The Antiproliferative and Proapoptotic Effects of Cucurbitacin B on BPH-1 Cells via the p53/MDM2 Axis
Abstract
:1. Introduction
2. Results
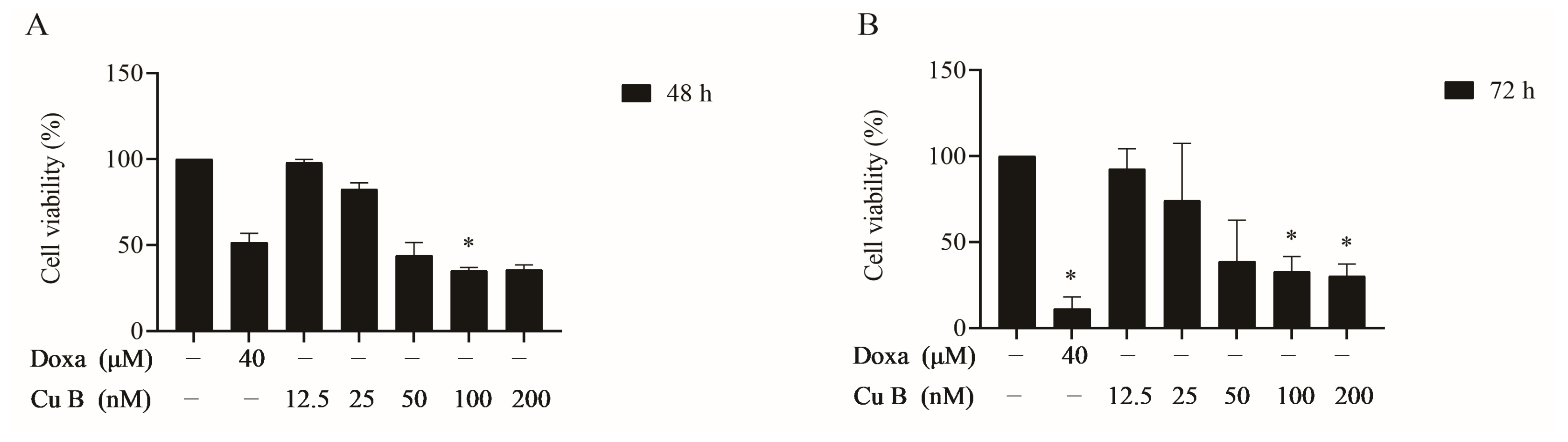
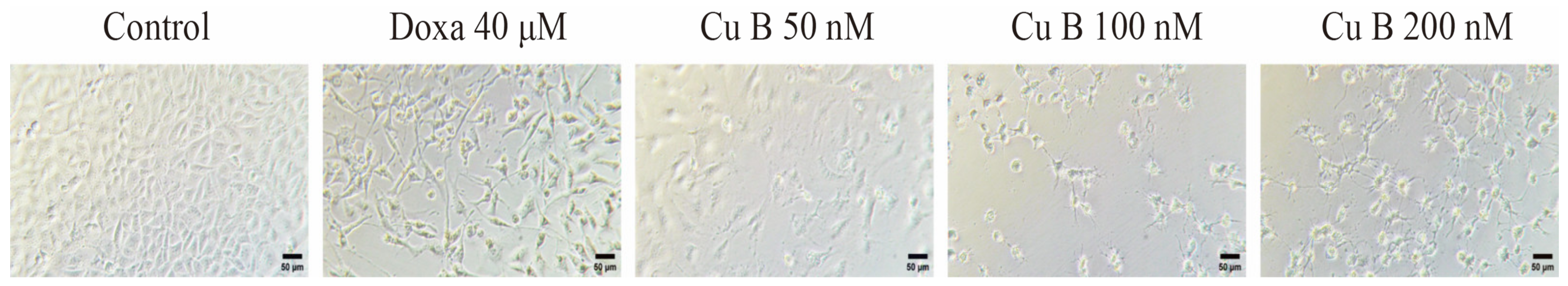
2.1. Cu B Exhibited Antiproliferative Effects on BPH-1 Cells
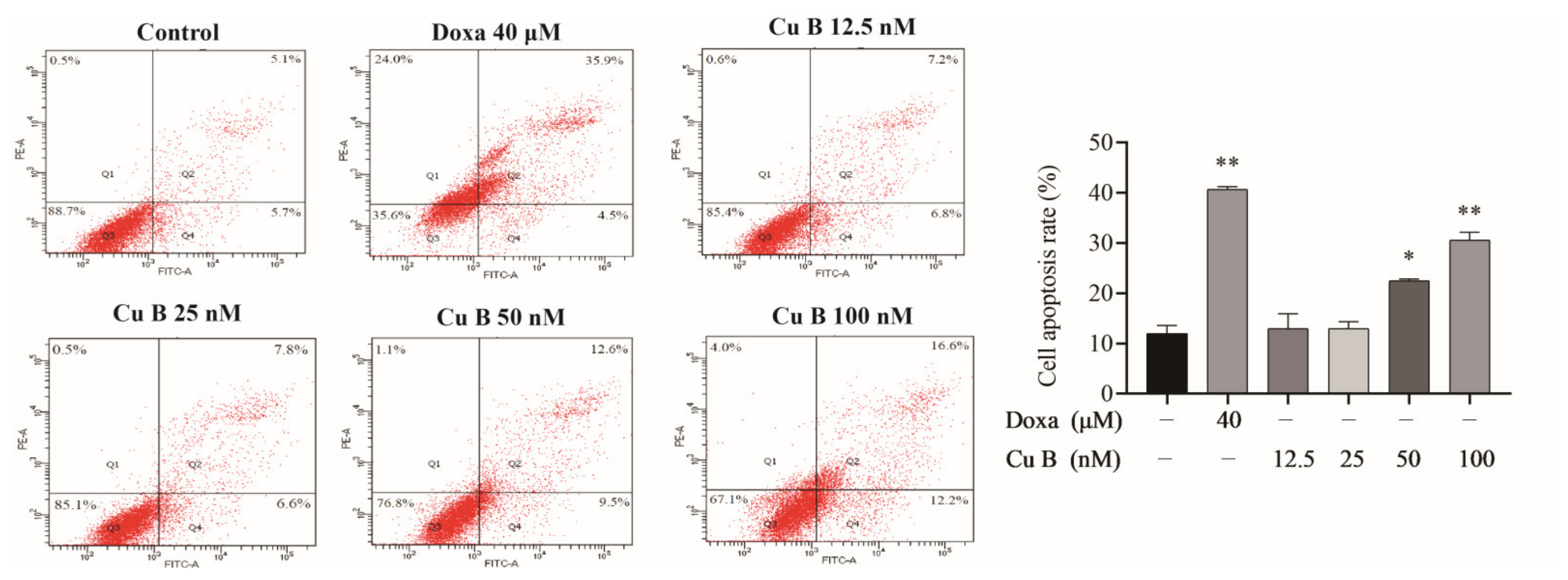
2.2. Cu B Induced Apoptosis of BPH-1 Cells
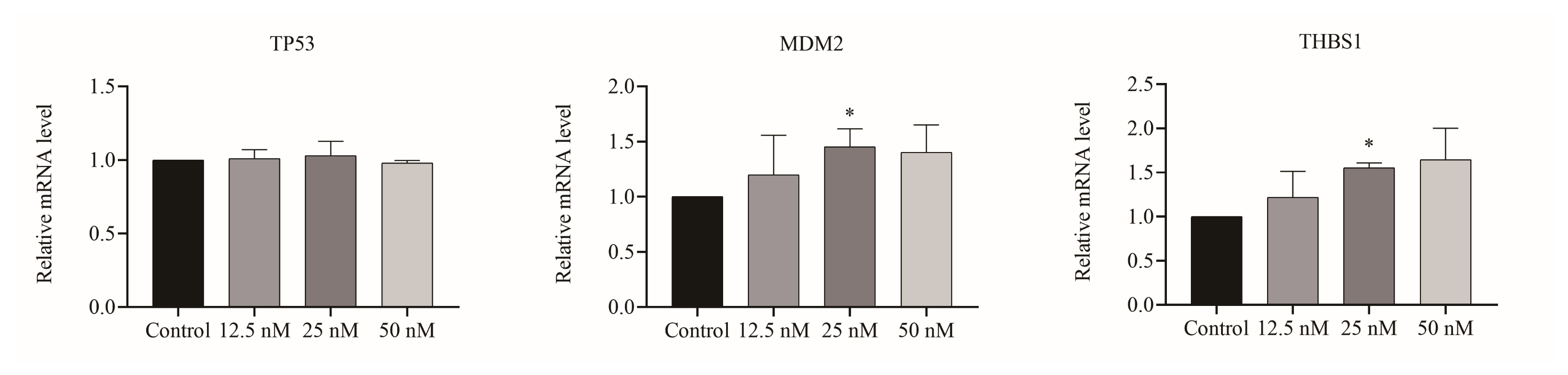
2.3. Cu B Regulated Gene Levels of the p53/MDM2 Signaling Axis
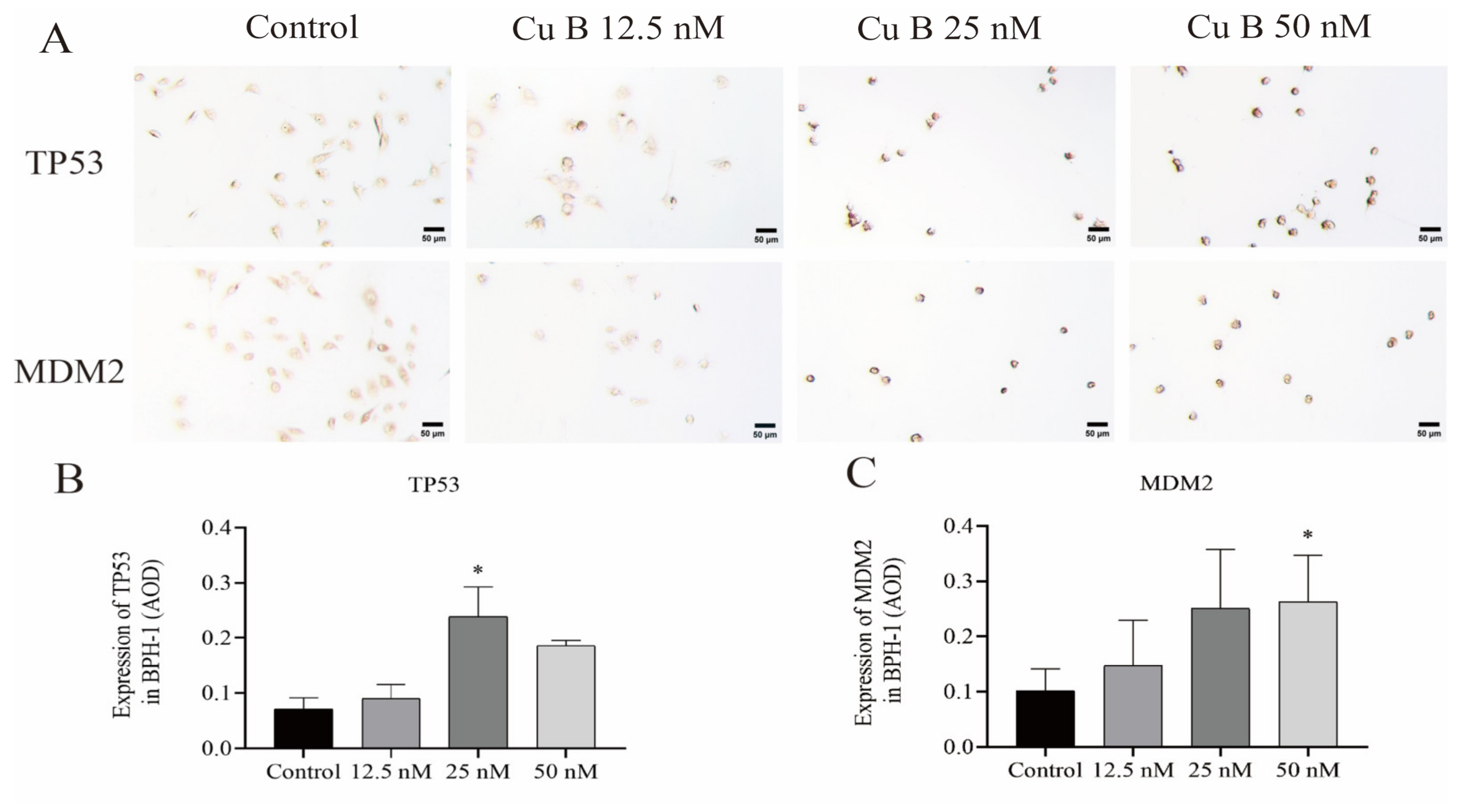
2.4. Cu B Upregulated TP53, MDM2, and THBS1 Protein Expressions in BPH-1 Cells
2.5. Cu B Downregulated COX-2 Expression in BPH-1 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Viability and Cell Morphology
4.4. Annexin V-FITC/PI Staining
4.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.6. Immunocytochemistry
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayward, S.W.; Dahiya, R.; Cunha, G.R.; Bartek, J.; Deshpande, N.; Narayan, P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev. Biol. Anim. 1995, 31, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, Y.; Chen, L.; Shi, J.; Wang, C.Y.; Miao, L.; Klocker, H.; Park, I.; Lee, C.; Zhang, J. Benign prostatic hyperplasia (BPH) epithelial cell line BPH-1 induces aromatase expression in prostatic stromal cells via prostaglandin E2. J. Endocrinol. 2007, 195, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, C.; Zhao, X.; Ma, C.; Fu, K.; Liu, Y.; Peng, C.; Li, Y. Cucurbitacin B: A review of its pharmacology, toxicity, and pharmacokinetics. Pharmacol. Res. 2023, 187, 106587. [Google Scholar] [CrossRef]
