Bisphosphonates as Radiopharmaceuticals: Spotlight on the Development and Clinical Use of DOTAZOL in Diagnostics and Palliative Radionuclide Therapy
Abstract
:1. Introduction
2. Bisphosphonates: From Conventional Drugs to Radiopharmaceuticals
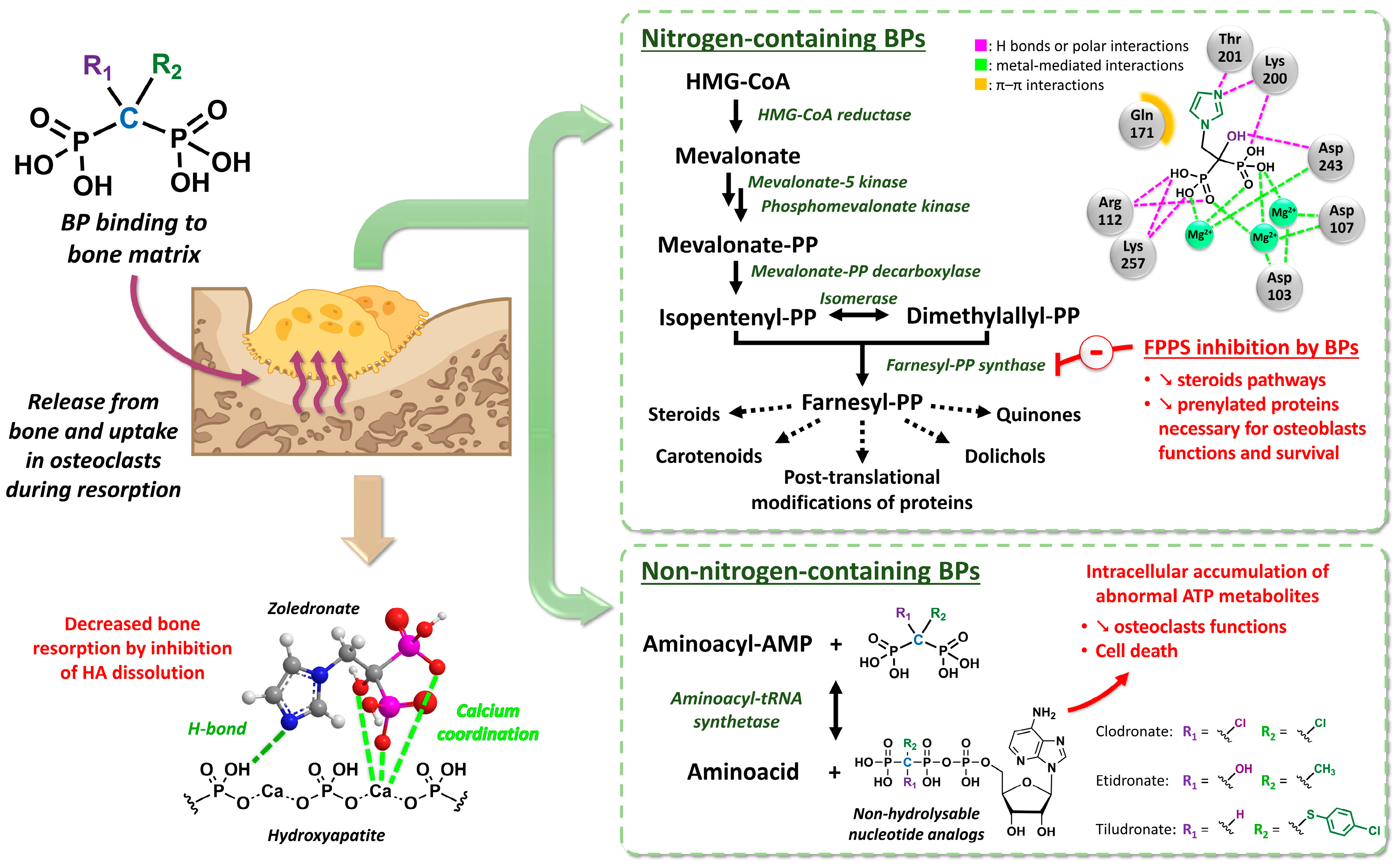
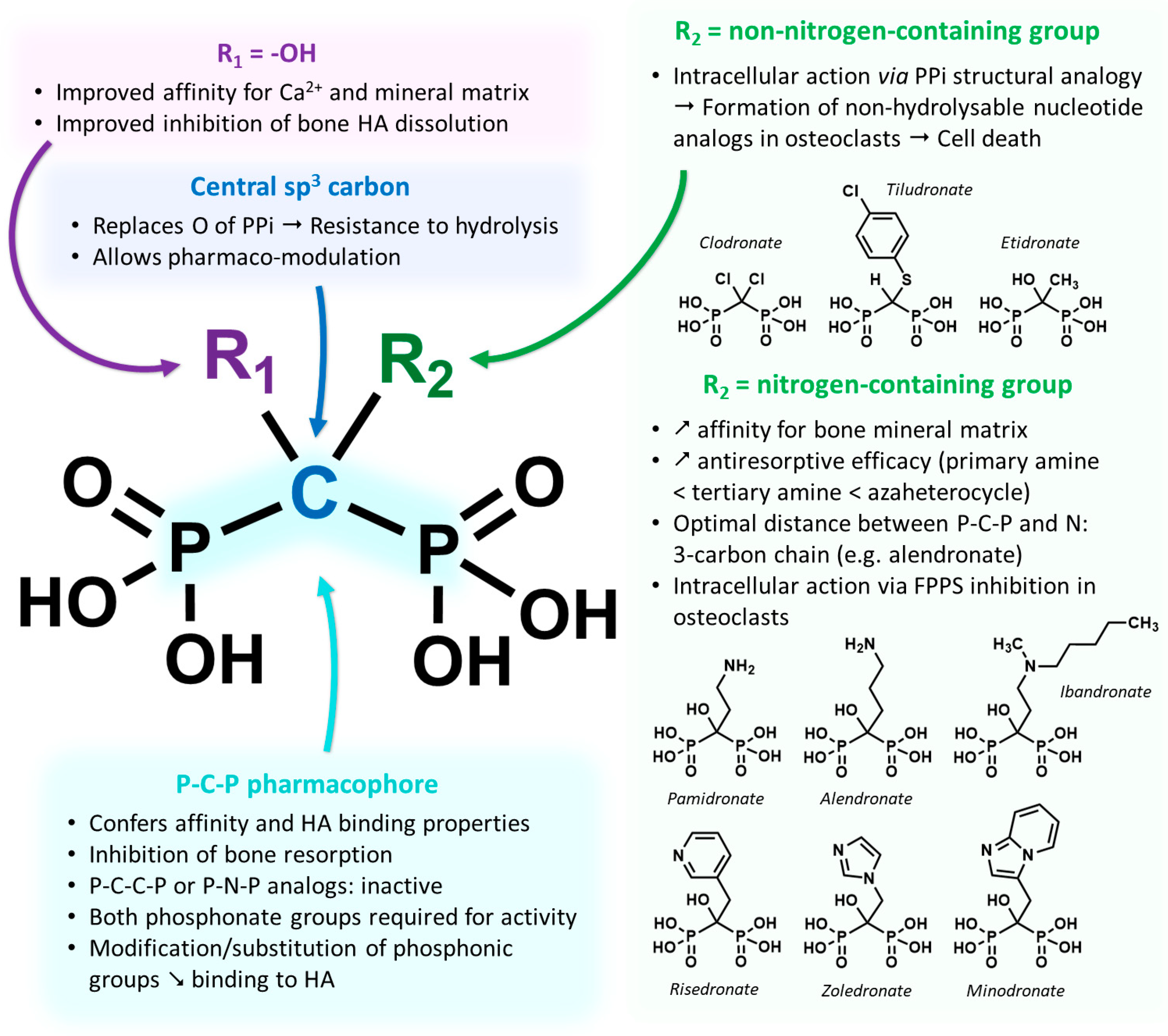
2.1. Development and Pharmacology Basics of Bisphosphonates
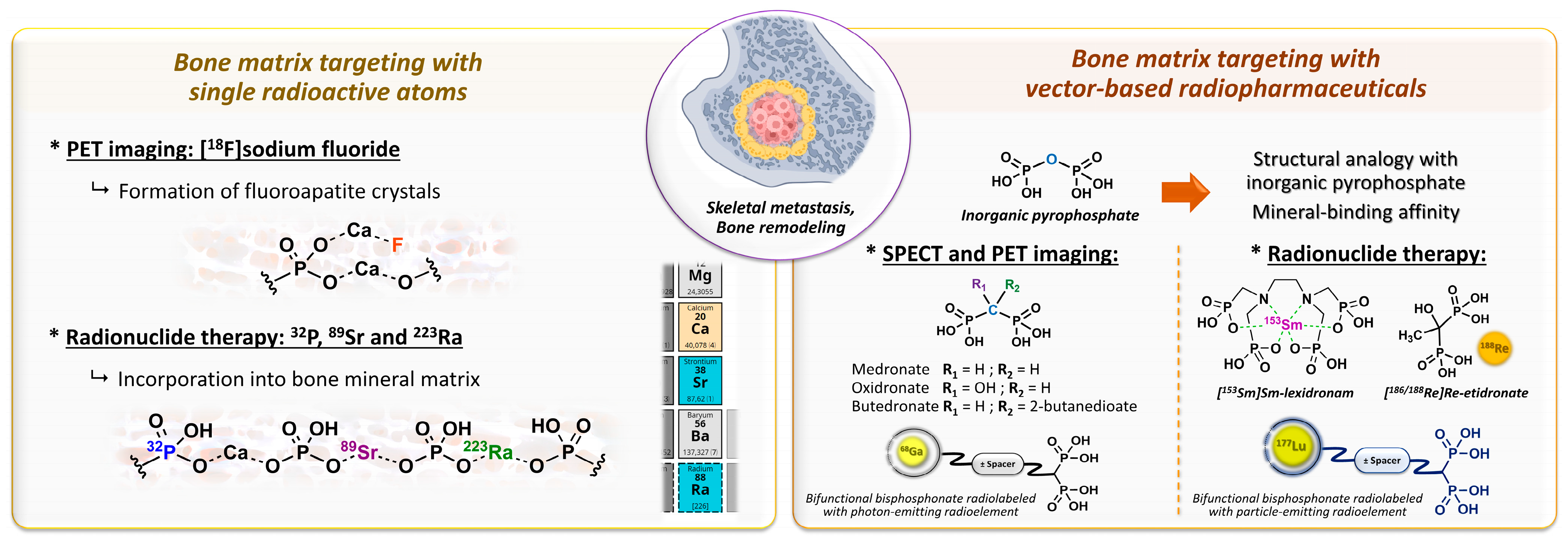
2.2. Bone Metastases Molecular Targeting and Early Bisphosphonate-Based Radiopharmaceuticals
- Specific bone targeting based on the electronic properties of a free radioactive atom: two isotopes of the same atom do not differ in their electronic structure and therefore have identical chemical properties, which allows skeletal targeting by the isotopes of atoms with natural affinities for bone. This applies to [18F]fluoride ions, which replace hydroxide anions in hydroxyapatite crystals to form fluoroapatite in the bone mineral [82]. The [18F]NaF PET imaging agent tends to be more accurate than bone scintigraphy for the detection of skeletal metastatic lesions in several types of cancer [83,84,85,86]. In therapy, [32P]orthophosphate (t1/2 = 14.3 days; Eβ-max = 1.71 MeV) is also incorporated into hydroxyapatite crystals [87] and was historically used to treat painful osteoblastic metastases [88]. This treatment showed moderate efficacy with the disappearance of pain symptoms in almost half of patients [89] but caused common bone marrow toxicity, especially in patients with renal impairment. Likewise, the chemical elements in the same group/column of the periodic table of elements are characterized by the same number of valence electrons, and therefore, by usually comparable chemical reactivity. Part of the same group as calcium, 89Sr (t1/2 = 50.5 days; Eβ-max = 1.49 MeV) is an alkaline earth metal that accumulates in lesions with high osteoblastic activity and has been used under its dichloride salt form in the palliative treatment of pain associated with bone metastases [90], especially in prostate cancer. The initial clinical trials with this radiopharmaceutical in monotherapy showed modest effects on pain control in bone metastases associated with substantial bone marrow toxicity [91,92,93], while its use in patients treated with doxorubicin [94] or docetaxel [95] tended to improve both the symptoms associated with bone metastases and survival. Similarly, alpha-emitting radionuclide 223Ra (t1/2 = 11.4 days; Eα = 5.0 to 7.5 MeV [95.3%]; Eβ-max = 1.37 MeV and 1.42 MeV [3.6%]) showed an overall survival benefit in patients with metastatic prostate cancer, with a significant 9-month delay in bone-related events when associated with a bone-protecting agent (e.g., denosumab) [96]. To date, radium-223 dichloride (Xofigo®, Bayer, Leverkusen, Germany) is the only FDA- and EMA-approved targeted alpha therapy available.
