Extracellular Self- and Non-Self DNA Involved in Damage Recognition in the Mistletoe Parasitism of Mesquite Trees
Abstract
:1. Introduction
| Specie | DAMP Type | Time of Response | Responses |
|---|---|---|---|
| Arabidopsis thaliana | Self-leaf extract | 20–60 min | Increase in JA level [28] |
| Phaseolus lunatus | Self-leaf extract | 20–60 min | Decrease in secretion of EFN [28] |
| Increase in JA level [28] | |||
| Upregulation of JA-related genes [28] | |||
| Downregulation photosynthesis genes [28] | |||
| Solanum lycopersicum | Self-leaf extract | 20–60 min | Increase in JA level [28] |
| Fragaria sp. | Self-leaf extract | 20–60 min | Increase in JA level [28] |
| Sesamun indicum | Self-leaf extract | 20–60 min | Increase in JA level [28] |
| Zea mays | Self-leaf extract | 20–60 min | Increase in JA level [28] |
| Phaseolus vulgaris | Bean-leaf homogenate | 2 h | Increase in secretion of EFN [29] |
| H2O2 accumulation [29] | |||
| 24 h | Resistance to pathogens [29] | ||
| Non-bean-leaf homogenate | 24 h | Resistance to pathogens [29] | |
| Nicotiana benthamiana | Cuscuta homogenates | 1 h | Increase in ET level [62] |
| Solanum lycopersicum | Cuscuta Cell-wall homogenates | 1 h | Increase in ET levels [62] |
| Increase in ET levels [62] | |||
| Increase lignification in application site [30,63] | |||
| Vitis vinifera | Self-xyloglucan from cell walls | 5 min | MAPKs activation [64] |
| 20 min | H2O2 accumulation [64] | ||
| 1 h | Overexpression of PAL and stillbene synthase (STS) genes [64] | ||
| Arabidopsis thaliana | eATP | 0 h | O2•−and H2O2 accumulation [31,34] |
| Activation of Ca2+ channels [34] | |||
| Upregulation of MPK3 [34] | |||
| 30 min | Upregulation of genes respond to MeJA [37] | ||
| Upregulation of RBOHD [37] | |||
| 1–2 h | Upregulation of SA-related genes [37] | ||
| Upregulation of RBOHD and PAL [31] | |||
| 12 h | Inhibition of vesicular trafficking [35] | ||
| 24 h | Activation of ET signaling [65] | ||
| Reduction of cell viability [35] | |||
| 10 d | Reduction of JAZ1 stability [37] | ||
| Resistance to pathogens [37] | |||
| Decrease in leaf area and root length [39] | |||
| NPTs (ATP, GTP, CTP) | 5 s | Elevation in cytosolic Ca2+ [40] | |
| O2•− accumulation [31] | |||
| Nicotiana tabacum | eATP | 3–5 d | Upregulation of SA-related genes [33] |
| Resistance to pathogens [33] | |||
| Phaseolus vulgaris | eATP | 2h | H2O2 accumulation [38] |
| Increase in CAT and PPO activities [38] | |||
| Increase in malondialdehyde content [38] | |||
| 4 h | H2O2 level restored [38] | ||
| CAT activity restored [38] | |||
| Solanum lycopersicum | eATP | 30 min | NO accumulation [32] |
| Dimocarpus longan | eATP | 1–5 d | Polyphenols and flavonoids accumulation [36] |
| Decrease in PPO activity and total sugar content [36] | |||
| Populus euphratica | eATP | 30 min | Elevation of cytosolic Ca2+ [66] |
| Reduction of cell viability [66] | |||
| 12 h | H2O2 and NO accumulation [66] | ||
| Chlamydomonas reinhardtii | Self-exDNA | 168 h | Inhibition of root growth [49] |
| Forming aggregates [49] | |||
| Nannochloropsis gaditana | Self-exDNA | 168 h | Inhibition of root growth [49] |
| Forming aggregates [49] | |||
| Neochloris oleoabundans | Self-exDNA | 15–60 min | Increase in PEX activity [54] |
| Polyphenols and lipids accumulation [54] | |||
| 24–48 h | Increase in Cks and GA level [54] | ||
| Arabidopsis thaliana | Self-exDNA | 15 min | H2O2 accumulation [58] |
| 1 h | Increase in JA level [56,58] | ||
| 2 h | Upregulation of MPK3, MPK6, OXI1, and CML37 [47] | ||
| 8–10 h | Upregulation RBOHF, LOX3, MYC2, and JAZ1 [56] | ||
| cAMP production [53] | |||
| Accumulation of RNA constituents [53] | |||
| 16 h | Increase in root hair density [48] | ||
| Upregulation of CK’s-related genes [48] | |||
| 24 h | Downregulation ABA-related genes [48] | ||
| Increase in SA level [58] | |||
| 5–10 d | Resistance to pathogens [47] | ||
| Inhibition of root growth [56] | |||
| Necrosis and chlorosis in root and leaf, respectively [48] | |||
| Non-self exDNA (A. thaliana ecotypes) | 15 min | H2O2 accumulation [58] | |
| 1 h | Increase in JA level [56,58] | ||
| 5 d | Upregulation of MPK3, MPK6, OXI1, and CML37 [47] | ||
| Inhibition of root growth [56] | |||
| Non-self- exDNA (Arabidopsis pumila) | 1 h | H2O2 accumulation [56] | |
| 5 d | Inhibition of root growth [56] | ||
| Non-self exDNA (Brassica) | 1 h | H2O2 production [56] | |
| Upregulation of MPK3, MPK6, OXI1, and CML37 [47] | |||
| 24 h | Increase in SA level [58] | ||
| 5 d | Inhibition of root growth [56] | ||
| Non-self exDNA (Phaseolus vulgaris) | 1 h | Upregulation of MPK3, MPK6, OXI1, and CML37 [47] | |
| Non-self exDNA (Citrus aurantium) | 1 h | Upregulation MPK3 and OXI1 [47] | |
| Non-self exDNA (Triticum aestivum and Solanum lycopersicum) | 1 h | No detectable effects [56] | |