- Han, K.; Lang, T.; Zhang, Z.; Zhang, Y.; Sun, Y.; Shen, Z.; Beuerman, R.W.; Zhou, L.; Min, D. Luteolin attenuates Wnt signaling via upregulation of FZD6 to suppress prostate cancer stemness revealed by comparative proteomics. Sci. Rep. 2018, 8, 8537. [Google Scholar] [CrossRef]
- Sikander, M.; Bin Hafeez, B.; Malik, S.; Alsayari, A.; Halaweish, F.T.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. Cucurbitacin D exhibits potent anti-cancer activity in cervical cancer. Sci. Rep. 2016, 6, 36594. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhao, C.; Li, Y.; Hu, X.; Wu, L.; Chen, M.; Tong, S. The miR-223-3p/MAP1B axis aggravates TGF-β-induced proliferation and migration of BPH-1 cells. Cell Signal 2021, 84, 110004. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jin, B.-R.; An, H.-J. Umbelliferone Ameliorates Benign Prostatic Hyperplasia by Inhibiting Cell Proliferation and G1/S Phase Cell Cycle Progression through Regulation of STAT3/E2F1 Axis. Int. J. Mol. Sci. 2021, 22, 9019. [Google Scholar] [CrossRef]
- Jain, A.K.; Barton, M.C. p53: Emerging roles in stem cells, development and beyond. Development 2018, 145, dev158360. [Google Scholar] [CrossRef]
- Mendoza, M.; Mandani, G.; Momand, J. The MDM2 gene family. Biomol. Concepts 2014, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Karni-Schmidt, O.; Lokshin, M.; Prives, C. The Roles of MDM2 and MDMX in Cancer. Annu. Rev. Pathol. 2016, 11, 617–644. [Google Scholar] [CrossRef] [PubMed]
