- -
- A reduction in Bacteroides and Parabacteroides.
- -
- An increase in Leuconostocaceae and a decrease in Collinsella, Parabacteroides, and Catabacter [101].
- -
- The non-modification of the Roseburia species [93].
- -
3.7. Therapy
4. Discussion
Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS ONE 2017, 12, e0179739. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, M.; Ceccarini, M.R.; Stoppini, F.; Cataldi, S.; Mazzeschi, C.; Delvecchio, E.; Albi, E.; Gizzi, G. Brain and gut microbiota disorders in the psychopathology of anorexia nervosa. Transl. Neurosci. 2022, 13, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Dhopatkar, N.; Keeler, J.L.; Mutwalli, H.; Whelan, K.; Treasure, J.; Himmerich, H. Gastrointestinal symptoms, gut microbiome, probiotics and prebiotics in anorexia nervosa: A review of mechanistic rationale and clinical evidence. Psychoneuroendocrinology 2023, 147, 105959. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.; Belheouane, M.; Schulz, N.; Dempfle, A.; Baines, J.F.; Herpertz-Dahlmann, B. The Impact of Starvation on the Microbiome and Gut-Brain Interaction in Anorexia Nervosa. Front. Endocrinol. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Dechelotte, P.; Ribet, D. Intestinal microbiota and Anorexia Nervosa. Clin. Nutr. Exp. 2019, 28, 11–21. [Google Scholar] [CrossRef]
- van der Gun, L.L. Elucidating the Role of the Anorectic Gut Microbiome in Value-Based Decision Making and Reward Signalling. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2023. Available online: https://studenttheses.uu.nl/bitstream/handle/20.500.12932/43509/vanderGun_LL_report_AN_microbiome_in_flexible_value-based_decision_making_and_reward_signaling.pdf?sequence=1 (accessed on 15 August 2023).
- Flint, H.J. Obesity and the gut microbiota. J. Clin. Gastroenterol. 2011, 45, S128–S132. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, L.L.; Lane, M.A.; Committee on adolescence. Identification and Management of Eating Disorders in Children and Adolescents. Pediatrics 2021, 147, e2020040279. [Google Scholar] [CrossRef] [PubMed]
- Grigoroiu-Şerbănescu, M.A. Brief inventory for assessing personality traits and disorders in children aged 8–11. Rev. Roum. Neurol. Psychiatr. 1987, 25, 41–55. [Google Scholar]
- Hoeck, H.W.; van Hoeken, D. Review of the prevalence and incidence of eating disorders. Int. J. Eat. Disord. 2003, 34, 383–396. [Google Scholar] [CrossRef]
- Kovács Krizbai, T.; Szabó, P. Prevalence of eating disorders in Romanian, Hungarian and Saxon secondary school students in Transylvania. Psychiatr. Hung. 2009, 24, 124–132. [Google Scholar]
- Herpertz-Dahlmann, B. Adolescent Eating Disorders: Definitions, Symptomatology, Epidemiology and Comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.A.; Crow, S.J.; Le Grange, D.; Swendsen, J.; Merikangas, K.R. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch. Gen. Psychiatry 2011, 68, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Zeiler, M.; Waldherr, K.; Philipp, J.; Truttmann, S.; Dür, W.; Treasure, J.L.; Karwautz, A.F.K. Mental health problems in Austrian adolescents: A nationwide, two-stage epidemiological study applying DSM-5 criteria. Eur. Child. Adolesc. Psychiatry 2017, 26, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Petkova, H.; Simic, M.; Nicholls, D.; Ford, T.; Prina, A.M.; Stuart, R.; Livingstone, N.; Kelly, G.; Macdonald, G.; Eisler, I.; et al. Incidence of anorexia nervosa in young people in the UK and Ireland: A national surveillance study. BMJ Open 2019, 9, e027339. [Google Scholar] [CrossRef] [PubMed]
- Langdon-Daly, J.; Serpell, L. Protective factors against disordered eating in family systems: A systematic review of research. J. Eat. Disord. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Neale, J.; Hudson, L.D. Anorexia nervosa in adolescents. Br. J. Hosp. Med. 2020, 81, 1–8. [Google Scholar] [CrossRef]
