Metabolomics in Animal Models of Bronchial Asthma and Its Translational Importance for Clinics
Abstract
:1. Introduction
2. Bronchial Asthma
2.1. Features of Bronchial Asthma
2.2. Diagnostic Tools in Asthma
2.3. Advances of “Omics” in Asthma
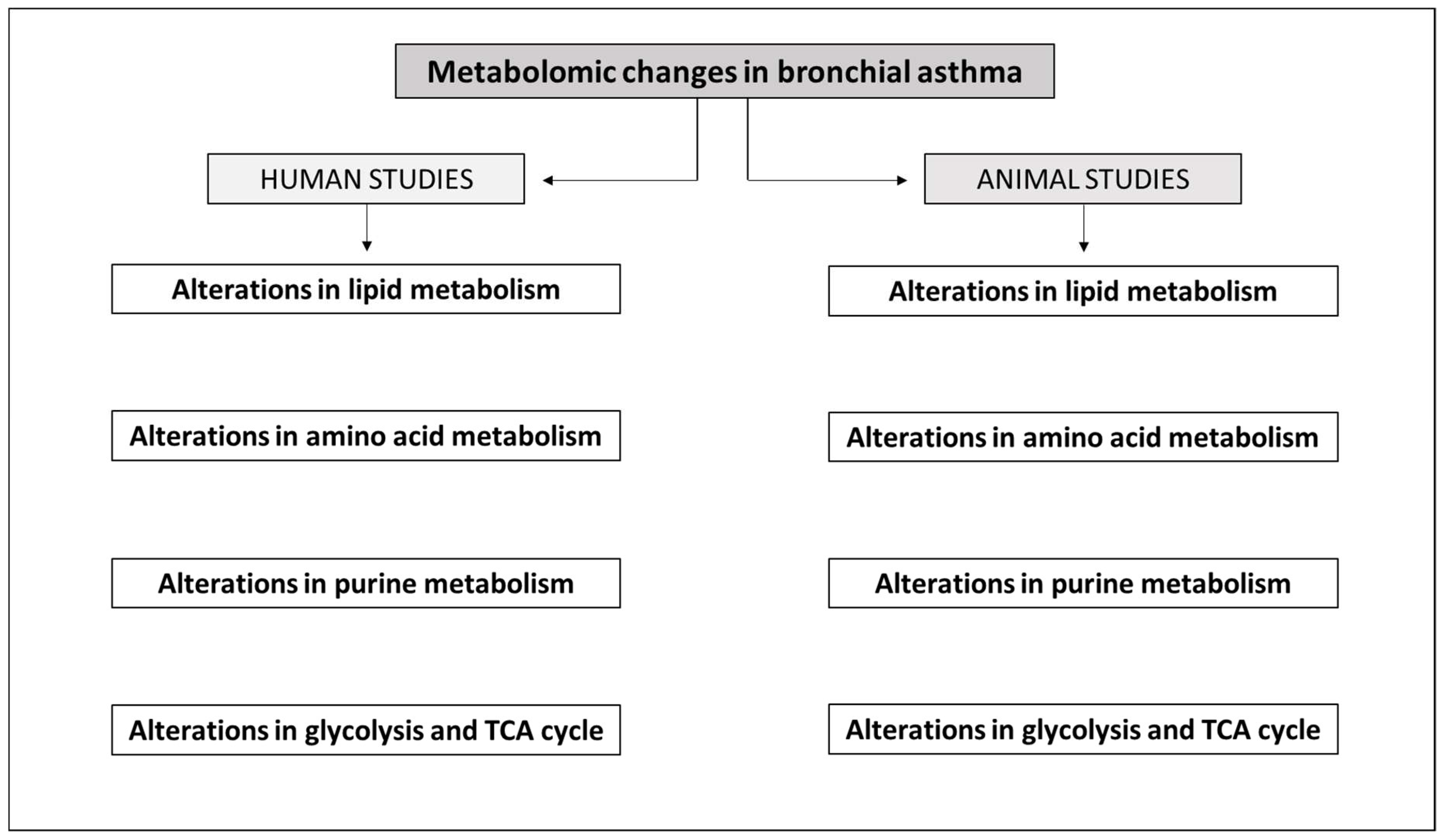
3. Metabolomics in Asthma
3.1. Metabolomics Data for Comparison between Asthma vs. Healthy Individuals
3.1.1. Human Studies
3.1.2. Animal Studies
3.2. Metabolomics Data for Comparison between Obesity-Associated Asthma vs. Healthy Individuals
3.2.1. Human Studies
3.2.2. Animal Studies
3.3. Metabolomics Data for Sex Comparisons
3.3.1. Human Studies
3.3.2. Animal Studies
3.4. Metabolomics Data for Age Groups Comparisons
3.4.1. Human Studies
3.4.2. Animal Studies
3.5. Metabolomics Data Demonstrating Effect of Lifestyle, Diet, and Gut Microbioma
3.5.1. Human Studies
3.5.2. Animal Studies
3.6. Metabolomics Data Demonstrating the Effect of Asthma Treatment
3.6.1. Human Studies
3.6.2. Animal Studies
| Asthma Population; Reference | Participants | Metabolomic Analysis; Samples | Main Results |
|---|---|---|---|
| Adults [52] | 18 Non-steroid treated asthma, 20 steroid-treated asthma, 13 controls | HPLC-QTOF-MS; BALF | ↑ LPC, TG, PC, PG, PS, SM in non-steroid treated asthma vs. controls; no difference between steroid-treated asthma vs. controls |
| Adults [55] | 57 Asthma | Targeted SPME/GCxGC-TOF/MS; urine | Lipid peroxidation metabolites associated with asthma severity, lung function and eosinophilic inflammation |
| Adults [29] | 54 Asthma/22 controls | Untargeted LC-MS/ Targeted LC/MS; serum | ↑ Ceramide, sphingomyelin, hexosylceramide, LTE4 in asthma vs. healthy; ↓ 14,15- DiHETE, 19,20- DiHDPA in asthma vs. healthy |
| Adults [51] | 15 Asthma, 15 controls | UHPLC-QTOF-MS; serum | ↑ 5(S)-HETE, 8(S)-HETE, 11(S)-HETE, 12(S)-HETE, 15(S)-HETE, 15(S)-HEPE, ProstGA2, ProstG B2, ProstG F1a, ProstG F2a, ProstG J2, 15-keto-ProstG F2a in asthma vs. control, ↓ palmitic acid, lauric acid in asthma vs. controls |
| Adults [103] | 25 Obese asthma, 30 obese non-asthma, 30 lean asthma | Untargeted NMR; EBC | Respiratory metabolic profile in obese asthmatics divergent from other patient groups; differences in methane, pyruvate, and glyoxylate and dicarboxylate pathways |
| Adults [44] | 13 Eosinophilic asthma (EA), 16 non-eosinophilic asthma (NEA), 15 healthy controls | Untargeted UPLC-MS; serum | Changes in glycerophospholipid, retinol, sphingolipid, galactose and inositol phosphate metabolisms for EA vs. NEA |
| Adults [39] | 33 Asthma, 28 healthy controls | LC-MS/MS-based lipidomics; plasma | ↑ PE (18:1p/22:6), PE (20:0/18:1), PE (38:1), SM (d18:1/18:1), TG (16:0/16:0/18:1) in asthma vs. healthy; ↓ PI (16:0/20:4), TG (17:0/18:1/18:1), PG (44:0), ceramide (d16:0/27:2), LPC (22:4) in asthma vs. controls |
| Children [179] | 50 asthma, 49 healthy controls | Targeted LC-MS; serum | ↓ Ascorbic acid, 2-isopropylmalic acid, shikimate-3-phosphate, 6-phospho-D-gluconate, and reduced glutathione in asthma vs. controls |
| Children [180] | 380 children with asthma | Targeted LC-MS; plasma | Glycerophospholipid, linoleic acid, and pyrimidine metabolisms associated with AHR, and pre- and postbronchodilator FEV1/FVC |
| Children [181] | 30 children with asthma, 30 controls | NMR; urine | ↓ 1-Methylnicotinamide and allantoin in asthma vs. controls |
| Children [64] | 13 asthma, 17 healthy controls | LC-MS/MS; serum | ↑ L-arginine, Β-alanine, Ƴ-amino-N-butyric acid, L-histidine, hydroxy-L-proline in asthma vs. controls; ↓ D,L-Β-Aminoisobutyric acid, taurine, L-tryptophan, L-valine in asthma vs. controls |
| Children [77] | 92 children with asthma, 73 controls | NMR; EBC | ↑ Lactate, formate, butyric acid, isobutyrate in asthma |
| Model of Asthma; Reference | Animals (Groups) | Metabolomic Analysis; Samples | Main Results |
|---|---|---|---|
| OVA-induced asthma [78] | Dunkin-Hartley female guinea pigs (controls, controls treated with DEX, OVA-sensitized, OVA-sensitized + challenged, sensitized + challenged treated with DEX) | NMR; urine | Urine metabolites correlated with airway dysfunction in asthma model |
| OVA-induced asthma [177] | BALB/c female mice (OVA-induced asthma, OVA-induced asthma treated with DEX, controls) | GC-MS, LC-MS; BALF | Alterations of energy metabolism, carbohydrate, lipid and sterol metabolisms in asthma model; partial reverse by DEX, but DEX ineffective in decreasing lactate, malate and creatinine |
| OVA-induced asthma [79] | BALB/c female mice (OVA-induced asthma model, controls) | Untargeted UPLC-Q-TOF/MS; plasma | Changes in purine, sphingolipid, glycerophospholipid, FAs, tryptophan and bile acid biosynthesis metabolism in asthma model |
| OVA-induced asthma [83] | BALB/c female mice (OVA-induced asthma, OVA-induced asthma treated with SPA, controls) | Untargeted UPLC-Q-TOF-MS; serum | Changed 32 metabolites in 9 metabolic pathways in asthma model; significant reverse after SPA treatment |
| OVA-induced asthma [82] | C57BL/6 female mice (OVA sensitized and/or challenged, controls) | Untargeted HPLC-TOF/MS, Targeted HPLC-MS; BALF and plasma | Changes in sphingolipid, glycerophospholipid, arginine and proline metabolisms, and neurotrophin signaling pathway in asthma model; AHR correlated with urea-1-carboxylate and ornithine; lung eosinophilia correlated with agmatine |
| OVA-induced asthma [81] | C57BL/6 female mice (OVA sensitized, controls) | GC-MS; plasma | Changes in 25 metabolites, including eight AAs, nine FAs and eight OAs; most significant changes in palmitic acid, methionine, pipecolic, lactic, α-ketoglutaric and linoleic acids |
| OVA-induced asthma [80] | BALB/c female mice (OVA-induced asthma, OVA-induced asthma treated with DEX or mKG, controls | Untargeted UPLC-Q-TOF/MS; lung tissue and plasma | Changes in 24 metabolites including myristic acid, sphinganine, and lysoPC in lung and plasma of asthma model; l-acetylcarnitine, thromboxane B2, 10-HDoHE, and 5-HETE as potential biomarkers; mKG and DEX influenced the biomarkers; DEX less effective |
| OVA-induced asthma [178] | Hartley male guinea pigs (OVA-sensitized, OVA-sensitized and treated with DEX or AST, controls) | UPLC-ESI-QTOF/MS; serum | AST therapy restored phospholipid, sphingolipid, purine, AAs and epinephrine levels back to normal control level; AST could alter the sphingolipid metabolism |
| OVA-induced asthma [85] | TRIK male guinea pigs (OVA-sensitized, controls) | Targeted UPLC/MS; plasma | Changes in 22 metabolites, ↓ PC, carnitine, dimethylarginine, dimethylarginine/arginine ratio, kynurenine/tryptophan ratio, ↑ tryptophan, taurine and methionine sulfoxide/methionine ratio in asthma model |
