Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery
Abstract
1. Introduction
2. Biology of Extracellular Vesicles
2.1. Biogenesis of Extracellular Vesicles
2.2. Composition of Extracellular Vesicles
| Homeostasis State | EVs Source | EVs Cargo | Physiological “Good” Effect | Pathological “Bad” Effect | Ref. |
|---|---|---|---|---|---|
| Urinary Tract Water-Salt Balance | Nephron collecting duct epithelial cell-derived EVs | AQP2 protein/miRNA | Balancing overall water and ion levels in response to blood osmolality | Diabetic nephropathy (AQP2-AQP5 interaction) and nephrogenic diabetes insipidus (AQP2 mutation) result in the inability to concentrate urine | [17] |
| Gastrointestinal Tract Gut–Brain–Microbiota Axis (GBMAx) | GIT-Microbiota (Bacteroidota—Gram negative/Firmicutes—Gram positive)-derived OMV/MV ratio | OMVs carrying LPS cross BBB | Low (Bacteroidota/Firmicutes)-derived OMV/MV ratio reduces GBMAx permeability, producing normal child brain development and function | High (Bacteroidota/Firmicutes)-derived OMV/MV ratio increases GBMAx permeability; therefore, children are vulnerable to ASDs | [18] |
| Musculoskeletal System Myogenesis | Muscle precursor satellite cell-derived EVs | MyomiR miR-206 | Upregulation of miR-206 targets ribosome binding protein 1 required for collagen synthesis along with dystrophin, which stimulates asymmetric division of satellite cells and will help repair muscle injury and reduce extracellular matrix deposition ideal for muscle remodeling | DMD is caused by a mutation in the dystrophin gene. Therefore, upregulation of miR-206 will further promote collagen synthesis at the expense of quiescent satellite cells, inflammatory cytokine secretion, and disturb calcium/mitochondrial homeostasis, contributing to the replacement of muscles with fibrous and adipose tissues | [19] |
| Reproductive Tract Semi-Allogeneic Fetus Tolerance and Self- Recognition | STB-derived EVs | NKG2D receptor binding MIC-related proteins; A, B, and UL16, pro-apoptotic proteins; FASL and TRAIL | STB-EV MICs and pro-apoptotics maintain semi-allogeneic fetus immune tolerance by suppressing immunity at the fetal–maternal interface via downregulating NKG2D NK cells and promoting Treg cell development through HSPEI and their miRNA cargo | STB-EVs carrying MICs can induce semi-allogeneic fetus rejection, i.e., miscarriage by cross-dressing maternal APCs, thereby activating NKG2D NK to attack fetal cells | [20] |
| Central Nervous System Sonic Hedgehog (SHH) Signaling Pathway | Cerebellar Purkinje Cell-derived AXL-RAB18-TMED10 (ART)-EVs | SHH protein | SHH protein stimulates proliferation of GCPs, a progenitor cell that generates granule neurons, the most abundant neuron in the brain | Loss-of-function (LOF) mutations in the ESCRT-III member, CHMP1A required for vesicular SHH secretion causes microcephaly with pontocerebellar hypoplasia and short stature in humans | [21] |
| Cardiovascular System Blood Coagulation | Platelet-derived EVs | TF-CD142 | Platelet EVs mediate the homeostasis necessary for embryogenesis, angiogenesis, and inflammation | Human Scott syndrome is a mild bleeding disorder caused by loss of Ca2+-dependent scramblase activity. Upon vascular damage, the perivascular TF and not the platelet EVs’ TF will initiate the coagulation process | [22] |
| Immune System Immune Tolerance versus Immune Regulation | APCs: DCs, BLs, and MP-derived EVs | MHC-I and -II versus immunoregulatory molecules: PD-L1, CTLA4, FASL, and TRAIL | The participation of EVs in the cross-presentation of exogenous antigens on MHC-I complexes to CD8+ T cells has an important role in immunity against viruses and tumors and in the immune response upon vaccination and induction of tolerance | EVs express immunoregulatory molecules: PDL1, CTLA4, FASL, and TRAIL, which interact with cognate ligands and receptors expressed T and NK cells, inhibit their activity, or induce apoptosis | [20] |
2.3. Physiological Roles of Extracellular Vesicles
2.3.1. Urinary Tract
2.3.2. Gastrointestinal Tract
2.3.3. Musculoskeletal System
2.3.4. Reproductive Tract
2.3.5. Central Nervous System
2.3.6. Cardiovascular System (Blood Pressure and Coagulation)
2.3.7. Immune System
2.4. Pathological Roles of Extracellular Vesicles
2.4.1. Urinary Tract
2.4.2. Gastrointestinal Tract
2.4.3. Musculoskeletal System
2.4.4. Reproductive Tract
2.4.5. Central Nervous System
2.4.6. Cardiovascular System (Blood Pressure and Coagulation)
2.4.7. Immune System
3. Pharmacology of Extracellular Vesicles
3.1. Pharmacodynamics of Extracellular Vesicles
3.1.1. Therapeutic Targeting of Extracellular Vesicles
3.1.2. Extracellular Vesicles as Therapeutics
Mesenchymal Stromal Cell-Derived Extracellular Vesicles (MSC-EVs)
Tumor Cell-Derived Extracellular Vesicles (TC-EVs)
Immune Cell-Derived Extracellular Vesicles (IC-EVs)
Human Microbiome-Derived Extracellular Vesicles (HMB-EVs)
Breast Milk-Derived Extracellular Vesicles (BM-EVs)
3.2. Pharmacokinetics of Extracellular Vesicles
3.2.1. Pharmacokinetics of Intrinsic Extracellular Vesicles
Extracellular Vesicle Release
Extracellular Vesicle Uptake
3.2.2. Pharmacokinetics of Extrinsic Extracellular Vesicles
Extracellular Vesicle Labelling
Extracellular Vesicle Engineering
4. Pharmaceutical Applications of Extracellular Vesicles
4.1. Applications of Lipid Bilayer Vesicles for Drug Delivery
4.1.1. Oral Delivery
4.1.2. Dermal Delivery
4.1.3. Parenteral Delivery
4.1.4. Pulmonal Delivery
4.1.5. Local Delivery
4.2. Applications of Hydrogel Platforms for Lipid Bilayer Vesicle Delivery
4.2.1. Wound Dressings
4.2.2. Microneedle Patches
4.2.3. Injectable Applications
4.2.4. Bioink-3D Bioprinting
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018(MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2023, 15, e1835. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C. A brief history of nearly EV-erything–The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular vesicles: Exosomes and microvesicles, integrators of homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Kosanović, M.; Milutinovic, B.; Glamočlija, S.; Morlans, I.M.; Ortiz, A.; Bozic, M. Extracellular Vesicles and Acute Kidney Injury: Potential Therapeutic Avenue for Renal Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 3792. [Google Scholar] [CrossRef]
- Kosanović, M.; Milutinović, B.; Kutzner, T.J.; Mouloud, Y.; Bozic, M. Clinical Prospect of Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles in Kidney Disease: Challenges and the Way Forward. Pharmaceutics 2023, 15, 1911. [Google Scholar] [CrossRef]
- Jin, Y.; Ma, L.; Zhang, W.; Yang, W.; Feng, Q.; Wang, H. Extracellular signals regulate the biogenesis of extracellular vesicles. Biol. Res. 2022, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Hirano, M.; Isoyama, J.; Nagayama, S.; Tomonaga, T.; Adachi, J. Comprehensive proteomic profiling of plasma and serum phosphatidylserine-positive extracellular vesicles reveals tissue-specific proteins. Iscience 2022, 25, 104012. [Google Scholar] [CrossRef] [PubMed]