- Argyrides, M.; Anastasiades, E.; Alexiou, E. Risk and Protective Factors of Disordered Eating in Adolescents Based on Gender and Body Mass Index. Int. J. Environ. Res. Public Health 2020, 17, 9238. [Google Scholar] [CrossRef]
- Barakat, S.; McLean, S.A.; Bryant, E.; Le, A.; Marks, P.; Touyz, S.; Maguire, S. Risk factors for eating disorders: Findings from a rapid review. J. Eat. Disord. 2023, 11, 8. [Google Scholar] [CrossRef]
- Freimer, D.; Yang, T.T.; Ho, T.C.; Tymofiyeva, O.; Leung, C. The gut microbiota, HPA axis, and brain in adolescent-onset depression: Probiotics as a novel treatment. Brain Behav. Immun.-Health 2022, 26, 100541. [Google Scholar] [CrossRef]
- Gorwood, P.; Blanchet-Collet, C.; Chartrel, N.; Duclos, J.; Dechelotte, P.; Hanachi, M.; Fetissov, S.; Godart, N.; Melchior, J.C.; Ramoz, N.; et al. New Insights in Anorexia Nervosa. Front. Neurosci. 2016, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Gagliardi, M.; Guarino, M.P.L.; Siniscalchi, M.; Ciacci, C.; Iovino, P. Eating disorders and gastrointestinal diseases. Nutrients 2019, 11, 3038. [Google Scholar] [CrossRef] [PubMed]
- Schwensen, H.F.; Kan, C.; Treasure, J.; Høiby, N.; Sjögren, M. A systematic review of studies on the faecal microbiota in anorexia nervosa: Future research may need to include microbiota from the small intestine. Eat. Weight Disord. EWD 2018, 23, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Blaser, M.J. Pathways in microbe-induced obesity. Cell Metab. 2013, 17, 883–894. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Sarkar, A.; Harty, S.; Lehto, S.M.; Moeller, A.H.; Dinan, T.G.; Dunbar, R.I.M.; Cryan, J.F.; Burnet, P.W.J. The Microbiome in Psychology and Cognitive Neuroscience. Trends Cogn. Sci. 2018, 22, 611–636. [Google Scholar] [CrossRef]
- Brett, B.E.; de Weerth, C. The microbiota-gut-brain axis: A promising avenue to foster healthy developmental outcomes. Dev. Psychobiol. 2019, 61, 772–782. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Vaher, K.; Bogaert, D.; Richardson, H.; Boardman, J.P. Microbiome-gut-brain axis in brain development, cognition and behaviour during infancy and early childhood. Dev. Rev. 2022, 66, 101038. [Google Scholar] [CrossRef]
- Karakuła-Juchnowicz, H.; Pankowicz, H.; Juchnowicz, D.; Valverde Piedra, J.L.; Małecka-Massalska, T. Intestinal microbiota—A key to understanding the pathophysiology of anorexia nervosa? Psychiatr. Pol. 2017, 51, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.M.; Barnett, J.A.; Gibson, D.L. A critical analysis of eating disorders and the gut microbiome. J. Eat. Disord. 2022, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behaviour, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Lafuse, W.P.; Galley, J.D.; Ali, M.M.; Ahmer, B.M.; Bailey, M.T. The intestinal microbiota is necessary for stressor-induced enhancement of splenic macrophage microbicidal activity. Brain Behav. Immun. 2012, 26, 371–382. [Google Scholar] [CrossRef]
- Gareau, M.G.; Silva, M.A.; Perdue, M.H. Pathophysiological mechanisms of stress-induced intestinal damage. Curr. Mol. Med. 2008, 8, 274–281. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Spectrum of mast cell activation disorders. Expert Rev. Clin. Immunol. 2014, 10, 729–739. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B.; Seitz, J.; Baines, J. Food matters: How the microbiome and gut-brain interaction might impact the development and course of anorexia nervosa. Eur. Child Adolesc. Psychiatry 2017, 26, 1031–1041. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef]