| OVA-induced asthma [134] | Guinea pigs (controls, OVA-sensitized, OVA-sensitized and treated by DEX or BCE) | UPLC-MS; serum and BALF | Sex-based differences in 39 metabolites, changes in 37 metabolites in asthma animals involving 17 metabolic pathways; BCE improved nerve and energy metabolism; sex-specific differences for BCE |
| HDM + ozone- induced-induced asthma [132] | BALB/c female and male mice (HDM + ozone-sensitized, controls) | LC-MS/MS; BALF and lung tissue | ↑ glycosphingolipids associated with ↑ AHR and airway inflammation in males and females, but more severe in females |
| PM2.5-induced asthma [92] | BALB/c female mice (controls, three concentrations of PM2.5) | GC-MS; lung tissue | Medium and high concentrations of PM2.5-induced asthma linked with changes in 13 metabolites associated with oxidative stress and metabolism |
4. Translational Value and Limitations of Animal Models of Asthma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.; Semprini, A.; Mitchell, E.A. Risk factors for asthma: Is prevention possible? Lancet 2015, 386, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Corren, J. Asthma phenotypes and endotypes: An evolving paradigm for classification. Discov. Med. 2013, 15, 243–249. [Google Scholar] [PubMed]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.; Demarche, S.; Louis, R. Biomarkers in the Management of Difficult Asthma. Curr. Top. Med. Chem. 2016, 16, 1561–1573. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Levine, S.J.; Brusselle, G.G. Treatment options in type-2 low asthma. Eur. Respir. J. 2021, 57, 2000528. [Google Scholar] [CrossRef]
- Svenningsen, S.; Nair, P. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Front. Med. 2017, 4, 158. [Google Scholar] [CrossRef]
- Muneswarao, J.; Hassali, M.A.; Ibrahim, B.; Saini, B.; Ali, I.A.H.; Verma, A.K. It is time to change the way we manage mild asthma: An update in GINA 2019. Respir. Res. 2019, 20, 183. [Google Scholar] [CrossRef]
- Chung, K.F. Personalised medicine in asthma: Time for action. Eur. Respir. Rev. 2017, 26, 170064. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Asthma phenotyping: A necessity for improved therapeutic precision and new targeted therapies. J. Intern. Med. 2016, 279, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Tiotiu, A. Biomarkers in asthma: State of the art. Asthma Res. Pract. 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Pité, H.; Morais-Almeida, M.; Rocha, S.M. Metabolomics in asthma: Where do we stand? Curr. Opin. Pulm. Med. 2018, 24, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, S.; Zhang, S.; Ouyang, Z.; Wang, G.; Wang, F. Research Progress of Metabolomics in Asthma. Metabolites 2021, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Panettieri, R.A., Jr.; Jude, J. Metabolomics in asthma: A platform for discovery. Mol. Asp. Med. 2022, 85, 100990. [Google Scholar] [CrossRef]
- Loewenthal, L.; Menzies-Gow, A. FeNO in Asthma. Semin. Respir. Crit. Care Med. 2022, 43, 635–645. [Google Scholar] [CrossRef]
- Rufo, J.C.; Madureira, J.; Fernandes, E.O.; Moreira, A. Volatile organic compounds in asthma diagnosis: A systematic review and meta-analysis. Allergy 2016, 71, 175–188. [Google Scholar] [CrossRef]
- Van Vliet, D.; Smolinska, A.; Jöbsis, Q.; Rosias, P.; Muris, J.; Dallinga, J.; Dompeling, E.; van Schooten, F.J. Can exhaled volatile organic compounds predict asthma exacerbations in children? J. Breath Res. 2017, 11, 016016. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Adachi, Y.; Pozo Beltrán, C.F.; El-Sayed, Z.A.; Gómez, R.M.; Hossny, E.; Filipovic, I.; Le Souef, P.; Morais-Almeida, M.; Miligkos, M.; et al. Utility of biomarkers in the diagnosis and monitoring of asthmatic children. World Allergy Organ. J. 2022, 16, 100727. [Google Scholar] [CrossRef] [PubMed]
- Szefler, S.J.; Wenzel, S.; Brown, R.; Erzurum, S.C.; Fahy, J.V.; Hamilton, R.G.; Hunt, J.F.; Kita, H.; Liu, A.H.; Panettieri, R.A., Jr.; et al. Asthma outcomes: Biomarkers. J. Allergy Clin. Immunol. 2012, 129, S9–S23. [Google Scholar] [CrossRef] [PubMed]
- Moitra, S.; Bandyopadhyay, A.; Lacy, P. Metabolomics of Respiratory Diseases. Handb. Exp. Pharmacol. 2023, 277, 339–365. [Google Scholar] [CrossRef] [PubMed]
- Amberg, A.; Riefke, B.; Schlotterbeck, G.; Ross, A.; Senn, H.; Dieterle, F.; Keck, M. NMR and MS Methods for Metabolomics. Methods Mol. Biol. 2017, 1641, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 1–24. [Google Scholar] [CrossRef]
- Stringer, K.A.; McKay, R.T.; Karnovsky, A.; Quémerais, B.; Lacy, P. Metabolomics and Its Application to Acute Lung Diseases. Front. Immunol. 2016, 7, 44. [Google Scholar] [CrossRef]
- German, J.B.; Hammock, B.D.; Watkins, S.M. Metabolomics: Building on a century of biochemistry to guide human health. Metabolomics 2005, 1, 3–9. [Google Scholar] [CrossRef]
- Kelly, R.S.; Dahlin, A.; McGeachie, M.J.; Qiu, W.; Sordillo, J.; Wan, E.S.; Wu, A.C.; Lasky-Su, J. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest 2017, 151, 262–277. [Google Scholar] [CrossRef]
- Reinke, S.N.; Gallart-Ayala, H.; Gómez, C.; Checa, A.; Fauland, A.; Naz, S.; Kamleh, M.A.; Djukanović, R.; Hinks, T.S.; Wheelock, C.E. Metabolomics analysis identifies different metabotypes of asthma severity. Eur. Respir. J. 2017, 49, 1601740. [Google Scholar] [CrossRef]
- Roshan Lal, T.; Cechinel, L.R.; Freishtat, R.; Rastogi, D. Metabolic Contributions to Pathobiology of Asthma. Metabolites 2023, 13, 212. [Google Scholar] [CrossRef]
- Kelly, R.S.; Sordillo, J.E.; Lutz, S.M.; Avila, L.; Soto-Quiros, M.; Celedón, J.C.; McGeachie, M.J.; Dahlin, A.; Tantisira, K.; Huang, M.; et al. Pharmacometabolomics of Bronchodilator Response in Asthma and the Role of Age-Metabolite Interactions. Metabolites 2019, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, J.E.; Lutz, S.M.; Kelly, R.S.; McGeachie, M.J.; Dahlin, A.; Tantisira, K.; Clish, C.; Lasky-Su, J.; Wu, A.C. Plasmalogens Mediate the Effect of Age on Bronchodilator Response in Individuals With Asthma. Front. Med. 2020, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Bong How, S.; Gummer, J.; Trengove, R.; Moodley, Y. Metabolomics in chronic lung diseases. Respirology 2020, 25, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Pite, H.; Aguiar, L.; Morello, J.; Monteiro, E.C.; Alves, A.C.; Bourbon, M.; Morais-Almeida, M. Metabolic Dysfunction and Asthma: Current Perspectives. J. Asthma Allergy 2020, 13, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Akbarshahi, H.; Uller, L. Translational value of animal models of asthma: Challenges and promises. Eur. J. Pharmacol. 2015, 759, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Dennis, E.A. The human plasma lipidome. N. Engl. J. Med. 2011, 365, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Jaishy, B.; Abel, E.D. Lipids, lysosomes, and autophagy. J. Lipid Res. 2016, 57, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, R.; Nag, K.; Orgeig, S.; Possmayer, F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta 1998, 1408, 90–108. [Google Scholar] [CrossRef]