- Slabakova, E.; Kharaishvili, G.; Smejova, M.; Pernicova, Z.; Suchankova, T.; Remsik, J.; Lerch, S.; Strakova, N.; Bouchal, J.; Kral, M.; et al. K. Opposite regulation of MDM2 and MDMX expression in acquisition of mesenchymal phenotype in benign and cancer cells. Oncotarget 2015, 6, 36156–36171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kryczek, I.; Li, S.; Li, X.; Aguilar, A.; Wei, S.; Grove, S.; Vatan, L.; Yu, J.; Yan, Y.; et al. The ubiquitin ligase MDM2 sustains STAT5 stability to control T cell-mediated antitumor immunity. Nat. Immunol. 2021, 22, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.J.; Kuo, N.L.; Eischen, C.M.; Stepanova, L.; Sherr, C.J.; Roussel, M.F. The RING domain of Mdm2 can inhibit cell proliferation. Cancer Res. 2002, 62, 1222–1230. [Google Scholar] [PubMed]
- Brown, D.R.; Thomas, C.A.; Deb, S.P. The human oncoprotein MDM2 arrests the cell cycle: Elimination of its cell-cycle-inhibitory function induces tumorigenesis. Embo J. 1998, 17, 2513–2525. [Google Scholar] [CrossRef]
- Kaur, S.; Bronson, S.M.; Pal-Nath, D.; Miller, T.W.; Soto-Pantoja, D.R.; Roberts, D.D. Functions of Thrombospondin-1 in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 4570. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ahuja, N.; Burger, P.C.; Issa, J.P.J. Methylation and silencing of the Thrombospondin-1 promoter in human cancer. Oncogene 1999, 18, 3284–3289. [Google Scholar] [CrossRef]
- Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell Mol. Med. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Zhou, T.; Song, H.; Hulsurkar, M.; Su, N.; Liu, Y.; Wang, Z.; Shao, L.; Ittmann, M.; et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat. Commun. 2018, 9, 4080. [Google Scholar] [CrossRef]