- Mack, I.; Cuntz, U.; Grämer, C.; Niedermaier, S.; Pohl, C.; Schwiertz, A.; Zimmermann, K.; Zipfel, S.; Enck, P.; Penders, J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched-chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 2016, 6, 26752. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Yang, L.; Yao, G.; Geng, S.; Ge, Q.; Bo, S.; Li, X. Features of gut microbiota in patients with anorexia nervosa. Chin. Med. J. 2022, 135, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Morkl, S.; Lackner, S.; Müller, W.; Gorkiewicz, G.; Kashofer, K.; Oberascher, A.; Painold, A.; Holl, A.; Holzer, P.; Meinitzer, A.; et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int. J. Eat. Disord. 2017, 50, 1421–1431. [Google Scholar] [CrossRef]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, S.C.; Glenny, E.M.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tsilimigras, M.C.B.; Fodor, A.A.; Bulik, C.M.; Carroll, I.M. Daily Changes in Composition and Diversity of the Intestinal Microbiota in Patients with Anorexia Nervosa: A Series of Three Cases. Eur. Eat. Disord. Rev. 2017, 25, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS ONE 2015, 10, e0145274. [Google Scholar] [CrossRef]
- Hanachi, M.; Manichanh, C.; Schoenenberger, A.; Pascal, V.; Levenez, F.; Cournède, N.; Doré, J.; Melchior, J.C. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: An explicative factor of functional intestinal disorders? Clin. Nutr. 2019, 38, 2304–2310. [Google Scholar] [CrossRef]
- Hata, T.; Miyata, N.; Takakura, S.; Yoshihara, K.; Asano, Y.; Kimura-Todani, T.; Yamashita, M.; Zhang, X.T.; Watanabe, N.; Mikami, K.; et al. The Gut Microbiome Derived From Anorexia Nervosa Patients Impairs Weight Gain and Behavioral Performance in Female Mice. Endocrinology 2019, 160, 2441–2452. [Google Scholar] [CrossRef]
- Prochazkova, P.; Roubalova, R.; Dvorak, J.; Kreisinger, J.; Hill, M.; Tlaskalova-Hogenova, H.; Tomasova, P.; Pelantova, H.; Cermakova, M.; Kuzma, M.; et al. The intestinal microbiota and metabolites in patients with anorexia nervosa. Gut Microbes 2021, 13, 1–25. [Google Scholar] [CrossRef]
- Roubalova, R.; Prochazkova, P.; Papezova, H.; Smitka, K.; Bilej, M.; Tlaskalova-Hogenov, H. Anorexia nervosa: Gut microbiota-immune-brain interactions. Clin. Nutr. 2020, 36, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered faecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. NeuroTherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Zhang, X.; Yu, Z.H.; Zhang, Z.; Deng, M.; Zhao, J.H.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Xia, X.; He, S.Y.; Zhang, X.L.; Wang, D.; He, Q.; Xiao, Q.A.; Yang, Y. The causality between gut microbiome and anorexia nervosa: A Mendelian randomization analysis. Front. Microbiol. 2023, 14, 1290246. [Google Scholar] [CrossRef]
- Calcaterra, V.; Rossi, V.; Massini, G.; Regalbuto, C.; Hruby, C.; Panelli, S.; Bandi, C.; Zuccotti, G. Precocious puberty and microbiota: The role of the sex hormone–gut microbiome axis. Front. Endocrinol. 2022, 13, 1000919. [Google Scholar] [CrossRef]
- del Castillo-Izquierdo, Á.; Mayneris-Perxachs, J.; Fernández-Rea, J.M. Bidirectional relationships between the gut microbiome and sexual traits. Am. J. Physiol. Cell Physiol. 2022, 322, C1223–C1229. [Google Scholar] [CrossRef]
- Tennoune, N.; Chan, P.; Breton, J.; Legrand, R.; Chabane, Y.N.; Akkermann, K.; Järv, A.; Ouelaa, W.; Takagi, K.; Ghouzali, I.; et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl. Psychiatry 2014, 4, e458. [Google Scholar] [CrossRef]
- Sinno, M.H.; Do Rego, J.C.; Coëffier, M.; Bole-Feysot, C.; Ducrotté, P.; Gilbert, D.; Tron, F.; Costentin, J.; Hökfelt, T.; Déchelotte, P.; et al. Regulation of feeding and anxiety by alpha-MSH reactive autoantibodies. Psychoneuroendocrinology 2009, 34, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Legrand, R.; Akkermann, K.; Järv, A.; Harro, J.; Déchelotte, P.; Fetissov, S.O. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int. J. Eat. Disord. 2016, 49, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Glenny, E.M.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.M.; Carroll, I.M. Eating Disorders and the Intestinal Microbiota: Mechanisms of Energy Homeostasis and Behavioral Influence. Curr. Psychiatry Rep. 2017, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Ghenciulescu, A.; Park, R.J.; Burnet, P.W.J. The Gut Microbiome in Anorexia Nervosa: Friend or Foe? Front. Psychiatry 2021, 11, 611677. [Google Scholar] [CrossRef] [PubMed]