- Kehrloesser, S.; Cast, O.; Elliott, T.S.; Ernst, R.J.; Machel, A.C.; Chen, J.-X.; Chin, J.W.; Miller, M.L. Cell-of-origin–specific proteomics of extracellular vesicles. PNAS Nexus 2023, 2, pgad107. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Buratta, S.; Urbanelli, L.; Tognoloni, A.; Latella, R.; Cerrotti, G.; Emiliani, C.; Chiaradia, E. Protein and Lipid Content of Milk Extracellular Vesicles: A Comparative Overview. Life 2023, 13, 401. [Google Scholar] [CrossRef]
- Clarke-Bland, C.E.; Bill, R.M.; Devitt, A. Emerging roles for AQP in mammalian extracellular vesicles. Biochim. Et Biophys. Acta(BBA)-Biomembr. 2022, 1864, 183826. [Google Scholar] [CrossRef]
- Roussin, L. Effect of Gut Microbiota from Children with Autism Spectrum Disorder(ASD) on Behavior and ASD-Related Biological Markers in Germ-Free Mice. Ph.D. Thesis, Université Paris-Saclay, Paris, France, 2023. [Google Scholar]
- Yedigaryan, L.; Sampaolesi, M. Extracellular vesicles and Duchenne muscular dystrophy pathology: Modulators of disease progression. Front. Physiol. 2023, 14, 204. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Coulter, M.E.; Dorobantu, C.M.; Lodewijk, G.A.; Delalande, F.; Cianferani, S.; Ganesh, V.S.; Smith, R.S.; Lim, E.T.; Xu, C.S.; Pang, S. The ESCRT-III protein CHMP1A mediates secretion of sonic hedgehog on a distinctive subtype of extracellular vesicles. Cell Rep. 2018, 24, 973–986. [Google Scholar] [CrossRef]
- Agbani, E.O.; Hers, I.; Poole, A.W. Platelet procoagulant membrane dynamics: A key distinction between thrombosis and hemostasis? Blood Adv. 2023, 7, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, G.; Lee, J.; Lee, Y.; Kim, J.-H. Secretome of stem cells: Roles of extracellular vesicles in diseases, stemness, differentiation, and reprogramming. Tissue Eng. Regen. Med. 2022, 19, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Riley, N.M.; Yang, A.C.; Kim, J.T.; Terrell, S.M.; Li, V.L.; Garcia-Contreras, M.; Bertozzi, C.R.; Long, J.Z. Cell type-selective secretome profiling in vivo. Nat. Chem. Biol. 2021, 17, 326–334. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Zhang, Y.; Zhang, S.; Wang, D.; Ye, H. Exosomes: Cell-free therapy for cardiovascular diseases. J. Cardiovasc. Transl. Res. 2020, 13, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, M.; Merlotti, G.; Colombatto, A.; Bruno, S.; Stasi, A.; Franzin, R.; Castellano, G.; Grossini, E.; Fanelli, V.; Cantaluppi, V. Stem Cell-Derived Extracellular Vesicles as Potential Therapeutic Approach for Acute Kidney Injury. Dysfunct. Immune Syst. Dur. Acute Kidney Inj. 2022, 13, 849891. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Yang, Y.; Liu, K.; Wu, J.; Gao, P.; Zhang, C. Exosomes in liver fibrosis: The role of modulating hepatic stellate cells and immune cells, and prospects for clinical applications. Front. Immunol. 2023, 14, 1133297. [Google Scholar] [CrossRef]
- Hering, C.; Shetty, A.K. Extracellular Vesicles Derived From Neural Stem Cells, Astrocytes, and Microglia as Therapeutics for Easing TBI-Induced Brain Dysfunction. Stem Cells Transl. Med. 2023, 12, 140–153. [Google Scholar] [CrossRef]
- Bian, D.; Wu, Y.; Song, G.; Azizi, R.; Zamani, A. The application of mesenchymal stromal cells(MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell Res. Ther. 2022, 13, 24. [Google Scholar] [CrossRef]
- Ghanam, J.; Chetty, V.K.; Barthel, L.; Reinhardt, D.; Hoyer, P.-F.; Thakur, B.K. DNA in extracellular vesicles: From evolution to its current application in health and disease. Cell Biosci. 2022, 12, 37. [Google Scholar] [CrossRef]
- Dellar, E.R.; Hill, C.; Melling, G.E.; Carter, D.R.; Baena-Lopez, L.A. Unpacking extracellular vesicles: RNA cargo loading and function. J. Extracell. Biol. 2022, 1, e40. [Google Scholar] [CrossRef]
- Vaka, R.; Parent, S.; Risha, Y.; Khan, S.; Courtman, D.; Stewart, D.J.; Davis, D.R. Extracellular vesicle microRNA and protein cargo profiling in three clinical-grade stem cell products reveals key functional pathways. Mol. Ther. Nucleic Acids 2023, 32, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Brunet, M.Y.; Fernandez-Rhodes, M.; Williams, S.; Heaney, L.M.; Gethings, L.A.; Federici, A.; Davies, O.G.; Hoey, D.; Cox, S.C. Epigenetic reprogramming enhances the therapeutic efficacy of osteoblast-derived extracellular vesicles to promote human bone marrow stem cell osteogenic differentiation. J. Extracell. Vesicles 2021, 10, e12118. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.H.; Feng, S.; Wilson, K.F.; Cerione, R.A.; Antonyak, M.A. Embryonic stem cell-derived extracellular vesicles maintain ESC stemness by activating FAK. Dev. Cell 2021, 56, 277–291.e6. [Google Scholar] [CrossRef] [PubMed]
- Ekram, S.; Khalid, S.; Ramzan, F.; Salim, A.; Bashir, I.; Durrieu, M.C.; Khan, I. Mesenchymal Stem Cell–Derived Extracellular Vesicles Protect Rat Nucleus Pulposus Cells from Oxidative Stress. Cartilage 2023. [Google Scholar] [CrossRef] [PubMed]
- Alasztics, B.; Kovács, Á.F.; Molvarec, A.; Koller, Á.; Szabó, G.; Fekete, N.; Buzás, E.I.; Pállinger, É.; Rigó Jr, J. Platelet-derived extracellular vesicles may contribute to the hypercoagulable state in preeclampsia. J. Reprod. Immunol. 2021, 148, 103380. [Google Scholar] [CrossRef] [PubMed]
- Delrue, C.; De Bruyne, S.; Speeckaert, R.; Speeckaert, M.M. Urinary Extracellular Vesicles in Chronic Kidney Disease: From Bench to Bedside? Diagnostics 2023, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M. Urinary Exosomes: A Promising Biomarker for Disease Diagnosis. Lab. Med. 2023, 54, 115–125. [Google Scholar] [CrossRef]
- Jain, G.; Das, P.; Ranjan, P.; Valderrama, F.; Cieza-Borrella, C. Urinary extracellular vesicles miRNA—A new era of prostate cancer biomarkers. Front. Genet. 2023, 14, 61. [Google Scholar] [CrossRef]
- Spanu, S.; van Roeyen, C.; Denecke, B.; Floege, J.; Mühlfeld, A. Urinary Exosomes: A Novel Means to Non-Invasively Assess Changes in Renal Gene. PLoS ONE 2014, 9, e109631. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef]
- Oshikawa-Hori, S.; Yokota-Ikeda, N.; Sonoda, H.; Sasaki, Y.; Ikeda, M. Reduced urinary release of AQP1-and AQP2-bearing extracellular vesicles in patients with advanced chronic kidney disease. Physiol. Rep. 2021, 9, e15005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Jose, P.A.; Yang, J.; Zeng, C. Importance of extracellular vesicles in hypertension. Exp. Biol. Med. 2021, 246, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Choi, C.; Yoo, T.-H. Extracellular vesicles in kidneys and their clinical potential in renal diseases. Kidney Res. Clin. Pract. 2021, 40, 194. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H. Extracellular vesicles in renal physiology and clinical applications for renal disease. Korean J. Intern. Med. 2019, 34, 470. [Google Scholar] [CrossRef] [PubMed]
