Changes in DNA Methylation and mRNA Expression in Lung Tissue after Long-Term Supplementation with an Increased Dose of Cholecalciferol
Abstract
:1. Introduction
2. Results
2.1. Plasma Vitamin D Concentration
2.2. Methyl-Seq
2.3. mRNA-Seq
2.4. Integration of the Methyl-Seq and mRNA-Seq Results
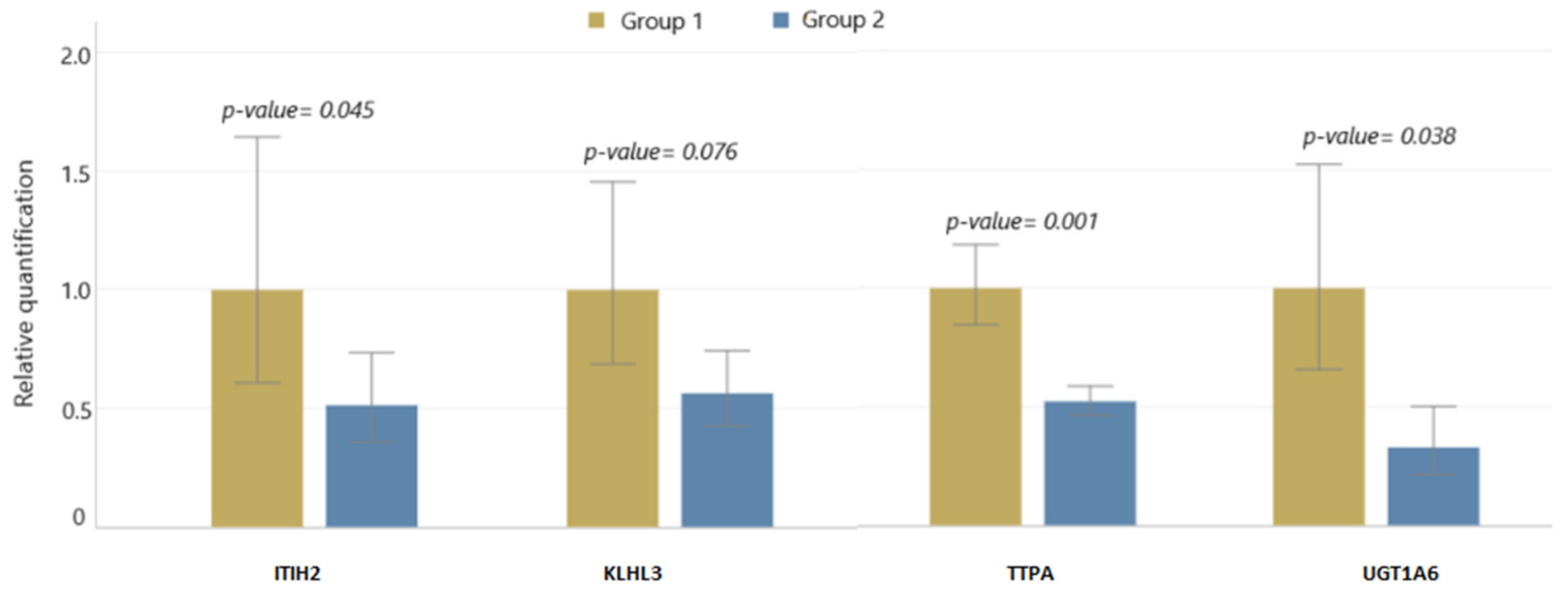
2.5. qPCR Validation
2.6. Functional Analysis
2.6.1. Methyl-Seq
2.6.2. mRNA-Seq
2.6.3. Integration of the Methyl-Seq and mRNA-Seq Results
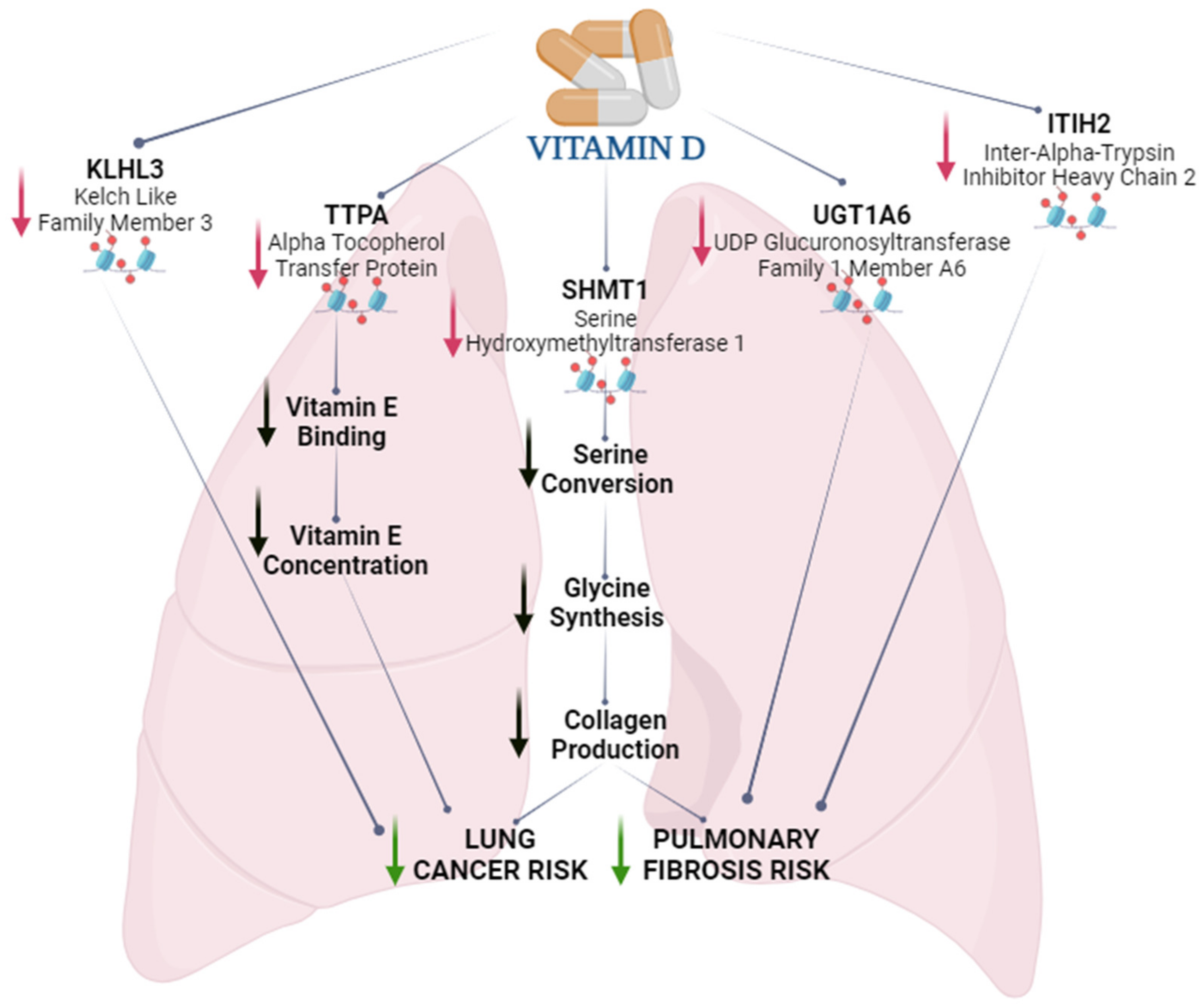
3. Discussion
4. Materials and Methods
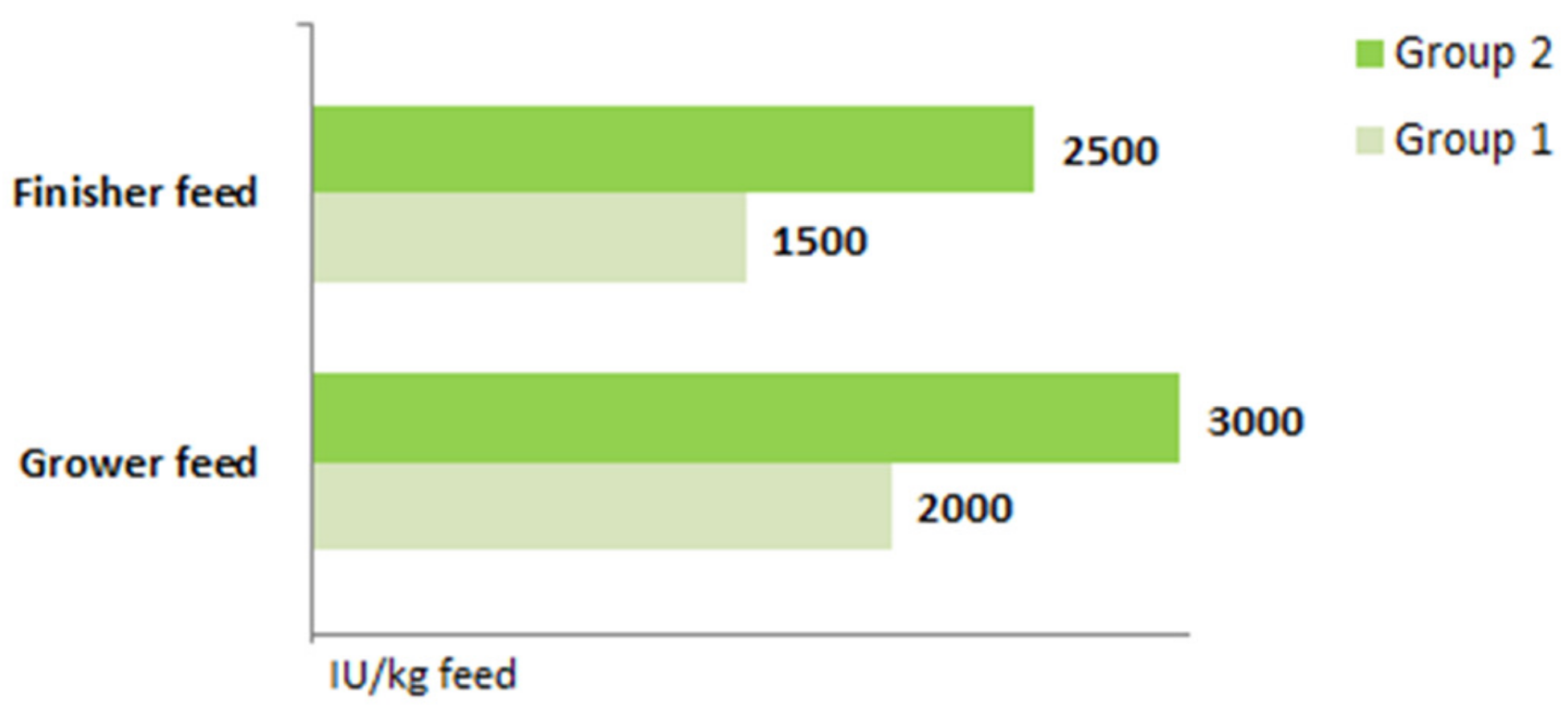
4.1. Animal, Diets, and 25(OH)D Blood Serum Concentration Measurements
4.2. DNA and RNA Isolations, Library Construction, and Sequencing
4.3. qPCR Validation
4.4. Statistical Analysis
4.4.1. Methyl-Seq
4.4.2. mRNA-Seq
4.4.3. Integration of the Methyl-Seq and mRNA-Seq Results
4.4.4. Functional Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wierzbicka, A.; Oczkowicz, M. Sex differences in vitamin D metabolism, serum levels and action. Br. J. Nutr. 2022, 128, 2115–2130. [Google Scholar] [CrossRef] [PubMed]
- Das, R.R.; Singh, M.; Naik, S.S. Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia. Cochrane Database Syst. Rev. 2023, 1, CD011597. [Google Scholar] [CrossRef] [PubMed]
- Mullin, M.L.L.; Milne, S. Vitamin D deficiency in chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2023, 29, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Gupta, K.; Gupta, G.; Mishra, N.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Dureja, H.; Zacconi, F.; Oliver, B.G. Vitamin D-A prominent immunomodulator to prevent COVID-19 infection. Int. J. Rheum. Dis. 2023, 26, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Mandell, E.; Ryan, S.; Seedorf, G.J.; Gonzalez, T.; Abman, S.H.; Fleet, J.C. Maternal vitamin D deficiency induces transcriptomic changes in newborn rat lungs. J. Steroid Biochem. Mol. Biol. 2020, 199, 105613. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.C.; Vigeland, M.D.; Hjorthaug, H.S.; Etholm, L.; Nome, C.G.; Taubøll, E.; Heuser, K.; Selmer, K.K. Neuronal and glial DNA methylation and gene expression changes in early epileptogenesis. PLoS ONE 2019, 14, e0226575. [Google Scholar] [CrossRef] [PubMed]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.; Carlberg, C. Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 2020, 173, 113595. [Google Scholar] [CrossRef]
- Alkafaas, S.S.; Abdallah, A.M.; Ghosh, S.; Loutfy, S.A.; Elkafas, S.S.; Abdel Fattah, N.F.; Hessien, M. Insight into the role of clathrin-mediated endocytosis inhibitors in SARS-CoV-2 infection. Rev. Med. Virol. 2023, 33, e2403. [Google Scholar] [CrossRef]
- O’Reilly, S. Epigenetics in fibrosis. Mol. Asp. Med. 2017, 54, 89–102. [Google Scholar] [CrossRef]
- Biao, H.; Gharaee-Kermani, M.; Wu, Z.; Phan, S.H. Essential Role of MeCP2 in the Regulation of Myofibroblast Differentiation during Pulmonary Fibrosis. Am. J. Pathol. 2011, 178, 1500–1508. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Wongtrakool, C.; Welch, T.; Steinmeyer, A.; Zügel, U.; Roman, J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J. Steroid Biochem. Mol. Biol. 2010, 118, 142–150. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Z.; Guo, X.; Wang, Y.; Zhou, Y.; Cai, W. Synergistic effects of nab-PTX and anti-PD-1 antibody combination against lung cancer by regulating the Pi3K/AKT pathway through the Serpinc1 gene. Front. Oncol. 2022, 12, 933646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Feng, J.; Li, Z.; Yu, H.; Ding, X.; Yang, F.; Linghu, E. Glycopatterns and Glycoproteins Changes in MCN and SCN: A Prospective Cohort Study. Biomed. Res. Int. 2019, 2019, 2871289. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Song, F.; Guo, W.; Tan, J.; Zhang, X.; Qiao, F.; Guo, J.; Zhang, L.; Jia, X. Potential Genes Associated with COVID-19 and Comorbidity. Int. J. Med. Sci. 2022, 19, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Curtis, E.M.; Krstic, N.; Cook, E.; D’Angelo, S.; Crozier, S.R.; Moon, R.J.; Murray, R.; Garratt, E.; Costello, P.; Cleal, J.; et al. Gestational Vitamin D Supplementation Leads to Reduced Perinatal RXRA DNA Methylation: Results from the MAVIDOS Trial. J. Bone Miner. Res. 2019, 34, 231–240. [Google Scholar] [CrossRef]
- Zhu, W.; Ding, Q.; Wang, L.; Xu, G.; Diao, Y.; Qu, S.; Chen, S.; Shi, Y. Vitamin D3 alleviates pulmonary fibrosis by regulating the MAPK pathway via targeting PSAT1 expression in vivo and in vitro. Int. Immunopharmacol. 2021, 101 Pt B, 108212. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Cai, J.; He, Z.; Yuan, J.; Zhu, X.; Li, Y.; Li, M.; Guan, H. PSAT1 regulates cyclin D1 degradation and sustains proliferation of non-small cell lung cancer cells. Int. J. Cancer 2015, 136, E39–E50. [Google Scholar] [CrossRef]
- Qian, W.; Xia, S.; Yang, X.; Yu, J.; Guo, B.; Lin, Z.; Wei, R.; Mao, M.; Zhang, Z.; Zhao, G.; et al. Complex Involvement of the Extracellular Matrix, Immune Effect, and Lipid Metabolism in the Development of Idiopathic Pulmonary Fibrosis. Front. Mol. Biosci. 2022, 8, 800747. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Su, Z.; Sun, Q.; Zhao, Y.; Feng, T.; Jiang, J.; Zhang, F.; Ma, H. Lipid metabolism gene-wide profile and survival signature of lung adenocarcinoma. Lipids Health Dis. 2020, 19, 222. [Google Scholar] [CrossRef]
- Cengiz, B.; Yumrutas, O.; Bozgeyik, E.; Borazan, E.; Igci, Y.Z.; Bozgeyik, I.; Oztuzcu, S. Differential expression of the UGT1A family of genes in stomach cancer tissues. Tumour Biol. 2015, 36, 5831–5837. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.N.K.; Vo, D.K.; Kim, H.; Balla, A.; Lee, Y.; Yoon, I.S.; Maeng, H.J. Differential Effects of 1α,25-Dihydroxyvitamin D3 on the Expressions and Functions of Hepatic CYP and UGT Enzymes and Its Pharmacokinetic Consequences In Vivo. Pharmaceutics 2020, 12, 1129. [Google Scholar] [CrossRef]