- Jiang, T.; Dai, L.; Li, P.; Zhao, J.; Wang, X.; An, L.; Liu, M.; Wu, S.; Wang, Y.; Peng, Y. Lipid metabolism and identification of biomarkers in asthma by lipidomic analysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158853. [Google Scholar] [CrossRef]
- Zuo, L.; Wijegunawardana, D. Redox Role of ROS and Inflammation in Pulmonary Diseases. Adv. Exp. Med. Biol. 2021, 1304, 187–204. [Google Scholar] [CrossRef]
- Hofford, J.M.; Milakofsky, L.; Pell, S.; Fish, J.E.; Peters, S.P.; Pollice, M.; Vogel, W.H. Levels of amino acids and related compounds in bronchoalveolar lavage fluids of asthmatic patients. Am. J. Respir. Crit. Care Med. 1997, 155, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Kim, S.D.; Kim, J.M.; Lee, H.Y.; Lee, S.Y.; Shim, J.W.; Yun, J.; Im, D.S.; Bae, Y.S. Lysophosphatidylglycerol stimulates chemotactic migration in human natural killer cells. Biochem. Biophys. Res. Commun. 2008, 372, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ried, J.S.; Baurecht, H.; Stückler, F.; Krumsiek, J.; Gieger, C.; Heinrich, J.; Kabesch, M.; Prehn, C.; Peters, A.; Rodriguez, E.; et al. Integrative genetic and metabolite profiling analysis suggests altered phosphatidylcholine metabolism in asthma. Allergy 2013, 68, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Wang, G.; Wang, C.; Zhang, W.; Liu, J.; Wang, F. Serum Metabolomics Analysis of Asthma in Different Inflammatory Phenotypes: A Cross-Sectional Study in Northeast China. Biomed. Res. Int. 2018, 2018, 2860521. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Zhang, L.; Dong, F.; Zhang, X.; Yao, L.; Chang, C. Serum sphingolipid profile in asthma. J. Leukoc. Biol. 2021, 110, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Worgall, T.S.; Veerappan, A.; Sung, B.; Kim, B.I.; Weiner, E.; Bholah, R.; Silver, R.B.; Jiang, X.C.; Worgall, S. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci. Transl. Med. 2013, 5, 186ra67. [Google Scholar] [CrossRef] [PubMed]
- Esteves, P.; Blanc, L.; Celle, A.; Dupin, I.; Maurat, E.; Amoedo, N.; Cardouat, G.; Ousova, O.; Gales, L.; Bellvert, F.; et al. Crucial role of fatty acid oxidation in asthmatic bronchial smooth muscle remodelling. Eur. Respir. J. 2021, 58, 2004252. [Google Scholar] [CrossRef]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef]
- Miyata, J.; Fukunaga, K.; Kawashima, Y.; Ohara, O.; Kawana, A.; Asano, K.; Arita, M. Dysregulated metabolism of polyunsaturated fatty acids in eosinophilic allergic diseases. Prostaglandins Other Lipid Mediat. 2020, 150, 106477. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Bian, X.; Sun, B.; Zheng, P.; Li, N.; Wu, J.L. Derivatization enhanced separation and sensitivity of long chain-free fatty acids: Application to asthma using targeted and non-targeted liquid chromatography-mass spectrometry approach. Anal. Chim. Acta 2017, 989, 59–70. [Google Scholar] [CrossRef]
- Kang, Y.P.; Lee, W.J.; Hong, J.Y.; Lee, S.B.; Park, J.H.; Kim, D.; Park, S.; Park, C.S.; Park, S.W.; Kwon, S.W. Novel approach for analysis of bronchoalveolar lavage fluid (BALF) using HPLC-QTOF-MS-based lipidomics: Lipid levels in asthmatics and corticosteroid-treated asthmatic patients. J. Proteome Res. 2014, 13, 3919–3929. [Google Scholar] [CrossRef]
- Calabrese, C.; Triggiani, M.; Marone, G.; Mazzarella, G. Arachidonic acid metabolism in inflammatory cells of patients with bronchial asthma. Allergy 2000, 55 (Suppl. S61), 27–30. [Google Scholar] [CrossRef]
- Comhair, S.A.; McDunn, J.; Bennett, C.; Fettig, J.; Erzurum, S.C.; Kalhan, S.C. Metabolomic Endotype of Asthma. J. Immunol. 2015, 195, 643–650. [Google Scholar] [CrossRef]
- Loureiro, C.C.; Oliveira, A.S.; Santos, M.; Rudnitskaya, A.; Todo-Bom, A.; Bousquet, J.; Rocha, S.M. Urinary metabolomic profiling of asthmatics can be related to clinical characteristics. Allergy 2016, 71, 1362–1365. [Google Scholar] [CrossRef]
- Daynes, R.A.; Jones, D.C. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2002, 2, 748–759. [Google Scholar] [CrossRef]
- McGarvey, L.P.; Butler, C.A.; Stokesberry, S.; Polley, L.; McQuaid, S.; Abdullah, H.; Ashraf, S.; McGahon, M.K.; Curtis, T.M.; Arron, J.; et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J. Allergy Clin. Immunol. 2014, 133, 704–712.e4. [Google Scholar] [CrossRef]
- Xu, W.; Ghosh, S.; Comhair, S.A.; Asosingh, K.; Janocha, A.J.; Mavrakis, D.A.; Bennett, C.D.; Gruca, L.L.; Graham, B.B.; Queisser, K.A.; et al. Increased mitochondrial arginine metabolism supports bioenergetics in asthma. J. Clin. Investig. 2016, 126, 2465–2481. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.H.; Lee, H.S.; Choi, G.S.; Jung, Y.S.; Ryu, D.H.; Park, H.S.; Hwang, G.S. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin. Exp. Allergy 2013, 43, 425–433. [Google Scholar] [CrossRef]
- Zimmermann, N.; Rothenberg, M.E. The arginine-arginase balance in asthma and lung inflammation. Eur. J. Pharmacol. 2006, 533, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kraj, L.; Krawiec, M.; Koter, M.; Graboń, W.; Kraj, G.; Chołojczyk, M.; Kulus, M.; Barańczyk-Kuźma, A. Altered L-arginine metabolism in children with controlled asthma. Allergy Asthma Proc. 2014, 35, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Comhair, S.A.A.; Janocha, A.J.; Lara, A.; Mavrakis, L.A.; Bennett, C.D.; Kalhan, S.C.; Erzurum, S.C. Arginine metabolic endotypes related to asthma severity. PLoS ONE 2017, 12, e0183066. [Google Scholar] [CrossRef] [PubMed]