- Ilea, A.; Andrei, V.; Feurdean, C.N.; Băbțan, A.-M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Ciui, B.; Tertiș, M.; Săndulescu, R. Saliva, a magic biofluid available for multilevel assessment and a mirror of general health—A systematic review. Biosensors 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; Seyed Alikhani, V.; Ekström, K.; Eldh, M.; Torregrosa Paredes, P.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 1–8. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Sturk, A.; van Tienen, L.M.; Schaap, M.C.; Nieuwland, R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood J. Am. Soc. Hematol. 2011, 117, 3172–3180. [Google Scholar] [CrossRef]
- Yu, Y.; Gool, E.; Berckmans, R.; Coumans, F.; Barendrecht, A.; Maas, C.; van der Wel, N.; Altevogt, P.; Sturk, A.; Nieuwland, R. Extracellular vesicles from human saliva promote hemostasis by delivering coagulant tissue factor to activated platelets. J. Thromb. Haemost. 2018, 16, 1153–1163. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kanai-Azuma, M.; Akimoto, Y.; Kawakami, H.; Yanoshita, R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol. Pharm. Bull. 2008, 31, 1059–1062. [Google Scholar] [CrossRef]
- Bajinka, O.; Tan, Y.; Darboe, A.; Ighaede-Edwards, I.G.; Abdelhalim, K.A. The gut microbiota pathway mechanisms of diabetes. AMB Express 2023, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Villa, T.; Sánchez-Pérez, A. The Gut Microbiome Affects Human Mood and Behavior. In Developmental Biology in Prokaryotes and Lower Eukaryotes; Springer: Cham, Switzerland, 2021; pp. 541–565. [Google Scholar]
- Wang, Y.; Liu, T.; Wan, Z.; Wang, L.; Hou, J.; Shi, M.; Tsui, S.K.W. Investigating causal relationships between the gut microbiota and allergic diseases: A mendelian randomization study. Front. Genet. 2023, 14, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Ren, F.; Dai, D.; Sun, N.; Qian, Y.; Song, P. The causality between intestinal flora and allergic diseases: Insights from a bi-directional two-sample Mendelian randomization analysis. Front. Immunol. 2023, 14, 1121273. [Google Scholar] [CrossRef] [PubMed]
- Ñahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota–host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, S.; Wang, L.; Cao, Z.; Zhang, M.; Zhang, Y.; Liu, R.; Liu, J. Versatility of bacterial outer membrane vesicles in regulating intestinal homeostasis. Sci. Adv. 2023, 9, eade5079. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Yedigaryan, L.; Sampaolesi, M. Skeletal Muscle–Extricated Extracellular Vesicles: Facilitators of Repair and Regeneration: Facilitators of Repair and Regeneration. In Handbook of Stem Cell Therapy; Springer: Cham, Switzerland, 2022; pp. 1–25. [Google Scholar]
- Ji, S.; Ma, P.; Cao, X.; Wang, J.; Yu, X.; Luo, X.; Lu, J.; Hou, W.; Zhang, Z.; Yan, Y. Myoblast-derived exosomes promote the repair and regeneration of injured skeletal muscle in mice. FEBS Open Bio 2022, 12, 2213–2226. [Google Scholar] [CrossRef]
- Florin, A.; Lambert, C.; Sanchez, C.; Zappia, J.; Durieux, N.; Tieppo, A.M.; Mobasheri, A.; Henrotin, Y. The secretome of skeletal muscle cells: A systematic review. Osteoarthr. Cartil. Open 2020, 2, 100019. [Google Scholar] [CrossRef]
- Lam, N.T.; Gartz, M.; Thomas, L.; Haberman, M.; Strande, J.L. Influence of microRNAs and exosomes in muscle health and diseases. J. Muscle Res. Cell Motil. 2020, 41, 269–284. [Google Scholar] [CrossRef]
- Coenen-Stass, A.M.; Betts, C.A.; Lee, Y.F.; Mäger, I.; Turunen, M.P.; El Andaloussi, S.; Morgan, J.E.; Wood, M.J.; Roberts, T.C. Selective release of muscle-specific, extracellular microRNAs during myogenic differentiation. Hum. Mol. Genet. 2016, 25, 3960–3974. [Google Scholar] [CrossRef]
- Mytidou, C.; Koutsoulidou, A.; Zachariou, M.; Prokopi, M.; Kapnisis, K.; Spyrou, G.M.; Anayiotos, A.; Phylactou, L.A. Age-related exosomal and endogenous expression patterns of miR-1, miR-133a, miR-133b, and miR-206 in skeletal muscles. Front. Physiol. 2021, 12, 708278. [Google Scholar] [CrossRef] [PubMed]
- Chidester, S.; Livinski, A.A.; Fish, A.F.; Joseph, P.V. The role of extracellular vesicles in β-cell function and viability: A scoping review. Front. Endocrinol. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, M.; Chen, Z.; Xu, L.; Chang, M.; Wang, K.; Deng, C.; Gu, Y.; Zhou, S.; Shen, Y. Biogenesis and function of extracellular vesicles in pathophysiological processes skeletal muscle atrophy. Biochem. Pharmacol. 2022, 114954. [Google Scholar] [CrossRef] [PubMed]
- Yahara, Y.; Nguyen, T.; Ishikawa, K.; Kamei, K.; Alman, B.A. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development 2022, 149, dev199908. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, W.; Li, M.; Zheng, A. Exosome-Based Carrier for RNA Delivery: Progress and Challenges. Pharmaceutics 2023, 15, 598. [Google Scholar] [CrossRef] [PubMed]
- Uenaka, M.; Yamashita, E.; Kikuta, J.; Morimoto, A.; Ao, T.; Mizuno, H.; Furuya, M.; Hasegawa, T.; Tsukazaki, H.; Sudo, T. Osteoblast-derived vesicles induce a switch from bone-formation to bone-resorption in vivo. Nat. Commun. 2022, 13, 1066. [Google Scholar] [CrossRef]
- Yuan, F.-L.; Wu, Q.-y.; Miao, Z.-N.; Xu, M.-H.; Xu, R.-S.; Jiang, D.-L.; Ye, J.-X.; Chen, F.-h.; Zhao, M.-D.; Wang, H.-j. Osteoclast-derived extracellular vesicles: Novel regulators of osteoclastogenesis and osteoclast–osteoblasts communication in bone remodeling. Front. Physiol. 2018, 9, 628. [Google Scholar] [CrossRef]
- Xu, L.; Qian, Z.; Wang, S.; Wang, R.; Pu, X.; Yang, B.; Zhou, Q.; Du, C.; Chen, Q.; Feng, Z. Galectin-3 Enhances Osteogenic Differentiation of Precursor Cells From Patients With Diffuse Idiopathic Skeletal Hyperostosis via Wnt/β-Catenin Signaling. J. Bone Miner. Res. 2022, 37, 724–739. [Google Scholar] [CrossRef]
- Anderson, H.C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J. Cell Biol. 1969, 41, 59–72. [Google Scholar] [CrossRef]
- Ali, S.Y.; Sajdera, S.; Anderson, H. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc. Natl. Acad. Sci. 1970, 67, 1513–1520. [Google Scholar] [CrossRef]
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 2018, 39, 292–332. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Kim, B.-J.; Kang, J.; Nam, T.-S.; Lim, J.M.; Kim, H.T.; Park, J.K.; Kim, Y.G.; Chae, S.-W.; Kim, U.-H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011, 4, ra31. [Google Scholar] [CrossRef] [PubMed]
- Aalberts, M.; Stout, T.; Stoorvogel, W. Prostasomes: Extracellular vesicles from the prostate. Reproduction 2014, 147, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Påhlson, C.; Bergquist, M.; Ronquist, G.; Stridsberg, M. Antibacterial activity of human prostasomes. Prostate 2000, 44, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Al-Dossary, A.A.; Strehler, E.E.; Martin-DeLeon, P.A. Expression and secretion of plasma membrane Ca2+-ATPase 4a(PMCA4a) during murine estrus: Association with oviductal exosomes and uptake in sperm. PLoS ONE 2013, 8, e80181. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liang, H.; Guo, H.; Wang, Z.; Deng, Q. PMCA4 gene expression is regulated by the androgen receptor in the mouse testis during spermatogenesis. Mol. Med. Rep. 2021, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Ravaux, B.; Favier, S.; Perez, E.; Gourier, C. Egg CD9 protein tides correlated with sperm oscillations tune the gamete fusion ability in mammal. J. Mol. Cell Biol. 2018, 10, 494–502. [Google Scholar] [CrossRef]