- Ruusunen, A.; Rocks, T.; Jacka, F.; Loughman, A. The gut microbiome in anorexia nervosa: Relevance for nutritional rehabilitation. Psychopharmacology 2019, 236, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Gröbner, E.M.; Zeiler, M.; Fischmeister, F.P.S.; Kollndorfer, K.; Schmelz, S.; Schneider, A.; Haid-Stecher, N.; Sevecke, K.; Wagner, G.; Keller, L.; et al. The effects of probiotics administration on the gut microbiome in adolescents with anorexia nervosa—A study protocol for a longitudinal, double-blind, randomized, placebo-controlled trial. Eur. Eat. Disord. Rev. 2022, 30, 61–74. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Mens Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota–gut–brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022, 79, 80. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Palmnä, M.S.A.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-Dose Aspartame Consumption Differentially Affects Gut Microbiota-Host Metabolic Interactions in the Diet-Induced Obese Rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Glick-Bauer, M.; Yeh, M.C. The Health Advantage of a Vegan Diet: Exploring the Gut Microbiota Connection. Nutrients 2014, 6, 4822–4838. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Figueroa, V.; Biscaia, J.M.; Mohedano, R.B.; Blanco-Fernandez, A.; Bailen, M.; Bressa, C.; Larrosa, M.; Gonzalez-Soltero, R. Can Gut Microbiota and Lifestyle Help Us in the Handling of Anorexia Nervosa Patients? Microorganisms 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Andrioaie, I.M.; Duhaniuc, A.; Nastase, E.V.; Iancu, L.S.; Lunca, C.; Trofin, F.; Anton-Păduraru, D.T.; Dorneanu, O.S. The Role of the Gut Microbiome in Psychiatric Disorders. Microorganisms 2022, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Tsai, P.; Anderson, E.J.; Hubbard, J.L.; Gallagher, K.; Soyka, L.A.; Miller, K.K.; Herzog, D.B.; Klibanski, A. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. Am. J. Clin. Nutr. 2006, 84, 698–706. [Google Scholar] [CrossRef]
- Chiurazzi, C.; Cioffi, I.; De Caprio, C.; De Filippo, E.; Marra, M.; Sammarco, R.; Di Guglielmo, M.L.; Contaldo, F.; Pasanisi, F. Adequacy of nutrient intake in women with restrictive anorexia nervosa. Nutrition 2017, 38, 80–84. [Google Scholar] [CrossRef]
- Patsalos, O.; Dalton, B.; Kyprianou, C.; Firth, J.; Shivappa, N.; Hébert, J.R.; Schmidt, U.; Himmerich, H. Nutrient Intake and Dietary Inflammatory Potential in Current and Recovered Anorexia Nervosa. Nutrients 2021, 13, 4400. [Google Scholar] [CrossRef]
- Ayton, A.K. Dietary polyunsaturated fatty acids and anorexia nervosa: Is there a link? Nutr. Neurosci. 2004, 7, 1–12. [Google Scholar] [CrossRef]
- Matzkin, V.B.; Geissler, C.; Coniglio, R.; Selles, J.; Bello, M. Cholesterol concentrations in patients with Anorexia Nervosa and in healthy controls. Int. J. Psychiatr. Nurs. Res. 2006, 11, 1283–1293. [Google Scholar]
- Rigaud, D.; Tallonneau, I.; Vergès, B. Hypercholesterolaemia in anorexia nervosa: Frequency and changes during refeeding. Diabetes Metab. 2009, 35, 57–63. [Google Scholar] [CrossRef]
