Direct and Indirect Evidence of Effects of Bacteroides spp. on Obesity and Inflammation
Abstract
:1. Introduction
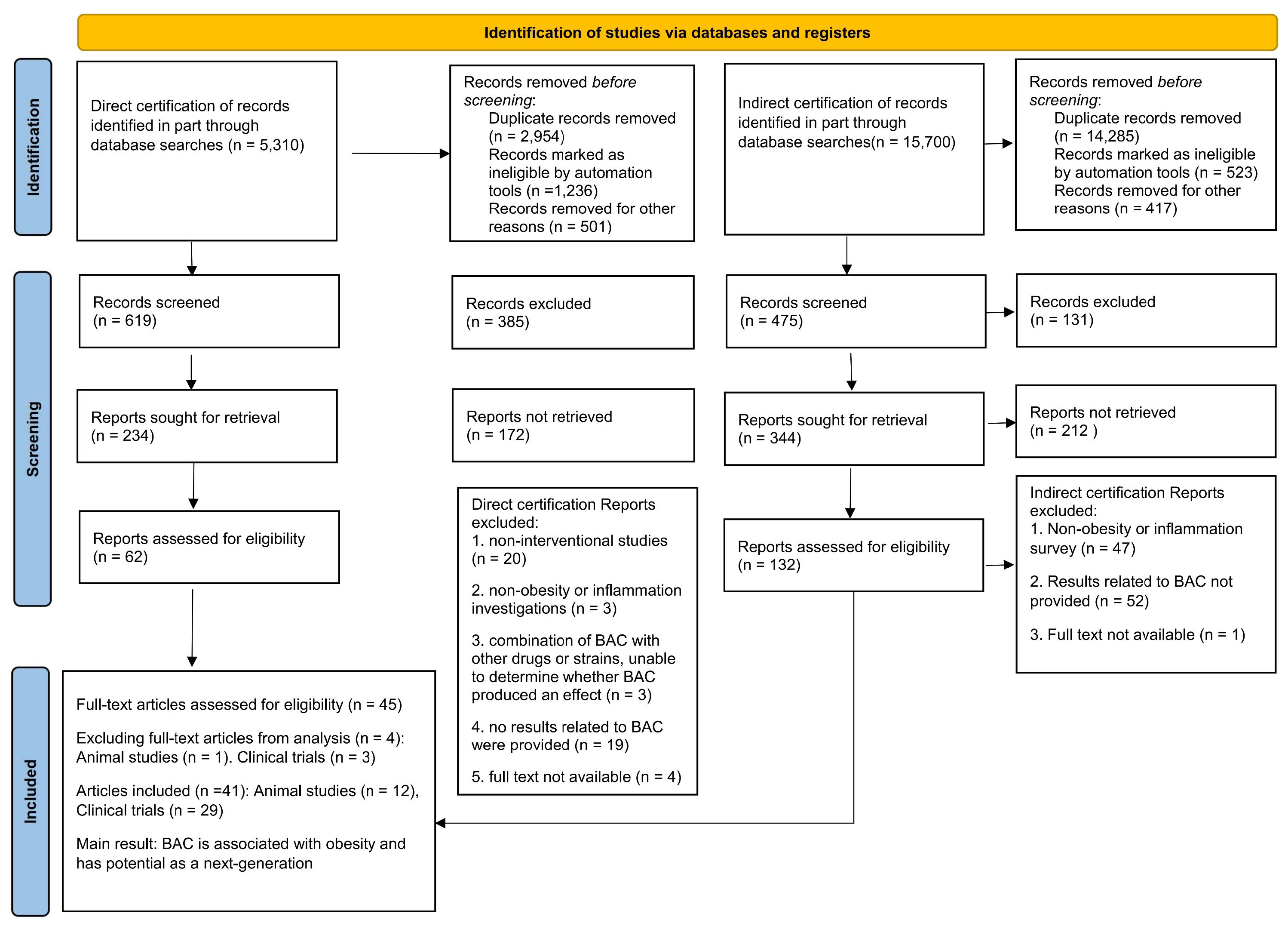
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Data Analysis
2.4. Quality Assessment
3. Results
3.1. Study Description
3.2. Indirect Evidence of the Anti-Obesity Effect of BAC from Clinical Studies
| Research Purpose | Numbers of Patients Randomized/ Analyzed | Intervention Type (Regimen) | Treatment Period | Main Outcome Measure | Main Conclusion | Reference |
|---|---|---|---|---|---|---|
| Exploration of the anti-obesity efficacy of steamed Rehmannia glutinosa root. | Middle-aged female participants with obesity (40–65 years) 12/12 | Nor group (n = 25) LCD group (n = 26) | 8 weeks | BAC↑ Waist circumference Roseburia↑ Dorea↓ | BAC levels are negatively correlated with waist circumference. | [28] |
| Assessment of metabolic markers related to obesity post-Schisandra chinensis fruit administration | Participants with obesity 40/28 | SCF group (n = 13) Placebo group (n = 15) | 12 weeks | BAC↑ Akkermansia↑ Roseburia↑ Prevotella↑ Bifidobacterium↑ Ruminococcus↓ Relationship with BAC: Fat mass | BAC levels are negatively correlated with fat mass and aspartate aminotransferase and/or alanine aminotransferase levels. | [29] |
| Examination of the correlation between gut microbial modulation and plasma lipopolysaccharide-binding protein (LBP) levels. | Participants who were overweight and obese 49/49 | Pomegranate extract (PE) Placebo | 3 weeks | BAC↑ Plasma LBP High-sensitivity C-reactive protein Faecalibacterium↑ Butyricicoccus↑ Odoribacter↑ Butyricimonas↑ | BAC levels are positively correlated with the levels of other beneficial bacteria and health indicators. | [30] |
| Determination of the effects of metformin on anthropometry and gut microbiota in non-diabetic women with obesity under a low-calorie regimen. | Women with obesity 46/36 | Metformin + LCD group (n = 20) Placebo + LCD group (n = 16) | 8 weeks | BAC↑ BMI Insulin concentration Escherichia/Shigella abundance Roseburia↑ Blautia↑ Butyrivibrio↑ | BAC levels are negatively correlated with BMI and insulin concentrations. | [31] |
| Evaluation of the role of daily avocado intake within a hypocaloric diet on weight management, inflammation biomarkers, and gut microbiota diversity. | Healthy men and women who were overweight/obese 63/51 | Avocado daily intake (n = 24) hypocaloric diet (n = 27) | 12 weeks | BAC↑ BW BMI Total body fat Visceral adipose tissue Serum glucose levels Serum hepatic growth factor levels IL-1β C-reactive protein Clostridium↑ Methanosphaera↑ Candidatus Soleaferrea↑ | BAC levels are negatively correlated with BW. | [32] |
| Assessment of the efficacy of probiotic and synbiotic supplementation on intestinal microbiota modulation in adults with prediabetes. | Patients with prediabetes 120/85 | Probiotic group (n = 27) Synbiotic group (n = 29) Placebo group (n = 28) | 6 weeks | Probiotics: B. fragilis/E. coli↑ Firmicutes/Bacteroidetes↓ | BAC levels are positively correlated with the levels of other beneficial bacteria. | [33] |
| Assessment of a low-fat vegan diet’s influence on gut microbiota and its correlation with weight and insulin resistance in an overweight cohort. | Participants Age: 25~75 years old BMI: 28~40 kg/m2 168/115 | Vegan group (n = 84) Control group (n = 84) | 16 weeks | Vegan group: B. fragilis↓ BW Fat mass Visceral fat PREDIM Faecalibacterium prausnitzii | BAC levels are negatively correlated with BW. | [34] |
| Assessment of the influence of a calorie-restricted Mediterranean diet on the gut microbial composition in individuals who are overweight and obese. | Patients with obesity 23/23 | NW OB | 3 weeks | B. cellulosilyticus↑ B. uniformis↑ BW Waist circumference Body mass index Fat mass Daily caloric intake Prevotella stercorea↑ | BAC levels are negatively correlated with BW. | [35] |
| Investigation of comparative gut microbiota profiles in individuals with dyslipidemia who were overweight and obese: Responses to orlistat and ezetimibe interventions. | 174 Volunteers (96 volunteers with dyslipidemia who were overweight and obese) 174/116 | Control group (n = 31) Patient group (n = 27) Orlistat group (n = 32) Ezetimibe group (n = 26) | 12 weeks | BAC↑ Waist circumference TG FBG Actinomyces↑ | BAC levels are negatively correlated with waist circumference. | [36] |
| Evaluation of the prebiotic impact of omega-3 supplementation on the gut microbiome. | Patients with obesity 69/69 | 20 g of inulin fiber 500 mg of omega-3 supplements daily | 6 weeks | BAC↑ Butyric acid Iso-butyric acid Iso-valeric acid Total fatty acids Total omega-3/total fatty acids Coprococcus spp.↑ | BAC levels are positively correlated with increased levels of SCFAs. | [37] |