- Tannetta, D.; Masliukaite, I.; Vatish, M.; Redman, C.; Sargent, I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J. Reprod. Immunol. 2017, 119, 98–106. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Jelenčić, V.; Polić, B. NKG2D: A master regulator of immune cell responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Awoyemi, T.; Cerdeira, A.S.; Zhang, W.; Jiang, S.; Rahbar, M.; Logenthiran, P.; Redman, C.; Vatish, M. Preeclampsia and syncytiotrophoblast membrane extracellular vesicles(STB-EVs). Clin. Sci. 2022, 136, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Guerby, P.; Swiader, A.; Augé, N.; Parant, O.; Vayssière, C.; Uchida, K.; Salvayre, R.; Negre-Salvayre, A. High glutathionylation of placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2019, 22, 101126. [Google Scholar] [CrossRef] [PubMed]
- Maraghechi, P.; Aponte, M.T.S.; Ecker, A.; Lázár, B.; Tóth, R.; Szabadi, N.T.; Gócza, E. Pluripotency-Associated microRNAs in Early Vertebrate Embryos and Stem Cells. Genes 2023, 14, 1434. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ma, Y.; Wu, H.; Wang, Y.-L. Placenta-derived microRNAs in the pathophysiology of human pregnancy. Front. Cell Dev. Biol. 2021, 9, 646326. [Google Scholar] [CrossRef] [PubMed]
- Cooke, W.R.; Cribbs, A.; Zhang, W.; Kandzija, N.; Motta-Mejia, C.; Dombi, E.; Ri, R.; Cerdeira, A.S.; Redman, C.; Vatish, M. Maternal circulating syncytiotrophoblast-derived extracellular vesicles contain biologically active 5’-tRNA halves. Biochem. Biophys. Res. Commun. 2019, 518, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cooke, W.R.; Jones, G.D.; Redman, C.W.; Vatish, M. Syncytiotrophoblast derived extracellular vesicles in relation to preeclampsia. Matern. -Fetal Med. 2021, 3, 151–160. [Google Scholar] [CrossRef]
- Yakovlev, A.A. Neuronal Exosomes as a New Signaling System. Biochemistry 2023, 88, 457–465. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Ferretti, M.T. Function and dysfunction of microglia during brain development: Consequences for synapses and neural circuits. Front. Synaptic Neurosci. 2017, 9, 9. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morozumi, A.; Hirokawa, N. Nodal flow transfers polycystin to determine mouse left-right asymmetry. Dev. Cell 2023, 58, 1447–1461.e6. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Xu, F.; Xu, N.; Sharifi, N.H.; Zhang, P.; Flores, J.J.; Wu, L.; He, Q.; Kanamaru, H. Fractalkine Enhances Hematoma Resolution and Improves Neurological Function via CX3CR1/AMPK/PPARγ Pathway After GMH. Stroke 2023, 54, 2420–2433. [Google Scholar] [CrossRef]
- Brown, M.; Johnson, L.A.; Leone, D.A.; Majek, P.; Vaahtomeri, K.; Senfter, D.; Bukosza, N.; Schachner, H.; Asfour, G.; Langer, B. Lymphatic exosomes promote dendritic cell migration along guidance cues. J. Cell Biol. 2018, 217, 2205–2221. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y. Systemic exosomal delivery of shRNA minicircles prevents parkinsonian pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, D.M.; Stojiljkovic, M.; Diezel, C.; Streicher, P.-E.; Röstel, F.; Lindner, J.; Weis, S.; Schmeer, C.; Marz, M. Peripheral blood exosomes pass blood-brain-barrier and induce glial cell activation. BioRxiv 2018, 471409. [Google Scholar]
- Luarte, A.; Nardocci, G.; Chakraborty, A.; Batiz, L.F.; Pino-Lagos, K.; Wyneken, Ú. Astrocyte-derived extracellular vesicles in stress-associated mood disorders. Does the immune system get astrocytic? Pharmacol. Res. 2023, 194, 106833. [Google Scholar] [CrossRef]
- Sharma, K.; Zhang, Y.; Paudel, K.R.; Kachelmeier, A.; Hansbro, P.M.; Shi, X. The Emerging Role of Pericyte-Derived Extracellular Vesicles in Vascular and Neurological Health. Cells 2022, 11, 3108. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, Y.; Li, Y.; Luo, L.; Zhao, Y.; Yao, Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020, 6, 68. [Google Scholar] [CrossRef]
- Murugesan, S.; Hussey, H.; Saravanakumar, L.; Sinkey, R.G.; Sturdivant, A.B.; Powell, M.F.; Berkowitz, D.E. Extracellular vesicles from women with severe preeclampsia impair vascular endothelial function. Anesth. Analg. 2022, 134, 713–723. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Ayers, L.; Nieuwland, R.; Kohler, M.; Kraenkel, N.; Ferry, B.; Leeson, P. Dynamic microvesicle release and clearance within the cardiovascular system: Triggers and mechanisms. Clin. Sci. 2015, 129, 915–931. [Google Scholar] [CrossRef]
- Torti, M.; Vismara, M.; Manfredi, M.; Zarà, M.; Trivigno, S.; Galgano, L.; Barbieri, S.; Canobbio, I.; Guidetti, G. Proteomic and functional profiling of platelet-derived extracellular vesicles released under physiological or tumor-associated conditions. Cell Death Discov. 2022, 8, 467. [Google Scholar]
- Gasecka, A.; Nieuwland, R.; Siljander, P.R.-M. Platelet-derived extracellular vesicles. In Platelets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 401–416. [Google Scholar]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular vesicles from activated platelets: A semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Puricelli, C.; Boggio, E.; Gigliotti, C.L.; Stoppa, I.; Sutti, S.; Giordano, M.; Dianzani, U.; Rolla, R. Platelets, Protean Cells with All-Around Functions and Multifaceted Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 4565. [Google Scholar] [CrossRef] [PubMed]
- Puhka, M.; Takatalo, M.; Nordberg, M.-E.; Valkonen, S.; Nandania, J.; Aatonen, M.; Yliperttula, M.; Laitinen, S.; Velagapudi, V.; Mirtti, T. Metabolomic profiling of extracellular vesicles and alternative normalization methods reveal enriched metabolites and strategies to study prostate cancer-related changes. Theranostics 2017, 7, 3824. [Google Scholar] [CrossRef] [PubMed]
- Reddy, E.C.; Rand, M.L. Procoagulant phosphatidylserine-exposing platelets in vitro and in vivo. Front. Cardiovasc. Med. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Jia, Z.; Zhang, Y.; Le, S.C.; Chen, J.; Yang, H. An inner activation gate controls TMEM16F phospholipids scrambling. Biophys. J. 2019, 116, 25a–26a. [Google Scholar] [CrossRef]
- Frühbeis, C.; Helmig, S.; Tug, S.; Simon, P.; Krämer-Albers, E.-M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J. Extracell. Vesicles 2015, 4, 28239. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Qin, Y.; Men, J.; Ren, J.; Li, X.; Xu, C.; Li, Q.; Li, Y.; Cui, W. MPs-ACT, an Assay to Evaluate the Procoagulant Activity of Microparticles. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231159374. [Google Scholar] [CrossRef]