| Synthetic ssODNs | 1.5 h | Stomatal closure [46] | |
| 4 h | Upregulation of MPK3, PROPEP1, and WRKY33 [46] | ||
| 24 h | Resistance to pathogens [46] | ||
| Acanthus mollis | Self-exDNA | Nd | Inhibition of root growth [41] |
| Non-self exDNA (A. thaliana, Quercus ilex) | Nd | No detectable effects [41] | |
| Phaseolus vulgaris | Self-exDNA | 30 min | H2O2 accumulation [45] |
| Increase in JA level [45] | |||
| 12 h | MAPKs activation [45] | ||
| 24 h | Increase in SA level [27] | ||
| Secretion of EFN [45] | |||
| Resistance to pathogens [27,45] | |||
| 4 d | Reduction of herbivore [27] | ||
| End growth cycle | Inhibition of root growth [45] | ||
| Enhance in seed production [27] | |||
| Non-self exDNA (P. lunatus and Acacia farnesiana) | 24 h | Increase in SA level [27] | |
| Induction of resistance to pathogens [27] | |||
| Self-exDNA | 30 min | Increase of cytosolic Ca2+ [43] | |
| Non-self exDNA (Zea mays) | 30 min | Increase of cytosolic Ca2+ [43] | |
| 1 h | H2O2 accumulation [42,56] | ||
| Polyphenols and flavonoids accumulation, CAT, SOD, and PAL activity [52] | |||
| Inhibition of root growth [56] | |||
| 2 h | JA-Ile production [56] | ||
| 24 h | Upregulation of PRRs, PIs ACC oxidase, and calcium-related genes [42] | ||
| 48 h | Downregulation of cat, polygalacturonase, and coumaric acid to CoA genes [42] | ||
| Increase in PAL activity [52] | |||
| 10 d | Upregulation LoxD, MYC2, JAZ1, and RBOH [56] | ||
| Resistance to pathogens [56] | |||
| Polyphenols and flavonoids accumulation [52] | |||
| Non-self exDNA (A. thaliana and T. aestivumand) | 1, 2, and 24 h | No detectable effects [56] | |
| Non-self exDNA (Latuca sativa, Apium graveolens and Cucumis sativus) | 0–48 h | Increase in CAT activity, polyphenols, and flavonoids accumulation [52] | |
| Lactuca sativa | Self exDNA | 5 d | Upregulation of SOD, CAT and PAL [44] |
| Polyphenols and flavonoids accumulation [44] | |||
| Inhibition of germination and root growth [44] | |||
| Non-self exDNA (Capsicum chinense) | 5 d | Upregulation of SOD, CAT, and PAL [44] | |
| Polyphenols and flavonoids accumulation [44] | |||
| Inhibition of germination and root growth [44] | |||
| Non-self exDNA (Acaciella angustissima) | 5 d | Upregulation of PAL [44] | |
| Polyphenols and flavonoids accumulation [44] | |||
| Prunus persica | Self-exDNA | 12 h | Upregulation of MAPK-related genes [57] |
| Increase in ethylene level [57] | |||
| Upregulation of ET-related genes [57] | |||
| 36 h | Resistance to pathogens [57] | ||
| 5 d | Decrease in sugar content [57] | ||
| Non-self exDNA (S. lycopersicum) | 36 h | Resistance to pathogens [57] | |
| 5 d | Decrease in sugar content [57] | ||
| Alnus glutinosa | Self-exDNA | 72 h | Root damage [50] |
| Non-self eDNA (Festuca drymeja) | 72 h | Root damage [50] | |
| Oryza sativa | Self-exDNA | 7 d | O2•− and H2O2 accumulation [55] |
| Inhibition of root growth [55] | |||
| Downregulation SOD and CAT [55] |
2. Results
2.1. Study System
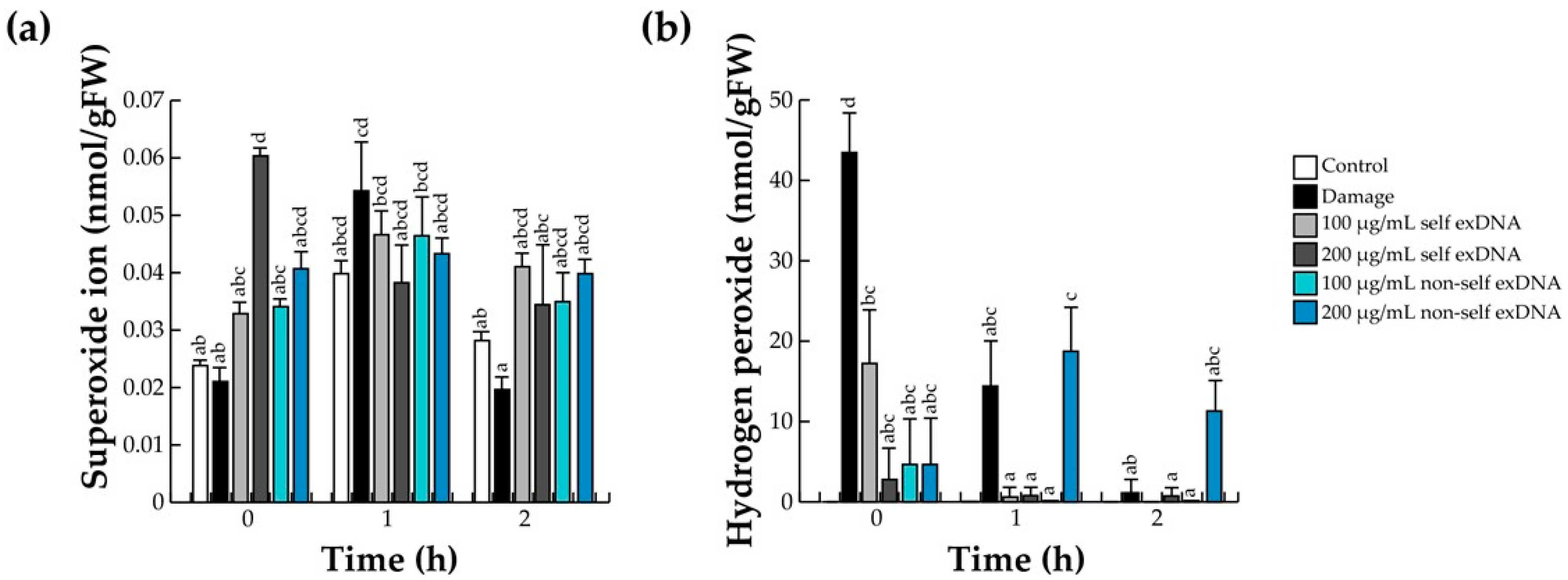
2.2. Effect of exDNA on the Oxidative Burst
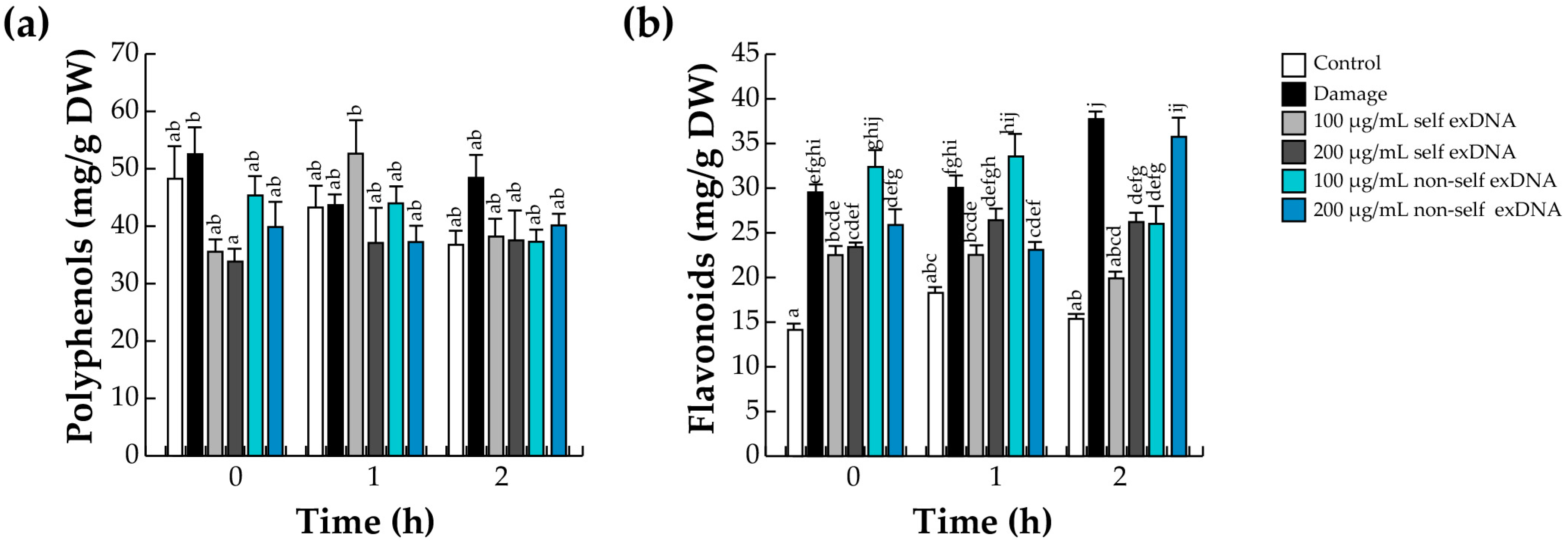
2.3. Accumulation of Polyphenols and Flavonoids
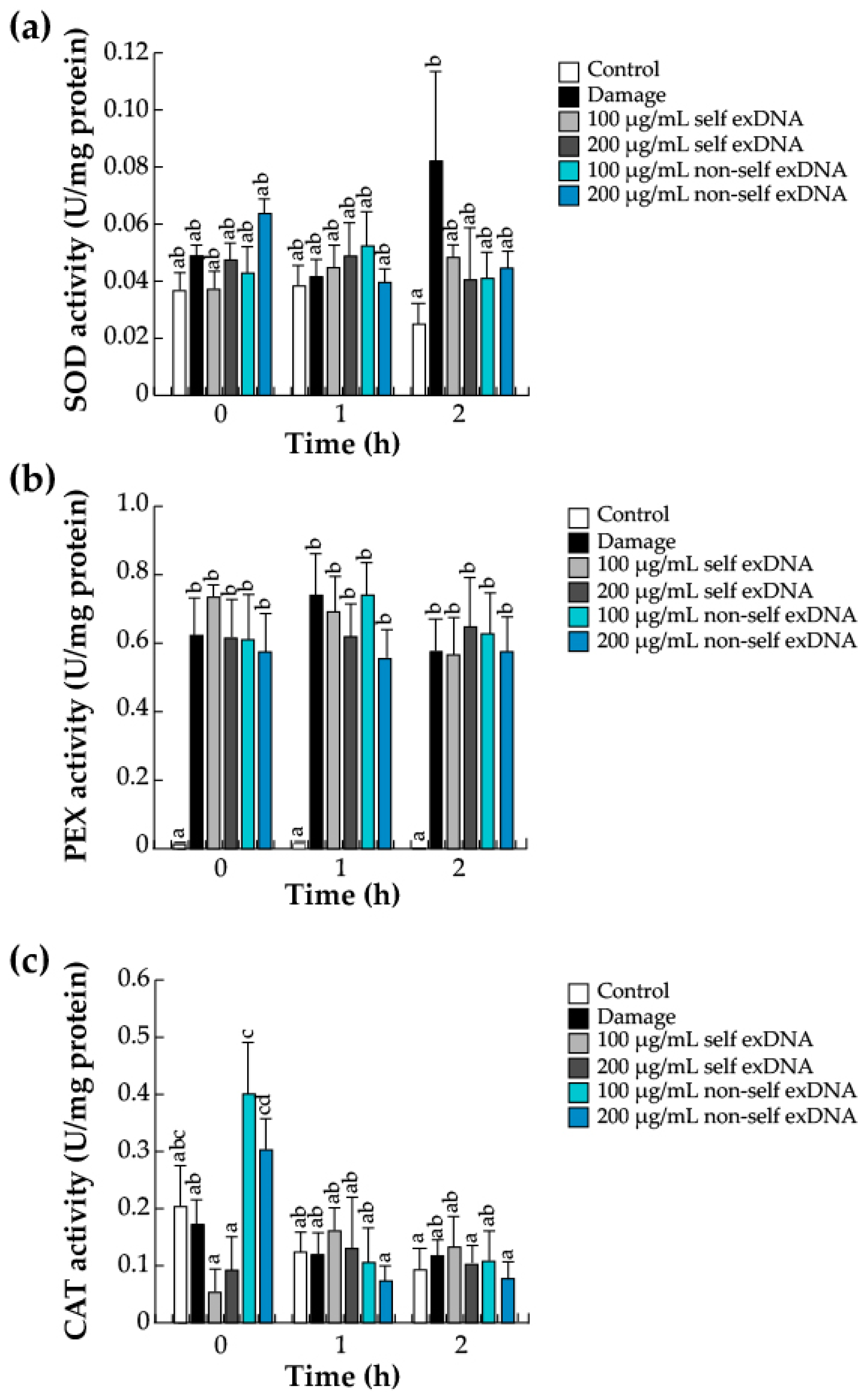
2.4. Antioxidant Enzymes
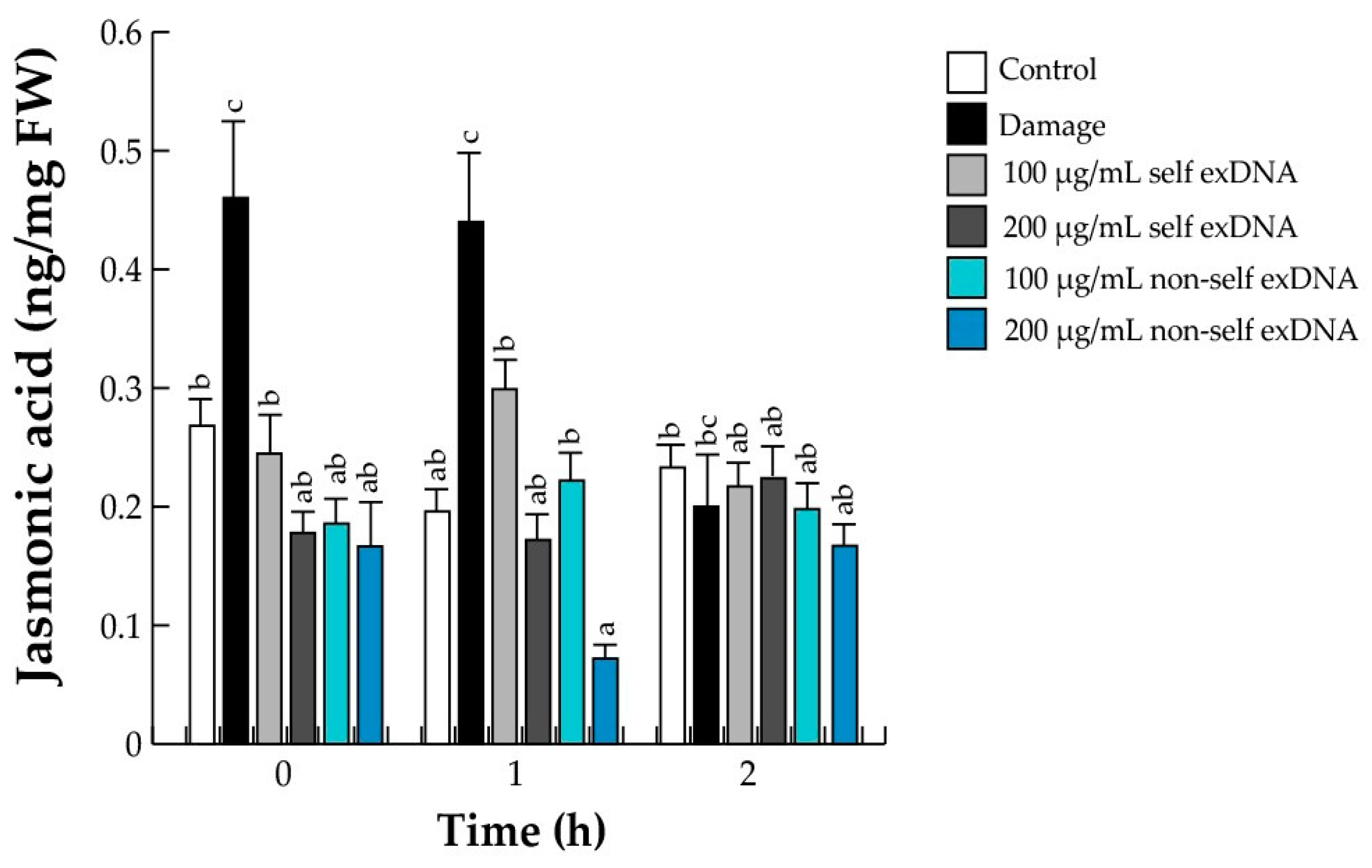
2.5. Activation of JA Signaling
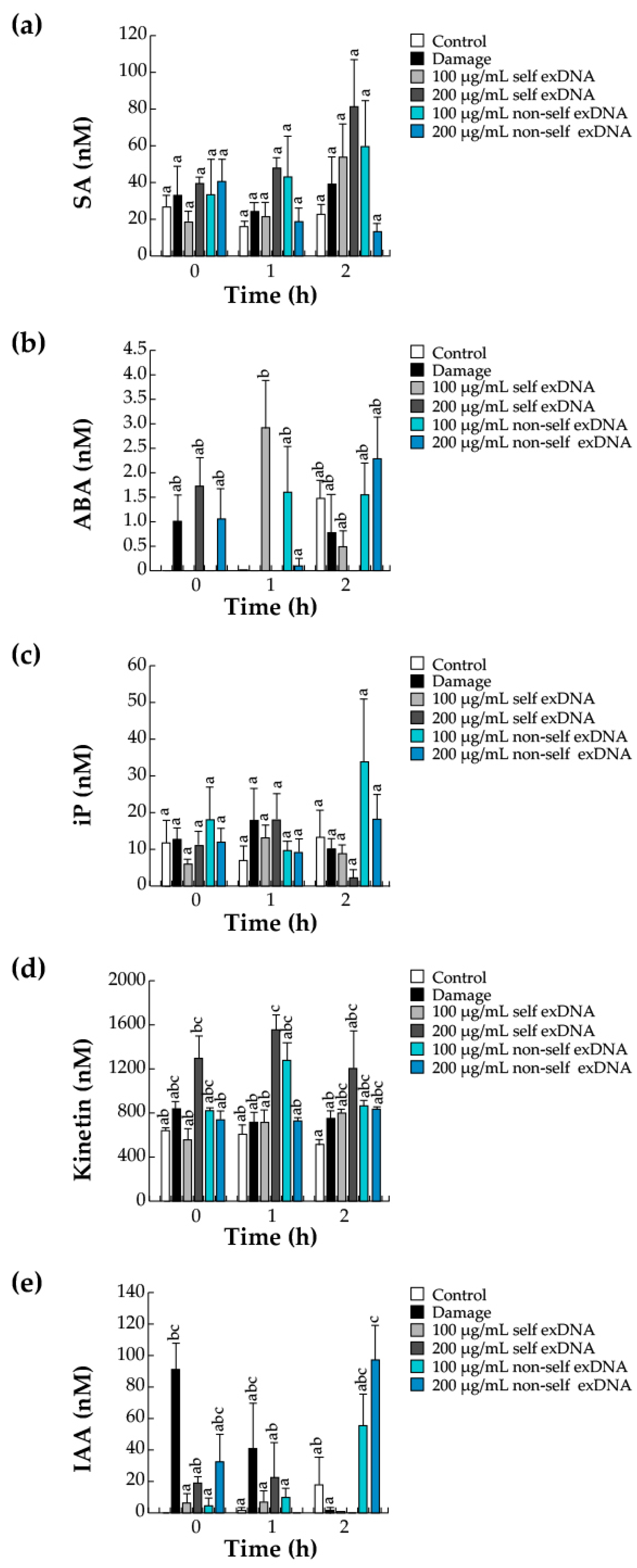
2.6. Hormonal Accumulation during the Perception of Self- and Non-Self exDNA
2.7. Mapk Gene Expression Levels by Self and Non-Self exDNA
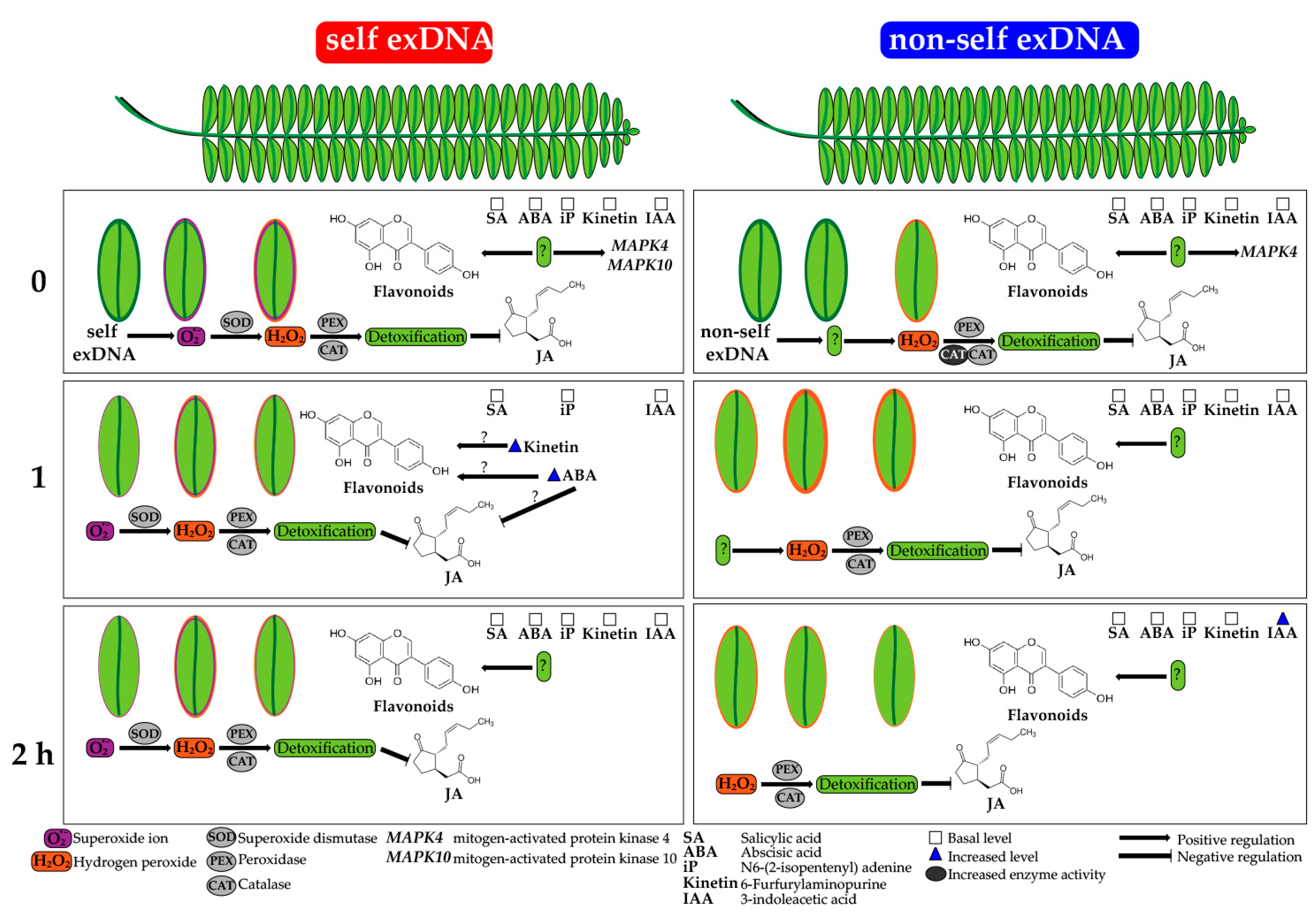
3. Discussion
Relationship of exDNA in the P. calyculatus Establishment
4. Materials and Methods
4.1. exDNA Generation
4.2. Study Site and exDNA Application
4.3. Quantification of O2•−
4.4. H2O2 Quantification
4.5. Jasmonic Acid Quantification
4.6. RNA Extraction from Mesquite Leaves Treated with exDNA
4.7. cDNA Synthesis and Analysis of MAPKs Gene Expression
4.8. Quantification of Polyphenols and Flavonoids
4.9. Antioxidant Enzyme Activities
4.10. Phytohormone Quantification
4.11. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauceda, E.N.R.; Martínez, G.E.R.; Valverde, B.R.; Ruiz, R.M.; Hermida, M.d.l.C.C.; Torres, S.M.M.; Ruiz, H.H.P. Análisis técnico del árbol del mezquite (Prosopis laevigata Humb. & Bonpl. ex Willd.) en México. Ra Ximhai 2014, 10, 173–193. [Google Scholar]
- Mudgil, D.; Barak, S. Mesquite gum (Prosopis gum): Structure, properties & applications—A review. Int. J. Biol. Macromol. 2020, 159, 1094–1102. [Google Scholar]
- Villalón-Mendoza, H.; Hernández-Hernández, E.E.; Manzanares-Miranda, N. Presence and Importance of Mesquite Prosopis laevigata (Humb. & Bonpl. ex Willd.) MC Johnst in Northeastern Mexico. In Sustainable Management of Natural Resources: Diversity, Ecology, Taxonomy and Sociology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 115–129. [Google Scholar]