| Investigation of predictive alterations in gut microbiota post-short-term low-carbohydrate dietary intervention in patients with obesity. | Participants who were overweight or obese 51/51 | ND group (n = 25) LCD group (n = 26) | 12 weeks | BAC↑ BW Ruminococcaceae Oscillospira↑ Odoribacteraceae Butyricimonas↑ Porphyromonadaceae Parabacteroides↑ | BAC levels are negatively correlated with BW. | [38] |
| Investigation of the combined effects of a Medical Food Therapy plant-based diet and intermittent energy restriction on glycemic parameters. | T2D patients without obesity 39/39 | 20 CMNT group (received a low-calorie human CMNT diet for five consecutive days, 10 days of habitual eating per cycle for 90 days) 19 Control group (continued normal diet) | 8 weeks | BAC↑ Hemoglobin A1C BMI FBG Postprandial blood glucose levels Medication usage Parabacteroides↑ Roseburia↑ | BAC levels are negatively correlated with BMI. | [39] |
| Comparative analysis of the effects of traditional vs. commercial kochujang on obesity metrics in adults who are overweight. | Participants 62/48 | High-dose Kochujang (n = 19) Low-dose Kochujang (n = 18) Commercial Kochujang (CK; n = 17) | 6 weeks | High-dose Kochujang and Low-dose Kochujang groups BAC↑ TC Low-density lipoprotein cholesterol High-density lipoprotein cholesterol Triglyceride levels Waist circumference Lactobacillus spp.↑ Bifidobacterium spp.↑ Lactococcus lactis↑ Enterococcus faecium↑ | BAC levels are positively correlated with positive health knots, such as reduced waist circumference. | [40] |
| Evaluation of the effect of Lactobacillus salivarius Ls-33 on the fecal microbiota composition in adolescents with obesity. | Adolescents with obesity 51/50 | 1 × 1010 CFU L. salivarius Ls-33ATCCSD5208 (n = 27) Placebo: maltodextrin (n = 23) | 12 weeks | B. fragilis↑ SCFAs↑ E. rectale↓ | BAC levels were positively correlated with the increase in SCFA levels. | [41] |
| Detailed analysis of propionate-induced alterations in glucose homeostasis, gut microbiota, plasma metabolome, and immune responses. | Non-diabetic adults who were overweight and obese 14/12 | Cellulose Inulin IPE | 42 days ×3 | Inulin and IPE: B. uniformis ↑ B. caccae ↑ B. xylanisolvens ↑ Insulin resistance IgG IL-8 | BAC levels are positively correlated with insulin sensitivity and inversely correlated with inflammatory indicators. | [42] |
| Microbiota shifts in individuals with obesity post very low-energy dietary intervention. | Finnish participants with obesity 16/16 | Participants with obesity (BMI > 30 kg/m2; six men and ten women) | 12 weeks | BAC↑ | BAC levels positively correlate with health indicators. | [43] |
| Examination of the interplay between Korean red ginseng’s effects on metabolic syndrome and gut microbiota alterations. | Patients with metabolic syndrome 60/50 | KRG group (n = 25) placebo group (n = 25) | 8 weeks | BAC↑ BMI Prevotella↑ Ruminococcaceae↑ Sutterella↑ Odobacter↑ | BAC levels are negatively correlated with the BMI and insulin concentrations. | [44] |
| Assessment of the impact of intermittent fasting on the composition of gut microbiome. | Health volunteers 9/9 | Female (n = 7) Male (n = 2) | B. fragilis ↑ A. muciniphila↑ Serum fasting glucose levels TC | BAC levels are positively correlated with other beneficial bacteria levels. | [45] |
| Research Purpose | Numbers of Patients Randomized/Analyzed | Intervention Type (Regimen) | Treatment Period | Main Outcome Measure | Main Conclusion | Reference |
|---|---|---|---|---|---|---|
| To investigate the effects of prebiotic supplementation on body composition, inflammatory markers, bile acids in stool samples, and gut microbiota composition in overweight or children with obesity. | Children who were overweight or obese 42/42 | Prebiotic group (n = 22) Placebo (n = 20) | 16 weeks | B. vulgatus↓ BW BMI Percent body fat Percent trunk fat Bifidobacterium↑ IL-6 | BAC levels are negatively correlated with the beneficial effects of prebiotic supplementation. | [46] |
| To investigate the impact of dietary inulin-type fructans (ITF prebiotics) on host metabolism through modulation of the gut microbiota in women with obesity. | Females with obesity 30/30 | Inulin/oligofructose 50/50 mix; (n = 15) placebo (maltodextrin; n = 15) | 12 weeks | B. intestinalis↓ B. vulgatus↓ Bifidobacterium↑ Faecalibacterium Rausnitzii↑ Propionibacterium↓ Serum lipopolysaccharide levels | BAC levels are inversely correlated with metabolic improvement due to ITF probiotics. | [47] |