- Hamm, A.; Veeck, J.; Bektas, N.; Wild, P.J.; Hartmann, A.; Heindrichs, U.; Kristiansen, G.; Werbowetski-Ogilvie, T.; Del Maestro, R.; Knuechel, R.; et al. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: A systematic expression analysis. BMC Cancer 2008, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Garantziotis, S.; Zudaire, E.; Trempus, C.S.; Hollingsworth, J.W.; Jiang, D.; Lancaster, L.H.; Richardson, E.; Zhuo, L.; Cuttitta, F.; Brown, K.K.; et al. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am. J. Respir. Crit. Care Med. 2008, 178, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Q.; Che, L.; Xie, Z.; Cai, X.; Gong, L.; Li, Z.; Liu, D.; Liu, S. Using biological information to analyze potential miRNA-mRNA regulatory networks in the plasma of patients with non-small cell lung cancer. BMC Cancer 2022, 22, 299. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Q.; Jin, X. Kelch-like protein 3 in human disease and therapy. Mol. Biol. Rep. 2022, 49, 9813–9824. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yan, Q.; Shang, Y.; Zhao, R.; Ding, X.; Gao, S.J.; Li, W.; Lu, C. A viral interferon regulatory factor degrades RNA-binding protein hnRNP Q1 to enhance aerobic glycolysis via recruiting E3 ubiquitin ligase KLHL3 and decaying GDPD1 mRNA. Cell Death Differ. 2022, 29, 2233–2246. [Google Scholar] [CrossRef] [PubMed]
- Alpha-Tocopherol; Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Lee, I.M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef]
- Huang, S.; Goplen, N.P.; Zhu, B.; Cheon, I.S.; Son, Y.; Wang, Z.; Li, C.; Dai, Q.; Jiang, L.; Xiang, M.; et al. Macrophage PPAR-γ suppresses long-term lung fibrotic sequelae following acute influenza infection. PLoS ONE 2019, 14, e0223430. [Google Scholar] [CrossRef]
| Gene | mRNA-Seq | Methyl-Seq | ||