- Rasanen, L.A.; Lindstrom, K. Effects of Biotic and Abiotic Constraints on the Symbiosis between Rhizobia and the Tropical Leguminous Trees Acacia and Prosopis; NISCAIR-CSIR: New Delhi, India, 2003. [Google Scholar]
- Herrera-Arreola, G.; Herrera, Y.; Reyes-Reyes, B.; Dendooven, L. Mesquite (Prosopis juliflora (Sw.) DC.), huisache (Acacia farnesiana (L.) Willd.) and catclaw (Mimosa biuncifera Benth.) and their effect on dynamics of carbon and nitrogen in soils of the semi-arid highlands of Durango Mexico. J. Arid. Environ. 2007, 69, 583–598. [Google Scholar] [CrossRef]
- García-Sánchez, R.; Camargo-Ricalde, S.L.; García-Moya, E.; Luna-Cavazos, M.; Romero-Manzanares, A.; Manuel Montaño, N. Prosopis laevigata and Mimosa biuncifera (Leguminosae), jointly influence plant diversity and soil fertility of a Mexican semiarid ecosystem. Rev. Biol. Trop. 2012, 60, 87–103. [Google Scholar] [CrossRef]
- Frías-Hernández, J.; Aguilar-Ledezma, A.; Olalde-Portugal, V.; Cinvestav-Ipn, J.; Balderas-Lopez, J.; Gutierrez-Juarez, G.; Alvarado-Gil, J.; Castro, J.; Vargas, H.; Albores, A. Research note soil characteristics in semiarid highlands of central Mexico as affected by mesquite trees (Prosopis laevigata). Arid. Soil Res. Rehabil. 1999, 13, 305–312. [Google Scholar] [CrossRef]
- Zuria, I.; Castellanos, I.; Gates, J.E. The influence of mistletoes on birds in an agricultural landscape of central Mexico. Acta Oecologica 2014, 61, 51–56. [Google Scholar] [CrossRef]
- Geils, B.; Hawksworth, F. Damage, effects, and importance of dwarf mistletoes. In Mistletoes of North American Conifers; Geils, B.W., Cibrián Tovar, J., Moody, B., Eds.; Gen. Tech. Rep. RMRS-GTR-98; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2002; Volume 98, pp. 57–65. [Google Scholar]
- Reid, N.; Yan, Z.; Fittler, J. Impact of mistletoes (Amyema miquelii) on host (Eucalyptus blakelyi and Eucalyptus melliodora) survival and growth in temperate Australia. For. Ecol. Manag. 1994, 70, 55–65. [Google Scholar] [CrossRef]
- Meinzer, F.; Woodruff, D.; Shaw, D. Integrated responses of hydraulic architecture, water and carbon relations of western hemlock to dwarf mistletoe infection. Plant Cell Environ. 2004, 27, 937–946. [Google Scholar] [CrossRef]
- Escher, P.; Peuke, A.D.; Bannister, P.; Fink, S.; Hartung, W.; Jiang, F.; Rennenberg, H. Transpiration, CO2 assimilation, WUE, and stomatal aperture in leaves of Viscum album (L.): Effect of abscisic acid (ABA) in the xylem sap of its host (Populus × euamericana). Plant Physiol. Biochem. 2008, 46, 64–70. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Nickrent, D.L.; Shaw, D.C.; Watson, D.M. Mistletoes: Pathology, systematics, ecology, and management. Plant Dis. 2008, 92, 988–1006. [Google Scholar] [CrossRef]
- Agne, M.C.; Shaw, D.C.; Woolley, T.J.; Queijeiro-Bolaños, M.E. Effects of dwarf mistletoe on stand structure of lodgepole pine forests 21-28 years post-mountain pine beetle epidemic in central Oregon. PLoS ONE 2014, 9, e107532. [Google Scholar] [CrossRef] [PubMed]
- Szmidla, H.; Tkaczyk, M.; Plewa, R.; Tarwacki, G.; Sierota, Z. Impact of common mistletoe (Viscum album L.) on Scots pine forests—A call for action. Forests 2019, 10, 847. [Google Scholar] [CrossRef]
- Pérez-Crespo, M.J.; Ornelas, J.F.; Martén-Rodríguez, S.; González-Rodríguez, A.; Lara, C. Reproductive biology and nectar production of the Mexican endemic Psittacanthus auriculatus (Loranthaceae), a hummingbird-pollinated mistletoe. Plant Biol. 2016, 18, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-González, A.; Hódar, J.A.; Zamora, R. Mistletoe versus host pine: Does increased parasite load alter the host chemical profile? J. Chem. Ecol. 2019, 45, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Rodríguez, E.; Ramírez-Rodríguez, A.G.; Ramírez-Chávez, E.; Molina-Torres, J.; Camacho-Coronel, X.; Esparza-Claudio, J.; Heil, M.; Orona-Tamayo, D. Biochemical traits in the flower lifetime of a mexican mistletoe parasitizing mesquite biomass. Front. Plant Sci. 2018, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Venegas, M.; Quintana-Rodríguez, E.; Aguilar-Hernández, V.; López-García, M.; Conejo-Dávila, E.; Brito-Argáez, L.; Loyola-Vargas, V.M.; Vega-Arreguín, J.; Orona-Tamayo, D. Protein Profiling of Psittacanthus calyculatus during Mesquite Infection. Plants 2023, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Yeo, I.-C.; Shan, L. Knowing me, knowing you: Self and non-self recognition in plant immunity. Essays Biochem. 2022, 66, 447–458. [Google Scholar]
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtell, M.J.; Westwood, J.H. Molecular dialog between parasitic plants and their hosts. Annu. Rev. Phytopathol. 2019, 57, 279–299. [Google Scholar] [CrossRef]