3.3. Comparison of Gut Microbiota between Individuals with Obesity and Healthy Individuals Demonstrates an Association between BAC and Obesity
| Research Purpose | Main Outcome Measure | Results (Relationship with BAC) | Main Conclusion | Reference |
|---|---|---|---|---|
| Objective: To characterize the gut microbiota and short-chain fatty acids (SCFAs) in participants with obesity from Xinjiang, China. Summary: This study highlights regional variations in gut microbial composition related to obesity. | 16S rRNA sequencing and microbial diversity and community analyses 68 individuals with obesity 31 controls | Individuals with obesity↑ B. fragilis↓ Gemmiger↑ Dialister↑ Megamonas↑ Anaerostipe↑ Blautia↑ Gemmiger↓ Bifidobacterium↓ Prevotella↓ | The gut microbiota profile of obese patients was characterized by enrichment of Lactobacillus and the reduction in the diversity and the depletion of Actinobacteria, BAC, Bifidobacterium, and B. fragilis. | [36] |
| Objective: To identify core gut microbiota in obese and lean twins and their association with obesity. Summary: This study investigated twins discordant in obesity to reveal key microbial players associated with obesity. | 16S rRNA gene sequencing 31 monozygotic twin pairs 23 dizygotic twin pairs and their mothers (n = 46) | Participants with obesity: BAC↓ Actinobacteria↑ Carbohydrate, lipid, and amino acid metabolism↓ | The deviations from a core microbiome at function level are associated with different physiological states. | [48] |
| FObjective: To determine the relationship between gut microbiota composition and obesity in a Japanese population. Summary: This study explores unique microbial patterns associated with obesity in the Japanese demographic. | T-RFLP analysis and 16S rRNA sequencing 23 participants without obesity BMI < 20 kg/m2 33 participants with obesity BMI ≥ 25 kg/m2 | Participants without obesity: B. faecichinchillae↑ B. thetaiotaomicron↑ Blautia wexlerae↑ Clostridium bolteae↑ Flavonifractor plautii↑ | Gut microbial properties differ between obese and non-obese subjects, suggesting that gut microbiota composition is related to obesity. | [49] |
| Objective: To elucidate the gut microbial composition in normal adolescents and those with obesity. Summary: This research uncovered microbial signatures specific to obesity during adolescence. | 16S rRNA gene sequencing 67 adolescents with obesity (BMI ≥ 30 kg/m2 or ≥ 99th BMI percentile) 67 normal adolescents (BMI < 25 kg/m2 or <85th BMI percentile) | Adolescents with obesity BAC↓ Prevotella↓ TG↑ TC↑ hs-CRP↑ | A significant association between the composition of several bacterial taxa and childhood obesity was revealed. | [50] |
| Objective: To understand the mechanisms of obesity development by comparing the gut microbiota of children with obesity with that of healthy controls. Summary: This research highlights distinct gut microbial features associated with obesity. | 16S rRNA gene sequencing, enterotypes Children with obesity aged 3 to 18 years (n = 87) healthy children aged 3 to 18 years (n = 56) | Children with obesity: BAC↓ Firmicutes/Bacteroidetes ↑ Enterococcus↑ Blautia↑ Sutterella↑ Klebsiella↑ Parabacteroides↓ Anaerotruncus↓ Coprobacillus↓ | BAC are associated with weight loss, and are recommended as a dietary prebiotic and probiotic supplement as an adjunct to obesity treatment. | [51] |
| Objective: To identify microbial and metabolic factors associated with obesity through MWAS and serum metabolomics profiling. Summary: This comprehensive approach uncovers microbial and metabolic contributors to obesity in young Chinese individuals. | shotgun sequencing 72 individuals with obesity (body mass index (BMI), 36.78 ± 4.46 kg/m2; age, 23.6 ± 3.7 years) 79 controls (BMI, 20.2 ± 1.3 kg/m2; age, 23.2 ± 1.8 years) | Controls B. thetaiotaomicron↑ B. uniformis↑ B. xylanisolvens↑ B. ovatus ↑ Akkermansia muciniphila↑ Fecalibacterium prausnitzii↑ | The abundance and diversity of BAC were significantly lower in obese individuals than in normal individuals. | [52] |
| Objective: To study gut microbiota alterations specific to childhood obesity. Summary: This research reveals microbial markers and shifts associated with obesity during childhood. | 16S rRNA gene sequencing Children with obesity aged 6 to 16 years (n = 42) Children of normal weight (n = 36) | Children with obesity: B. vulgatus↓ B. stercoris↓ Firmicutes/Bacteroidetes↑ (p < 0.0001) | Reduced numbers of BAC in the gut may be linked to childhood obesity. | [53] |