- Specific targeting based on a vector molecule with bone tropism: in scintigraphic imaging, medronate (MDP) [97] and oxidronate (HMDP) [98,99] were among the first bisphosphonates to be used as bone scintigraphy imaging vectors on humans after the pioneering application of [99mTc]Tc-etidronate [100,101,102,103] (Figure 5). These two compounds are characterized by their simple chemical structures, which do not contain dedicated chelation sites. Although their formulation in single-vial cold kits for radiopharmaceutical preparation makes 99mTc radiolabeling simple and ensures high radiochemical purity levels, [99mTc]Tc-MDP and [99mTc]Tc-HMDP complexes do not form a single defined chemical entity but are rather structured into a mixture of monomers, oxo-bridged dimers and oligomeric clusters of varying sizes, featuring diverse technetium-oxo core arrangements, oxidation states and ligand coordination numbers [104] with a composition that varies according to pH, technetium concentration and oxygen amount [105]. Lastly, the phosphonate groups of MDP and HMDP (as well as the hydroxyl group of HMDP) serve both as coordination sites with 99mTc and as recognition sites for the bone mineral matrix. Consequently, the bone affinity of the corresponding 99mTc complexes is intrinsically reduced [106]. Even so, these radiopharmaceuticals remain reference bone scintigraphy agents, either in oncology for cancer staging [107,108] and therapeutic response evaluation [109,110,111] or in benign bone disorders such as Paget disease [112,113] or primary hyperparathyroidism [114,115]. Interestingly, 99mTc-radiolabeled butedronate (2,3-dicarboxypropane-1,1-diphosphonate, DPD, Figure 5) [116] is another SPECT imaging agent with the same indications as [99mTc]Tc-MDP and [99mTc]Tc-HMDP but also has a particular role in the detection of cardiac amyloidosis [117,118,119]. Concerning therapy, a bisphosphonate-related derivative with an ethylenediamine tetraphosphonate structure (EDTMP, also named lexidronam) radiolabeled with 153Sm (t1/2 = 1.9 days; Eβ-max = 0.81 MeV) has also been used since the late 1980s [120,121] for the relief of pain resulting from bone metastases; two clinical trials demonstrated its efficacy in this indication versus the placebo and its improved toxicity profile compared to 89Sr and 32P [122,123]. Notably, etidronate was also selected for radionuclide therapy applications after radiolabeling with beta minus-emitting rhenium isotopes, either 186Re or 188Re [9,10,11,12,13,14,15,16,17,18,19,20,21], but with rather limited clinical use.
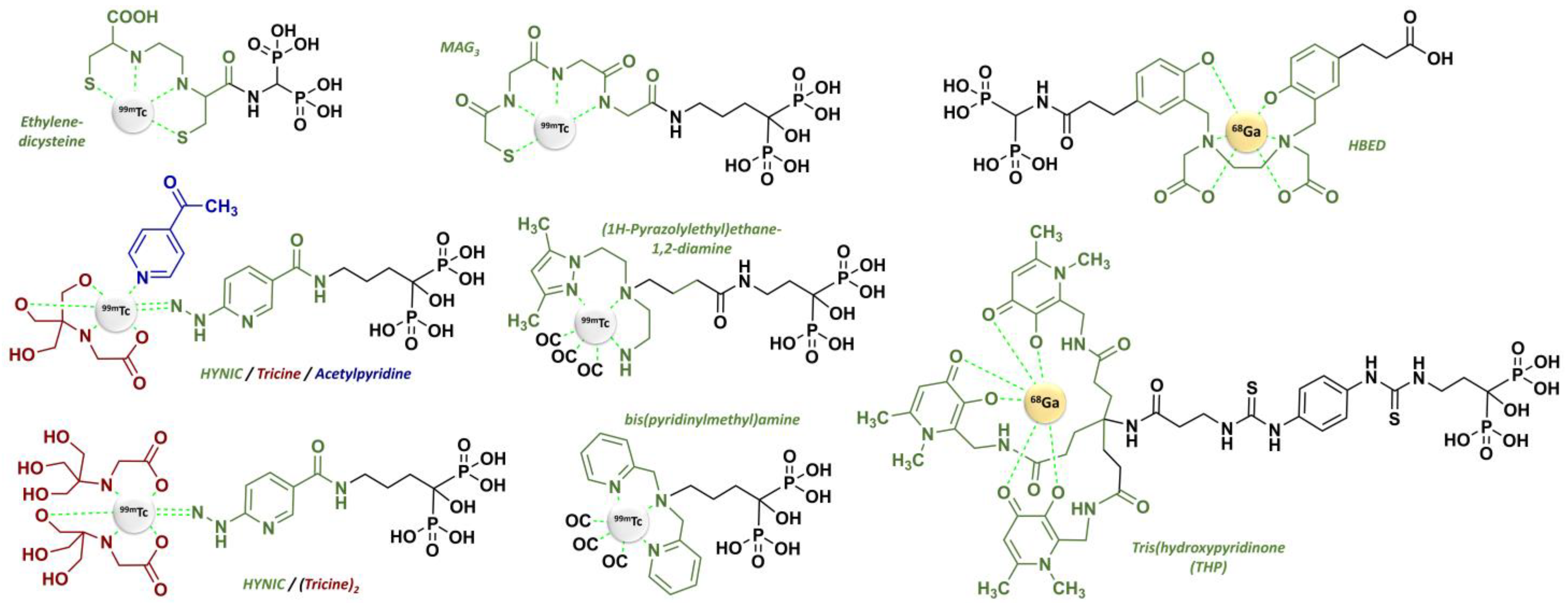
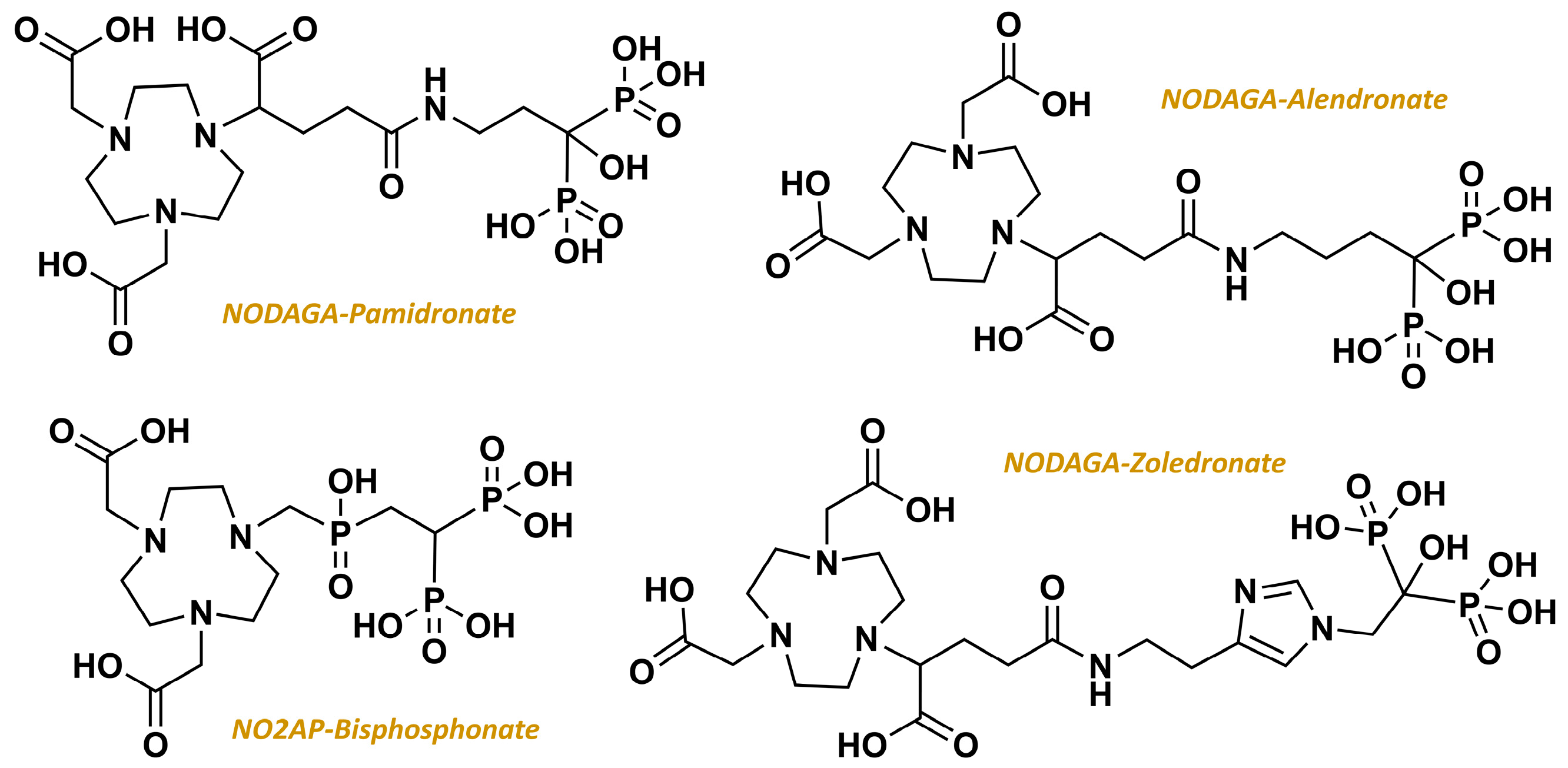
2.3. From Standard Bisphosphonates to Bifunctional Derivatives Optimized for Nuclear Medicine
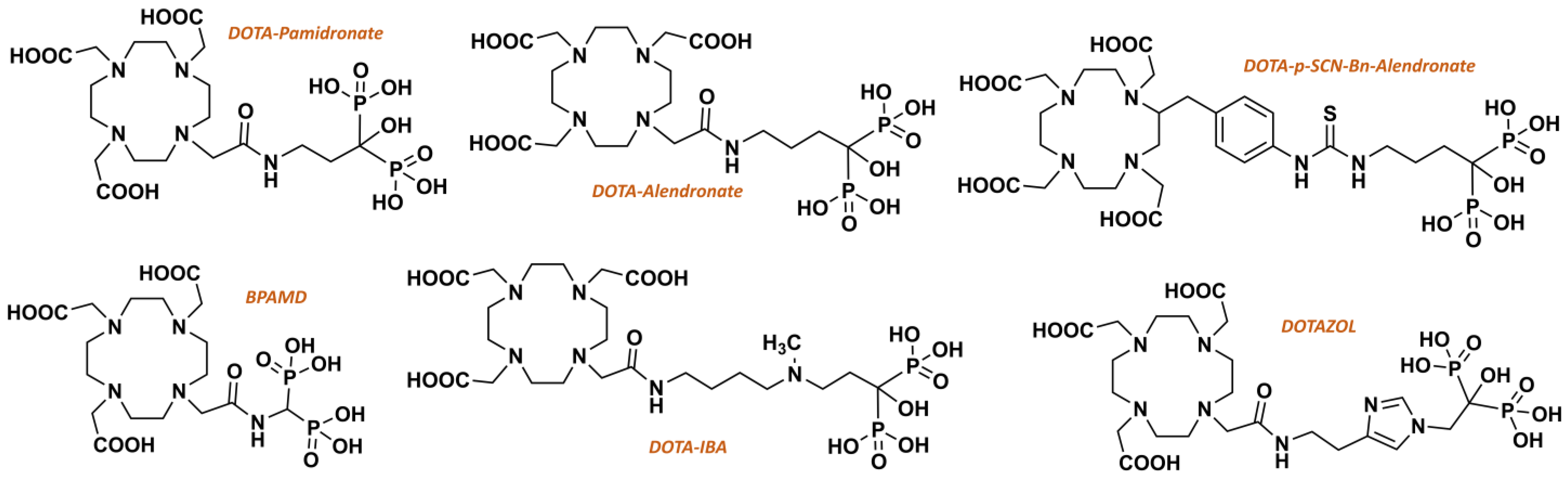
3. DOTAZOL: Chemistry and Radiochemistry Considerations
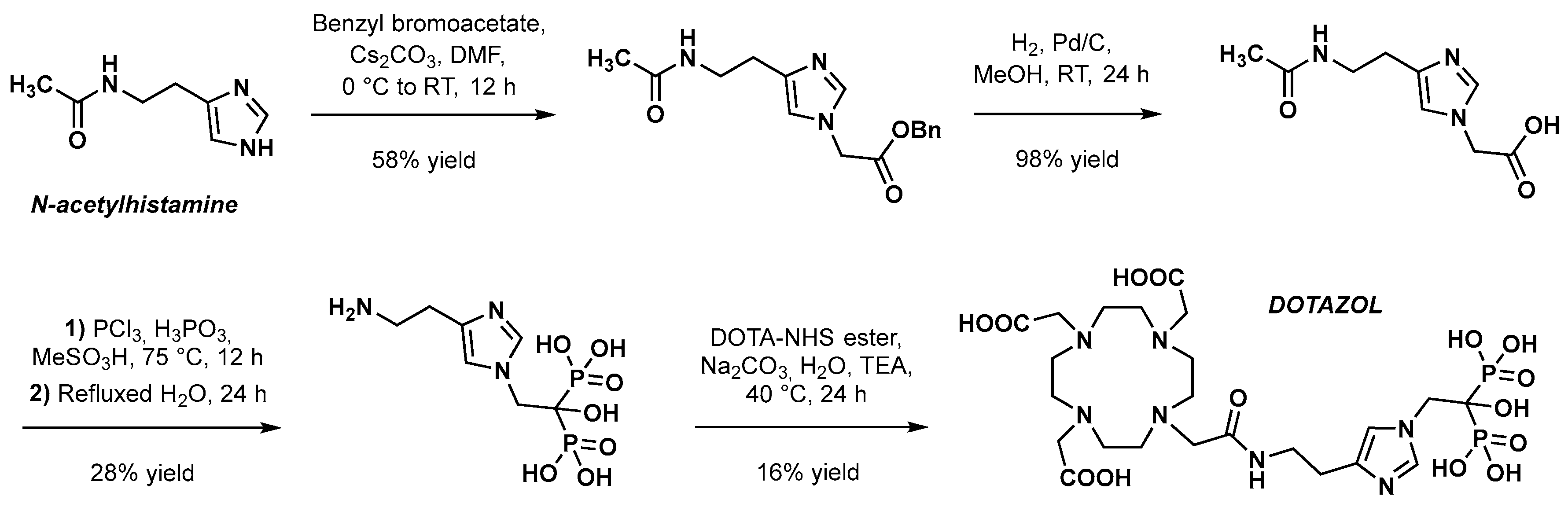
3.1. Chemical Synthesis of DOTAZOL
3.2. DOTAZOL Radiolabeling with 68Ga or 177Lu
3.3. Quality Controls of Radiolabeled DOTAZOL: A Critical Step
4. Preclinical Investigations on DOTAZOL
4.1. [68Ga]Ga-DOTAZOL
4.2. [177Lu]Lu-DOTAZOL
5. Clinical Uses of DOTAZOL
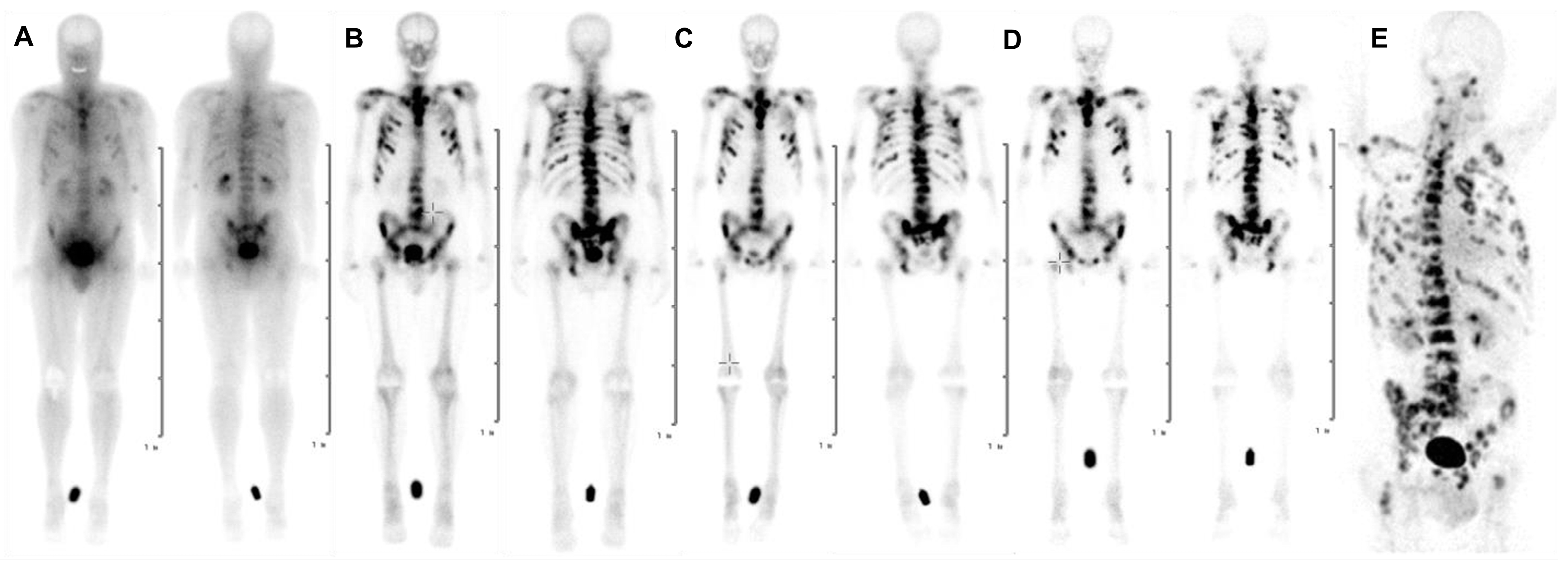
5.1. PET Imaging with [68Ga]Ga-DOTAZOL
5.2. Targeted Radionuclide Therapy with [177Lu]Lu-DOTAZOL
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ryan, C.; Stoltzfus, K.C.; Horn, S.; Chen, H.; Louie, A.V.; Lehrer, E.J.; Trifiletti, D.M.; Fox, E.J.; Abraham, J.A.; Zaorsky, N.G. Epidemiology of Bone Metastases. Bone 2022, 158, 115783. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone Metastases. Nat. Rev. Dis. Primers. 2020, 6, 83. [Google Scholar] [CrossRef]
- O’Mara, R.E. Skeletal Scanning in Neoplastic Disease. Cancer 1976, 37, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Libshitz, H.I.; Seabold, J.E. Osseous Metastases of Breast Cancer. Clinical, Biochemical, Radiographic, and Scintigraphic Evaluation of Response to Therapy. Cancer 1984, 53, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Vinholes, J.; Coleman, R.; Eastell, R. Effects of Bone Metastases on Bone Metabolism: Implications for Diagnosis, Imaging and Assessment of Response to Cancer Treatment. Cancer Treat. Rev. 1996, 22, 289–331. [Google Scholar] [CrossRef]
- Rybak, L.D.; Rosenthal, D.I. Radiological Imaging for the Diagnosis of Bone Metastases. Q. J. Nucl. Med. 2001, 45, 53–64. [Google Scholar]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Cianni, R.; Bagni, O. Theranostic Approaches in Nuclear Medicine: Current Status and Future Prospects. Expert. Rev. Med. Devices 2020, 17, 331–343. [Google Scholar] [CrossRef]
- Ogawa, K.; Saji, H. Advances in Drug Design of Radiometal-Based Imaging Agents for Bone Disorders. Int. J. Mol. Imaging. 2011, 2011, 537687. [Google Scholar] [CrossRef]
- de Klerk, J.M.; van Dijk, A.; van het Schip, A.D.; Zonnenberg, B.A.; van Rijk, P.P. Pharmacokinetics of Rhenium-186 after Administration of Rhenium-186-HEDP to Patients with Bone Metastases. J. Nucl. Med. 1992, 33, 646–651. [Google Scholar]
- Maxon, H.R.; Schroder, L.E.; Washburn, L.C.; Thomas, S.R.; Samaratunga, R.C.; Biniakiewicz, D.; Moulton, J.S.; Cummings, D.; Ehrhardt, G.J.; Morris, V. Rhenium-188(Sn)HEDP for Treatment of Osseous Metastases. J. Nucl. Med. 1998, 39, 659–663. [Google Scholar]
- Palmedo, H.; Guhlke, S.; Bender, H.; Sartor, J.; Schoeneich, G.; Risse, J.; Grünwald, F.; Knapp, F.F.; Biersack, H.J. Dose Escalation Study with Rhenium-188 Hydroxyethylidene Diphosphonate in Prostate Cancer Patients with Osseous Metastases. Eur. J. Nucl. Med. 2000, 27, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zhang, H.; Tian, M.; Wang, J.; Zheng, X. Rhenium-188 HEDP To Treat Painful Bone Metastases. Clin. Nucl. Med. 2001, 26, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; de Klerk, J.M.H.; Tan, S.; van het Schip, A.D.; Derksen, B.H.; van Dijk, A.; Kruitwagen, C.L.J.J.; Blijham, G.H.; van Rijk, P.P.; Zonnenberg, B.A. The Placorhen Study: A Double-Blind, Placebo-Controlled, Randomized Radionuclide Study with 186Re-Etidronate in Hormone-Resistant Prostate Cancer Patients with Painful Bone Metastases. J. Nucl. Med. 2002, 43, 1150–1156. [Google Scholar] [PubMed]
- Liepe, K.; Kropp, J.; Runge, R.; Kotzerke, J. Therapeutic Efficiency of Rhenium-188-HEDP in Human Prostate Cancer Skeletal Metastases. Br. J. Cancer. 2003, 89, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, M.; Li, S.; Liu, J.; Tanada, S.; Endo, K. Rhenium-188-HEDP Therapy for the Palliation of Pain Due to Osseous Metastases in Lung Cancer Patients. Cancer. Biother. Radiopharm. 2003, 18, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Liepe, K.; Hliscs, R.; Kropp, J.; Runge, R.; Knapp, F.F.; Franke, W.-G. Dosimetry of 188Re-Hydroxyethylidene Diphosphonate in Human Prostate Cancer Skeletal Metastases. J. Nucl. Med. 2003, 44, 953–960. [Google Scholar]
- Liepe, K.; Runge, R.; Kotzerke, J. The Benefit of Bone-Seeking Radiopharmaceuticals in the Treatment of Metastatic Bone Pain. J. Cancer. Res. Clin. Oncol. 2005, 131, 60–66. [Google Scholar] [CrossRef]
- Liepe, K.; Kotzerke, J. A Comparative Study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the Treatment of Painful Skeletal Metastases. Nucl. Med. Commun. 2007, 28, 623–630. [Google Scholar] [CrossRef]
- Cheng, A.; Chen, S.; Zhang, Y.; Yin, D.; Dong, M. The Tolerance and Therapeutic Efficacy of Rhenium-188 Hydroxyethylidene Diphosphonate in Advanced Cancer Patients with Painful Osseous Metastases. Cancer. Biother. Radiopharm. 2011, 26, 237–244. [Google Scholar] [CrossRef]