- Sachetto, A.T.; Archibald, S.J.; Hisada, Y.; Rosell, A.; Havervall, S.; van Es, N.; Nieuwland, R.; Campbell, R.A.; Middleton, E.A.; Rondina, M.T. Tissue factor activity of small and large extracellular vesicles in different diseases. Res. Pract. Thromb. Haemost. 2023, 7, 100124. [Google Scholar] [CrossRef]
- Hu, Y.; Repa, A.; Lisman, T.; Yerlikaya-Schatten, G.; Hau, C.; Pabinger, I.; Ay, C.; Nieuwland, R.; Thaler, J. Extracellular vesicles from amniotic fluid, milk, saliva, and urine expose complexes of tissue factor and activated factor VII. J. Thromb. Haemost. 2022, 20, 2306–2312. [Google Scholar] [CrossRef]
- Hong, C.-W. Extracellular vesicles of neutrophils. Immune Netw. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xie, F.; Wang, L.; Zhang, L.; Zhang, S.; Fang, M.; Zhou, F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell. Mol. Immunol. 2020, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Mehrani, Y.; Rahimi Junqani, R.; Morovati, S.; Mehrani, H.; Karimi, N.; Ghasemi, S. The Importance of Neutrophils in Osteoarthritis: Current Concepts and Therapeutic Perspectives. Immuno 2023, 3, 250–272. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, H.; Li, L.; Zhang, X.; Zheng, C.; Gao, X.; Yang, Y.; Sun, B. An injectable, activated neutrophil-derived exosome mimetics/extracellular matrix hybrid hydrogel with antibacterial activity and wound healing promotion effect for diabetic wound therapy. J. Nanobiotechnol. 2023, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-P.; Wang, X.-Y.; Pan, X.-Y.; Hu, W.-W.; Cai, S.-T.; Joshi, K.; Deng, L.-H.; Ma, D. Circulating neutrophil-derived microparticles associated with the prognosis of patients with sepsis. J. Inflamm. Res. 2020, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.J.; McFadyen, J.D.; Peter, K. Caught at the scene of the crime: Platelets and neutrophils are conspirators in thrombosis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Zimmermann, A.-K.; Rivieccio, F.; Visser, C.; Blango, M.G. Host-derived extracellular vesicles for antimicrobial defense. MicroLife 2021, 2, uqab003. [Google Scholar] [CrossRef]
- Kolonics, F.; Kajdácsi, E.; Farkas, V.J.; Veres, D.S.; Khamari, D.; Kittel, Á.; Merchant, M.L.; McLeish, K.R.; Lőrincz, Á.M.; Ligeti, E. Neutrophils produce proinflammatory or anti-inflammatory extracellular vesicles depending on the environmental conditions. J. Leucoc. Biol. 2021, 109, 793–806. [Google Scholar] [CrossRef]
- Lim, K.; Hyun, Y.-M.; Lambert-Emo, K.; Capece, T.; Bae, S.; Miller, R.; Topham, D.J.; Kim, M. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 2015, 349, aaa4352. [Google Scholar] [CrossRef]
- Popēna, I.; Ābols, A.; Saulīte, L.; Pleiko, K.; Zandberga, E.; Jēkabsons, K.; Endzeliņš, E.; Llorente, A.; Linē, A.; Riekstiņa, U. Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Commun. Signal. 2018, 16, 17. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Nolte-‘t Hoen, E.N.; Buschow, S.I.; Anderton, S.M.; Stoorvogel, W.; Wauben, M.H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood J. Am. Soc. Hematol. 2009, 113, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Hatami, Z.; Hashemi, Z.S.; Eftekhary, M.; Amiri, A.; Karpisheh, V.; Nasrollahi, K.; Jafari, R. Natural killer cell-derived exosomes for cancer immunotherapy: Innovative therapeutics art. Cancer Cell Int. 2023, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.; Motta-Mejia, C.; Kandzija, N.; Cooke, W.; Zhang, W.; Cerdeira, A.S.; Bastie, C.; Redman, C.; Vatish, M. Placental syncytiotrophoblast-derived extracellular vesicles carry active NEP(neprilysin) and are increased in preeclampsia. Hypertension 2019, 73, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Buca, D.; Bologna, G.; D’Amico, A.; Cugini, S.; Musca, F.; Febbo, M.; D’Arcangelo, D.; Buca, D.; Simeone, P.; Liberati, M. Extracellular vesicles in feto–maternal crosstalk and pregnancy disorders. Int. J. Mol. Sci. 2020, 21, 2120. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hsueh, Y.-W.; Chang, C.-W.; Hsu, H.-C.; Yang, T.-C.; Lin, W.-C.; Chang, H.-M. Establishment of the fetal-maternal interface: Developmental events in human implantation and placentation. Front. Cell Dev. Biol. 2023, 11, 1200330. [Google Scholar] [CrossRef]
- Göhner, C.; Plösch, T.; Faas, M.M. Immune-modulatory effects of syncytiotrophoblast extracellular vesicles in pregnancy and preeclampsia. Placenta 2017, 60, S41–S51. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, J.; Xie, R.; Gao, L.; Yang, Y.; Fan, H.; Qian, K. Exosomal-like vesicles with immune-modulatory features are present in human plasma and can induce CD4+ T-cell apoptosis in vitro. Transfusion 2011, 51, 1002–1011. [Google Scholar] [CrossRef]
- Karlsson, M.; Lundin, S.; Dahlgren, U.; Kahu, H.; Pettersson, I.; Telemo, E. “Tolerosomes” are produced by intestinal epithelial cells. Eur. J. Immunol. 2001, 31, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Marazuela, E.G.; Segura, E.; Fernandez-Garcia, H.; Villalba, M.; Théry, C.; Rodríguez, R.A.; Batanero, E. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J. Immunol. 2008, 181, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Hiltbrunner, S.; Larssen, P.; Eldh, M.; Martinez-Bravo, M.-J.; Wagner, A.K.; Karlsson, M.C.; Gabrielsson, S. Exosomal cancer immunotherapy is independent of MHC molecules on exosomes. Oncotarget 2016, 7, 38707. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Lauzurica-Valdemoros, R.; Borràs, F.E. Tolerance in organ transplantation: From conventional immunosuppression to extracellular vesicles. Front. Immunol. 2014, 5, 416. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, Y.; Zheng, J.C. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Dalla, P.V.; Santos, J.; Milthorpe, B.K.; Padula, M.P. Selectively-packaged proteins in breast cancer extracellular vesicles involved in metastasis. Int. J. Mol. Sci. 2020, 21, 4990. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, E.; Rodosthenous, R.S.; Kujala, V.; Karalis, K.; Spanos, M.; Lehmann, H.I.; Oliveira, G.O.P.D.; Shi, M.; Fleming, T.W.M.; Li, G. Circulating Extracellular Vesicles in Human Cardiorenal Syndrome Promote Renal Injury. medRxiv 2023. medRxiv:2023.02.07.23285599. [Google Scholar]
- Wang, C.; Li, S.-W.; Zhong, X.; Liu, B.-C.; Lv, L.-L. An update on renal fibrosis: From mechanisms to therapeutic strategies with a focus on extracellular vesicles. Kidney Res. Clin. Pract. 2023, 42, 174. [Google Scholar] [CrossRef]
- Mazzariol, M.; Camussi, G.; Brizzi, M.F. Extracellular vesicles tune the immune system in renal disease: A focus on systemic lupus erythematosus, antiphospholipid syndrome, thrombotic microangiopathy and ANCA-Vasculitis. Int. J. Mol. Sci. 2021, 22, 4194. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, R.; Gu, X.; Wang, X.; Xi, P.; Chen, X. Exosomes from tubular epithelial cells undergoing epithelial-to-mesenchymal transition promote renal fibrosis by M1 macrophage activation. FASEB BioAdv. 2023, 5, 101–113. [Google Scholar] [CrossRef]