| Objective: To identify unique gut microbiota characteristics in patients who were overweight/obese and compare them with those in normal weight controls in Sardinia. Summary: This research uncovers microbial signatures specific to obesity in this geographical region. | 16S rRNA gene sequencing Patients who were overweight/obese (n = 46) normal weight participants (n = 46) | Patients who were overweight/obese: BAC↓ Flavobacteriaceae↓ Porphyromonadaceae↓ Sphingobacteriaceae↓ Flavobacterium↓ Rikenella spp.↓ Pedobacter spp.↓ Parabacteroides spp.↓ | Body fatness and waist circumference were negatively correlated with BAC. | [54] |
| Objective: To explore interactions between the gut microbiome, metabolites, and brain network metrics. Summary: This investigation sheds light on how the gut microbiota and its metabolic products influence brain function, potentially linking gut–brain interactions to obesity. | 16S rRNA gene sequencing 287 participants with obesity and without obesity (male n = 99, female n = 198) | Participants with obesity: BAC↓ Microbial diversity↓ Prevotella/BAC↑ Fecal tryptophan↓ | Observed significant taxonomic changes at the genus level associated with obesity. BAC↓ (p < 0.001) | [55] |
3.4. In Vivo Direct Evidence of the Anti-Obesity Effects of BAC
| Bacteroides Strain | Source | Subject | Intervention Type (Regimen) | Treatment Period | Main Outcome Measure | Main Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| B. acidifaciens JCM10556 | Human feces | C57BL/6, CD11c-Cre, Villine-Cre, Atg7f/f and LysM-Cre mice | HFD + PBS HFD + 5 × 109 cfu B. acidifaciens JCM10556 | 10 weeks | BW and fat mass Insulin Bile acid-TGR5-PPARα axis Dipeptidyl peptidase-4 Glucagon-like peptide-1 | B. acidifaciens JCM10556 helps prevent metabolic disorders such as diabetes and obesity. | [14] |
| B. uniformis CECT7771 | Infant stool | Male C57BL/6J mice | Nor HFD Nor + 5.0 × 108 cfu B. uniformis CECT 7771 HFD + 5.0 × 108 cfu B. uniformis CECT 7771 | 7 weeks | BW Adipose tissue weight Hepatic steatosis Macrophage functionality Dendritic cell functionality | B. uniformis CECT7771 improves metabolic and immune dysfunction associated with intestinal ecological dysregulation. | [56] |
| B. uniformis CECT7771 | Infant stool | Adult male Wistar Kyoto rats (170–200 g) | SD IF IF + 1 × 108 cfu B. uniformis CECT 7771 | 18 days | Caloric intake Anxiety-like behavior Dopamine | B. uniformis CECT 7771 helps control compulsive eating by affecting brain reward systems. | [57] |
| B. uniformis CBA7346 | Human feces | Male C57BL/6J mice | Nor + PBS Nor + 1 × 106 cfu B. uniformis HFD + PBS HFD + 1 × 106 cfu B. uniformis | 12 weeks | Body and liver weights Blood lipids Liver injury and steatosis Hepatic lipid metabolism | B. uniformis CBA7346 mitigates HFD-induced NAFLD, thereby modulating LPS release, lipid-related proteins, and insulin sensitivity. | [59] |
| B. dorei DSM17855 and B. vulgatus ATCC8482 | Human feces | Male C57BL/6J mice | Nor HFD HFD + 2.5 × 109 cfu B. dorei + B. vulgatus mix | 12 weeks | BW Blood glucose BAT weight UCP1 Macrophage number | B. dorei DSM17855 and B. vulgatus ATCC8482 enhance branched-chain amino acid catabolism in brown adipose tissue | [60] |
3.5. Studies on Direct Anti-Inflammatory Therapy with BAC
| Bacteroides Strain | Source | Subject | Intervention Type (Regimen) | Treatment Period | Main Outcome Measure | Main Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| B. fragilis NCTC9343 | Human feces | Male C57BL/6J mice | DSS DSS + 5 × 109~7 × 109 CFU B. fragilis NCTC9343 | 8 days | IL-1β CCR5 Number of colonic tumors TLR2 signaling | This study highlights B. fragilis’ protective role against weight loss, colonic histopathological changes, and inflammation in colitis-associated colon cancer. | [62] |
| B. thetaiotaomicron DSM 2079 | Human feces | Female and male C57BL/6J mice | Control DSS DSS + 3 × 1010 CFU B. thetaiotaomicron DSM 2079 | 8–14 days | inflammation BW Colon length | This study demonstrates that B. ovatus monotherapy is a consistent and effective approach for colitis management, surpassing traditional FMT. | [63] |
| B. fragilis (HCK-B3, ATCC25285) and B. ovatus (ELH-B2, JCM5824) | Human feces | Female C57BL/6J mice | 1 × 109 CFU B. fragilis HCK 1 × 109 CFU B. fragilis 25285 1 × 109 CFU B. ovatus ELH 1 × 109 CFU B. ovatus JCM5824 | 5 days | TNF IL-10 FITC-Dextran NF-κB | This research underscores the promise of BAC as a therapeutic agent for intestinal inflammatory diseases. | [64] |
| B. ovatus ATCC 8483 | Human feces | Male C57BL/6J mice | B. ovatus B. thetaiotaomicron B. vulgatus B. ovatus + B. thetaiotaomicron + B. vulgatus mix | 9 days | Increased proliferation of epithelial cells Hyperplastic crypts inflammation | This study demonstrates that B. ovatus monotherapy is a consistent and effective approach for colitis management, surpassing traditional FMT. | [65] |