- Jhu, M.-Y.; Sinha, N.R. Cuscuta species: Model organisms for haustorium development in stem holoparasitic plants. Front. Plant Sci. 2022, 13, 1086384. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Ferrari, S.; Cervone, F.; Okun, E. Extracellular DAMPs in plants and mammals: Immunity, tissue damage and repair. Trends Immunol. 2018, 39, 937–950. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Sede, A.R.; Mutterer, J.; Boutant, E.; Heinlein, M. Suppression of a dsRNA-induced plant immunity pathway by viral movement protein. BioRxiv 2021. [Google Scholar] [CrossRef]
- Chambard, M.; Plasson, C.; Derambure, C.; Coutant, S.; Tournier, I.; Lefranc, B.; Leprince, J.; Kiefer-Meyer, M.-C.; Driouich, A.; Follet-Gueye, M.-L. New insights into plant extracellular DNA. A study in soybean root extracellular trap. Cells 2021, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Durán-Flores, D.; Heil, M. The CpG-dependent plant immune response to self-DNA triggers defence hormone signalling and improves fitness. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Heil, M.; Ibarra-Laclette, E.; Adame-Alvarez, R.M.; Martinez, O.; Ramirez-Chavez, E.; Molina-Torres, J.; Herrera-Estrella, L. How Plants Sense Wounds: Damaged-Self Recognition Is Based on Plant-Derived Elicitors and Induces Octadecanoid Signaling. PLoS ONE 2012, 7, e30537. [Google Scholar] [CrossRef] [PubMed]
- Duran-Flores, D.; Heil, M. Damaged-self recognition in common bean (Phaseolus vulgaris) shows taxonomic specificity and triggers signaling via reactive oxygen species (ROS). Front. Plant Sci. 2014, 5, 585. [Google Scholar] [CrossRef] [PubMed]
- Hegenauer, V.; Fürst, U.; Kaiser, B.; Smoker, M.; Zipfel, C.; Felix, G.; Stahl, M.; Albert, M. Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science 2016, 353, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232. [Google Scholar] [CrossRef]
- Foresi, N.P.; Laxalt, A.M.; Tonón, C.V.; Casalongué, C.A.; Lamattina, L. Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol. 2007, 145, 589–592. [Google Scholar] [CrossRef]
- Chivasa, S.; Murphy, A.M.; Hamilton, J.M.; Lindsey, K.; Carr, J.P.; Slabas, A.R. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009, 60, 436–448. [Google Scholar] [CrossRef]
- Demidchik, V.; Shang, Z.; Shin, R.; Thompson, E.; Rubio, L.; Laohavisit, A.; Mortimer, J.C.; Chivasa, S.; Slabas, A.R.; Glover, B.J. Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. 2009, 58, 903–913. [Google Scholar] [CrossRef]
- Deng, S.; Sun, J.; Zhao, R.; Ding, M.; Zhang, Y.; Sun, Y.; Wang, W.; Tan, Y.; Liu, D.; Ma, X. Populus euphratica APYRASE2 enhances cold tolerance by modulating vesicular trafficking and extracellular ATP in Arabidopsis plants. Plant Physiol. 2015, 169, 530–548. [Google Scholar] [CrossRef]
- Chen, M.; Lin, H.; Zhang, S.; Lin, Y.; Chen, Y.; Lin, Y. Effects of adenosine triphosphate (ATP) treatment on postharvest physiology, quality and storage behavior of longan fruit. Food Bioprocess Technol. 2015, 8, 971–982. [Google Scholar] [CrossRef]
- Tripathi, D.; Zhang, T.; Koo, A.J.; Stacey, G.; Tanaka, K. Extracellular ATP acts on jasmonate signaling to reinforce plant defense. Plant Physiol. 2018, 176, 511–523. [Google Scholar] [CrossRef]
- Wang, Q.-W.; Jia, L.-Y.; Shi, D.-L.; Wang, R.-f.; Lu, L.-N.; Xie, J.-J.; Sun, K.; Feng, H.-Q.; Li, X. Effects of extracellular ATP on local and systemic responses of bean (Phaseolus vulgaris L.) leaves to wounding. Biosci. Biotechnol. Biochem. 2019, 83, 417–428. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, H.; Zhang, Y.; Jia, L.; Pang, H.; Feng, H.; Wang, X. The involvement of extracellular ATP in regulating the stunted growth of Arabidopsis plants by repeated wounding. BMC Plant Biol. 2022, 22, 279. [Google Scholar] [CrossRef]
- Tanaka, K.; Swanson, S.J.; Gilroy, S.; Stacey, G. Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol. 2010, 154, 705–719. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Cartenì, F.; Bonanomi, G.; Senatore, M.; Termolino, P.; Giannino, F.; Incerti, G.; Rietkerk, M.; Lanzotti, V.; Chiusano, M.L. Inhibitory effects of extracellular self-DNA: A general biological process? New Phytol. 2015, 206, 127–132. [Google Scholar] [CrossRef]
- Barbero, F.; Guglielmotto, M.; Islam, M.; Maffei, M.E. Extracellular fragmented self-DNA is involved in plant responses to biotic stress. Front. Plant Sci. 2021, 1558, 686121. [Google Scholar] [CrossRef]
- Barbero, F.; Guglielmotto, M.; Capuzzo, A.; Maffei, M.E. Extracellular self-DNA (esDNA), but not heterologous plant or insect DNA (etDNA), induces plasma membrane depolarization and calcium signaling in lima bean (Phaseolus lunatus) and maize (Zea mays). Int. J. Mol. Sci. 2016, 17, 1659. [Google Scholar] [CrossRef]
- Vega-Muñoz, I.; Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Guevara-González, R. Exogenous fragmented DNA acts as a damage-associated molecular pattern (DAMP) inducing changes in CpG DNA methylation and defence-related responses in Lactuca sativa. Funct. Plant Biol. 2018, 45, 1065–1072. [Google Scholar] [CrossRef]