|---|---|---|---|---|
| Log2FoldChange | Base Mean | Meth. Diff. | Consequence | |
| ITIH2 | −2.164 | 1543.602 | 30.606 | upstream gene |
| HSD17B6 | −1.796 | 318.769 | −32.363 | intron |
| CYP3A22 | −1.884 | 1599.78 | −30.897 | intron |
| TTPA | −2.297 | 201.53 | −29.991 | intron |
| SHMT1 | −1.72 | 1007.481 | −28.281 | intron |
| MIPEP | −1.021 | 196.128 | −27.29 | intron |
| PSMA1 | 1.658 | 41.835 | 25.747 | intron |
| HDLBP | −2.089 | 291.196 | 30.216 | intron |
| KLHL3 | −2.213 | 227.073 | 30.429 | intron |
| BHMT | −2.227 | 1523.91 | 38.036 | intron |
| UGT1A6 | −1.603 | 260.41 | 50.914 | intron |
| Base | Term Description | No. of Genes | Strength | FDR |
|---|---|---|---|---|
| GO molecular function | GTPase regulator activity | 55 | 0.45 | 3.3 × 10−7 |
| GO molecular function | Cytoskeletal protein binding | 84 | 0.31 | 1.34 × 10−6 |
| GO molecular function | Enzyme regulator activity | 92 | 0.28 | 4.21 × 10−6 |
| GO molecular function | Kinase binding | 60 | 0.35 | 7.62 × 10−6 |
| GO molecular function | Guanyl-nucleotide exchange factor activity | 28 | 0.47 | 0.00025 |
| GO molecular function | Actin binding | 43 | 0.36 | 0.00031 |
| GO biological processes | Actin filament-based process | 51 | 0.35 | 0.00035 |
| GO cellular component | Actin cytoskeleton | 41 | 0.35 | 0.00072 |
| GO biological processes | Actin cytoskeleton organization | 47 | 0.35 | 0.00075 |
| Base | Term Description | No. of Genes | Strength | FDR |
|---|---|---|---|---|
| KEGG | Chemical carcinogenesis | 12 | 1.55 | 1.66 × 10−12 |
| KEGG | Metabolism of xenobiotics by cytochrome P450 | 11 | 1.57 | 9.57 × 10−12 |
| KEGG | Retinol metabolism | 10 | 1.52 | 2.77 × 10−10 |
| KEGG | Drug metabolism–cytochrome P450 | 9 | 1.5 | 3.59 × 10−9 |
| GO biological process | Negative regulation of blood coagulation | 8 | 1.53 | 2.94 × 10−7 |
| GO biological process | Regulation of blood coagulation | 9 | 1.4 | 2.94 × 10−7 |