- Roubalova, R.; Prochazkova, P.; Papezova, H. Linking Anorexia Nervosa with the Gut Microbiota. A New Narrative. Eat. Disord. 2022, 487–512. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Antibiotic-Therapy-Induced Gut Dysbiosis Affecting Gut Microbiota-Brain Axis and Cognition: Restoration by Intake of Probiotics and Synbiotics. Int. J. Mol. Sci. 2023, 24, 3074. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Löfmark, S.; Jernberg, C.; Jansson, J.K.; Edlund, C. Clindamycin-induced enrichment and long-term persistence of resistant Bacteroides spp. and resistance genes. J. Antimicrob. Chemother. 2006, 58, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.U.; Zaura, E.; Buijs, M.J.; Keijser, B.J.; Crielaard, W.; Nord, C.E.; Weintraub, A. Determining the Long-term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S77–S84. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Kim, A.H.; Lee, Y.; Kim, E.; Ji, S.C.; Chung, J.Y.; Cho, J.Y. Assessment of Oral Vancomycin-Induced Alterations in Gut Bacterial Microbiota and Metabolome of Healthy Men. Front. Cell. Infect. Microbiol. 2021, 11, 629438. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Mondot, S.; Lachkar, L.; Doré, J.; Blottière, H.M.; Hanachi, M. Roseburia, a decreased bacterial taxon in the gut microbiota of patients suffering from anorexia nervosa. Eur. J. Clin. Nutr. 2022, 76, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Di Lodovico, L.; Mondot, S.; Doré, J.; Mack, I.; Hanachi, M.; Gorwood, P. Anorexia nervosa and gut microbiota: A systematic review and quantitative synthesis of pooled microbiological data. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110114. [Google Scholar] [CrossRef]
- Schulz, N.; Belheouane, M.; Dahmen, B.; Ruan, V.A.; Specht, H.E.; Dempfle, A.; Herpertz- Dahlmann, B.; Baines, J.F.; Seitz, J. Gut microbiota alteration in adolescent anorexia nervosa does not normalize with short-term weight restoration. Int. J. Eat. Disord. 2021, 54, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef]
- Faraj, J.; Takanti, V.; Tavakoli, H.R. The Gut-Brain Axis: Literature Overview and Psychiatric Applications. Fed. Pract. 2021, 38, 356–362. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Troisi, J.; Fasano, A.; Dalle Grave, R.; Marciello, F.; Serena, G.; Calugi, S.; Scala, G.; Corrivetti, G.; Cascino, G.; et al. Multi-omics data integration in anorexia nervosa patients before and after weight regain: A microbiome-metabolomics investigation. Clin. Nutr. 2021, 40, 1137–1146. [Google Scholar] [CrossRef]
- Davis, D.J.; Hecht, P.M.; Jasarevic, E.; Beversdorf, D.Q.; Will, M.J.; Fritsche, K.; Gillespie, C.H. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behaviour of socially-isolated mice. Brain Behav. Immun. 2017, 59, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; El Aidy, S.; Crispie, F.; O’Sullivan, O.; Cotter, P.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. N-3 Polyunsaturated Fatty Acids (PUFAs) Reverse the Impact of Early-Life Stress on the Gut Microbiota. PLoS ONE 2015, 10, e0139721. [Google Scholar] [CrossRef]
- Garcia, N.; Gutierrez, E. Anorexia nervosa and microbiota: Systematic review and critical appraisal. Eat. Weight Disord. EWD 2023, 28, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Cao, Y.; Wang, C.; Zhao, C.; Wang, H.; Cui, G.; Wang, M.; Pan, Y.; Shi, Y.; et al. Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int. J. Med. Sci. 2019, 16, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Specht, H.E.; Mannig, N.; Belheouane, M.; Andreani, N.A.; Tenbrock, K.; Biemann, R.; Borucki, K.; Dahmen, B.; Dempfle, A.; Baines, J.F.; et al. Lower serum levels of IL-1β and IL-6 cytokines in adolescents with anorexia nervosa and their association with gut microbiota in a longitudinal study. Front. Psychiatry 2022, 13, 920665. [Google Scholar] [CrossRef]
- Tindall, A.M.; McLimans, C.J.; Petersen, K.S.; Kris-Etherton, P.M.; Lamendella, R. Walnuts and Vegetable Oils Containing Oleic Acid Differentially Affect the Gut Microbiota and Associations with Cardiovascular Risk Factors: Follow-up of a Randomized, Controlled, Feeding Trial in Adults at Risk for Cardiovascular Disease. J. Nutr. 2020, 150, 806–817. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