- Asosingh, K.; Lauruschkat, C.D.; Alemagno, M.; Frimel, M.; Wanner, N.; Weiss, K.; Kessler, S.; Meyers, D.A.; Bennett, C.; Xu, W.; et al. Arginine metabolic control of airway inflammation. JCI Insight 2020, 5, e127801. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Klupczynska, A.; Packi, K.; Mackowiak-Jakubowska, A.; Bręborowicz, A.; Pawlicka, O.; Olejniczak, K.; Kokot, Z.J.; Matysiak, J. Alterations in Serum-Free Amino Acid Profiles in Childhood Asthma. Int. J. Environ. Res. Public Health 2020, 17, 4758. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zhou, J.; Wang, Y.; Jin, F.; Chen, X.; Yang, J.; Chen, Z. Urinary Metabolomic Profiling Reveals Biological Pathways and Predictive Signatures Associated with Childhood Asthma. J. Asthma Allergy 2020, 13, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Wang, C.J.; Tsai, M.H.; Lin, G. Integrated metabolic and microbial analysis reveals host-microbial interactions in IgE-mediated childhood asthma. Sci. Rep. 2021, 11, 23407. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Choudhury, P.; Kaushik, S.R.; Arya, R.; Nanda, R.; Bhattacharyya, P.; Roychowdhury, S.; Banerjee, R.; Chaudhury, K. Metabolomic fingerprinting and systemic inflammatory profiling of asthma COPD overlap (ACO). Respir. Res. 2020, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Wypych, T.P.; Pattaroni, C.; Perdijk, O.; Yap, C.; Trompette, A.; Anderson, D.; Creek, D.J.; Harris, N.L.; Marsland, B.J. Microbial metabolism of L-tyrosine protects against allergic airway inflammation. Nat. Immunol. 2021, 22, 279–286. [Google Scholar] [CrossRef]
- Gostner, J.M.; Becker, K.; Kofler, H.; Strasser, B.; Fuchs, D. Tryptophan Metabolism in Allergic Disorders. Int. Arch. Allergy Immunol. 2016, 169, 203–215. [Google Scholar] [CrossRef]
- Stapleton, P.P.; O’Flaherty, L.; Redmond, H.P.; Bouchier-Hayes, D.J. Host defense—A role for the amino acid taurine? J. Parenter. Enter. Nutr. 1998, 22, 42–48. [Google Scholar] [CrossRef]
- Kool, M.; Willart, M.A.; van Nimwegen, M.; Bergen, I.; Pouliot, P.; Virchow, J.C.; Rogers, N.; Osorio, F.; Reis e Sousa, C.; Hammad, H.; et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 2011, 34, 527–540. [Google Scholar] [CrossRef]
- Liang, Y.; Gai, X.Y.; Chang, C.; Zhang, X.; Wang, J.; Li, T.T. Metabolomic Profiling Differences among Asthma, COPD, and Healthy Subjects: A LC-MS-based Metabolomic Analysis. Biomed. Environ. Sci. 2019, 32, 659–672. [Google Scholar] [CrossRef]
- Tao, J.L.; Chen, Y.Z.; Dai, Q.G.; Tian, M.; Wang, S.C.; Shan, J.J.; Ji, J.J.; Lin, L.L.; Li, W.W.; Yuan, B. Urine metabolic profiles in paediatric asthma. Respirology 2019, 24, 572–581. [Google Scholar] [CrossRef]
- Chang, C.; Guo, Z.G.; He, B.; Yao, W.Z. Metabolic alterations in the sera of Chinese patients with mild persistent asthma: A GC-MS-based metabolomics analysis. Acta Pharmacol. Sin. 2015, 36, 1356–1366. [Google Scholar] [CrossRef]
- Turi, K.N.; Michel, C.R.; Manke, J.; Doenges, K.A.; Reisdorph, N.; Bauer, A.K. Multi-Omics Analysis of Lung Tissue Demonstrates Changes to Lipid Metabolism during Allergic Sensitization in Mice. Metabolites 2023, 13, 406. [Google Scholar] [CrossRef]
- Ostroukhova, M.; Goplen, N.; Karim, M.Z.; Michalec, L.; Guo, L.; Liang, Q.; Alam, R. The role of low-level lactate production in airway inflammation in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L300–L307. [Google Scholar] [CrossRef]
- Chang-Chien, J.; Huang, H.Y.; Tsai, H.J.; Lo, C.J.; Lin, W.C.; Tseng, Y.L.; Wang, S.L.; Ho, H.Y.; Cheng, M.L.; Yao, T.C. Metabolomic differences of exhaled breath condensate among children with and without asthma. Pediatr. Allergy Immunol. 2021, 32, 264–272. [Google Scholar] [CrossRef]
- Saude, E.J.; Obiefuna, I.P.; Somorjai, R.L.; Ajamian, F.; Skappak, C.; Ahmad, T.; Dolenko, B.K.; Sykes, B.D.; Moqbel, R.; Adamko, D.J. Metabolomic biomarkers in a model of asthma exacerbation: Urine nuclear magnetic resonance. Am. J. Respir. Crit. Care Med. 2009, 179, 25–34. [Google Scholar] [CrossRef]
- Yu, M.; Cui, F.X.; Jia, H.M.; Zhou, C.; Yang, Y.; Zhang, H.W.; Ding, G.; Zou, Z.M. Aberrant purine metabolism in allergic asthma revealed by plasma metabolomics. J. Pharm. Biomed. Anal. 2016, 120, 181–189. [Google Scholar] [CrossRef]
- Yu, M.; Jia, H.M.; Cui, F.X.; Yang, Y.; Zhao, Y.; Yang, M.H.; Zou, Z.M. The Effect of Chinese Herbal Medicine Formula mKG on Allergic Asthma by Regulating Lung and Plasma Metabolic Alternations. Int. J. Mol. Sci. 2017, 18, 602. [Google Scholar] [CrossRef]
- Seo, C.; Hwang, Y.H.; Lee, H.S.; Kim, Y.; Shin, T.H.; Lee, G.; Son, Y.J.; Kim, H.; Yee, S.T.; Park, A.K.; et al. Metabolomic study for monitoring of biomarkers in mouse plasma with asthma by gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1063, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.D.; Schedel, M.; Nkrumah-Elie, Y.; Joetham, A.; Armstrong, M.; Cruickshank-Quinn, C.; Reisdorph, R.; Gelfand, E.W.; Reisdorph, N. Dysregulation of metabolic pathways in a mouse model of allergic asthma. Allergy 2017, 72, 1327–1337. [Google Scholar] [CrossRef]
- Su, L.; Shi, L.; Liu, J.; Huang, L.; Huang, Y.; Nie, X. Metabolic profiling of asthma in mice and the interventional effects of SPA using liquid chromatography and Q-TOF mass spectrometry. Mol. Biosyst. 2017, 13, 1172–1181. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, C.; Hwang, Y.H.; Shin, T.H.; Park, H.J.; Kim, Y.; Ji, M.; Min, J.; Choi, S.; Kim, H.; et al. Metabolomic approaches to polyamines including acetylated derivatives in lung tissue of mice with asthma. Metabolomics 2019, 15, 8. [Google Scholar] [CrossRef]
- Kertys, M.; Grendar, M.; Kosutova, P.; Mokra, D.; Mokry, J. Plasma based targeted metabolomic analysis reveals alterations of phosphatidylcholines and oxidative stress markers in guinea pig model of allergic asthma. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165572. [Google Scholar] [CrossRef]
- Christmann, U.; Page, A.E.; Horohov, D.W.; Adams, A.A.; Chapman, S.E.; Hancock, C.L.; Emery, A.L.; Poovey, J.R.; Hagg, C.; Ortega Morales, S.M.; et al. Lipidomic analysis of surfactant and plasma from horses with asthma and age-matched healthy horses. Am. J. Vet. Res. 2022, 83, ajvr.21.11.0179. [Google Scholar] [CrossRef]
- Ho, W.E.; Xu, Y.J.; Cheng, C.; Peh, H.Y.; Tannenbaum, S.R.; Wong, W.S.F.; Ong, C.N. Metabolomics Reveals Inflammatory-Linked Pulmonary Metabolic Alterations in a Murine Model of House Dust Mite-Induced Allergic Asthma. J. Proteome Res. 2014, 13, 3771–3782. [Google Scholar] [CrossRef]
- Van de Wetering, C.; Manuel, A.M.; Sharafi, M.; Aboushousha, R.; Qian, X.; Erickson, C.; MacPherson, M.; Chan, G.; Adcock, I.M.; ZounematKermani, N.; et al. Glutathione-S-transferase P promotes glycolysis in asthma in association with oxidation of pyruvate kinase M2. Redox Biol. 2021, 47, 102160. [Google Scholar] [CrossRef]
- Moore, W.C.; Hastie, A.T.; Li, X.; Li, H.; Busse, W.W.; Jarjour, N.N.; Wenzel, S.E.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R.; et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol. 2014, 133, 1557–1563.e5. [Google Scholar] [CrossRef]