| GO biological process | Regulation of wound-healing | 10 | 1.21 | 8.14 × 10−7 |
| GO biological process | Blood coagulation | 10 | 1.17 | 0.0000017 |
| GO biological process | Retinoid metabolic process | 8 | 1.29 | 7.68 × 10−6 |
| KEGG | Tyrosine metabolism | 5 | 1.34 | 0.00022 |
| Base | Term Description | No. of Genes | Strength | FDR |
|---|---|---|---|---|
| KEGG | Steroid hormone biosynthesis | 2 | 2.03 | 0.0387 |
| KEGG | Glycine, serine, and threonine metabolism | 2 | 2.11 | 0.0387 |
| KEGG | Retinol metabolism | 2 | 2 | 0.0387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicka, A.; Semik-Gurgul, E.; Świątkiewicz, M.; Szmatoła, T.; Steg, A.; Oczkowicz, M. Changes in DNA Methylation and mRNA Expression in Lung Tissue after Long-Term Supplementation with an Increased Dose of Cholecalciferol. Int. J. Mol. Sci. 2024, 25, 464. https://doi.org/10.3390/ijms25010464
Wierzbicka A, Semik-Gurgul E, Świątkiewicz M, Szmatoła T, Steg A, Oczkowicz M. Changes in DNA Methylation and mRNA Expression in Lung Tissue after Long-Term Supplementation with an Increased Dose of Cholecalciferol. International Journal of Molecular Sciences. 2024; 25(1):464. https://doi.org/10.3390/ijms25010464
Chicago/Turabian StyleWierzbicka, Alicja, Ewelina Semik-Gurgul, Małgorzata Świątkiewicz, Tomasz Szmatoła, Anna Steg, and Maria Oczkowicz. 2024. "Changes in DNA Methylation and mRNA Expression in Lung Tissue after Long-Term Supplementation with an Increased Dose of Cholecalciferol" International Journal of Molecular Sciences 25, no. 1: 464. https://doi.org/10.3390/ijms25010464
APA StyleWierzbicka, A., Semik-Gurgul, E., Świątkiewicz, M., Szmatoła, T., Steg, A., & Oczkowicz, M. (2024). Changes in DNA Methylation and mRNA Expression in Lung Tissue after Long-Term Supplementation with an Increased Dose of Cholecalciferol. International Journal of Molecular Sciences, 25(1), 464. https://doi.org/10.3390/ijms25010464