- Shinto, A.; Mallia, M.; Kameswaran, M.; Kamaleshwaran, K.; Joseph, J.; Radhakrishnan, E.; Upadhyay, I.; Subramaniam, R.; Sairam, M.; Banerjee, S.; et al. Clinical Utility of 188Rhenium-Hydroxyethylidene-1,1-Diphosphonate as a Bone Pain Palliative in Multiple Malignancies. World J. Nucl. Med. 2018, 17, 228–235. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Gui, J.; Liu, C.; Wang, Y.; Zhang, G.; Kuai, D.; Wu, Y.; Liu, Z.; Zuo, C.; et al. Efficacy and Safety of 188Re-HEDP in Lung Cancer Patients with Bone Metastases: A Randomized, Multicenter, Multiple-Dose Phase IIa Study. Int. J. Clin. Oncol. 2021, 26, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.; Overbeek, F.; de Klerk, J.; Pasker-de Jong, P.; van den Berk, A.; ter Heine, R.; Rodenburg, C.; Kooistra, A.; Hendrikse, N.H.; Bloemendal, H. Treatment of painful bone metastases in prostate and breast cancer patients with the therapeutic radiopharmaceutical rhenium-188-HEDP: Clinical benefit in a real-world study. Nuklearmedizin 2016, 55, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kleynhans, J.; Duatti, A.; Bolzati, C. Fundamentals of Rhenium-188 Radiopharmaceutical Chemistry. Molecules 2023, 28, 1487. [Google Scholar] [CrossRef] [PubMed]
- Lepareur, N.; Lacœuille, F.; Bouvry, C.; Hindré, F.; Garcion, E.; Chérel, M.; Noiret, N.; Garin, E.; Knapp, F.F.R. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 132. [Google Scholar] [CrossRef]
- Lepareur, N. Cold Kit Labeling: The Future of 68Ga Radiopharmaceuticals? Front. Med. 2022, 9, 812050. [Google Scholar] [CrossRef] [PubMed]
- Meisenheimer, M.; Saenko, Y.; Eppard, E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider. In Medical Isotopes; Ali Raza Naqvi, S., Babar Imrani, M., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83880-627-9. [Google Scholar]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. (Russ) Lutetium-177 Therapeutic Radiopharmaceuticals: Linking Chemistry, Radiochemistry, and Practical Applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [68Ga]Ga-PSMA-11: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 713. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Blomen, L.J.M.J. Discovery and History of the Non-Medical Uses of Bisphosphonates. In Bisphosphonates on Bone; Chapter 7; Bijvoet, O., Fleisch, H.A., Canfield, R.E., Russell, R.G.G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1995; pp. 111–124. [Google Scholar]
- Menschutkin, N. Ueber die Einwirkung des Chloracetyls auf phosphorige Säure. Ann. Chem. Pharm. 1865, 133, 317–320. [Google Scholar] [CrossRef]
- Francis, M.D.; Graham, R.; Russell, G.; Fleisch, H. Diphosphonates Inhibit Formation of Calcium Phosphate Crystals in Vitro and Pathological Calcification in Vivo. Science 1969, 165, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Russell, R.G.; Francis, M.D. Diphosphonates Inhibit Hydroxyapatite Dissolution in Vitro and Bone Resorption in Tissue Culture and in Vivo. Science 1969, 165, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.; Mühlbauer, R.C.; Bisaz, S.; Williams, D.A.; Fleisch, H. The Influence of Pyrophosphate, Condensed Phosphates, Phosphonates and Other Phosphate Compounds on the Dissolution of Hydroxyapatite in Vitro and on Bone Resorption Induced by Parathyroid Hormone in Tissue Culture and in Thyroparathyroidectomised Rats. Calcif. Tissue. Res. 1970, 6, 183–196. [Google Scholar] [CrossRef]
- Quimby, O.T.; Prentice, J.B.; Nicholson, D.A. Tetrasodium Carbonyldiphosphonate. Synthesis, Reactions, and Spectral Properties. J. Org. Chem. 1967, 32, 4111–4114. [Google Scholar] [CrossRef]
- Ebrahimpour, A.; Francis, M.D. Bisphosphonate Therapy in Acute and Chronic Bone Loss: Physical Chemical Considerations in Bisphosphonate-Related Therapies. In Bisphosphonates on Bone; Bijvoet, O., Fleisch, H.A., Canfield, R.E., Russell, R.G.G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1995; pp. 125–136. [Google Scholar]
- Baeyer, H.V.; Hofmann, K.A. Acetodiphosphorige Säure. Berichte. Dtsch. Chem. Ges. 1897, 30, 1973–1978. [Google Scholar] [CrossRef]
- Bijvoet, O.L.M.; Nollen, A.J.G.; Slooff, T.J.J.H.; Feith, R. Effect of a Diphosphonate on Para-Articular Ossification after Total Hip Replacement. Acta Orthop. Scand. 1974, 45, 926–934. [Google Scholar] [CrossRef]
- Pelorgeas, S.; Martin, J.-B.; Satre, M. Cytotoxicity of Dichloromethane Diphosphonate and of 1-Hydroxyethane-1,1-Diphosphonate in the Amoebae of the Slime Mould Dictyostelium Discoideum. Biochem. Pharmacol. 1992, 44, 2157–2163. [Google Scholar] [CrossRef]
- Frith, J.C.; Mönkkönen, J.; Blackburn, G.M.; Russell, R.G.G.; Rogers, M.J. Clodronate and Liposome-Encapsulated Clodronate Are Metabolized to a Toxic ATP Analog, Adenosine 5′-(β,γ-Dichloromethylene) Triphosphate, by Mammalian Cells In Vitro. J. Bone Min. Res. 1997, 12, 1358–1367. [Google Scholar] [CrossRef]
- Rogers, M.J.; Brown, R.J.; Hodkin, V.; Blackburn, G.M.; Russell, R.G.G.; Watts, D.J. Bisphosphonates Are Incorporated into Adenine Nucleotides by Human Aminoacyl-tRNA Synthetase Enzymes. Biochem. Biophys. Res. Commun. 1996, 224, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Auriola, S.; Frith, J.; Rogers, M.J.; Koivuniemi, A.; Mönkkönen, J. Identification of Adenine Nucleotide-Containing Metabolites of Bisphosphonate Drugs Using Ion-Pair Liquid Chromatography–Electrospray Mass Spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1997, 704, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Xiong, X.; Ji, X.; Mönkkönen, J.; Russell, R.G.G.; Williamson, M.P.; Ebetino, F.H.; Watts, D.J. Inhibition of Growth of Dictyostelium Discoideum Amoebae by Bisphosphonate Drugs Is Dependent on Cellular Uptake. Pharm. Res. 1997, 14, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.C.; Mönkkönen, J.; Auriola, S.; Mönkkönen, H.; Rogers, M.J. The Molecular Mechanism of Action of the Antiresorptive and Antiinflammatory Drug Clodronate: Evidence for the Formation in Vivo of a Metabolite That Inhibits Bone Resorption and Causes Osteoclast and Macrophage Apoptosis. Arthritis. Rheum. 2001, 44, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Lehenkari, P.P.; Kellinsalmi, M.; Näpänkangas, J.P.; Ylitalo, K.V.; Mönkkönen, J.; Rogers, M.J.; Azhayev, A.; Väänänen, H.K.; Hassinen, I.E. Further Insight into Mechanism of Action of Clodronate: Inhibition of Mitochondrial ADP/ATP Translocase by a Nonhydrolyzable, Adenine-Containing Metabolite. Mol. Pharmacol. 2002, 61, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, H.; Adamek, G.; Felix, R.; Fleisch, H.; Schenk, R.; Hagan, P. Structure-Activity Relationships of Various Bisphosphonates. Calcif. Tissue. Int. 1983, 35, 87–99. [Google Scholar] [CrossRef]
- Schenk, R.; Eggli, P.; Fleisch, H.; Rosini, S. Quantitative Morphometric Evaluation of the Inhibitory Activity of New Aminobisphosphonates on Bone Resorption in the Rat. Calcif. Tissue. Int. 1986, 38, 342–349. [Google Scholar] [CrossRef]
- Russell, R.G.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of Action of Bisphosphonates: Similarities and Differences and Their Potential Influence on Clinical Efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Amin, D.; Cornell, S.A.; Gustafson, S.K.; Needle, S.J.; Ullrich, J.W.; Bilder, G.E.; Perrone, M.H. Bisphosphonates Used for the Treatment of Bone Disorders Inhibit Squalene Synthase and Cholesterol Biosynthesis. J. Lipid. Res. 1992, 33, 1657–1663. [Google Scholar] [CrossRef]
- Amin, D.; Cornell, S.A.; Perrone, M.H.; Bilder, G.E. 1-Hydroxy-3-(Methylpentylamino)-Propylidene-1,1-Bisphosphonic Acid as a Potent Inhibitor of Squalene Synthase. Arzneimittelforschung 1996, 46, 759–762. [Google Scholar]