- Shen, A.-R.; Lv, L.-L. Tubule epithelial cells and fibroblasts communication: Vicious cycle of renal fibrosis. EBioMedicine 2022, 86, 104360. [Google Scholar] [CrossRef] [PubMed]
- Kosanović, M.; Llorente, A.; Glamočlija, S.; Valdivielso, J.M.; Bozic, M. Extracellular Vesicles and Renal Fibrosis: An Odyssey toward a New Therapeutic Approach. Int. J. Mol. Sci. 2021, 22, 3887. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.-y.; Li, Z.; Chu, L.; Liu, Y.; He, J.-r.; Xin, Y.; Li, A.-m.; Zhang, H. Iron metabolism and chronic inflammation in IgA nephropathy. Ren. Fail. 2023, 45, 2195012. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, T.; Zhao, Y.; Yin, Y.; Huang, Y.; Cheng, Z.; Wang, B.; Liu, S.; Pan, M.; Sun, D. PPARγ Mediates the Anti-Epithelial-Mesenchymal Transition Effects of FGF1ΔHBS in Chronic Kidney Diseases via Inhibition of TGF-β1/SMAD3 Signaling. Front. Pharmacol. 2021, 12, 690535. [Google Scholar] [CrossRef]
- Gomes, R.N.; Manuel, F.; Nascimento, D.S. The bright side of fibroblasts: Molecular signature and regenerative cues in major organs. NPJ Regen. Med. 2021, 6, 43. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Ortega, A.; Redon, J.; Cortes, R. Therapeutic potential of extracellular vesicles in hypertension-associated kidney disease. Hypertension 2021, 77, 28–38. [Google Scholar] [CrossRef]
- Rigalli, J.P.; Barros, E.R.; Sommers, V.; Bindels, R.J.; Hoenderop, J.G. Novel aspects of extracellular vesicles in the regulation of renal physiological and pathophysiological processes. Front. Cell Dev. Biol. 2020, 8, 244. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, Y.; Li, H.; Shao, S. Helicobacter pylori promotes gastric cancer progression through the tumor microenvironment. Appl. Microbiol. Biotechnol. 2022, 106, 4375–4385. [Google Scholar] [CrossRef]
- Usui, Y.; Taniyama, Y.; Endo, M.; Koyanagi, Y.N.; Kasugai, Y.; Oze, I.; Ito, H.; Imoto, I.; Tanaka, T.; Tajika, M. Helicobacter pylori, homologous-recombination genes, and gastric cancer. N. Engl. J. Med. 2023, 388, 1181–1190. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori virulence factors—Mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef]
- Bucci, P.; Barbaglia, Y.; Tedeschi, F.; Zalazar, F. Helicobacter pylori infection: A balance between bacteria and host. Rev. Argent. De Microbiol. 2023, 55, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-I.; Choi, J.-P.; Seo, J.; Kim, B.J.; Rho, M.; Han, J.K.; Kim, J.G. Helicobacter pylori-derived extracellular vesicles increased in the gastric juices of gastric adenocarcinoma patients and induced inflammation mainly via specific targeting of gastric epithelial cells. Exp. Mol. Med. 2017, 49, e330. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, P.; Xi, Y.; Sheng, J. From trash to treasure: The role of bacterial extracellular vesicles in gut health and disease. Front. Immunol. 2023, 14, 1274295. [Google Scholar] [CrossRef] [PubMed]
- Navashenaq, J.G.; Shabgah, A.G.; Banach, M.; Jamialahmadi, T.; Penson, P.E.; Johnston, T.P.; Sahebkar, A. The interaction of Helicobacter pylori with cancer immunomodulatory stromal cells: New insight into gastric cancer pathogenesis. Semin. Cancer Biol. 2022, 86, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ye, Y.; Gu, L.; Jian, Z.; Stary, C.M.; Xiong, X. Extracellular vesicle-derived miRNA as a novel regulatory system for bi-directional communication in gut-brain-microbiota axis. J. Transl. Med. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Kotsiliti, E. Gut microbiome and autism spectrum disorder. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 6. [Google Scholar] [CrossRef]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.M.; Podder, B.R.; Ara Mimi, A.; Aktar Suchi, S. Role of gut microbiome in autism spectrum disorder and its therapeutic regulation. Front. Cell. Infect. Microbiol. 2022, 12, 998. [Google Scholar] [CrossRef]
- Cuesta, C.M.; Guerri, C.; Ureña, J.; Pascual, M. Role of microbiota-derived extracellular vesicles in gut-brain communication. Int. J. Mol. Sci. 2021, 22, 4235. [Google Scholar] [CrossRef]
- Zaidi, S.; Ali, K.; Khan, A.U. It’s all relative: Analyzing microbiome compositions, its significance, pathogenesis and microbiota derived biofilms: Challenges and opportunities for disease intervention. Arch. Microbiol. 2023, 205, 257. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia—Understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef]
- Vitucci, D.; Martone, D.; Alfieri, A.; Buono, P. Muscle-derived exosomes and exercise in cancer prevention. Front. Mol. Med. 2023, 3, 16. [Google Scholar] [CrossRef]
- Catapano, F.; Scaglioni, D.; Maresh, K.; Ala, P.; Domingos, J.; Selby, V.; Ricotti, V.; Phillips, L.; Servais, L.; Seferian, A. Novel free-circulating and extracellular vesicle-derived miRNAs dysregulated in Duchenne muscular dystrophy. Epigenomics 2020, 12, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Hirai, Y.; Hashido, K.; Okada, T. Therapeutic Application of Extracellular Vesicles-Capsulated Adeno-Associated Virus Vector via nSMase2/Smpd3, Satellite, and Immune Cells in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2022, 23, 1551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, B.; Meng, W.; Hu, J. Elucidating a fresh perspective on the interplay between exosomes and rheumatoid arthritis. Front. Cell Dev. Biol. 2023, 11, 1177303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhuang, Y.; Fang, L.; Yuan, C.; Wang, X.; Lin, K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact. Mater. 2023, 22, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Scheck, S.; Paterson, E.S.; Henry, C.E. A promising future for endometriosis diagnosis and therapy: Extracellular vesicles-a systematic review. Reprod. Biol. Endocrinol. 2022, 20, 174. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, P.; Han, C.; Li, J.; Liu, L.; Zhao, Z.; Gao, Y.; Qin, Y.; Xu, Q.; Yan, Y. Association of placenta-derived extracellular vesicles with pre-eclampsia and associated hypercoagulability: A clinical observational study. BJOG: Int. J. Obstet. Gynaecol. 2021, 128, 1037–1046. [Google Scholar] [CrossRef]
- Reyes, A.B.; Burger, D. Small extracellular vesicles: A new player in GDM pathogenesis. Clin. Sci. 2022, 136, 1873–1875. [Google Scholar] [CrossRef]
- Kulkarni, R.; Teves, M.E.; Han, A.X.; McAllister, J.M.; Strauss III, J.F. Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. J. Endocr. Soc. 2019, 3, 2204–2223. [Google Scholar] [CrossRef]