- Duran-Flores, D.; Heil, M. Extracellular self-DNA as a damage-associated molecular pattern (DAMP) that triggers self-specific immunity induction in plants. Brain Behav. Immun. 2018, 72, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Toum, L.; Conti, G.; Guerriero, F.C.; Conforte, V.P.; Garolla, F.A.; Asurmendi, S.; Vojnov, A.A.; Gudesblat, G.E. Single-stranded oligodeoxynucleotides induce plant defence in Arabidopsis thaliana. Ann. Bot. 2020, 126, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Rassizadeh, L.; Cervero, R.; Flors, V.; Gamir, J. Extracellular DNA as an elicitor of broad-spectrum resistance in Arabidopsis thaliana. Plant Sci. 2021, 312, 111036. [Google Scholar] [CrossRef] [PubMed]
- Chiusano, M.L.; Incerti, G.; Colantuono, C.; Termolino, P.; Palomba, E.; Monticolo, F.; Benvenuto, G.; Foscari, A.; Esposito, A.; Marti, L. Arabidopsis thaliana response to extracellular DNA: Self versus nonself exposure. Plants 2021, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Palomba, E.; Chiaiese, P.; Termolino, P.; Paparo, R.; Filippone, E.; Mazzoleni, S.; Chiusano, M.L. Effects of extracellular self-and nonself-DNA on the freshwater microalga Chlamydomonas reinhardtii and on the marine microalga Nannochloropsis gaditana. Plants 2022, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Termolino, P.; De Micco, V.; Mazzoleni, S. Field evidence for litter and self-DNA inhibitory effects on Alnus glutinosa roots. New Phytol. 2022, 236, 399–412. [Google Scholar] [CrossRef]
- Carbajal-Valenzuela, I.A.; Medina-Ramos, G.; Caicedo-Lopez, L.H.; Jiménez-Hernández, A.; Ortega-Torres, A.E.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Guevara-González, R.G. Extracellular DNA: Insight of a signal molecule in crop protection. Biology 2021, 10, 1022. [Google Scholar] [CrossRef]
- Carbajal-Valenzuela, I.A.; Guzmán-Cruz, R.; González-Chavira, M.M.; Medina-Ramos, G.; Serrano-Jamaica, L.M.; Torres-Pacheco, I.; Vázquez, L.; Feregrino-Pérez, A.A.; Rico-García, E.; Guevara-González, R.G. Response of Plant Immunity Markers to Early and Late Application of Extracellular DNA from Different Sources in Tomato (Solanum lycopersicum). Agriculture 2022, 12, 1587. [Google Scholar] [CrossRef]
- Lanzotti, V.; Grauso, L.; Mangoni, A.; Termolino, P.; Palomba, E.; Anzano, A.; Incerti, G.; Mazzoleni, S. Metabolomics and molecular networking analyses in Arabidopsis thaliana show that extracellular self-DNA affects nucleoside/nucleotide cycles with accumulation of cAMP, cGMP and N6-methyl-AMP. Phytochemistry 2022, 204, 113453. [Google Scholar] [CrossRef]
- Zárate-López, M.A.; Quintana-Rodríguez, E.; Orona-Tamayo, D.; Aguilar-Hernández, V.; Araujo-León, J.A.; Brito-Argáez, L.; Molina-Torres, J.; Hernández-Flores, J.L.; Loyola-Vargas, V.M.; Lozoya-Pérez, N.E. Metabolic Responses of the Microalga Neochloris oleoabundans to Extracellular Self-and Nonself-DNA. Int. J. Mol. Sci. 2023, 24, 14172. [Google Scholar] [CrossRef] [PubMed]
- Tjia, T.; Meitha, K.; Septiani, P.; Awaludin, R.; Sumardi, D. Extracellular self-DNA induces local inhibition of growth, regulates production of reactive oxygen species, and gene expression in rice roots. Biol. Plant 2023, 67, 9–18. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, H.; Zhang, X.; Khashi u Rahman, M.; Mazzoleni, S.; Du, M.; Wu, F. Plant extracellular self-DNA inhibits growth and induces immunity via the jasmonate signaling pathway. Plant Physiol. 2023, 192, 2475–2491. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, K.; Zou, Y.; Lei, C.; Chen, Z.; Zheng, Y. Extracellular self-DNA induced a PTI-related local defence against Rhizopus rot in postharvest peach fruit. Postharvest Biol. Technol. 2023, 200, 112306. [Google Scholar] [CrossRef]
- Vega-Muñoz, I.; Herrera-Estrella, A.; Martínez-de la Vega, O.; Heil, M. ATM and ATR, two central players of the DNA damage response, are involved in the induction of systemic acquired resistance by extracellular DNA, but not the plant wound response. Front. Immunol. 2023, 14, 2385. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr. How the immune system works to protect the host from infection: A personal view. Proc. Natl. Acad. Sci. USA 2001, 98, 7461–7468. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Land, W.G.; Tör, M. Wound recognition across the tree of life. Front. Plant Sci. 2016, 7, 1319. [Google Scholar] [CrossRef]
- Gallucci, S.; Maffei, M.E. DNA sensing across the tree of life. Trends Immunol. 2017, 38, 719–732. [Google Scholar] [CrossRef]
- Hegenauer, V.; Slaby, P.; Körner, M.; Bruckmüller, J.-A.; Burggraf, R.; Albert, I.; Kaiser, B.; Löffelhardt, B.; Droste-Borel, I.; Sklenar, J. The tomato receptor CuRe1 senses a cell wall protein to identify Cuscuta as a pathogen. Nat. Commun. 2020, 11, 5299. [Google Scholar] [CrossRef]