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Russell, R.G.G.; Rogers, M.J. Nitrogen-Containing Bisphosphonates Inhibit the Mevalonate Pathway and Prevent Post-Translational Prenylation of GTP-Binding Proteins, Including Ras. J. Bone Min. Res. 1998, 13, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.E.; Rogers, M.J.; Halasy, J.M.; Luckman, S.P.; Hughes, D.E.; Masarachia, P.J.; Wesolowski, G.; Russell, R.G.; Rodan, G.A.; Reszka, A.A. Alendronate Mechanism of Action: Geranylgeraniol, an Intermediate in the Mevalonate Pathway, Prevents Inhibition of Osteoclast Formation, Bone Resorption, and Kinase Activation in Vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, E.; Pieterman, E.; Cohen, L.; Löwik, C.; Papapoulos, S. Nitrogen-Containing Bisphosphonates Inhibit Isopentenyl Pyrophosphate Isomerase/Farnesyl Pyrophosphate Synthase Activity with Relative Potencies Corresponding to Their Antiresorptive Potenciesin Vitroandin Vivo. Biochem. Biophys. Res. Commun. 1999, 255, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Dunford, J.E.; Thompson, K.; Coxon, F.P.; Luckman, S.P.; Hahn, F.M.; Poulter, C.D.; Ebetino, F.H.; Rogers, M.J. Structure-Activity Relationships for Inhibition of Farnesyl Diphosphate Synthase in Vitro and Inhibition of Bone Resorption in Vivo by Nitrogen-Containing Bisphosphonates. J. Pharmacol. Exp. Ther. 2001, 296, 235–242. [Google Scholar]
- Kavanagh, K.L.; Guo, K.; Dunford, J.E.; Wu, X.; Knapp, S.; Ebetino, F.H.; Rogers, M.J.; Russell, R.G.G.; Oppermann, U. The Molecular Mechanism of Nitrogen-Containing Bisphosphonates as Antiosteoporosis Drugs. Proc. Natl. Acad. Sci. USA 2006, 103, 7829–7834. [Google Scholar] [CrossRef]
- Luckman, S.P.; Coxon, F.P.; Ebetino, F.H.; Russell, R.G.G.; Rogers, M.J. Heterocycle-Containing Bisphosphonates Cause Apoptosis and Inhibit Bone Resorption by Preventing Protein Prenylation: Evidence from Structure-Activity Relationships in J774 Macrophages. J. Bone. Min. Res. 1998, 13, 1668–1678. [Google Scholar] [CrossRef]
- Sahni, M.; Guenther, H.L.; Fleisch, H.; Collin, P.; Martin, T.J. Bisphosphonates Act on Rat Bone Resorption through the Mediation of Osteoblasts. J. Clin. Invest. 1993, 91, 2004–2011. [Google Scholar] [CrossRef]
- Nishikawa, M.; Akatsu, T.; Katayama, Y.; Yasutomo, Y.; Kado, S.; Kugai, N.; Yamamoto, M.; Nagata, N. Bisphosphonates Act on Osteoblastic Cells and Inhibit Osteoclast Formation in Mouse Marrow Cultures. Bone 1996, 18, 9–14. [Google Scholar] [CrossRef]
- Giuliani, N.; Pedrazzoni, M.; Passeri, G.; Girasole, G. Pass Bisphosphonates Inhibit IL-6 Production by Human Osteoblast-like Cells. Scand. J. Rheumatol. 1998, 27, 38–41. [Google Scholar] [CrossRef]
- D’Aoust, P.; McCulloch, C.A.G.; Tenenbaum, H.C.; Lekic, P.C. Etidronate (HEBP) Promotes Osteoblast Differentiation and Wound Closure in Rat Calvaria. Cell Tissue Res. 2000, 302, 353–363. [Google Scholar] [CrossRef]
- Itoh, F.; Aoyagi, S.; Furihata-Komatsu, H.; Aoki, M.; Kusama, H.; Kojima, M.; Kogo, H. Clodronate Stimulates Osteoblast Differentiation in ST2 and MC3T3-E1 Cells and Rat Organ Cultures. Eur. J. Pharmacol. 2003, 477, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Im, G.-I.; Qureshi, S.A.; Kenney, J.; Rubash, H.E.; Shanbhag, A.S. Osteoblast Proliferation and Maturation by Bisphosphonates. Biomaterials 2004, 25, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Plotkin, L.I. Novel Actions of Bisphosphonates in Bone: Preservation of Osteoblast and Osteocyte Viability. Bone 2011, 49, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Mori, K.; Orita, M.; Takeuchi, M. Computational Insights into Binding of Bisphosphates to Farnesyl Pyrophosphate Synthase. CMC 2011, 18, 220–233. [Google Scholar] [CrossRef]
- Rogers, M.J.; Mönkkönen, J.; Munoz, M.A. Molecular Mechanisms of Action of Bisphosphonates and New Insights into Their Effects Outside the Skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef]
- Ebetino, F.H.; Sun, S.; Cherian, P.; Roshandel, S.; Neighbors, J.D.; Hu, E.; Dunford, J.E.; Sedghizadeh, P.P.; McKenna, C.E.; Srinivasan, V.; et al. Bisphosphonates: The Role of Chemistry in Understanding Their Biological Actions and Structure-Activity Relationships, and New Directions for Their Therapeutic Use. Bone 2022, 156, 116289. [Google Scholar] [CrossRef]
- Varela, I.; Pereira, S.; Ugalde, A.P.; Navarro, C.L.; Suárez, M.F.; Cau, P.; Cadiñanos, J.; Osorio, F.G.; Foray, N.; Cobo, J.; et al. Combined Treatment with Statins and Aminobisphosphonates Extends Longevity in a Mouse Model of Human Premature Aging. Nat. Med. 2008, 14, 767–772. [Google Scholar] [CrossRef]
- Misra, J.; Mohanty, S.T.; Madan, S.; Fernandes, J.A.; Hal Ebetino, F.; Russell, R.G.G.; Bellantuono, I. Zoledronate Attenuates Accumulation of DNA Damage in Mesenchymal Stem Cells and Protects Their Function. Stem. Cells 2016, 34, 756–767. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Massaro, J.; D’Agostino, R.B.; Shappell, H.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.H.; Nazarian, A.; et al. Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children With Hutchinson-Gilford Progeria Syndrome. Circulation 2016, 134, 114–125. [Google Scholar] [CrossRef]
- Eidtmann, H.; De Boer, R.; Bundred, N.; Llombart-Cussac, A.; Davidson, N.; Neven, P.; Von Minckwitz, G.; Miller, J.; Schenk, N.; Coleman, R. Efficacy of Zoledronic Acid in Postmenopausal Women with Early Breast Cancer Receiving Adjuvant Letrozole: 36-Month Results of the ZO-FAST Study. Ann. Oncol. 2010, 21, 2188–2194. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Heck, D.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Pristauz, G.; et al. Adjuvant Endocrine Therapy plus Zoledronic Acid in Premenopausal Women with Early-Stage Breast Cancer: 62-Month Follow-up from the ABCSG-12 Randomised Trial. Lancet Oncol. 2011, 12, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M.; Harker, W.G.; Beck, J.T.; Bosserman, L.; Vogel, C.; Seidler, C.; Jin, L.; Warsi, G.; Argonza-Aviles, E.; Hohneker, J.; et al. Final 5-Year Results of Z-FAST Trial: Adjuvant Zoledronic Acid Maintains Bone Mass in Postmenopausal Breast Cancer Patients Receiving Letrozole. Cancer 2012, 118, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Friedl, T.W.P.; Fehm, T.; Müller, V.; Lichtenegger, W.; Blohmer, J.; Lorenz, R.; Forstbauer, H.; Fink, V.; Bekes, I.; Huober, J.; et al. Prognosis of Patients With Early Breast Cancer Receiving 5 Years vs 2 Years of Adjuvant Bisphosphonate Treatment: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1149. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Adjuvant Bisphosphonate Treatment in Early Breast Cancer: Meta-Analyses of Individual Patient Data from Randomised Trials. Lancet 2015, 386, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Adjuvant Bone-Targeted Therapy to Prevent Metastasis: Lessons from the AZURE Study. Curr. Opin. Support. Palliat. Care 2012, 6, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Brown, J.; Holen, I. Bone Metastases. In Abeloff’s Clinical. Oncology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 809–830.e3. ISBN 978-0-323-47674-4. [Google Scholar]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Coleman, R.E. Skeletal Complications of Malignancy. Cancer 1997, 80, 1588–1594. [Google Scholar] [CrossRef]
- Czernin, J.; Satyamurthy, N.; Schiepers, C. Molecular Mechanisms of Bone 18 F-NaF Deposition. J. Nucl. Med. 2010, 51, 1826–1829. [Google Scholar] [CrossRef]
- Even-Sapir, E.; Metser, U.; Flusser, G.; Zuriel, L.; Kollender, Y.; Lerman, H.; Lievshitz, G.; Ron, I.; Mishani, E. Assessment of Malignant Skeletal Disease: Initial Experience with 18F-Fluoride PET/CT and Comparison between 18F-Fluoride PET and 18F-Fluoride PET/CT. J. Nucl. Med. 2004, 45, 272–278. [Google Scholar]