| B. fragilis NCTC9343 | Human feces | WT (129S6/SvEvTac) and Rag2−/− (129S6/SvEvTac-Rag2tm1Fwa) mice | WT + B. fragilis WT + PSA Rag + PSA WT + PBS Rag + PBS | 21 days | T-cell populations IL-10 IFNγ | This research underscores the protective role of B. fragilis NCTC9343 against nervous system inflammatory diseases. | [66] |
| B. vulgatus FJS7K1 | Human feces | Male C57BL/6J mice | Control LPS LPS + B. vulgatus 5K1 LPS + B. vulgatus 7K1 LPS + B. vulgatus 11B4 LPS + B. vulgatus 51K1 | 5 days | Number of Treg cells Intestinal epithelial integrity IL-6 IL-10 TNF SCFAs | This study highlights B. vulgatus FTJS7K1 as a potential agent for mitigating acute inflammation and intestinal injury through microbial community modulation and cytokine regulation. | [67] |
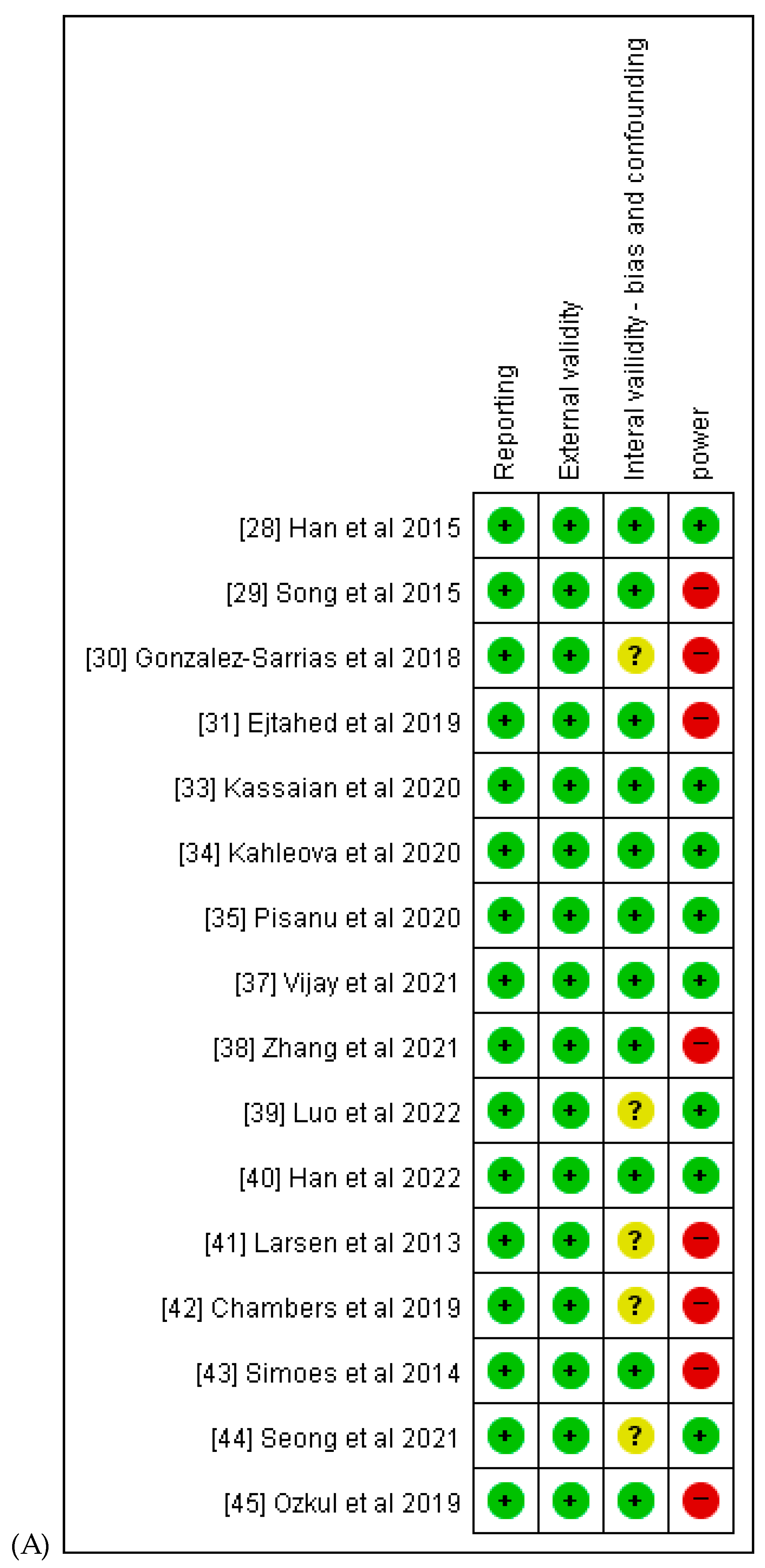
3.6. Quality Assessment and Scores
| Larsen et al. [41] | Simoes et al. [43] | Han et al. [28] | Song et al. [29] | Gonzalez-Sarrias et al. [30] | Chambers et al. [42] | Ejtahed et al. [31] | Henning et al. [32] | Ozkul et al. [45] | Kassaian et al. [33] | Kahleova et al. [34] | Pisanu et al. [35] | Seong et al. [44] | Vijay et al. [37] | Zhang et al. [38] | Luo et al. [39] | Han et al. [40] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Hypothesis/aim/objective clearly described | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | Main outcomes in Introduction or Methods | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q3 | Patient characteristics clearly described | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q4 | Interventions of interest clearly described | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q5 | Principal confounders clearly described | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q6 | Main findings clearly described | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q7 | Estimates of random variability provided for main outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q8 | All adverse events of intervention reported | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Q9 | Characteristics of patients lost to follow-up described | 0 | - | 1 | - | 0 | - | 1 | 1 | - | 1 | 1 | - | 1 | 0 | 1 | 0 | 1 |
| Q10 | Probability values reported for main outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q11 | Subjects Study participants asked to participate were representative of the source population | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q12 | Subjects Study participants prepared to participate were representative of the source population | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q13 | Location and delivery of study treatment were representative of the source population | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q14 | Study participants blinded to treatment | 1 | - | - | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | - | 1 | 0 | 1 | 1 | 1 |
| Q15 | Blinded outcome assessment | 1 | - | - | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 |
| Q16 | Any data dredging clearly described | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q17 | Analyses adjusted for differing lengths of follow-up | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Q18 | Appropriate statistical tests performed | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q19 | Compliance with interventions was reliable | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q20 | Outcome measures were reliable and valid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q21 | All participants recruited from the same source population | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q22 | All participants recruited over the same time period | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q23 | Participants randomized to treatment(s) | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 |
| Q24 | Allocation of treatment concealed from investigators and participants | 1 | - | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 0 | 1 | - | 1 | 0 | 1 |
| Q25 | Adequate adjustment for confounding | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Q26 | Losses to follow-up taken into account | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Q27 | Sufficient power to detect treatment effect at a significance level of 0.05 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| TOTAL | 22 | 20 | 26 | 25 | 24 | 24 | 26 | 27 | 19 | 27 | 26 | 23 | 26 | 19 | 26 | 24 | 27 |