- Cui, C.; Wang, J.; Han, X.; Wang, Q.; Zhang, S.; Liang, S.; Li, H.; Meng, L.; Zhang, C.; Chen, H. Identification of small extracellular vesicle-linked miRNA specifically derived from intrafollicular cells in women with polycystic ovary syndrome. Reprod. Biomed. Online 2021, 42, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Koiou, E.; Tziomalos, K.; Katsikis, I.; Papadakis, E.; Kandaraki, E.A.; Panidis, D. Platelet-derived microparticles in overweight/obese women with the polycystic ovary syndrome. Gynecol. Endocrinol. 2013, 29, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.; Connolly, K.; Ladell, K.; Davies, T.; Guschina, I.; Ramji, D.; Miners, K.; Price, D.; Clayton, A.; James, P. Young women with polycystic ovary syndrome have raised levels of circulating annexin V-positive platelet microparticles. Hum. Reprod. 2014, 29, 2756–2763. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steegers, E.A.; Von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Awoyemi, T.; Jiang, S.; Bjarkadottir, B.; Rahbar, M.; Logenthiran, P.; Collett, G.; Zhang, W.; Cribbs, A.; Cerdeira, A.S.; Vatish, M. Identification of Novel Syncytiotrophoblast Membrane Extracellular Vesicles Derived Protein Biomarkers in Preeclampsia: A Cross-Sectional Study. medRxiv 2023. medRxiv:2023.06.03.23290935. [Google Scholar]
- Han, C.; Wang, C.; Chen, Y.; Wang, J.; Xu, X.; Hilton, T.; Cai, W.; Zhao, Z.; Wu, Y.; Li, K. Placenta-derived extracellular vesicles induce preeclampsia in mouse models. Haematologica 2020, 105, 1686. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Guanzon, D.; Scholz-Romero, K.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental exosomes as early biomarker of preeclampsia: Potential role of exosomal microRNAs across gestation. J. Clin. Endocrinol. Metab. 2017, 102, 3182–3194. [Google Scholar] [CrossRef]
- James-Allan, L.B.; Rosario, F.J.; Barner, K.; Lai, A.; Guanzon, D.; McIntyre, H.D.; Lappas, M.; Powell, T.L.; Salomon, C.; Jansson, T. Regulation of glucose homeostasis by small extracellular vesicles in normal pregnancy and in gestational diabetes. FASEB J. 2020, 34, 5724–5739. [Google Scholar] [CrossRef]
- Bathla, T.; Abolbaghaei, A.; Reyes, A.B.; Burger, D. Extracellular vesicles in gestational diabetes mellitus: A scoping review. Diabetes Vasc. Dis. Res. 2022, 19, 14791641221093901. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Cao, Y.; Jia, H.; Li, X.; Li, F.; Zhang, S.; Zhang, J. Neuroinflammation of traumatic brain injury: Roles of extracellular vesicles. Front. Immunol. 2023, 13, 1088827. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Qi, B.; Jiang, Y.; Lian, N.; Zhuang, X.; Yu, Y. Brain-derived extracellular vesicles mediated coagulopathy, inflammation and apoptosis after sepsis. Thromb. Res. 2021, 207, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Marostica, G.; Gelibter, S.; Gironi, M.; Nigro, A.; Furlan, R. Extracellular vesicles in neuroinflammation. Front. Cell Dev. Biol. 2021, 8, 623039. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Mustapic, M.; Diaz-Arrastia, R.; Lange, R.; Gulyani, S.; Diehl, T.; Motamedi, V.; Osier, N.; Stern, R.A.; Kapogiannis, D. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 2018, 32, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, F.; Bai, X.; Jia, H.; Wang, C.; Li, P.; Zhang, Q.; Guan, S.; Peng, R.; Zhang, S. Circulating extracellular vesicles from patients with traumatic brain injury induce cerebrovascular endothelial dysfunction. Pharmacol. Res. 2023, 192, 106791. [Google Scholar] [CrossRef] [PubMed]
- Pistono, C.; Osera, C.; Cuccia, M.; Bergamaschi, R. Roles of Extracellular Vesicles in Multiple Sclerosis: From Pathogenesis to Potential Tools as Biomarkers and Therapeutics. Sclerosis 2023, 1, 91–112. [Google Scholar] [CrossRef]
- Yas, A. Preparation and characterization of L–Dopa loaded chitosan–based dry powder: Rescue/continuous supplement in Parkinson’s disease via inhalation. World J. Pharm. Sci. 2016, 23–36. [Google Scholar]
- Iba, M.; Guo, J.L.; McBride, J.D.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M.-Y. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci. 2013, 33, 1024–1037. [Google Scholar] [CrossRef]
- Ruan, Z.; Pathak, D.; Venkatesan Kalavai, S.; Yoshii-Kitahara, A.; Muraoka, S.; Bhatt, N.; Takamatsu-Yukawa, K.; Hu, J.; Wang, Y.; Hersh, S. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain 2021, 144, 288–309. [Google Scholar] [CrossRef]
- Aulston, B.; Liu, Q.; Mante, M.; Florio, J.; Rissman, R.A.; Yuan, S.H. Extracellular vesicles isolated from familial Alzheimer’s disease neuronal cultures induce aberrant tau phosphorylation in the wild-type mouse brain. J. Alzheimer’s Dis. 2019, 72, 575–585. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Stuendl, A.; Kunadt, M.; Kruse, N.; Bartels, C.; Moebius, W.; Danzer, K.M.; Mollenhauer, B.; Schneider, A. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain 2016, 139, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Piao, H.; Wang, Y.; Zheng, D.; Wang, W. Circulating exosomes in cardiovascular disease: Novel carriers of biological information. Biomed. Pharmacother. 2021, 135, 111148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Cheng, Q.; Bai, L.; Huang, S.; Gao, J. Extracellular vesicles in cardiovascular diseases: Diagnosis and therapy. Front. Cell Dev. Biol. 2022, 10, 875376. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Ahmed, S.; Johnson, M.; Saeedullah, U.; De Leon, J. Exosomes in Cardiovascular Disease: From Mechanism to Therapeutic Target. Metabolites 2023, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Buffolo, F.; Monticone, S.; Camussi, G.; Aikawa, E. Role of extracellular vesicles in the pathogenesis of vascular damage. Hypertension 2022, 79, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Otani, K.; Okada, M.; Yamawaki, H. Plasma small extracellular vesicles in hypertensive rats impair reactivity of isolated blood vessels. J. Vet. Med. Sci. 2020, 82, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Drożdż, D.; Drożdż, M.; Wójcik, M. Endothelial dysfunction as a factor leading to arterial hypertension. Pediatr. Nephrol. 2022, 38, 2973–2985. [Google Scholar] [CrossRef]
- Barsom, S.H.; Glasstetter, L.M.; Siddiqi, S.; Rajagopalan, K.S.; Eirin, A.; Lerman, L.O. Emergent players in renovascular disease. Clin. Sci. 2022, 136, 239–256. [Google Scholar] [CrossRef]
- Rui, R.; Yang, H.; Liu, Y.; Zhou, Y.; Xu, X.; Li, C.; Liu, S. Effects of berberine on atherosclerosis. Front. Pharmacol. 2021, 3074, 764175. [Google Scholar] [CrossRef]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, M.; Lu, Y.; Han, X.; Yu, L.; Zhang, W.; Liu, S.; Liu, Y. Advances in Molecular Pathology of Obstructive Sleep Apnea. Molecules 2022, 27, 8422. [Google Scholar]
- Jang, S.; Palzer, E.F.; Rudser, K.D.; Fox, C.K.; Hebbel, R.P.; Dengel, D.R.; Milbauer, L.; Kelly, A.S.; Ryder, J.R. Relationship of Endothelial Microparticles to Obesity and Cardiovascular Disease Risk in Children and Adolescents. J. Am. Heart Assoc. 2022, 11, e026430. [Google Scholar] [CrossRef] [PubMed]
- Nik Ibrahim, N.N.I.; Abdul Rahman, R.; Azlan, M.; Abd Aziz, A.; Ghulam Rasool, A.H. Endothelial Microparticles as Potential Biomarkers in the Assessment of Endothelial Dysfunction in Hypercholesterolemia. Medicina 2022, 58, 824. [Google Scholar] [CrossRef]