- Withofs, N.; Grayet, B.; Tancredi, T.; Rorive, A.; Mella, C.; Giacomelli, F.; Mievis, F.; Aerts, J.; Waltregny, D.; Jerusalem, G.; et al. 18F-Fluoride PET/CT for Assessing Bone Involvement in Prostate and Breast Cancers. Nucl. Med. Commun. 2011, 32, 168–176. [Google Scholar] [CrossRef]
- Damle, N.A.; Bal, C.; Bandopadhyaya, G.P.; Kumar, L.; Kumar, P.; Malhotra, A.; Lata, S. The Role of 18F-Fluoride PET-CT in the Detection of Bone Metastases in Patients with Breast, Lung and Prostate Carcinoma: A Comparison with FDG PET/CT and 99mTc-MDP Bone Scan. Jpn. J. Radiol. 2013, 31, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Lindenberg, L.; Shih, J.H.; Mena, E.; Kim, J.W.; Park, J.C.; Alikhani, A.; McKinney, Y.Y.; Weaver, J.; Turkbey, B.; et al. Prospective Study Evaluating Na 18F PET/CT in Predicting Clinical Outcomes and Survival in Advanced Prostate Cancer. J. Nucl. Med. 2016, 57, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.B. Teletherapy and Radiopharmaceutical Therapy of Painful Bone Metastases. Semin. Nucl. Med. 2005, 35, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.B. The Treatment of Painful Osseous Metastases with Phosphorus-32-Labeled Phosphates. Semin. Oncol. 1993, 20, 10–21. [Google Scholar] [PubMed]
- Silberstein, E.B.; Elgazzar, A.H.; Kapilivsky, A. Phosphorus-32 Radiopharmaceuticals for the Treatment of Painful Osseous Metastases. Semin. Nucl. Med. 1992, 22, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, E.B.; Williams, C. Strontium-89 Therapy for the Pain of Osseous Metastases. J. Nucl. Med. 1985, 26, 345–348. [Google Scholar] [PubMed]
- Porter, A.T.; McEwan, A.J.B.; Powe, J.E.; Reid, R.; McGowan, D.G.; Lukka, H.; Sathyanarayana, J.R.; Yakemchuk, V.N.; Thomas, G.M.; Erlich, L.E.; et al. Results of a Randomized Phase-III Trial to Evaluate the Efficacy of Strontium-89 Adjuvant to Local Field External Beam Irradiation in the Management of Endocrine Resistant Metastatic Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 805–813. [Google Scholar] [CrossRef]
- Smeland, S.; Erikstein, B.; Aas, M.; Skovlund, E.; Hess, S.L.; Fosså, S.D. Role of Strontium-89 as Adjuvant to Palliative External Beam Radiotherapy Is Questionable: Results of a Double-Blind Randomized Study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1397–1404. [Google Scholar] [CrossRef]
- Oosterhof, G.O.N.; Roberts, J.T.; De Reijke, T.M.; Engelholm, S.A.; Horenblas, S.; Von Der Maase, H.; Neymark, N.; Debois, M.; Collette, L. Strontium-89 Chloride versus Palliative Local Field Radiotherapy in Patients with Hormonal Escaped Prostate Cancer: A Phase III Study of the European Organisation for Research and Treatment of Cancer Genitourinary Group. Eur. Urol. 2003, 44, 519–526. [Google Scholar] [CrossRef]
- Tu, S.-M.; Millikan, R.E.; Mengistu, B.; Delpassand, E.S.; Amato, R.J.; Pagliaro, L.C.; Daliani, D.; Papandreou, C.N.; Smith, T.L.; Kim, J.; et al. Bone-Targeted Therapy for Advanced Androgen-Independent Carcinoma of the Prostate: A Randomised Phase II Trial. Lancet 2001, 357, 336–341. [Google Scholar] [CrossRef]
- James, N.D.; Pirrie, S.J.; Pope, A.M.; Barton, D.; Andronis, L.; Goranitis, I.; Collins, S.; Daunton, A.; McLaren, D.; O’Sullivan, J.; et al. Clinical Outcomes and Survival Following Treatment of Metastatic Castrate-Refractory Prostate Cancer with Docetaxel Alone or With Strontium-89, Zoledronic Acid, or Both: The TRAPEZE Randomized Clinical Trial. JAMA Oncol. 2016, 2, 493. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; McAfee, J.G.; Blair, R.J.; Kallfelz, F.A.; Thomas, F.D. Technetium-99m-Methylene Diphosphonate--a Superior Agent for Skeletal Imaging: Comparison with Other Technetium Complexes. J. Nucl. Med. 1975, 16, 744–755. [Google Scholar]
- Bevan, J.A.; Tofe, A.J.; Benedict, J.J.; Francis, M.D.; Barnett, B.L. Tc-99m HMDP (Hydroxymethylene Diphosphonate): A Radiopharmaceutical for Skeletal and Acute Myocardial Infarct Imaging. I. Synthesis and Distribution in Animals. J. Nucl. Med. 1980, 21, 961–966. [Google Scholar] [PubMed]
- Domstad, P.A.; Coupal, J.J.; Kim, E.E.; Blake, J.S.; DeLand, F.H. 99mTc-Hydroxymethane Diphosphonate: A New Bone Imaging Agent with a Low Tin Content. Radiology 1980, 136, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Castronovo, F.P.; Callahan, R.J. New Bone Scanning Agent: 99mTc-Labeled 1-Hydroxy-Ethylidene-1, 1-Disodium Phosphonate. J. Nucl. Med. 1972, 13, 823–827. [Google Scholar] [PubMed]
- Subramanian, G.; McAfee, J.G.; Blair, R.J.; Mehter, A.; Connor, T. 99m Tc-EHDP: A Potential Radiopharmaceutical for Skeletal Imaging. J. Nucl. Med. 1972, 13, 947–950. [Google Scholar]
- Yano, Y.; McRae, J.; Van Dyke, D.C.; Anger, H.O. Technetium-99m-Labeled Stannous Ethane-1-Hydroxy-1 1-Diphosphonate: A New Bone Scanning Agent. J. Nucl. Med. 1973, 14, 73–78. [Google Scholar]
- Pendergrass, H.P.; Potsaid, M.S.; Castronovo, F.P. The Clinical Use of 99m Tc-Diphosphonate (HEDSPA). A New Agent for Skeletal Imaging. Radiology 1973, 107, 557–562. [Google Scholar] [CrossRef]
- Wilson, G.M.; Pinkerton, T.C. Determination of Charge and Size of Technetium Diphosphonate Complexes by Anion-Exchange Liquid Chromatography. Anal. Chem. 1985, 57, 246–253. [Google Scholar] [CrossRef]
- Tanabe, S.; Zodda, J.P.; Deutsch, E.; Heineman, W.R. Effect of pH on the Formation of Tc(NaBH4)-MDP Radiopharmaceutical Analogues. Int. J. Appl. Radiat. Isot. 1983, 34, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Libson, K.; Deutsch, E.; Barnett, B.L. Structural Characterization of a Technetium-99-Diphosphonate Complex. Implications for the Chemistry of Technetium-99m Skeletal Imaging Agents. J. Am. Chem. Soc. 1980, 102, 2476–2478. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, R.; Singh, H.; Bal, C.; Julka, P.K.; Thulkar, S.; Malhotra, A. Indeterminate Lesions on Planar Bone Scintigraphy in Lung Cancer Patients: SPECT, CT or SPECT-CT? Skelet. Radiol. 2012, 41, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Chen, J.-H.; Liang, J.-A.; Lin, C.-C.; Yang, K.-T.; Cheng, K.-Y.; Yeh, J.-J.; Kao, C.-H. Meta-Analysis: Comparison of F-18 Fluorodeoxyglucose-Positron Emission Tomography and Bone Scintigraphy in the Detection of Bone Metastasis in Patients with Lung Cancer. Acad. Radiol. 2012, 19, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Furuta, T.; Sakuda, T.; Ochi, M.; Adachi, N. Conventional 99mTc-(Hydroxy) Methylene Diphosphate Remains Useful to Predict Osteosarcoma Response to Neoadjuvant Chemotherapy: Individual Patient Data and Aggregate Data Meta-Analyses. Medicine 2018, 97, e13308. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Byun, B.H.; Lim, I.; Kim, B.I.; Kong, C.-B.; Song, W.S.; Cho, W.H.; Koh, J.-S.; Lim, S.M. Comparison of 99mTc-Methyl Diphosphonate Bone Scintigraphy and 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography to Predict Histologic Response to Neoadjuvant Chemotherapy in Patients with Osteosarcoma. Medicine 2018, 97, e12318. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.R.; Jia, X.; Mezheritskiy, I.S.; Stephenson, R.D.; Schoder, H.; Fox, J.J.; Heller, G.; Scher, H.I.; Larson, S.M.; Morris, M.J. Bone Scan Index: A Quantitative Treatment Response Biomarker for Castration-Resistant Metastatic Prostate Cancer. J. Clin. Oncol. 2012, 30, 519–524. [Google Scholar] [CrossRef]
- Rubini, G.; Lauriero, F.; Rubini, D.; D’Addabbo, A. 99Tcm-MDP Global Skeletal Uptake and Markers of Bone Metabolism in Patients with Bone Diseases. Nucl. Med. Commun. 1993, 14, 567–572. [Google Scholar] [CrossRef]