- Hulsurkar, M.; Li, Z.; Zhang, Y.; Li, X.; Zheng, D.; Li, W. Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene 2017, 36, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Maitisha, G.; Aimaiti, M.; An, Z.; Li, X. Allicin induces cell cycle arrest and apoptosis of breast cancer cells in vitro via modulating the p53 pathway. Mol. Biol. Rep. 2021, 48, 7261–7272. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Blando, J.M.; Perez, C.J.; Lal, P.; Feldman, M.D.; Smyth, E.M.; Ricciotti, E.; Grosser, T.; Benavides, F.; Kazanietz, M.G. COX-2 mediates pro-tumorigenic effects of PKCε in prostate cancer. Oncogene 2018, 37, 4735–4749. [Google Scholar] [CrossRef] [PubMed]
- Raafat, M.; Kamel, A.A.; Shehata, A.H.; Ahmed, A.F.; Bayoumi, A.M.A.; Moussa, R.A.; Abourehab, M.A.S.; El-Daly, M. Aescin Protects against Experimental Benign Prostatic Hyperplasia and Preserves Prostate Histomorphology in Rats via Suppression of Inflammatory Cytokines and COX-2. Pharmaceuticals 2022, 15, 130. [Google Scholar] [CrossRef]
- Park, E.; Lee, M.Y.; Seo, C.S.; Jeon, W.Y.; Shin, H.K. Yongdamsagan-tang, a traditional herbal formula, inhibits cell growth through the suppression of proliferation and inflammation in benign prostatic hyperplasia epithelial-1 cells. J. Ethnopharmacol. 2017, 209, 230–235. [Google Scholar] [CrossRef]
- Wahid, S.; Khan, R.A.; Feroz, Z.; Ikram, R. Analgesic, anti-inflammatory and toxic effects of ethanol extracts of Cucumis melo and Citrullus lanatus seeds. Pak. J. Pharm. Sci. 2020, 33, 1049–1055. [Google Scholar]
- Abdelwahab, S.I.; Hassan, L.E.A.; Sirat, H.M.; Yagi, S.M.A.; Koko, W.S.; Mohan, S.; Taha, M.M.E.; Ahmad, S.; Chuen, C.S.; Narrima, P.; et al. Anti-inflammatory activities of cucurbitacin E isolated from Citrullus lanatus var. citroides: Role of reactive nitrogen species and cyclooxygenase enzyme inhibition. Fitoterapia 2011, 82, 1190–1197. [Google Scholar] [CrossRef]

| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| TP53 | CACTAAGCGAGCACTGCCCAACA | GCCTCATTCAGCTCTCGGAACATCT |
| MDM2 | TTGGCGTGCCAAGCTTCTCTGTG | ACCTGAGTCCGATGATTCCTGCTGA |
| THBS1 | ATGGAGAATGCTGTCCTCGCTGTTG | CGGTTGTTGAGGCTATCGCAGGAG |
| GAPDH | CAGGAGGCATTGCTGATGAT | GAAGGCTGGGGCTCATTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Huang, S.; Shao, C.; Huang, D.; Hu, Y.; Su, X.; Yang, R.; Jiang, J.; Wu, J. The Antiproliferative and Proapoptotic Effects of Cucurbitacin B on BPH-1 Cells via the p53/MDM2 Axis. Int. J. Mol. Sci. 2024, 25, 442. https://doi.org/10.3390/ijms25010442
Zhou P, Huang S, Shao C, Huang D, Hu Y, Su X, Yang R, Jiang J, Wu J. The Antiproliferative and Proapoptotic Effects of Cucurbitacin B on BPH-1 Cells via the p53/MDM2 Axis. International Journal of Molecular Sciences. 2024; 25(1):442. https://doi.org/10.3390/ijms25010442
Chicago/Turabian StyleZhou, Ping, Sisi Huang, Congcong Shao, Dongyan Huang, Yingyi Hu, Xin Su, Rongfu Yang, Juan Jiang, and Jianhui Wu. 2024. "The Antiproliferative and Proapoptotic Effects of Cucurbitacin B on BPH-1 Cells via the p53/MDM2 Axis" International Journal of Molecular Sciences 25, no. 1: 442. https://doi.org/10.3390/ijms25010442
APA StyleZhou, P., Huang, S., Shao, C., Huang, D., Hu, Y., Su, X., Yang, R., Jiang, J., & Wu, J. (2024). The Antiproliferative and Proapoptotic Effects of Cucurbitacin B on BPH-1 Cells via the p53/MDM2 Axis. International Journal of Molecular Sciences, 25(1), 442. https://doi.org/10.3390/ijms25010442