- Zhang, J.; Fulgar, C.C.; Mar, T.; Young, D.E.; Zhang, Q.; Bein, K.J.; Cui, L.; Castañeda, A.; Vogel, C.F.A.; Sun, X.; et al. TH17-Induced Neutrophils Enhance the Pulmonary Allergic Response Following BALB/c Exposure to House Dust Mite Allergen and Fine Particulate Matter from California and China. Toxicol. Sci. 2018, 164, 627–643. [Google Scholar] [CrossRef]
- Castañeda, A.R.; Vogel, C.F.A.; Bein, K.J.; Hughes, H.K.; Smiley-Jewell, S.; Pinkerton, K.E. Ambient particulate matter enhances the pulmonary allergic immune response to house dust mite in a BALB/c mouse model by augmenting Th2- and Th17-immune responses. Physiol. Rep. 2018, 6, e13827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, S.; Xie, J.; Li, R. Identification of multiple dysregulated metabolic pathways by GC-MS-based profiling of lung tissue in mice with PM2.5-induced asthma. Chemosphere 2019, 220, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, J.; Geng, N.; Shan, Y.; Zhang, B.; Zhao, B.; Ni, Y.; Liang, Z.; Chen, J.; Zhang, L.; et al. Multi-omics analysis to reveal disorders of cell metabolism and integrin signaling pathways induced by PM2.5. J. Hazard. Mater. 2022, 424, 127573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, B.; Zhou, L.; Song, C.; Kang, T.; Xu, Y.; Liu, Y.; Han, Y.; Zhao, W.; Jia, H.; et al. PM2.5 exposure promotes asthma in aged Brown-Norway rats: Implication of multiomics analysis. Ecotoxicol. Environ. Saf. 2023, 263, 115393. [Google Scholar] [CrossRef]
- Orfanos, S.; Jude, J.; Deeney, B.T.; Cao, G.; Rastogi, D.; van Zee, M.; Pushkarsky, I.; Munoz, H.E.; Damoiseaux, R.; Di Carlo, D.; et al. Obesity increases airway smooth muscle responses to contractile agonists. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L673–L681. [Google Scholar] [CrossRef]
- Miethe, S.; Guarino, M.; Alhamdan, F.; Simon, H.U.; Renz, H.; Dufour, J.F.; Potaczek, D.P.; Garn, H. Effects of obesity on asthma: Immunometabolic links. Pol. Arch. Intern. Med. 2018, 128, 469–477. [Google Scholar] [CrossRef]
- Varraso, R.; Siroux, V.; Maccario, J.; Pin, I.; Kauffmann, F. Epidemiological Study on the Genetics and Environment of Asthma. Asthma severity is associated with body mass index and early menarche in women. Am. J. Respir. Crit. Care Med. 2005, 171, 334–339. [Google Scholar] [CrossRef]
- Weiss, S.T. Obesity: Insight into the origins of asthma. Nat. Immunol. 2005, 6, 537–539. [Google Scholar] [CrossRef]
- Chen, Y.; Dales, R.; Tang, M.; Krewski, D. Obesity may increase the incidence of asthma in women but not in men: Longitudinal observations from the Canadian National Population Health Surveys. Am. J. Epidemiol. 2002, 155, 191–197. [Google Scholar] [CrossRef]
- Sposato, B.; Scalese, M.; Scichilone, N.; Pammolli, A.; Balducci, M.T.; Migliorini, M.G.; Scala, R. BMI can influence adult males’ and females’ airway hyperresponsiveness differently. Multidiscip. Respir. Med. 2012, 7, 45. [Google Scholar] [CrossRef]
- Ait-Hadad, W.; Bédard, A.; Delvert, R.; Orsi, L.; Chanoine, S.; Dumas, O.; Laouali, N.; Le Moual, N.; Leynaert, B.; Siroux, V.; et al. Plant-Based Diets and the Incidence of Asthma Symptoms among Elderly Women, and the Mediating Role of Body Mass Index. Nutrients 2022, 15, 52. [Google Scholar] [CrossRef]
- Shore, S.A.; Cho, Y. Obesity and Asthma: Microbiome-Metabolome Interactions. Am. J. Respir. Cell. Mol. Biol. 2016, 54, 609–617. [Google Scholar] [CrossRef]
- Maniscalco, M.; Paris, D.; Melck, D.J.; D’Amato, M.; Zedda, A.; Sofia, M.; Stellato, C.; Motta, A. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J. Allergy Clin. Immunol. 2017, 139, 1536–1547.e5. [Google Scholar] [CrossRef]
- Manni, M.L.; Heinrich, V.A.; Buchan, G.J.; O’Brien, J.P.; Uvalle, C.; Cechova, V.; Koudelka, A.; Ukani, D.; Rawas-Qalaji, M.; Oury, T.D.; et al. Nitroalkene fatty acids modulate bile acid metabolism and lung function in obese asthma. Sci. Rep. 2021, 11, 17788. [Google Scholar] [CrossRef]
- Xu, S.; Karmacharya, N.; Cao, G.; Guo, C.; Gow, A.; Panettieri, R.A., Jr.; Jude, J.A. Obesity elicits a unique metabolomic signature in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 323, L297–L307. [Google Scholar] [CrossRef]
- Lutz, T.A.; Woods, S.C. Overview of animal models of obesity. Curr. Protoc. Pharmacol. 2012, 58, 1–18. [Google Scholar] [CrossRef]
- Showalter, M.R.; Nonnecke, E.B.; Linderholm, A.L.; Cajka, T.; Sa, M.R.; Lönnerdal, B.; Kenyon, N.J.; Fiehn, O. Obesogenic diets alter metabolism in mice. PLoS ONE 2018, 13, e0190632. [Google Scholar] [CrossRef]
- Jovicic, N.; Jeftic, I.; Jovanovic, I.; Radosavljevic, G.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS ONE 2015, 10, e0134089. [Google Scholar] [CrossRef]
- Calixto, M.C.; Lintomen, L.; Schenka, A.; Saad, M.J.; Zanesco, A.; Antunes, E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br. J. Pharmacol. 2010, 159, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Dietze, J.; Böcking, C.; Heverhagen, J.T.; Voelker, M.N.; Renz, H. Obesity lowers the threshold of allergic sensitization and augments airway eosinophilia in a mouse model of asthma. Allergy 2012, 67, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.C.; Oliveira, E.E.; Gouveia, A.C.C.; Brugiolo, A.S.S.; Alves, C.C.; Correa, J.O.A.; Gameiro, J.; Mattes, J.; Teixeira, H.C.; Ferreira, A.P. Obesity promotes prolonged ovalbumin-induced airway inflammation modulating T helper type 1 (Th1), Th2 and Th17 immune responses in BALB/c mice. Clin. Exp. Immunol. 2017, 189, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Everaere, L.; Ait-Yahia, S.; Molendi-Coste, O.; Vorng, H.; Quemener, S.; LeVu, P.; Fleury, S.; Bouchaert, E.; Fan, Y.; Duez, C.; et al. Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J. Allergy Clin. Immunol. 2016, 138, 1309–1318.e11. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Takahashi, K.; Sadamatsu, H.; Kato, G.; Kurata, K.; Kimura, S.; Sueoka-Aragane, N. Saturated Fatty Acid Increases Lung Macrophages and Augments House Dust Mite-Induced Airway Inflammation in Mice Fed with High-Fat Diet. Inflammation 2017, 40, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, V.A.; Uvalle, C.; Manni, M.L.; Li, K.; Mullett, S.J.; Donepudi, S.R.; Clader, J.; Fitch, A.; Ellgass, M.; Cechova, V.; et al. Meta-omics profiling of the gut-lung axis illuminates metabolic networks and host-microbial interactions associated with elevated lung elastance in a murine model of obese allergic asthma. Front. Microbiomes 2023, 2, 1153691. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Barosova, R.; Mokry, J. Sex-Based Differences in Bronchial Asthma: What are the Mechanisms behind Them? Appl. Sci. 2023, 13, 2694. [Google Scholar] [CrossRef]