- Griffith, K.; Pearson, D.; Parker, C.; Thorpe, S.; Vincent, R.M.; Hosking, D.J. The Use of a Whole Body Index with Bone Scintigraphy to Monitor the Response to Therapy in Paget’s Disease. Nucl. Med. Commun. 2001, 22, 1069–1075. [Google Scholar] [CrossRef]
- Israel, O.; Front, D.; Hardoff, R.; Ish-Shalom, S.; Jerushalmi, J.; Kolodny, G.M. In Vivo SPECT Quantitation of Bone Metabolism in Hyperparathyroidism and Thyrotoxicosis. J. Nucl. Med. 1991, 32, 1157–1161. [Google Scholar]
- Brenner, A.I.; Koshy, J.; Morey, J.; Lin, C.; DiPoce, J. The Bone Scan. Semin. Nucl. Med. 2012, 42, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Kloss, G. Technetium-99m DPD—A New Skeletal Imaging Agent. J. Nucl. Med. 1981, 22, P77. [Google Scholar]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.T.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Quarta, C.C.; Guidalotti, P.L.; Longhi, S.; Pettinato, C.; Leone, O.; Ferlini, A.; Salvi, F.; Gallo, P.; Gagliardi, C.; et al. Usefulness and Limitations of 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy in the Aetiological Diagnosis of Amyloidotic Cardiomyopathy. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.C.; Zheng, J.; Hutt, D.; Grigore, S.F.; Manwani, R.; Sachchithanantham, S.; Mahmood, S.A.; Whelan, C.J.; Fontana, M.; Martinez-Naharro, A.; et al. 99mTc-DPD Scintigraphy in Immunoglobulin Light Chain (AL) Cardiac Amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1304–1311. [Google Scholar] [CrossRef]
- Turner, J.H.; Martindale, A.A.; Sorby, P.; Hetherington, E.L.; Fleay, R.F.; Hoffman, R.F.; Claringbold, P.G. Samarium-153 EDTMP Therapy of Disseminated Skeletal Metastasis. Eur. J. Nucl. Med. 1989, 15, 784–795. [Google Scholar] [CrossRef]
- Turner, J.H.; Claringbold, P.G.; Hetherington, E.L.; Sorby, P.; Martindale, A.A. A Phase I Study of Samarium-153 Ethylenediaminetetramethylene Phosphonate Therapy for Disseminated Skeletal Metastases. J. Clin. Oncol. 1989, 7, 1926–1931. [Google Scholar] [CrossRef]
- Serafini, A.N.; Houston, S.J.; Resche, I.; Quick, D.P.; Grund, F.M.; Ell, P.J.; Bertrand, A.; Ahmann, F.R.; Orihuela, E.; Reid, R.H.; et al. Palliation of Pain Associated with Metastatic Bone Cancer Using Samarium-153 Lexidronam: A Double-Blind Placebo-Controlled Clinical Trial. JCO 1998, 16, 1574–1581. [Google Scholar] [CrossRef]
- Sartor, O.; Reid, R.H.; Hoskin, P.J.; Quick, D.P.; Ell, P.J.; Coleman, R.E.; Kotler, J.A.; Freeman, L.M.; Olivier, P. Samarium-153-Lexidronam Complex for Treatment of Painful Bone Metastases in Hormone-Refractory Prostate Cancer. Urology 2004, 63, 940–945. [Google Scholar] [CrossRef]
- Verbeke, K.; Rozenski, J.; Cleynhens, B.; Vanbilloen, H.; De Groot, T.; Weyns, N.; Bormans, G.; Verbruggen, A. Development of a Conjugate of 99m Tc-EC with Aminomethylenediphosphonate in the Search for a Bone Tracer with Fast Clearance from Soft Tissue. Bioconjug. Chem. 2002, 13, 16–22. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Inoue, Y.; Ono, M.; Saji, H. Development of a Novel 99mTc-Chelate-Conjugated Bisphosphonate with High Affinity for Bone as a Bone Scintigraphic Agent. J. Nucl. Med. 2006, 47, 2042–2047. [Google Scholar] [PubMed]
- Ogawa, K.; Mukai, T.; Kawai, K.; Takamura, N.; Hanaoka, H.; Hashimoto, K.; Shiba, K.; Mori, H.; Saji, H. Usefulness of Competitive Inhibitors of Protein Binding for Improving the Pharmacokinetics of 186Re-MAG3-Conjugated Bisphosphonate (186Re-MAG3-HBP), an Agent for Treatment of Painful Bone Metastases. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhong, G.; Wei, Y.; Zhang, M.; Wang, X. Synthesis and Biological Evaluation of a Novel 99mTc Complex of HYNIC-Conjugated Aminomethylenediphosphonate as a Potential Bone Imaging Agent. J. Radioanal. Nucl. Chem. 2011, 288, 467–473. [Google Scholar] [CrossRef]
- Yazdani, A.; Bilton, H.; Vito, A.; Genady, A.R.; Rathmann, S.M.; Ahmad, Z.; Janzen, N.; Czorny, S.; Zeglis, B.M.; Francesconi, L.C.; et al. A Bone-Seeking Trans-Cyclooctene for Pretargeting and Bioorthogonal Chemistry: A Proof of Concept Study Using 99mTc- and 177Lu-Labeled Tetrazines. J. Med. Chem. 2016, 59, 9381–9389. [Google Scholar] [CrossRef]
- Palma, E.; Oliveira, B.L.; Correia, J.D.G.; Gano, L.; Maria, L.; Santos, I.C.; Santos, I. A New Bisphosphonate-Containing (99m)Tc(I) Tricarbonyl Complex Potentially Useful as Bone-Seeking Agent: Synthesis and Biological Evaluation. J. Biol. Inorg. Chem. 2007, 12, 667–679. [Google Scholar] [CrossRef]
- Palma, E.; Correia, J.D.G.; Oliveira, B.L.; Gano, L.; Santos, I.C.; Santos, I. 99mTc(CO)3-Labeled Pamidronate and Alendronate for Bone Imaging. Dalton Trans. 2011, 40, 2787–2796. [Google Scholar] [CrossRef]
- Torres Martin de Rosales, R.; Finucane, C.; Foster, J.; Mather, S.J.; Blower, P.J. 188Re(CO)3-Dipicolylamine-Alendronate: A New Bisphosphonate Conjugate for the Radiotherapy of Bone Metastases. Bioconjug. Chem. 2010, 21, 811–815. [Google Scholar] [CrossRef]
- Keeling, G.P.; Sherin, B.; Kim, J.; San Juan, B.; Grus, T.; Eykyn, T.R.; Rösch, F.; Smith, G.E.; Blower, P.J.; Terry, S.Y.A.; et al. [68Ga]Ga-THP-Pam: A Bisphosphonate PET Tracer with Facile Radiolabeling and Broad Calcium Mineral Affinity. Bioconjug. Chem. 2021, 32, 1276–1289. [Google Scholar] [CrossRef]
- Hong, H.; Ploessl, K.; Zha, Z.; Wang, H.; Guo, R.; Xie, Q.; Zhu, H.; Yang, Z.; Zhu, L.; Kung, H.F. Development and Validation of a Kit Formulation of [68Ga]Ga-P15-041 as a Bone Imaging Agent. Appl. Radiat. Isot. 2021, 169, 109485. [Google Scholar] [CrossRef]
- Zha, Z.; Wu, Z.; Choi, S.R.; Ploessl, K.; Smith, M.; Alexoff, D.; Zhu, L.; Kung, H.F. A New [68Ga]Ga-HBED-CC-Bisphosphonate as a Bone Imaging Agent. Mol. Pharm. 2020, 17, 1674–1684. [Google Scholar] [CrossRef]
- Alexoff, D.; Doot, R.; Pryma, D.; Schubert, E.; Wu, Z.; Zha, Z.; Choi, S.; Ploessl, K.; Lee, S.; Kao, C.; et al. Preliminary Kinetic Analysis of [68Ga]P15-041, a Novel 68Ga Labeled Bisphosphonate, from First-in-Human Studies. J. Nucl. Med. 2017, 58, 388. [Google Scholar]
- Doot, R.K.; Young, A.J.; Daube-Witherspoon, M.E.; Alexoff, D.; Labban, K.J.; Lee, H.; Wu, Z.; Zha, Z.; Choi, S.R.; Ploessl, K.H.; et al. Biodistribution, Dosimetry, and Temporal Signal-to-Noise Ratio Analyses of Normal and Cancer Uptake of [68Ga]Ga-P15-041, a Gallium-68 Labeled Bisphosphonate, from First-in-Human Studies. Nucl. Med. Biol. 2020, 86, 1–8. [Google Scholar] [CrossRef]
- Guo, R.; Meng, X.; Wang, F.; Yu, J.; Xie, Q.; Zhao, W.; Zhu, L.; Kung, H.F.; Yang, Z.; Li, N. 68Ga-P15-041, A Novel Bone Imaging Agent for Diagnosis of Bone Metastases. Front. Oncol. 2021, 11, 766851. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Satake, M.; Suwada, J.; Oshikiri, S.; Ashino, H.; Dozono, H.; Hino, A.; Kasahara, H.; Minamizawa, T. Synthesis and Evaluation of a Novel 68Ga-Chelate-Conjugated Bisphosphonate as a Bone-Seeking Agent for PET Imaging. Nucl. Med. Biol. 2011, 38, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Ashhar, Z.; Yusof, N.A.; Ahmad Saad, F.F.; Mohd Nor, S.M.; Mohammad, F.; Bahrin Wan Kamal, W.H.; Hassan, M.H.; Ahmad Hassali, H.; Al-Lohedan, H.A. Preparation, Characterization, and Radiolabeling of [68Ga]Ga-NODAGA-Pamidronic Acid: A Potential PET Bone Imaging Agent. Molecules 2020, 25, 2668. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.; Meckel, M.; Kubíček, V.; Rösch, F.; Hermann, P. Gallium(III) Complexes of NOTA-Bis (Phosphonate) Conjugates as PET Radiotracers for Bone Imaging. Contrast Media. Mol. Imaging 2015, 10, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Passah, A.; Tripathi, M.; Ballal, S.; Yadav, M.P.; Kumar, R.; Roesch, F.; Meckel, M.; Sarathi Chakraborty, P.; Bal, C. Evaluation of Bone-Seeking Novel Radiotracer 68Ga-NO2AP-Bisphosphonate for the Detection of Skeletal Metastases in Carcinoma Breast. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Pfannkuchen, N.; Bergmann, R.; Pietzsch, J.; Bachmann, M.; Roesch, F. DOTAZOL and NODAGAZOL for Theranostics of Bone Metastases. J. Nucl. Med. 2017, 58, 324. [Google Scholar]