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afrizal, A.; Hitch, T.C.; Viehof, A.; Treichel, N.; Riedel, T.; Abt, B.; Buhl, E.M.; Kohlheyer, D.; Overmann, J.; Clavel, T. Anaerobic single-cell dispensing facilitates the cultivation of human gut bacteria. Environ. Microbiol. 2022, 24, 3861–3881. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.A.; Smidt, H.; de Vos, W.M. Reconstructing functional networks in the human intestinal tract using synthetic microbiomes. Curr. Opin. Biotechnol. 2019, 58, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S.; Wollam, A.; Shah, N.; Wang, C.; Magrini, V.; Wilson, R.K.; et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef] [PubMed]
- Kulagina, E.V.; Efimov, B.A.; Maximov, P.Y.; Kafarskaia, L.I.; Chaplin, A.V.; Shkoporov, A.N. Species composition of Bacteroidales order bacteria in the feces of healthy people of various ages. Biosci. Biotechnol. Biochem. 2012, 76, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Maier, T.; Urban, E.; Terhes, G.; Kostrzewa, M.; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Species identification of clinical isolates of Bacteroides by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 2009, 15, 796–802. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Sánchez, E.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal Bacteroides species associated with coeliac disease. J. Clin. Pathol. 2010, 63, 1105–1111. [Google Scholar] [CrossRef]
- Salyers, A. Bacteroides of the human lower intestinal tract. Annu. Rev. Microbiol. 1984, 38, 293–313. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Modulation of gut microbiota in the management of metabolic disorders: The prospects and challenges. Int. J. Mol. Sci. 2014, 15, 4158–4188. [Google Scholar] [CrossRef]
- Jung, S.; Lee, Y.J.; Kim, M.; Kim, M.; Kwak, J.H.; Lee, J.-W.; Ahn, Y.-T.; Sim, J.-H.; Lee, J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduced body adiposity and Lp-PLA2 activity in overweight subjects. J. Funct. Foods 2015, 19, 744–752. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.C.; Hosaka, T.; Nguyen, T.T.; Kassu, A.; Dang, T.O.; Tran, H.B.; Pham, T.P.; Tran, Q.B.; Le, T.H.H.; Da Pham, X. Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed. Res. 2015, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Chordà, J.; Del Pulgar, E.M.G.; Carrasco-Luna, J.; Benítez-Páez, A.; Sanz, Y.; Codoñer-Franch, P. Bifidobacterium pseudocatenulatum CECT 7765 supplementation improves inflammatory status in insulin-resistant obese children. Eur. J. Nutr. 2019, 58, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, Q.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. A potential species of next-generation probiotics? The dark and light sides of Bacteroides fragilis in health. Food Res. Int. 2019, 126, 108590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Duan, Y.; Cai, F.; Cao, D.; Wang, L.; Qiao, Z.; Hong, Q.; Li, N.; Zheng, Y.; Su, M.; et al. Next-Generation Probiotics: Microflora Intervention to Human Diseases. BioMed Res. Int. 2022, 2022, 5633403. [Google Scholar] [CrossRef] [PubMed]
- Surana, N.K.; Kasper, D.L. The yin yang of bacterial polysaccharides: Lessons learned from B. fragilis PSA. Immunol. Rev. 2012, 245, 13–26. [Google Scholar] [CrossRef]
- Schwalm, N.D.; Groisman, E.A. Navigating the gut buffet: Control of polysaccharide utilization in Bacteroides spp. Trends Microbiol. 2017, 25, 1005–1015. [Google Scholar] [CrossRef]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef]
- Ambesh, P.; Stroud, S.; Franzova, E.; Gotesman, J.; Sharma, K.; Wolf, L.; Kamholz, S. Recurrent Lactobacillus bacteremia in a patient with leukemia. J. Investig. Med. High Impact Case Rep. 2017, 5, 2324709617744233. [Google Scholar] [CrossRef]
- Mikucka, A.; Deptuła, A.; Bogiel, T.; Chmielarczyk, A.; Nurczyńska, E.; Gospodarek-Komkowska, E. Bacteraemia Caused by Probiotic Strains of Lacticaseibacillus rhamnosus—Case Studies Highlighting the Need for Careful Thought before Using Microbes for Health Benefits. Pathogens 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-promoting properties of lactobacilli in fermented dairy products. Front. Microbiol. 2021, 12, 673890. [Google Scholar] [CrossRef] [PubMed]
- King, S.J.; McCole, D.F. Epithelial-microbial diplomacy: Escalating border tensions drive inflammation in inflammatory bowel disease. Intest. Res. 2019, 17, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Carrow, H.C.; Batachari, L.E.; Chu, H. Strain diversity in the microbiome: Lessons from Bacteroides fragilis. PLoS Pathog. 2020, 16, e1009056. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Kainulainen, V.; Suutarinen, M.; Heini, T.; Bowers, J.R.; Jasso-Selles, D.; Lemmer, D.; Valentine, M.; Barnes, R.; Engelthaler, D.M. Isolation of anti-inflammatory and epithelium reinforcing Bacteroides and Parabacteroides spp. from a healthy fecal donor. Nutrients 2020, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Bose, S.; Kim, Y.M.; Chin, Y.W.; Kim, B.S.; Wang, J.H.; Lee, J.H.; Kim, H. Rehmannia glutinosa reduced waist circumferences of Korean obese women possibly through modulation of gut microbiota. Food Funct. 2015, 6, 2684–2692. [Google Scholar] [CrossRef]