- Audano, M.; Maldini, M.; De Fabiani, E.; Mitro, N.; Caruso, D. Gender-related metabolomics and lipidomics: From experimental animal models to clinical evidence. J. Proteom. 2018, 178, 82–91. [Google Scholar] [CrossRef]
- Jenkins, C.R.; Boulet, L.P.; Lavoie, K.L.; Raherison-Semjen, C.; Singh, D. Personalized Treatment of Asthma: The Importance of Sex and Gender Differences. J. Allergy Clin. Immunol. Pract. 2022, 10, 963–971.e3. [Google Scholar] [CrossRef]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef]
- Vaarhorst, A.A.; Beekman, M.; Suchiman, E.H.; van Heemst, D.; Houwing-Duistermaat, J.J.; Westendorp, R.G.; Slagboom, P.E.; Heijmans, B.T.; Leiden Longevity Study (LLS) Group. Lipid metabolism in long-lived families: The Leiden Longevity Study. Age 2011, 33, 219–227. [Google Scholar] [CrossRef]
- Gonzalez-Covarrubias, V.; Beekman, M.; Uh, H.W.; Dane, A.; Troost, J.; Paliukhovich, I.; van der Kloet, F.M.; Houwing-Duistermaat, J.; Vreeken, R.J.; Hankemeier, T.; et al. Lipidomics of familial longevity. Aging Cell 2013, 12, 426–434. [Google Scholar] [CrossRef]
- Ekpruke, C.D.; Silveyra, P. Sex Differences in Airway Remodeling and Inflammation: Clinical and Biological Factors. Front. Allergy 2022, 3, 875295. [Google Scholar] [CrossRef]
- Weir, J.M.; Wong, G.; Barlow, C.K.; Greeve, M.A.; Kowalczyk, A.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Jowett, J.B.; Shaw, J.; et al. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 2013, 54, 2898–2908. [Google Scholar] [CrossRef]
- Zheng, H.; Yde, C.C.; Arnberg, K.; Mølgaard, C.; Michaelsen, K.F.; Larnkjær, A.; Bertram, H.C. NMR-based metabolomic profiling of overweight adolescents: An elucidation of the effects of inter-/intraindividual differences, gender, and pubertal development. Biomed. Res. Int. 2014, 2014, 537157. [Google Scholar] [CrossRef]
- Song, Z.; Yan, W.; Abulikemu, M.; Wang, J.; Xing, Y.; Zhou, Q.; Ma, S.; Chang, C. Sphingolipid profiles and their relationship with inflammatory factors in asthmatic patients of different sexes. Chronic Dis. Transl. Med. 2021, 7, 199–205. [Google Scholar] [CrossRef]
- Kachroo, P.; Sordillo, J.E.; Lutz, S.M.; Weiss, S.T.; Kelly, R.S.; McGeachie, M.J.; Wu, A.C.; Lasky-Su, J.A. Pharmaco-Metabolomics of Inhaled Corticosteroid Response in Individuals with Asthma. J. Pers. Med. 2021, 11, 1148. [Google Scholar] [CrossRef]
- Leskanicova, A.; Chovancova, O.; Babincak, M.; Verboova, L.; Benetinova, Z.; Macekova, D.; Kostolny, J.; Smajda, B.; Kiskova, T. Sexual Dimorphism in Energy Metabolism of Wistar Rats Using Data Analysis. Molecules 2020, 25, 2353. [Google Scholar] [CrossRef] [PubMed]
- Stanley, E.G.; Bailey, N.J.; Bollard, M.E.; Haselden, J.N.; Waterfield, C.J.; Holmes, E.; Nicholson, J.K. Sexual dimorphism in urinary metabolite profiles of Han Wistar rats revealed by nuclear-magnetic-resonance-based metabonomics. Anal. Biochem. 2005, 343, 195–202. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Mostafa, D.H.D.; Spicer, V.; Piyadasa, H.; Maestre-Batlle, D.; Bolling, A.K.; Halayko, A.J.; Carlsten, C.; Mookherjee, N. Sex Dimorphism of Allergen-Induced Secreted Proteins in Murine and Human Lungs. Front. Immunol. 2022, 13, 923986. [Google Scholar] [CrossRef]
- Barrett, A.; Humeniuk, P.; Drevinge, C.; Corciulo, C.; Weidner, J.; Rådinger, M.; Carlsten, H.; Scheffler, J.M.; Islander, U. Physiological estrogen levels are dispensable for the sex difference in immune responses during allergen-induced airway inflammation. Immunobiology 2023, 228, 152360. [Google Scholar] [CrossRef]
- Mostafa, D.H.D.; Hemshekhar, M.; Piyadasa, H.; Altieri, A.; Halayko, A.J.; Pascoe, C.D.; Mookherjee, N. Characterization of sex-related differences in allergen house dust mite-challenged airway inflammation, in two different strains of mice. Sci. Rep. 2022, 12, 20837. [Google Scholar] [CrossRef]
- Barosova, R.; Baranovicova, E.; Adamcakova, J.; Prso, K.; Hanusrichterova, J.; Mokra, D. Sex differences in plasma metabolites in a guinea pig model of allergic asthma. Physiol. Res. 2023, 72. in press. [Google Scholar]
- Stevens, N.C.; Brown, V.J.; Domanico, M.C.; Edwards, P.C.; Van Winkle, L.S.; Fiehn, O. Alteration of glycosphingolipid metabolism by ozone is associated with exacerbation of allergic asthma characteristics in mice. Toxicol. Sci. 2023, 191, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Pederson, W.P.; Ellerman, L.M.; Jin, Y.; Gu, H.; Ledford, J.G. Metabolomic Profiling in Mouse Model of Menopause-Associated Asthma. Metabolites 2023, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, J.; Jiang, H.; Zhang, S.; Tang, S.; Yang, R.; Dong, X.; Zhang, L. Dual-Directional Regulation of Belamcanda chinensis Extract on Ovalbumin-Induced Asthma in Guinea Pigs of Different Sexes Based on Serum Metabolomics. Evid. Based Complement. Alternat. Med. 2022, 2022, 5266350. [Google Scholar] [CrossRef] [PubMed]
- Forkuo, G.S.; Guthrie, M.L.; Yuan, N.Y.; Nieman, A.N.; Kodali, R.; Jahan, R.; Stephen, M.R.; Yocum, G.T.; Treven, M.; Poe, M.M.; et al. Development of GABAA Receptor Subtype-Selective Imidazobenzodiazepines as Novel Asthma Treatments. Mol. Pharm. 2016, 13, 2026–2038. [Google Scholar] [CrossRef]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty acids, inflammation, and asthma. J. Allergy Clin. Immunol. 2014, 133, 1255–1264. [Google Scholar] [CrossRef]
- Rüdiger, M.; Kolleck, I.; Putz, G.; Wauer, R.R.; Stevens, P.; Rüstow, B. Plasmalogens effectively reduce the surface tension of surfactant-like phospholipid mixtures. Am. J. Physiol. 1998, 274, L143–L148. [Google Scholar] [CrossRef]
- Montoliu, I.; Scherer, M.; Beguelin, F.; DaSilva, L.; Mari, D.; Salvioli, S.; Martin, F.P.; Capri, M.; Bucci, L.; Ostan, R.; et al. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging 2014, 6, 9–25. [Google Scholar] [CrossRef]
- Mack, S.; Shin, J.; Ahn, Y.; Castaneda, A.R.; Peake, J.; Fulgar, C.; Zhang, J.; Cho, Y.H.; Pinkerton, K.E. Age-dependent pulmonary reactivity to house dust mite allergen: A model of adult-onset asthma? Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L757–L763. [Google Scholar] [CrossRef]
- Ford, M.L.; Ruwanpathirana, A.; Lewis, B.W.; Britt, R.D., Jr. Aging-Related Mechanisms Contribute to Corticosteroid Insensitivity in Elderly Asthma. Int. J. Mol. Sci. 2023, 24, 6347. [Google Scholar] [CrossRef]