- Lawal, I.; Ebenhan, T.; Mahapane, J.; Vorster, M.; Mokoala, K.; Meckel, M.; Rosch, F.; Sathekge, M. Assessment of Skeletal Metastasis in Prostate Cancer Staging: An Intra-Individual Comparison of 68Ga-PSMA PET/CT, 68Ga-NODAGA-Zoledronate PET/CT, and 99mTc-MDP Bone Scan. J. Nucl. Med. 2020, 61, 1256. [Google Scholar]
- Lawal, I.O.; Mokoala, K.M.G.; Mahapane, J.; Kleyhans, J.; Meckel, M.; Vorster, M.; Ebenhan, T.; Rösch, F.; Sathekge, M.M. A Prospective Intra-Individual Comparison of [68Ga]Ga-PSMA-11 PET/CT, [68Ga]Ga-NODAGAZOL PET/CT, and [99mTc]Tc-MDP Bone Scintigraphy for Radionuclide Imaging of Prostate Cancer Skeletal Metastases. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 134–142. [Google Scholar] [CrossRef]
- Ndlovu, H.; Lawal, I.; Popoola, G.; Brits, B.; Mokoala, K.; Mahapane, J.; Davis, C.; Sathekge, M. PET Imaging of Atherosclerotic Plaque Calcification with [68Ga]Ga-NODAGAZOL: Correlation of Uptake with Cardiovascular Risk Profile of Patients. J. Nucl. Med. 2022, 63, 2222. [Google Scholar]
- Ndlovu, H.; Lawal, I.O.; Popoola, G.O.; Brits, B.; Mokoala, K.M.G.; Maserumule, L.C.; Hlongwa, K.N.; Mahapane, J.; Davis, C.; Sathekge, M.M. [68Ga]Ga-NODAGAZOL Uptake in Atherosclerotic Plaques Correlates with the Cardiovascular Risk Profile of Patients. Ann. Nucl. Med. 2022, 36, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Fersing, C.; Bouhlel, A.; Cantelli, C.; Garrigue, P.; Lisowski, V.; Guillet, B. A Comprehensive Review of Non-Covalent Radiofluorination Approaches Using Aluminum [18F]Fluoride: Will [18F]AlF Replace 68Ga for Metal Chelate Labeling? Molecules 2019, 24, 2866. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Othman, M.F.; Abdul Razak, H.R.; Zakaria, Z.A.; Ahmad Saad, F.F.; Osman, M.A.; Yi, L.H.; Ashhar, Z.; Idris, J.; Abdul Hamid, M.H.N.; et al. Preparation, Optimisation, and In Vitro Evaluation of [18F]AlF-NOTA-Pamidronic Acid for Bone Imaging PET. Molecules 2022, 27, 7969. [Google Scholar] [CrossRef] [PubMed]
- Fersing, C.; Masurier, N.; Rubira, L.; Deshayes, E.; Lisowski, V. AAZTA-Derived Chelators for the Design of Innovative Radiopharmaceuticals with Theranostic Applications. Pharmaceuticals 2022, 15, 234. [Google Scholar] [CrossRef]
- Wu, Z.; Zha, Z.; Choi, S.R.; Plössl, K.; Zhu, L.; Kung, H.F. New 68Ga-PhenA Bisphosphonates as Potential Bone Imaging Agents. Nucl. Med. Biol. 2016, 43, 360–371. [Google Scholar] [CrossRef]
- Kubícek, V.; Rudovský, J.; Kotek, J.; Hermann, P.; Vander Elst, L.; Muller, R.N.; Kolar, Z.I.; Wolterbeek, H.T.; Peters, J.A.; Lukes, I. A Bisphosphonate Monoamide Analogue of DOTA: A Potential Agent for Bone Targeting. J. Am. Chem. Soc. 2005, 127, 16477–16485. [Google Scholar] [CrossRef]
- Fellner, M.; Biesalski, B.; Bausbacher, N.; Kubícek, V.; Hermann, P.; Rösch, F.; Thews, O. 68Ga-BPAMD: PET-Imaging of Bone Metastases with a Generator Based Positron Emitter. Nucl. Med. Biol. 2012, 39, 993–999. [Google Scholar] [CrossRef]
- Fellner, M.; Baum, R.P.; Kubícek, V.; Hermann, P.; Lukes, I.; Prasad, V.; Rösch, F. PET/CT Imaging of Osteoblastic Bone Metastases with 68Ga-Bisphosphonates: First Human Study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 834. [Google Scholar] [CrossRef]
- Mueller, D.; Klette, I.; Baum, R. Clinical Routine Production of 177Lu-BPAMD. J. Nucl. Med. 2013, 54, 1191. [Google Scholar]
- Meckel, M.; Nauth, A.; Timpe, J.; Zhernosekov, K.; Puranik, A.D.; Baum, R.P.; Rösch, F. Development of a [177Lu]BPAMD Labeling Kit and an Automated Synthesis Module for Routine Bone Targeted Endoradiotherapy. Cancer Biother. Radiopharm. 2015, 30, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, R.; Meckel, M.; Kubíček, V.; Pietzsch, J.; Steinbach, J.; Hermann, P.; Rösch, F. 177Lu-Labelled Macrocyclic Bisphosphonates for Targeting Bone Metastasis in Cancer Treatment. EJNMMI Res. 2016, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Fellner, M.; Baum, R.; Kubicek, V.; Hermann, P.; Roesch, F. 177Lu-BPAMD—From Bone Imaging to Therapy with a Macrocycle-Bisphosphonate Ligand. J. Nucl. Med. 2010, 51, 1164. [Google Scholar]
- Pfannkuchen, N.; Meckel, M.; Bergmann, R.; Bachmann, M.; Bal, C.; Sathekge, M.; Mohnike, W.; Baum, R.P.; Rösch, F. Novel Radiolabeled Bisphosphonates for PET Diagnosis and Endoradiotherapy of Bone Metastases. Pharmaceuticals 2017, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Yousefnia, H.; Zolghadri, S.; Sadeghi, H.R.; Naderi, M.; Jalilian, A.R.; Shanehsazzadeh, S. Preparation and Biological Assessment of 177Lu-BPAMD as a High Potential Agent for Bone Pain Palliation Therapy: Comparison with 177Lu-EDTMP. J. Radioanal. Nucl. Chem. 2016, 307, 1243–1251. [Google Scholar] [CrossRef]
- Meckel, M.; Kubíček, V.; Hermann, P.; Miederer, M.; Rösch, F. A DOTA Based Bisphosphonate with an Albumin Binding Moiety for Delayed Body Clearance for Bone Targeting. Nucl. Med. Biol. 2016, 43, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Prospective of 68Ga-Radiopharmaceutical Development. Theranostics 2013, 4, 47–80. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Chen, Z.; Yang, J.; Liu, H.; Peng, D.; Lei, L.; Liu, L.; Wang, L.; Xing, N.; et al. Preparation, Biological Characterization and Preliminary Human Imaging Studies of 68Ga-DOTA-IBA. Front. Oncol. 2022, 12, 1027792. [Google Scholar] [CrossRef]
- Yang, J.; Deng, J.; Fan, D.; Chen, G.; Lu, Z.; Liu, H.; Mok, G.S.P.; Chen, Y. Biodistribution and Internal Dosimetry of 68Ga-DOTA-IBA PET Imaging for Patients with Bone Metastases. Clin. Nucl. Med. 2023, 48, 847–852. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Wang, Y.; Liu, H.; Feng, Y.; Qiu, L.; Chen, Y. Lutetium177-Labeled DOTA-Ibandronate: A Novel Radiopharmaceutical for Targeted Treatment of Bone Metastases. Mol. Pharm. 2023, 20, 1788–1795. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Y.; Liu, H.; Wang, Q.; Chen, L.; Liu, L.; Wang, L.; Feng, Y.; Chen, Y. Safety and Efficacy of 68Ga- or 177Lu-Labeled DOTA-IBA as a Novel Theranostic Radiopharmaceutical for Bone Metastases: A Phase 0/I Study. Clin. Nucl. Med. 2023, 48, 489. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, T.; Hua, Q.; Wang, L.; Chen, Y. 177Lu-DOTA-IBA Therapy in Prostate Cancer With Bone Metastases. Clin. Nucl. Med. 2023, 48, 740. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Qu, G.; Liu, G.; Wang, L.; Chen, Y. A New Radiopharmaceutical 225Ac-DOTA-IBA in the Treatment of a Case of Bone Metastases. Clin. Nucl. Med. 2023, 48, 650. [Google Scholar] [CrossRef] [PubMed]
- Meckel, M.; Bergmann, R.; Miederer, M.; Roesch, F. Bone Targeting Compounds for Radiotherapy and Imaging: *Me(III)-DOTA Conjugates of Bisphosphonic Acid, Pamidronic Acid and Zoledronic Acid. EJNMMI Radiopharm. Chem. 2017, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kawashima, H.; Shiba, K.; Washiyama, K.; Yoshimoto, M.; Kiyono, Y.; Ueda, M.; Mori, H.; Saji, H. Development of [(90)Y]DOTA-Conjugated Bisphosphonate for Treatment of Painful Bone Metastases. Nucl. Med. Biol. 2009, 36, 129–135. [Google Scholar] [CrossRef]