- Jhu, M.-Y.; Farhi, M.; Wang, L.; Philbrook, R.N.; Belcher, M.S.; Nakayama, H.; Zumstein, K.S.; Rowland, S.D.; Ron, M.; Shih, P.M. Lignin-based resistance to Cuscuta campestris parasitism in Heinz resistant tomato cultivars. bioRxiv 2019, 706861. [Google Scholar] [CrossRef]
- Claverie, J.; Balacey, S.; Lemaître-Guillier, C.; Brulé, D.; Chiltz, A.; Granet, L.; Noirot, E.; Daire, X.; Darblade, B.; Héloir, M.-C. The cell wall-derived xyloglucan is a new DAMP triggering plant immunity in Vitis vinifera and Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Deng, C.; Yao, J.; Zhang, H.; Wang, Y.; Deng, S. A salt-signaling network involving ethylene, extracellular ATP, hydrogen peroxide, and calcium mediates K+/Na+ homeostasis in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 8683. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.L.; Deng, S.R.; Lu, C.F.; Shen, X.; Zhou, X.Y.; Zheng, X.J.; Hu, Z.M.; Chen, S.L. An ATP signalling pathway in plant cells: Extracellular ATP triggers programmed cell death in Populus euphratica. Plant Cell Environ. 2012, 35, 893–916. [Google Scholar] [CrossRef]
- Serrano-Jamaica, L.M.; Villordo-Pineda, E.; González-Chavira, M.M.; Guevara-González, R.G.; Medina-Ramos, G. Effect of fragmented DNA from plant pathogens on the protection against wilt and root rot of Capsicum annuum L. Plants. Front. Plant Sci. 2021, 11, 581891. [Google Scholar] [CrossRef]
- Marino, M.; Pellegrini, M.; La Rosa, P.; Acconcia, F. Susceptibility of estrogen receptor rapid responses to xenoestrogens: Physiological outcomes. Steroids 2012, 77, 910–917. [Google Scholar] [CrossRef]
- Sun, L.R.; Zhao, Z.J.; Hao, F.S. NADPH oxidases, essential players of hormone signalings in plant development and response to stresses. Plant Signal. Behav. 2019, 14, 1657343. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Recent Insights Into the Double Role of Hydrogen Peroxide in Plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Alon, M.; Malka, O.; Eakteiman, G.; Elbaz, M.; Moyal Ben Zvi, M.; Vainstein, A.; Morin, S. Activation of the Phenylpropanoid pathway in Nicotiana tabacum improves the performance of the whitefly Bemisia tabaci via reduced jasmonate signaling. PLoS ONE 2013, 8, e76619. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Kobarfard, F.; Ata, A.; Ayatollahi, S.A.; Khosravi-Dehaghi, N.; Jugran, A.K.; Tomas, M.; Capanoglu, E.; Matthews, K.R.; Popović-Djordjević, J. Prosopis plant chemical composition and pharmacological attributes: Targeting clinical studies from preclinical evidence. Biomolecules 2019, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Forner, S.; Strnad, M.; Hause, B. Jasmonates in flower and seed development. Biochimie 2013, 95, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- López-Ruiz, B.A.; Zluhan-Martínez, E.; Sánchez, M.d.l.P.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. Interplay between hormones and several abiotic stress conditions on Arabidopsis thaliana primary root development. Cells 2020, 9, 2576. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.; Scortecci, K. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Ishida, J.K.; Wakatake, T.; Yoshida, S.; Takebayashi, Y.; Kasahara, H.; Wafula, E.; Depamphilis, C.W.; Namba, S.; Shirasu, K. Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates haustorium development in the parasitic plant Phtheirospermum japonicum. Plant Cell 2016, 28, 1795–1814. [Google Scholar] [CrossRef]
- Du, Y.; Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2018, 69, 155–167. [Google Scholar] [CrossRef]
- Cavallari, N.; Artner, C.; Benkova, E. Auxin-regulated lateral root organogenesis. Cold Spring Harb. Perspect. Biol. 2021, 13, a039941. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, J.F.; Gándara, E.; Vásquez-Aguilar, A.A.; Ramírez-Barahona, S.; Ortiz-Rodriguez, A.E.; González, C.; Mejía Saules, M.T.; Ruiz-Sanchez, E. A mistletoe tale: Postglacial invasion of Psittacanthus schiedeanus (Loranthaceae) to Mesoamerican cloud forests revealed by molecular data and species distribution modeling. BMC Evol. Biol. 2016, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Mourão, F.A.; Jacobi, C.M.; Figueira, J.E.C.; Batista, E.K.L. Effects of the parasitism of Struthanthus flexicaulis (Mart.) Mart. (Loranthaceae) on the fitness of Mimosa calodendron Mart.(Fabaceae), an endemic shrub from rupestrian fields over ironstone outcrops, Minas Gerais State, Brazil. Acta Bot. Bras. 2009, 23, 820–825. [Google Scholar] [CrossRef]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mendoza, D.; Mendez-Trujillo, V.; Grimaldo-Juarez, O.; Ceceña-Duran, C.; Tzintzun-Camacho, O.; Gutierrez-Miceli, F.; Sanchez-Viveros, G.; Aviles Marin, M. Changes of photochemical efficiency and epidermal polyphenols content of Prosopis glandulosa and Prosopis juliflora leaves exposed to cadmium and copper. Open Life Sci. 2017, 12, 373–378. [Google Scholar] [CrossRef]