- Brandenberger, C.; Li, N.; Jackson-Humbles, D.N.; Rockwell, C.E.; Wagner, J.G.; Harkema, J.R. Enhanced allergic airway disease in old mice is associated with a Th17 response. Clin. Exp. Allergy 2014, 44, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Kaspy, M.S.; Semnani-Azad, Z.; Malik, V.S.; Jenkins, D.J.A.; Hanley, A.J. Metabolomic profile of combined healthy lifestyle behaviours in humans: A systematic review. Proteomics 2022, 22, e2100388. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.; Kelly, R.S.; Lasky-Su, J.; Kachroo, P.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Laranjo, N.; et al. Dietary and Plasma Polyunsaturated Fatty Acids Are Inversely Associated with Asthma and Atopy in Early Childhood. J. Allergy Clin. Immunol. Pract. 2019, 7, 529–538.e8. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Linseisen, J. Dietary intake of fatty acids, antioxidants and selected food groups and asthma in adults. Eur. J. Clin. Nutr. 2005, 59, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Boushey, H.A. The microbiome in asthma. J. Allergy Clin. Immunol. 2015, 135, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Frati, F.; Salvatori, C.; Incorvaia, C.; Bellucci, A.; Di Cara, G.; Marcucci, F.; Esposito, S. The Role of the Microbiome in Asthma: The Gut-Lung Axis. Int. J. Mol. Sci. 2018, 20, 123. [Google Scholar] [CrossRef]
- Budden, K.F.; Shukla, S.D.; Rehman, S.F.; Bowerman, K.L.; Keely, S.; Hugenholtz, P.; Armstrong-James, D.P.H.; Adcock, I.M.; Chotirmall, S.H.; Chung, K.F.; et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir. Med. 2019, 7, 907–920. [Google Scholar] [CrossRef]
- Durack, J.; Huang, Y.J.; Nariya, S.; Christian, L.S.; Ansel, K.M.; Beigelman, A.; Castro, M.; Dyer, A.M.; Israel, E.; Kraft, M.; et al. Bacterial biogeography of adult airways in atopic asthma. Microbiome 2018, 6, 104. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, C.C.; Duarte, I.F.; Gomes, J.; Carrola, J.; Barros, A.S.; Gil, A.M.; Bousquet, J.; Bom, A.T.; Rocha, S.M. Urinary metabolomic changes as a predictive biomarker of asthma exacerbation. J. Allergy Clin. Immunol. 2014, 133, 261–263.e5. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S2), S150–S156. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mande, S.S. Diet, Microbiota and Gut-Lung Connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.U.; Bratt, J.M.; Jiang, X.; Pedersen, T.L.; Grapov, D.; Adkins, Y.; Kelley, D.S.; Newman, J.W.; Kenyon, N.J.; Stephensen, C.B. Dietary long-chain omega-3 fatty acids do not diminish eosinophilic pulmonary inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2014, 50, 626–636. [Google Scholar] [CrossRef]
- Heras, A.; Gomi, R.; Young, M.; Chang, C.L.; Wasserman, E.; Sharma, A.; Wu, W.; Gu, J.; Balaji, U.; White, R.; et al. Dietary long-chain omega 3 fatty acids modify sphingolipid metabolism to facilitate airway hyperreactivity. Sci. Rep. 2022, 12, 19735. [Google Scholar] [CrossRef]
- Fussbroich, D.; Zimmermann, K.; Göpel, A.; Eickmeier, O.; Trischler, J.; Zielen, S.; Schubert, R.; Beermann, C. A specific combined long-chain polyunsaturated fatty acid supplementation reverses fatty acid profile alterations in a mouse model of chronic asthma. Lipids Health Dis. 2019, 18, 16. [Google Scholar] [CrossRef]
- Siddiquee, A.; Patel, M.; Rajalingam, S.; Narke, D.; Kurade, M.; Ponnoth, D.S. Effect of omega-3 fatty acid supplementation on resolvin (RvE1)-mediated suppression of inflammation in a mouse model of asthma. Immunopharmacol. Immunotoxicol. 2019, 41, 250–257. [Google Scholar] [CrossRef]
- Flesher, R.P.; Herbert, C.; Kumar, R.K. Resolvin E1 promotes resolution of inflammation in a mouse model of an acute exacerbation of allergic asthma. Clin. Sci. 2014, 126, 805–814. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, L.; Pang, W.; Liu, W.; Li, J.; Wang, H.; Shi, G. Dietary Fiber Intake Regulates Intestinal Microflora and Inhibits Ovalbumin-Induced Allergic Airway Inflammation in a Mouse Model. PLoS ONE 2016, 11, e0147778. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Zhao, X.; Wang, J.; Wang, Q. Plasma Metabolites and Gut Microbiota Are Associated With T cell Imbalance in BALB/c Model of Eosinophilic Asthma. Front. Pharmacol. 2022, 13, 819747. [Google Scholar] [CrossRef] [PubMed]
- Alharris, E.; Mohammed, A.; Alghetaa, H.; Zhou, J.; Nagarkatti, M.; Nagarkatti, P. The Ability of Resveratrol to Attenuate Ovalbumin-Mediated Allergic Asthma Is Associated With Changes in Microbiota Involving the Gut-Lung Axis, Enhanced Barrier Function and Decreased Inflammation in the Lungs. Front. Immunol. 2022, 13, 805770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, H.; Wang, T.; Zhao, X.; Wang, J.; Wang, Q. Anti-Inflammatory and Anti-asthmatic Effects of TMDCT Decoction in Eosinophilic Asthma Through Treg/Th17 Balance. Front. Pharmacol. 2022, 13, 819728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, L.; Zhang, H.; Zhang, H.; Liu, J.; Zhao, X.; Wang, J.; Wang, Q. Guominkang formula alleviate inflammation in eosinophilic asthma by regulating immune balance of Th1/2 and Treg/Th17 cells. Front. Pharmacol. 2022, 13, 978421. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Gaffin, J.M.; Israel, E.; Phipatanakul, W. Asthma and Corticosteroid Responses in Childhood and Adult Asthma. Clin. Chest Med. 2019, 40, 163–177. [Google Scholar] [CrossRef]
- Kannisto, S.; Laatikainen, A.; Taivainen, A.; Savolainen, K.; Tukiainen, H.; Voutilainen, R. Serum dehydroepiandrosterone sulfate concentration as an indicator of adrenocortical suppression during inhaled steroid therapy in adult asthmatic patients. Eur. J. Endocrinol. 2004, 150, 687–690. [Google Scholar] [CrossRef]
- Kachroo, P.; Stewart, I.D.; Kelly, R.S.; Stav, M.; Mendez, K.; Dahlin, A.; Soeteman, D.I.; Chu, S.H.; Huang, M.; Cote, M.; et al. Metabolomic profiling reveals extensive adrenal suppression due to inhaled corticosteroid therapy in asthma. Nat. Med. 2022, 28, 814–822. [Google Scholar] [CrossRef]
- Daley-Yates, P.; Keppler, B.; Brealey, N.; Shabbir, S.; Singh, D.; Barnes, N. Inhaled glucocorticoid-induced metabolome changes in asthma. Eur. J. Endocrinol. 2022, 187, 413–427. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013, 131, 636–645. [Google Scholar] [CrossRef]
- Berry, M.; Morgan, A.; Shaw, D.E.; Parker, D.; Green, R.; Brightling, C.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007, 62, 1043–1049. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Park, Y.; Brown, L.A.; Jones, D.P. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J. Allergy Clin. Immunol. 2014, 133, 258–261.e8. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Fitzpatrick, A.M.; Medriano, C.A.; Jones, D.P. High-resolution metabolomics to identify urine biomarkers in corticosteroid-resistant asthmatic children. J. Allergy Clin. Immunol. 2017, 139, 1518–1524.e4. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, J.; Herrera-Luis, E.; Lorenzo-Diaz, F.; González, M.; Sardón, O.; Villar, J.; Pino-Yanes, M. Precision Medicine in Childhood Asthma: Omic Studies of Treatment Response. Int. J. Mol. Sci. 2020, 21, 2908. [Google Scholar] [CrossRef] [PubMed]