- Song, M.Y.; Wang, J.H.; Eom, T.; Kim, H. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: A randomized, double-blind placebo-controlled study. Nutr. Res. 2015, 35, 655–663. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Romo-Vaquero, M.; Garcia-Villalba, R.; Cortes-Martin, A.; Selma, M.V.; Espin, J.C. The Endotoxemia Marker Lipopolysaccharide-Binding Protein is Reduced in Overweight-Obese Subjects Consuming Pomegranate Extract by Modulating the Gut Microbiota: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2018, 62, e1800160. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Tito, R.Y.; Siadat, S.D.; Hasani-Ranjbar, S.; Hoseini-Tavassol, Z.; Rymenans, L.; Verbeke, K.; Soroush, A.R.; Raes, J.; Larijani, B. Metformin induces weight loss associated with gut microbiota alteration in non-diabetic obese women: A randomized double-blind clinical trial. Eur. J. Endocrinol. 2019, 180, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Woo, S.L.; Lee, R.-P.; Huang, J.; Rasmusen, A.; Carpenter, C.L.; Thames, G.; Gilbuena, I.; Tseng, C.-H.; et al. Hass avocado inclusion in a weight-loss diet supported weight loss and altered gut microbiota: A 12-week randomized, parallel-controlled trial. Curr. Dev. Nutr. 2019, 3, nzz068. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Feizi, A.; Rostami, S.; Aminorroaya, A.; Yaran, M.; Amini, M. The effects of 6 mo of supplementation with probiotics and synbiotics on gut microbiota in the adults with prediabetes: A double blind randomized clinical trial. Nutrition 2020, 79–80, 110854. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, S.; Palmas, V.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Boi, F.; Loviselli, A.; et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients 2020, 12, 2707. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cheng, R.; Ren, Y.; Shen, X.; Wang, J.; Xue, Y.; Zhang, H.; Jia, X.; Li, T.; He, F.; et al. Distinctive Gut Microbiota in Patients with Overweight and Obesity with Dyslipidemia and its Responses to Long-term Orlistat and Ezetimibe Intervention: A Randomized Controlled Open-label Trial. Front. Pharmacol. 2021, 12, 732541. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes 2021, 13, 1863133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, P.; Tian, Y.; Liu, B.; Huang, L.; Liu, Z.; Lin, N.; Xu, N.; Ruan, Y.; Zhang, Z.; et al. Gut Microbiota Serves a Predictable Outcome of Short-Term Low-Carbohydrate Diet (LCD) Intervention for Patients with Obesity. Microbiol. Spectr. 2021, 9, e0022321. [Google Scholar] [CrossRef]
- Luo, W.; Zhou, J.; Yang, X.; Wu, R.; Liu, H.; Shao, H.; Huang, B.; Kang, X.; Yang, L.; Liu, D. A Chinese medical nutrition therapy diet accompanied by intermittent energy restriction alleviates type 2 diabetes by enhancing pancreatic islet function and regulating gut microbiota composition. Food Res. Int. 2022, 161, 111744. [Google Scholar] [CrossRef]
- Han, A.L.; Jeong, S.J.; Ryu, M.S.; Yang, H.J.; Jeong, D.Y.; Park, D.S.; Lee, H.K. Anti-Obesity Effects of Traditional and Commercial Kochujang in Overweight and Obese Adults: A Randomized Controlled Trial. Nutrients 2022, 14, 2783. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gobel, R.J.; Michaelsen, K.F.; Forssten, S.D.; Lahtinen, S.J.; Jakobsen, M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin. Nutr. 2013, 32, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [PubMed]
- Simoes, C.D.; Maukonen, J.; Scott, K.P.; Virtanen, K.A.; Pietilainen, K.H.; Saarela, M. Impact of a very low-energy diet on the fecal microbiota of obese individuals. Eur. J. Nutr. 2014, 53, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Seong, E.; Bose, S.; Han, S.Y.; Song, E.J.; Lee, M.; Nam, Y.D.; Kim, H. Positive influence of gut microbiota on the effects of Korean red ginseng in metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. EPMA J. 2021, 12, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Yalinay, M.; Karakan, T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: A preliminary study on intermittent fasting. Turk. J. Gastroenterol. 2019, 30, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef]