- González-Mendoza, D.; Troncoso-Rojas, R.; Gonzalez-Soto, T.; Grimaldo-Juarez, O.; Cecena-Duran, C.; Duran-Hernandez, D.; Gutierrez-Miceli, F. Changes in the phenylalanine ammonia lyase activity, total phenolic compounds, and flavonoids in Prosopis glandulosa treated with cadmium and copper. An. Acad. Bras. Ciências 2018, 90, 1465–1472. [Google Scholar] [CrossRef]
- Pontiggia, D.; Benedetti, M.; Costantini, S.; De Lorenzo, G.; Cervone, F. Dampening the DAMPs: How plants maintain the homeostasis of cell wall molecular patterns and avoid hyper-immunity. Front. Plant Sci. 2020, 11, 613259. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Malamy, J.; Carr, J.P.; Klessig, D.F.; Raskin, I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990, 250, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, N.; Pineda, A.; Pieterse, C.M.; Dicke, M.; Van Loon, J.J. Two-way plant mediated interactions between root-associated microbes and insects: From ecology to mechanisms. Front. Plant Sci. 2013, 4, 414. [Google Scholar] [CrossRef] [PubMed]
- Aleman, F.; Yazaki, J.; Lee, M.; Takahashi, Y.; Kim, A.Y.; Li, Z.; Kinoshita, T.; Ecker, J.R.; Schroeder, J.I. An ABA-increased interaction of the PYL6 ABA receptor with MYC2 transcription factor: A putative link of ABA and JA signaling. Sci. Rep. 2016, 6, 28941. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Hisano, H.; Hojo, Y.; Matsuura, T.; Ikeda, Y.; Mori, I.C.; Senthil-Kumar, M. Global profiling of phytohormone dynamics during combined drought and pathogen stress in Arabidopsis thaliana reveals ABA and JA as major regulators. Sci. Rep. 2017, 7, 4017. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, X.; Li, Z.; Wang, M. Jasmonate positively regulates cold tolerance by promoting ABA biosynthesis in tomato. Plants 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Heil, M. Damage-associated molecular patterns (DAMPs) in plant innate immunity: Applying the danger model and evolutionary perspectives. Annu. Rev. Phytopathol. 2021, 59, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 2016, 16, 232. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Shan, Z.; Hao, Q.; Zhang, C.; Yang, Z.; Zhang, X.; Yuan, S.; Qiu, D.; Chen, S. Herbivore defense responses and associated herbivore defense mechanism as revealed by comparing a resistant wild soybean with a susceptible cultivar. Crop J. 2015, 3, 451–467. [Google Scholar] [CrossRef]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK machinery in plants: Recognition and response to different stresses through multiple signal transduction pathways. Plant Signal. Behav. 2010, 5, 1370–1378. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Liu, Y.; Yang, M.; Wang, L.; Liu, X.; Zhang, Y.; Chen, Q.; Li, M.; Lin, Y. MAPK5 and MAPK10 overexpression influences strawberry fruit ripening, antioxidant capacity and resistance to Botrytis cinerea. Planta 2022, 255, 19. [Google Scholar] [CrossRef] [PubMed]
- Ferrusquía-Jiménez, N.I.; Serrano-Jamaica, L.M.; Martínez-Camacho, J.E.; Sáenz de la O, D.; Villagomez-Aranda, A.L.; González-Chavira, M.M.; Guerrero-Aguilar, B.Z.; Torres-Pacheco, I.; Feregrino-Pérez, A.A.; Medina-Ramos, G. Extracellular self-DNA plays a role as a damage-associated molecular pattern (DAMP) delaying zoospore germination rate and inducing stress-related responses in Phytophthora capsici. Plant Pathol. 2022, 71, 1066–1075. [Google Scholar] [CrossRef]
- Galletti, R.; Ferrari, S.; De Lorenzo, G. Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide-or flagellin-induced resistance against Botrytis cinerea. Plant Physiol. 2011, 157, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Hunter, K.; Vaahtera, L.; Tran, H.C.; Citterico, M.; Vaattovaara, A.; Rokka, A.; Stolze, S.C.; Harzen, A.; Meißner, L. CRK2 and C-terminal phosphorylation of NADPH oxidase RBOHD regulate reactive oxygen species production in Arabidopsis. Plant Cell 2020, 32, 1063–1080. [Google Scholar] [CrossRef]
- Seong, S.-Y.; Matzinger, P.; Land, W.G. DAMPs across the tree of life. Front. Immunol. 2022, 12, 844315. [Google Scholar] [CrossRef]
- Orona-Tamayo, D.; Wielsch, N.; Escalante-Pérez, M.; Svatos, A.; Molina-Torres, J.; Muck, A.; Ramirez-Chávez, E.; Ádame-Alvarez, R.M.; Heil, M. Short-term proteomic dynamics reveal metabolic factory for active extrafloral nectar secretion by A cacia cornigera ant-plants. Plant J. 2013, 73, 546–554. [Google Scholar] [CrossRef]
- Creelman, R.A.; Tierney, M.L.; Mullet, J.E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc. Natl. Acad. Sci. USA 1992, 89, 4938–4941. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, W.; Gu, Z.; Wu, S.; E, Y.; Zhou, W.; Lin, J.; Xu, L. Roles of the wound hormone jasmonate in plant regeneration. J. Exp. Bot. 2023, 74, 1198–1206. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Duran-Flores, D.; Heil, M.; Camacho-Coronel, X. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci. Hortic. 2018, 237, 207–220. [Google Scholar] [CrossRef]