- McGeachie, M.J.; Dahlin, A.; Qiu, W.; Croteau-Chonka, D.C.; Savage, J.; Wu, A.C.; Wan, E.S.; Sordillo, J.E.; Al-Garawi, A.; Martinez, F.D.; et al. The metabolomics of asthma control: A promising link between genetics and disease. Immun. Inflamm. Dis. 2015, 3, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Quan-Jun, Y.; Jian-Ping, Z.; Jian-Hua, Z.; Yong-Long, H.; Bo, X.; Jing-Xian, Z.; Bona, D.; Yuan, Z.; Cheng, G. Distinct Metabolic Profile of Inhaled Budesonide and Salbutamol in Asthmatic Children during Acute Exacerbation. Basic Clin. Pharmacol. Toxicol. 2017, 120, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Daley-Yates, P.; Keppler, B.; Baines, A.; Bardsley, G.; Fingleton, J. Metabolomic changes related to airway inflammation, asthma pathogenesis and systemic activity following inhaled fluticasone furoate/vilanterol: A randomized controlled trial. Respir. Res. 2022, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.E.; Xu, Y.J.; Xu, F.; Cheng, C.; Peh, H.Y.; Tannenbaum, S.R.; Wong, W.S.; Ong, C.N. Metabolomics reveals altered metabolic pathways in experimental asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.J.; Li, M.H.; Chan, C.O.; Shen, Q.; Chen, S.B.; An, B.C.; Yuen, A.C.; Wu, W.F.; Tang, H.H.; Cao, S.W.; et al. Altered metabolites in guinea pigs with allergic asthma after acupoint sticking therapy: New insights from a metabolomics approach. Phytomedicine 2019, 54, 182–194. [Google Scholar] [CrossRef]
- Checkley, W.; Deza, M.P.; Klawitter, J.; Romero, K.M.; Klawitter, J.; Pollard, S.L.; Wise, R.A.; Christians, U.; Hansel, N.N. Identifying biomarkers for asthma diagnosis using targeted metabolomics approaches. Respir. Med. 2016, 121, 59–66. [Google Scholar] [CrossRef]
- Kelly, R.S.; Virkud, Y.; Giorgio, R.; Celedón, J.C.; Weiss, S.T.; Lasky-Su, J. Metabolomic profiling of lung function in Costa-Rican children with asthma. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1590–1595. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Lin, G.; Cheng, M.L.; Chiang, M.H.; Tsai, M.H.; Su, K.W.; Hua, M.C.; Liao, S.L.; Lai, S.H.; Yao, T.C.; et al. Longitudinal urinary metabolomic profiling reveals metabolites for asthma development in early childhood. Pediatr. Allergy Immunol. 2018, 29, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Aun, M.V.; Bonamichi-Santos, R.; Arantes-Costa, F.M.; Kalil, J.; Giavina-Bianchi, P. Animal models of asthma: Utility and limitations. J. Asthma Allergy 2017, 10, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Takeda, K.; Gelfand, E.W. Understanding asthma using animal models. Allergy Asthma Immunol. Res. 2009, 1, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Nials, A.T.; Uddin, S. Mouse models of allergic asthma: Acute and chronic allergen challenge. Dis. Models Mech. 2008, 1, 213–220. [Google Scholar] [CrossRef]
- Kumar, R.K.; Herbert, C.; Foster, P.S. Mouse models of acute exacerbations of allergic asthma. Respirology 2016, 21, 842–849. [Google Scholar] [CrossRef]
- Johnson, J.R.; Wiley, R.E.; Fattouh, R.; Swirski, F.K.; Gajewska, B.U.; Coyle, A.J.; Gutierrez-Ramos, J.C.; Ellis, R.; Inman, M.D.; Jordana, M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 2004, 169, 378–385. [Google Scholar] [CrossRef]
- Fattouh, R.; Pouladi, M.A.; Alvarez, D.; Johnson, J.R.; Walker, T.D.; Goncharova, S.; Inman, M.D.; Jordana, M. House dust mite facilitates ovalbumin-specific allergic sensitization and airway inflammation. Am. J. Respir. Crit. Care Med. 2005, 172, 314–321. [Google Scholar] [CrossRef]
- Lan, F.; Liu, K.; Zhang, J.; Qi, Y.; Li, K.; Lin, P. Th17 response is augmented in OVA-induced asthmatic mice exposed to HDM. Med. Sci. Monit. 2011, 17, BR132–BR138. [Google Scholar] [CrossRef]
- Couetil, L.; Cardwell, J.M.; Leguillette, R.; Mazan, M.; Richard, E.; Bienzle, D.; Bullone, M.; Gerber, V.; Ivester, K.; Lavoie, J.P.; et al. Equine Asthma: Current Understanding and Future Directions. Front. Vet. Sci. 2020, 7, 450. [Google Scholar] [CrossRef]
- Couëtil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.-P.; Léguillette, R.; Richard, E.A. Inflammatory airway disease of horses—Revised consensus statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef]
- Bullone, M.; Lavoie, J.P. Asthma “of horses and men”—How can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol. Immunol. 2015, 66, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, M.; Laghi, L.; Zhu, C.; Magi, G.E.; Tesei, B.; Laus, F. Respiratory metabolites in bronchoalveolar lavage fluid (BALF) and exhaled breath condensate (EBC) can differentiate horses affected by severe equine asthma from healthy horses. BMC Vet. Res. 2020, 16, 233. [Google Scholar] [CrossRef]
- Höglund, N.; Nieminen, P.; Mustonen, A.M.; Käkelä, R.; Tollis, S.; Koho, N.; Holopainen, M.; Ruhanen, H.; Mykkänen, A. Fatty acid fingerprints in bronchoalveolar lavage fluid and its extracellular vesicles reflect equine asthma severity. Sci. Rep. 2023, 13, 9821. [Google Scholar] [CrossRef]
- Karagianni, A.E.; Eaton, S.L.; Kurian, D.; Cillán-Garcia, E.; Twynam-Perkins, J.; Raper, A.; Wishart, T.M.; Pirie, R.S. Application across species of a one health approach to liquid sample handling for respiratory based -omics analysis. Sci. Rep. 2021, 11, 14292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barosova, R.; Baranovicova, E.; Hanusrichterova, J.; Mokra, D. Metabolomics in Animal Models of Bronchial Asthma and Its Translational Importance for Clinics. Int. J. Mol. Sci. 2024, 25, 459. https://doi.org/10.3390/ijms25010459
Barosova R, Baranovicova E, Hanusrichterova J, Mokra D. Metabolomics in Animal Models of Bronchial Asthma and Its Translational Importance for Clinics. International Journal of Molecular Sciences. 2024; 25(1):459. https://doi.org/10.3390/ijms25010459
Chicago/Turabian StyleBarosova, Romana, Eva Baranovicova, Juliana Hanusrichterova, and Daniela Mokra. 2024. "Metabolomics in Animal Models of Bronchial Asthma and Its Translational Importance for Clinics" International Journal of Molecular Sciences 25, no. 1: 459. https://doi.org/10.3390/ijms25010459
APA StyleBarosova, R., Baranovicova, E., Hanusrichterova, J., & Mokra, D. (2024). Metabolomics in Animal Models of Bronchial Asthma and Its Translational Importance for Clinics. International Journal of Molecular Sciences, 25(1), 459. https://doi.org/10.3390/ijms25010459