- Hu, H.J.; Park, S.G.; Jang, H.B.; Choi, M.K.; Park, K.H.; Kang, J.H.; Park, S.I.; Lee, H.J.; Cho, S.H. Obesity Alters the Microbial Community Profile in Korean Adolescents. PLoS ONE 2015, 10, e0134333. [Google Scholar] [CrossRef]
- Hou, Y.P.; He, Q.Q.; Ouyang, H.M.; Peng, H.S.; Wang, Q.; Li, J.; Lv, X.F.; Zheng, Y.N.; Li, S.C.; Liu, H.L.; et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. Biomed. Res. Int. 2017, 2017, 7585989. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Guan, M.; Mayer, E.A.; Stains, J.; Liu, C.; Vora, P.; Jacobs, J.P.; Lagishetty, V.; Chang, L.; Barry, R.L.; et al. Obesity is associated with a distinct brain-gut microbiome signature that connects Prevotella and Bacteroides to the brain’s reward center. Gut Microbes 2022, 14, 2051999. [Google Scholar] [CrossRef] [PubMed]
- Gauffin Cano, P.; Santacruz, A.; Moya, A.; Sanz, Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE 2012, 7, e41079. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Campillo, I.; Balzano, T.; Benitez-Paez, A.; Lopez-Almela, I.; Romani-Perez, M.; Forteza, J.; Felipo, V.; Avena, N.M.; Sanz, Y. Bacteroides uniformis CECT 7771 Modulates the Brain Reward Response to Reduce Binge Eating and Anxiety-Like Behavior in Rat. Mol. Neurobiol. 2021, 58, 4959–4979. [Google Scholar] [CrossRef]
- You, H.J.; Si, J.; Kim, J.; Yoon, S.; Cha, K.H.; Yoon, H.S.; Lee, G.; Yu, J.; Choi, J.S.; Jung, M.; et al. Bacteroides vulgatus SNUG 40005 Restores Akkermansia Depletion by Metabolite Modulation. Gastroenterology 2023, 164, 103–116. [Google Scholar] [CrossRef]
- Lee, H.B.; Do, M.H.; Jhun, H.; Ha, S.K.; Song, H.S.; Roh, S.W.; Chung, W.H.; Nam, Y.D.; Park, H.Y. Amelioration of Hepatic Steatosis in Mice through Bacteroides uniformis CBA7346-Mediated Regulation of High-Fat Diet-Induced Insulin Resistance and Lipogenesis. Nutrients 2021, 13, 2989. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T.; et al. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. iScience 2021, 24, 103342. [Google Scholar] [CrossRef]
- Garg, S.S.; Kushwaha, K.; Dubey, R.; Gupta, J. Association between obesity, inflammation and insulin resistance: Insights into signaling pathways and therapeutic interventions. Diabetes Res. Clin. Pract. 2023, 200, 110691. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Mehrabian, P.; Boyajian, S.; Wu, W.L.; Selicha, J.; Vonderfecht, S.; Mazmanian, S.K. The Protective Role of Bacteroides fragilis in a Murine Model of Colitis-Associated Colorectal Cancer. mSphere 2018, 3, e00587-18. [Google Scholar] [CrossRef] [PubMed]
- Delday, M.; Mulder, I.; Logan, E.T.; Grant, G. Bacteroides thetaiotaomicron Ameliorates Colon Inflammation in Preclinical Models of Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Novel strains of Bacteroides fragilis and Bacteroides ovatus alleviate the LPS-induced inflammation in mice. Appl. Microbiol. Biotechnol. 2019, 103, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Ihekweazu, F.D.; Fofanova, T.Y.; Queliza, K.; Nagy-Szakal, D.; Stewart, C.J.; Engevik, M.A.; Hulten, K.G.; Tatevian, N.; Graham, D.Y.; Versalovic, J.; et al. Bacteroides ovatus ATCC 8483 monotherapy is superior to traditional fecal transplant and multi-strain bacteriotherapy in a murine colitis model. Gut Microbes 2019, 10, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Kujawski, M.; Chu, H.; Li, L.; Mazmanian, S.K.; Cantin, E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiao, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 2022, 36, 27–37. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Reese, A.T.; Chadaideh, K.S.; Diggins, C.E.; Schell, L.D.; Beckel, M.; Callahan, P.; Ryan, R.; Emery Thompson, M.; Carmody, R.N. Effects of domestication on the gut microbiota parallel those of human industrialization. eLife 2021, 10, e60197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Park, S.-H.; Kim, H. Direct and Indirect Evidence of Effects of Bacteroides spp. on Obesity and Inflammation. Int. J. Mol. Sci. 2024, 25, 438. https://doi.org/10.3390/ijms25010438
Wu L, Park S-H, Kim H. Direct and Indirect Evidence of Effects of Bacteroides spp. on Obesity and Inflammation. International Journal of Molecular Sciences. 2024; 25(1):438. https://doi.org/10.3390/ijms25010438
Chicago/Turabian StyleWu, Liangliang, Seo-Hyun Park, and Hojun Kim. 2024. "Direct and Indirect Evidence of Effects of Bacteroides spp. on Obesity and Inflammation" International Journal of Molecular Sciences 25, no. 1: 438. https://doi.org/10.3390/ijms25010438
APA StyleWu, L., Park, S.-H., & Kim, H. (2024). Direct and Indirect Evidence of Effects of Bacteroides spp. on Obesity and Inflammation. International Journal of Molecular Sciences, 25(1), 438. https://doi.org/10.3390/ijms25010438