- Fishman, M.R.; Shirasu, K. How to resist parasitic plants: Pre-and post-attachment strategies. Curr. Opin. Plant Biol. 2021, 62, 102004. [Google Scholar] [CrossRef] [PubMed]
- Bawin, T.; Didriksen, A.; Faehn, C.; Olsen, S.; Sørensen, I.; Rose, J.K.; Krause, K. Cuscuta campestris fine-tunes gene expression during haustoriogenesis as an adaptation to different hosts. Plant Physiol. 2023, kiad505. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Laclette, E.; Venancio-Rodríguez, C.A.; Vásquez-Aguilar, A.A.; Alonso-Sánchez, A.G.; Pérez-Torres, C.-A.; Villafán, E.; Ramírez-Barahona, S.; Galicia, S.; Sosa, V.; Rebollar, E.A. Transcriptional basis for haustorium formation and host establishment in hemiparasitic Psittacanthus schiedeanus Mistletoes. Front. Genet. 2022, 13, 929490. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Hernández, C.; Quintana-Rodríguez, E.; Lozoya-Gloria, E.; Orona-Tamayo, D. Defensive responses by Prosopis laevigata tree against the mistletoe Psittacanthus calyculatus during early infection stage. in progress.
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation. Version II. Plant Mol. Biol. Report. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Grellet Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Fonseca-García, C.; López-García, C.M.; Pacheco, R.; Armada, E.; Nava, N.; Pérez-Aguilar, R.; Solis-Miranda, J.; Quinto, C. Metallothionein1A regulates rhizobial infection and nodulation in Phaseolus vulgaris. Int. J. Mol. Sci. 2022, 23, 1491. [Google Scholar] [CrossRef]
- Malamy, J.E.; Benfey, P.N. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 1997, 124, 33–44. [Google Scholar] [CrossRef]
- Pluskota, W.E.; Qu, N.; Maitrejean, M.; Boland, W.; Baldwin, I.T. Jasmonates and its mimics differentially elicit systemic defence responses in Nicotiana attenuata. J. Exp. Bot. 2007, 58, 4071–4082. [Google Scholar] [CrossRef]
- Meng, L.; Feldman, L. A rapid TRIzol-based two-step method for DNA-free RNA extraction from Arabidopsis siliques and dry seeds. Biotechnol. J. 2010, 5, 183–186. [Google Scholar] [CrossRef]
- Wong, W.H.; Lee, W.X.; Ramanan, R.N.; Tee, L.H.; Kong, K.W.; Galanakis, C.M.; Sun, J.; Prasad, K.N. Two level half factorial design for the extraction of phenolics, flavonoids and antioxidants recovery from palm kernel by-product. Ind. Crop. Prod. 2015, 63, 238–248. [Google Scholar] [CrossRef]
- Méndez-Gómez, M.; Castro-Mercado, E.; Alexandre, G.; García-Pineda, E. Oxidative and antioxidative responses in the wheat-Azospirillum brasilense interaction. Protoplasma 2016, 253, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Patykowski, J.; Kołodziejek, J. Changes in Antioxidant Enzyme Activities of European Mistletoe (Viscum album L. subsp. Album) Leaves as a Response to Environmental Stress Caused by Pollution of the Atmosphere by Nitrogen Dioxide. Pol. J. Environ. Stud. 2016, 25, 725–732. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-García, C.M.; Ávila-Hernández, C.A.; Quintana-Rodríguez, E.; Aguilar-Hernández, V.; Lozoya-Pérez, N.E.; Rojas-Raya, M.A.; Molina-Torres, J.; Araujo-León, J.A.; Brito-Argáez, L.; González-Sánchez, A.A.; et al. Extracellular Self- and Non-Self DNA Involved in Damage Recognition in the Mistletoe Parasitism of Mesquite Trees. Int. J. Mol. Sci. 2024, 25, 457. https://doi.org/10.3390/ijms25010457
López-García CM, Ávila-Hernández CA, Quintana-Rodríguez E, Aguilar-Hernández V, Lozoya-Pérez NE, Rojas-Raya MA, Molina-Torres J, Araujo-León JA, Brito-Argáez L, González-Sánchez AA, et al. Extracellular Self- and Non-Self DNA Involved in Damage Recognition in the Mistletoe Parasitism of Mesquite Trees. International Journal of Molecular Sciences. 2024; 25(1):457. https://doi.org/10.3390/ijms25010457
Chicago/Turabian StyleLópez-García, Claudia Marina, César Alejandro Ávila-Hernández, Elizabeth Quintana-Rodríguez, Víctor Aguilar-Hernández, Nancy Edith Lozoya-Pérez, Mariana Atzhiry Rojas-Raya, Jorge Molina-Torres, Jesús Alfredo Araujo-León, Ligia Brito-Argáez, Avel Adolfo González-Sánchez, and et al. 2024. "Extracellular Self- and Non-Self DNA Involved in Damage Recognition in the Mistletoe Parasitism of Mesquite Trees" International Journal of Molecular Sciences 25, no. 1: 457. https://doi.org/10.3390/ijms25010457
APA StyleLópez-García, C. M., Ávila-Hernández, C. A., Quintana-Rodríguez, E., Aguilar-Hernández, V., Lozoya-Pérez, N. E., Rojas-Raya, M. A., Molina-Torres, J., Araujo-León, J. A., Brito-Argáez, L., González-Sánchez, A. A., Ramírez-Chávez, E., & Orona-Tamayo, D. (2024). Extracellular Self- and Non-Self DNA Involved in Damage Recognition in the Mistletoe Parasitism of Mesquite Trees. International Journal of Molecular Sciences, 25(1), 457. https://doi.org/10.3390/ijms25010457