| No. | Study | Author | Year | Lot | Prevalence | Reference |
|---|---|---|---|---|---|---|
| 1 | Assessing disorders in children | Grigoroiu Ṣerbănescu | 1987 | 8–11 years | 0.01% girls; 0% boys | [9] |
| 2 | Incidence of AN | Hoeck et al. | 2003 | 0.01% | [10] | |
| 3 | Prevalence of eating disorders in secondary school students | Kovacs Krizbai | 2009 | secondary school students | 0.2% girls | [11] |
| 4 | Current trends in epidemiology of eating disorders (including AN) | Herpertz-Dahlmann et al. | 2009 | 12–18 years | 1–4% | [12] |
| 5 | Prevalence of eating disorders in adolescents | Swanson et al. | 2011 | 13–18 years | 0.30% | [13] |
| 6 | Assessment of AN | Zipfel et al. | 2015 | males, females | 1% | [14] |
| 7 | To assess the prevalence of mental disorders | Wagner et al. | 2017 | 10–18 years | 1.44% | [15] |
| 8 | To estimate the incidence of AN | Petkova et al. | 2019 | 8–17 years | 0.01% | [16] |
| Authors | Armougom et al. [41] | Mack et al. [42] | Yuan et al. [43] | Borgo et al. [1] | Morkl et al. [44] | Million et al. [45] | Kleiman et al. [46] | Kleiman et al. [47] | Morita et al. [48] |
| Year | 2009 | 2016 | 2022 | 2017 | 2017 | 2013 | 2017 | 2015 | 2015 |
| Study type | cross-sectional | longitudinal | cross-sectional | cross-sectional | cross-sectional | cross-sectional | longitudinal, case series | longitudinal | cross-sectional |
| Data analysis method | Chao index, Shannon index, Unifrac, Bray–Curtis | Mann–Whitney U test/Chao/Shannon/Sobs/Ace index/Adonis analysis | Chao index | Shannon index | Chao index, UniFrac distances | Kruskal–Wallis, Wilcoxon, Mann–Whitney | |||
| Study group size | 9 | 44 | 30 | 15 | 18 | 15 | 3 | 16 | 25 |
| Control group size tents) | 20 | 55 | 30 | 15 | 20 | 76 | absent | 12 | 21 |
| Age (years) | 19–36 | 23.8 ± 6.8 | 16 | 22.44 ± 3.52 | 27.3 ± 10.8 | 23.3 | 28 ± 11.7 | 30 ± 10.2 | |
| Increased level | Bacteroidetes, M. smithii | Firmicutes, Actinobacteria, Proteobacteria, Verrucomicrobia, Methanobrevibacter, Clostridium cluster XI | Lachnospiraceae, Streptococcaceae, Bacteroidaceae, Coriobacteriaceae, Rikenellaceae, Enterobacteriaceae, | Proteobacteria, Enterobacteriaaceae | Coriobacteriaceae Roseburia, M. smithii, Clostridium | Bacteroidetes, M. smithii | Coriobacteriales, Lactobacillales | Clostridioides difficile | |
| Decreased level | Lactobacillus | Bacteroidetes | Ruminococcaceae, Bifidobacteriaceae, Peptostreptococcaceae, Oscillospiraceae, Burkholderiaceae | Firmicutes, Ruminococcaceae, Ruminococcus, Roseburia, Clostridium | Clostridia, Anaerostipes, Faecalibacterium | Clostridium coccoides, Clostridium leptum, Bacteroides fragilis, Streptococcus, Lactobacillus plantarum | |||
| Type of dysbiosis | Alpha and beta | Alpha and beta | Only beta | Alpha and beta | Alpha and beta | Alpha and beta | Alpha and beta | Alpha and beta | Alpha and beta |
| Other associated biomarkers | elevated concentrations of branched-chain fatty acids | reduction in total short-chain fatty acids, butyrate, propionate | lower CRP, higher ASAT and ALAT | ||||||
| Sample | faeces | faeces | faeces | faeces | faeces | faeces | faeces | faeces | faeces |
| Authors | Study | Year | Results | Reference |
|---|---|---|---|---|
| Palmnäs et al. | How aspartame influences gut microbial composition | 2014 | Aspartame consumption correlates with an increase in the prevalence of Enterobacteriaceae | [71] |
| Suez et al. | Consumption of non-caloric artificial sweetners inducts compositional and functional alterations to the intestinal microbiota. | 2014 | Existence of a connection between the use of non-caloric artificial sweeteners and the proliferation of Bacteroides vulgatus, Bacteroides fragilis, Akkermansia muciniphyla, Lactobacillus reuteri, as well as alterations in the Bacteroides/Firmicutes ratio | [72] |