- Ogawa, K.; Takai, K.; Kanbara, H.; Kiwada, T.; Kitamura, Y.; Shiba, K.; Odani, A. Preparation and Evaluation of a Radiogallium Complex-Conjugated Bisphosphonate as a Bone Scintigraphy Agent. Nucl. Med. Biol. 2011, 38, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Jalilian, A.R.; Johari-Daha, F.; Shafiee-Ardestani, M.; Khalaj, A. Preparation and Biological Study of 68Ga-DOTA-Alendronate. Asia. Ocean J. Nucl. Med. Biol. 2016, 4, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, B.J.; Li, L.; Ciminera, A.K.; Chea, J.; Poku, E.; Bading, J.R.; Weist, M.R.; Miller, M.M.; Colcher, D.M.; Shively, J.E. Diagnostic PET Imaging of Mammary Microcalcifications Using 64 Cu-DOTA-Alendronate in a Rat Model of Breast Cancer. J. Nucl. Med. 2017, 58, 1373–1379. [Google Scholar] [CrossRef]
- Ballinger, J.R. 68Ga-DOTA-Zoledronate. In PET Radiopharmaceuticals; Springer International Publishing: Cham, Switzerland, 2022; pp. 62–63. ISBN 978-3-031-10270-7. [Google Scholar]
- Grun, A.; Kovacs, R.; Nagy, D.I.; Garadnay, S.; Greiner, I.; Keglevich, G. Efficient Synthesis of Benzidronate Applying of Phosphorus Trichloride and Phosphorous Acid. Lett. Drug. Des. Discov. 2015, 12, 78–84. [Google Scholar] [CrossRef]
- Grus, T.; Lahnif, H.; Klasen, B.; Moon, E.-S.; Greifenstein, L.; Roesch, F. Squaric Acid-Based Radiopharmaceuticals for Tumor Imaging and Therapy. Bioconjug. Chem. 2021, 32, 1223–1231. [Google Scholar] [CrossRef]
- Greifenstein, L.; Engelbogen, N.; Máthé, D.; Grus, T.; Rösch, F.; Bergmann, R. Squaric Acid Bisphposphonates for Theranostics of Bone Metastasis—The Easy DOTA-Zoledronate. Front. Nucl. Med. 2022, 2, 870910. [Google Scholar] [CrossRef]
- Khawar, A.; Eppard, E.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Preliminary Results of Biodistribution and Dosimetric Analysis of [68Ga]Ga-DOTAZOL: A New Zoledronate-Based Bisphosphonate for PET/CT Diagnosis of Bone Diseases. Ann. Nucl. Med. 2019, 33, 404–413. [Google Scholar] [CrossRef]
- Meisenheimer, M.; Kürpig, S.; Essler, M.; Eppard, E. DOTA-ZOL: A Promising Tool in Diagnosis and Palliative Therapy of Bone Metastasis-Challenges and Critical Points in Implementation into Clinical Routine. Molecules 2020, 25, 2988. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.A.; Chobisa, D.; Urimi, D.; Ravindra, N. Pharmaceutical Glass Interactions: A Review of Possibilities. J. Pharm. Sci. 2016, 8, 103–111. [Google Scholar]
- Meckel, M.; Fellner, M.; Thieme, N.; Bergmann, R.; Kubicek, V.; Rösch, F. In Vivo Comparison of DOTA Based 68Ga-Labelled Bisphosphonates for Bone Imaging in Non-Tumour Models. Nucl. Med. Biol. 2013, 40, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Eppard, E.; Meisenheimer, M.; Fuente, A.D.L.; Kurpig, S.; Essler, M.; Roesch, F. Radiolabelling of DOTAMZOL with 68Ga and 44Sc for Clinical Application. EJEA 2016, 47, OC34. [Google Scholar] [CrossRef]
- Khawar, A.; Eppard, E.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Biodistribution and Post-Therapy Dosimetric Analysis of [177Lu]Lu-DOTAZOL in Patients with Osteoblastic Metastases: First Results. EJNMMI Res. 2019, 9, 102. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Meckel, M.; Roesch, F.; Bal, C. [177Lu]Lu-DOTA-ZOL Bone Pain Palliation in Patients with Skeletal Metastases from Various Cancers: Efficacy and Safety Results. EJNMMI Res. 2020, 10, 130. [Google Scholar] [CrossRef]
- Kreppel, B.; Gaertner, F.C.; Ahmadzadehfar, H.; Khawar, A.; Roesch, F.; Kürpig, S.; Meisenheimer, M.; Essler, M.; Bundschuh, R.A. [177Lu]Lu-DOTA-Zoledronate Therapy—First Application in a Patient with Primary Osseous Metastatic Bronchial Carcinoma. Nuklearmedizin 2020, 59, 281–283. [Google Scholar] [CrossRef]
- Clarke, E.T.; Martell, A.E. Stabilities of Trivalent Metal Ion Complexes of the Tetraacetate Derivatives of 12-, 13- and 14-Membered Tetraazamacrocycles. Inorganica Chim. Acta. 1991, 190, 37–46. [Google Scholar] [CrossRef]
- Kubíček, V.; Havlíčková, J.; Kotek, J.; Tircsó, G.; Hermann, P.; Tóth, É.; Lukeš, I. Gallium(III) Complexes of DOTA and DOTA−Monoamide: Kinetic and Thermodynamic Studies. Inorg. Chem. 2010, 49, 10960–10969. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, K.; Bakar, H.E.; Kilbas, B. Novel Developed HPLC Analyses of [68Ga]Ga/[177Lu]Lu-EDTMP and [68Ga]Ga/[177Lu]Lu-DOTA-Zoledronate. J. Label Comp. Radiopharm. 2022, 65, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Pfannkuchen, N.; Bausbacher, N.; Pektor, S.; Miederer, M.; Rosch, F. In Vivo Evaluation of [225Ac]Ac-DOTAZOL for α-Therapy of Bone Metastases. Curr. Radiopharm. 2018, 11, 223–230. [Google Scholar] [CrossRef]
- Park, E.A.; Graves, S.A.; Menda, Y. The Impact of Radiopharmaceutical Therapy on Renal Function. Semin. Nucl. Med. 2022, 52, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Singh, A.; Kulkarni, H.R.; Schuchardt, C.; Müller, D.; Wester, H.-J.; Maina, T.; Rösch, F.; van der Meulen, N.P.; Müller, C.; et al. From Bench to Bedside—The Bad Berka Experience With First-in-Human Studies. Semin. Nucl. Med. 2019, 49, 422–437. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Pain Palliation of Bone Metastases: Production, Quality Control and Dosimetry of Radiopharmaceuticals, IAEA Radioisotopes and Radiopharmaceuticals Series No.9 [IAEA Preprint]. 2021. Available online: https://Preprint.Iaea.Org/Search.Aspx?orig_q=RN:54079457 (accessed on 3 December 2023).
- Kabasakal, L.; AbuQbeitah, M.; Aygün, A.; Yeyin, N.; Ocak, M.; Demirci, E.; Toklu, T. Pre-Therapeutic Dosimetry of Normal Organs and Tissues of 177Lu-PSMA-617 Prostate-Specific Membrane Antigen (PSMA) Inhibitor in Patients with Castration-Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1976–1983. [Google Scholar] [CrossRef]
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W.; et al. The 68Ga/177Lu Theragnostic Concept in PSMA Targeting of Castration-Resistant Prostate Cancer: Correlation of SUVmax Values and Absorbed Dose Estimates. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 788–800. [Google Scholar] [CrossRef]
- Fernández, R.; Eppard, E.; Lehnert, W.; Jiménez-Franco, L.D.; Soza-Ried, C.; Ceballos, M.; Ribbeck, J.; Kluge, A.; Rösch, F.; Meckel, M.; et al. Evaluation of Safety and Dosimetry of 177Lu-DOTA-ZOL for Therapy of Bone Metastases. J. Nucl. Med. 2021, 62, 1126–1132. [Google Scholar] [CrossRef]
- Rubira, L.; Deshayes, E.; Santoro, L.; Kotzki, P.O.; Fersing, C. 225Ac-Labeled Somatostatin Analogs in the Management of Neuroendocrine Tumors: From Radiochemistry to Clinic. Pharmaceutics 2023, 15, 1051. [Google Scholar] [CrossRef]




| Mobile Phase | Rf [68Ga]Ga-DOTAZOL | Rf [68Ga]Ga3+ | Rf 68Ga-Colloids |
|---|---|---|---|
| TBAP 60 mM/MeOH (9:1) | 0.7–0.8 | 0.1–0.3 | 0.1–0.2 |
| Citrate buffer pH 4 | 0–0.1 | 0.7–1 | 0.1–0.2 |
| Acetylacetone/acetone (1:1) | 0–0.1 | 0.7–0.8 | 0–0.1/0.5–0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souche, C.; Fouillet, J.; Rubira, L.; Donzé, C.; Deshayes, E.; Fersing, C. Bisphosphonates as Radiopharmaceuticals: Spotlight on the Development and Clinical Use of DOTAZOL in Diagnostics and Palliative Radionuclide Therapy. Int. J. Mol. Sci. 2024, 25, 462. https://doi.org/10.3390/ijms25010462
Souche C, Fouillet J, Rubira L, Donzé C, Deshayes E, Fersing C. Bisphosphonates as Radiopharmaceuticals: Spotlight on the Development and Clinical Use of DOTAZOL in Diagnostics and Palliative Radionuclide Therapy. International Journal of Molecular Sciences. 2024; 25(1):462. https://doi.org/10.3390/ijms25010462
Chicago/Turabian StyleSouche, Céleste, Juliette Fouillet, Léa Rubira, Charlotte Donzé, Emmanuel Deshayes, and Cyril Fersing. 2024. "Bisphosphonates as Radiopharmaceuticals: Spotlight on the Development and Clinical Use of DOTAZOL in Diagnostics and Palliative Radionuclide Therapy" International Journal of Molecular Sciences 25, no. 1: 462. https://doi.org/10.3390/ijms25010462