| Glick-Bauer et al. | The Health Advantage of a Vegan Diet: Exploring the Gut Microbiota Connection | 2014 | The prevalence of Prevotella spp. and a decrease in Bacteroides in vegetarians | [73] |
| Borgo et al. | Microbiota in AN | 2017 | The reduction in Roseburia species, particularly Roseburia inulinivorans, is associated with diminished propionate production, and the concentrations of butyrate and Clostridium spp. are inversely correlated with the severity of anxiety and depression | [1] |
| Herpertz-Dahlmann et al. | Composition of the diet influences gut microbiota | 2017 | Low carbohydrate and low-fat dietary regimens are associated with increased levels of Firmicutes and Proteobacteria, accompanied by decreased levels of Bacteroidetes. | [40] |
| Mendez-Figueroa et al. | Influence of food intake on host-gut microbiota in AN | 2019 | Bifidobacterium, Bacteroides, and Verrucomicrobia, which metabolize non-digestible carbohydrates, exhibit altered dynamics in patients with AN | [74] |
| Kim et al. | Diet composition and gut microbiota | 2020 | Fruits containing polysaccharides correlate with increased Bifidobacterium levels, while vegetable intake exhibits inverse associations with Proteobacteria and Thermi populations. | [68] |
| Berding et al. | Diet as a major factor involved in shaping the gut microbiota composition | 2021 | Dietary patterns centred on fruits, vegetables, and vegetarian regimes contribute to enhanced microbial flora diversity, with a predominant representation of Bacteroidetes and Actinobacteria species. Augmented fibre intake is associated with heightened microbiota diversity and elevated levels of beneficial bacteria, including Bifidobacterium, Lactobacillus, Bacteroides, Roseburia, and Prevotella. | [30] |
| Fruits containing polysaccharides correlate with increased Bifidobacterium levels, while vegetable intake exhibits inverse associations with Proteobacteria and Thermi populations. | [30] | |||
| Consumption of whole-grain fibre is linked to an increased abundance of Actinobacteria, Bifidobacterium, Clostridium, Akkermansia, and Roseburia | [30] | |||
| Polyphenols contribute to the augmentation of bifidobacteria and lactobacilli while decreasing the presence of Clostridium perfringens and C. histolyticum species. | [30] | |||
| Andrioaie et al. | Influence of diet in psychiatric disorders | 2022 | An increase in both Prevotella and Bacteroides in vegetarians | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anton-Păduraru, D.-T.; Trofin, F.; Nastase, E.V.; Miftode, R.S.; Miftode, I.-L.; Trandafirescu, M.F.; Cojocaru, E.; Țarcă, E.; Mindru, D.E.; Dorneanu, O.S. The Role of the Gut Microbiota in Anorexia Nervosa in Children and Adults—Systematic Review. Int. J. Mol. Sci. 2024, 25, 41. https://doi.org/10.3390/ijms25010041
Anton-Păduraru D-T, Trofin F, Nastase EV, Miftode RS, Miftode I-L, Trandafirescu MF, Cojocaru E, Țarcă E, Mindru DE, Dorneanu OS. The Role of the Gut Microbiota in Anorexia Nervosa in Children and Adults—Systematic Review. International Journal of Molecular Sciences. 2024; 25(1):41. https://doi.org/10.3390/ijms25010041
Chicago/Turabian StyleAnton-Păduraru, Dana-Teodora, Felicia Trofin, Eduard Vasile Nastase, Radu Stefan Miftode, Ionela-Larisa Miftode, Mioara Florentina Trandafirescu, Elena Cojocaru, Elena Țarcă, Dana Elena Mindru, and Olivia Simona Dorneanu. 2024. "The Role of the Gut Microbiota in Anorexia Nervosa in Children and Adults—Systematic Review" International Journal of Molecular Sciences 25, no. 1: 41. https://doi.org/10.3390/ijms25010041
APA StyleAnton-Păduraru, D.-T., Trofin, F., Nastase, E. V., Miftode, R. S., Miftode, I.-L., Trandafirescu, M. F., Cojocaru, E., Țarcă, E., Mindru, D. E., & Dorneanu, O. S. (2024). The Role of the Gut Microbiota in Anorexia Nervosa in Children and Adults—Systematic Review. International Journal of Molecular Sciences, 25(1), 41. https://doi.org/10.3390/ijms25010041