- Grange, C.; Bussolati, B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022, 18, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhao, B.; Gui, L.; Sun, X.; Zhang, Z.; Huang, L. The role and mechanism of miR-92a in endothelial autophagy. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, X.; Liu, Q.; Yang, T.; Qu, H.; Zhou, H. Extracellular vesicles mediate biological information delivery: A double-edged sword in cardiac remodeling after myocardial infarction. Front. Pharmacol. 2023, 14, 1067992. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Wang, C.; Xiong, J.; Zhao, J.; Yang, K. Activated platelets, the booster of chronic kidney disease and cardiovascular complications. Kidney Dis. 2022, 8, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Figuer, A.; Alique, M.; Valera, G.; Serroukh, N.; Ceprían, N.; de Sequera, P.; Morales, E.; Carracedo, J.; Ramírez, R.; Bodega, G. New mechanisms involved in the development of cardiovascular disease in chronic kidney disease. Nefrología 2023, 43, 63–80. [Google Scholar] [CrossRef]
- Li, N.; Wu, B.; Wang, J.; Yan, Y.; An, P.; Li, Y.; Liu, Y.; Hou, Y.; Qing, X.; Niu, L. Differential proteomic patterns of plasma extracellular vesicles show potential to discriminate β-thalassemia subtypes. Iscience 2023, 26, 106048. [Google Scholar] [CrossRef]
- Carandina, A.; Favero, C.; Sacco, R.M.; Hoxha, M.; Torgano, G.; Montano, N.; Bollati, V.; Tobaldini, E. The Role of Extracellular Vesicles in Ischemic Stroke Severity. Biology 2022, 11, 1489. [Google Scholar] [CrossRef]
- Suades, R.; Padró, T.; Vilahur, G.; Badimon, L. Platelet-released extracellular vesicles: The effects of thrombin activation. Cell. Mol. Life Sci. 2022, 79, 190. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud, M.F.; Abdelmaksoud, S.S.; Abdelsamee, H.F.; Ezzelregal, H.G.; Alfeky, M.A. Platelets derived microparticles in COVID-19: Correlation to inflammatory and coagulation state. J. Appl. Hematol. 2021, 12, 195. [Google Scholar]
- Ebeyer-Masotta, M.; Eichhorn, T.; Weiss, R.; Lauková, L.; Weber, V. Activated platelets and platelet-derived extracellular vesicles mediate COVID-19-associated immunothrombosis. Front. Cell Dev. Biol. 2022, 10, 914891. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Bozbas, E.; Tannetta, S.; Alroqaiba, N.; Zhou, R.; Crawley, J.; Gibbins, J.; Jones, C.; Ahnström, J.; Yaqoob, P. Mode of induction of platelet-derived extracellular vesicles is a critical determinant of their phenotype and function. Sci. Rep. 2020, 10, 18061. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Pruner, I.; Antovic, A.; Taxiarchis, A.; Vila, Z.P.; Soutari, N.; Mobarrez, F.; Chaireti, R.; Widengren, J.; Piguet, J. Phosphatidylserine positive microparticles improve hemostasis in in-vitro hemophilia A plasma models. Sci. Rep. 2020, 10, 7871. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, R.I.; Nabiullina, R.M.; Zubairova, L.D.; Shakurova, M.A.; Andrianova, I.A.; Weisel, J.W. Lytic susceptibility, structure, and mechanical properties of fibrin in systemic lupus erythematosus. Front. Immunol. 2019, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S.; Kim, S. Shedding Light on the Cell Biology of Platelet-Derived Extracellular Vesicles and Their Biomedical Applications. Life 2023, 13, 1403. [Google Scholar] [CrossRef]
- Eustes, A.S.; Dayal, S. The role of platelet-derived extracellular vesicles in immune-mediated thrombosis. Int. J. Mol. Sci. 2022, 23, 7837. [Google Scholar] [CrossRef]
- Mabrouk, M.; Guessous, F.; Naya, A.; Merhi, Y.; Zaid, Y. The Pathophysiological Role of Platelet-Derived Extracellular Vesicles. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2022. [Google Scholar]
- Garcia, C.; Compagnon, B.; Poëtte, M.; Gratacap, M.-P.; Lapébie, F.-X.; Voisin, S.; Minville, V.; Payrastre, B.; Vardon-Bounes, F.; Ribes, A. Platelet versus megakaryocyte: Who is the real bandleader of thromboinflammation in sepsis? Cells 2022, 11, 1507. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q. Platelet-Derived Microparticles and Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 10275. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Żuber, Z.; Wojtkiewicz, M.; Wojtkiewicz, J. Role of microparticles in the pathogenesis of inflammatory joint diseases. Int. J. Mol. Sci. 2019, 20, 5453. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Zhao, Z.; Yang, Y.; Meng, Z.; Qin, L. Effects of the interactions between platelets with other cells in tumor growth and progression. Front. Immunol. 2023, 14, 1165989. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Hisada, Y. Therapeutic potential of granulocyte microvesicles in sepsis. Blood J. Am. Soc. Hematol. 2022, 139, 2269–2271. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, R.; Xing, W.; Chen, X.; Wan, J.; Wang, S.; Xiong, L.; Nandakumar, K.S.; Holmdahl, R.; Geng, H. Platelets derived citrullinated proteins and microparticles are potential autoantibodies ACPA targets in RA patients. Front. Immunol. 2023, 14, 1084283. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, S.; Feldbrügge, L.; Csizmadia, E.; Mitsuhashi, M.; Robson, S.C.; Moss, A.C. Luminal extracellular vesicles (EVs) in inflammatory bowel disease(IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm. Bowel Dis. 2016, 22, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Digby, J.E.; Cahill, T.J.; Tavare, A.N.; Corbin, A.L.; Saluja, S.; Dawkins, S.; Edgar, L.; Rawlings, N.; Ziberna, K. Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight 2017, 2, e93344. [Google Scholar] [CrossRef]
- Jansen, F.; Nickenig, G.; Werner, N. Extracellular vesicles in cardiovascular disease: Potential applications in diagnosis, prognosis, and epidemiology. Circ. Res. 2017, 120, 1649–1657. [Google Scholar] [CrossRef]
- Atehortúa, L.; Rojas, M.; Vásquez, G.; Muñoz-Vahos, C.H.; Vanegas-García, A.; Posada-Duque, R.A.; Castaño, D. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Zhang, A.; Wang, M.; Fang, Z.; Zhang, J. Exosomes: An emerging factor in atherosclerosis. Biomed. Pharmacother. 2019, 115, 108951. [Google Scholar] [CrossRef]
- Blonda, M.; Amoruso, A.; Martino, T.; Avolio, C. New insights into immune cell-derived extracellular vesicles in multiple sclerosis. Front. Neurol. 2018, 9, 604. [Google Scholar] [CrossRef]
- Casella, G.; Colombo, F.; Finardi, A.; Descamps, H.; Ill-Raga, G.; Spinelli, A.; Podini, P.; Bastoni, M.; Martino, G.; Muzio, L. Extracellular vesicles containing IL-4 modulate neuroinflammation in a mouse model of multiple sclerosis. Mol. Ther. 2018, 26, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hohjoh, H.; Yamamura, T. The role for exosomal microRNAs in disruption of regulatory T cell homeostasis in multiple sclerosis. J. Exp. Neurosci. 2018, 12, 1179069518764892. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S. Evolving story of autoantibodies in systemic lupus erythematosus. J. Autoimmun. 2020, 110, 102356. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Luo, S.; Xiao, Y.; Xia, Y.; Li, X.; Huang, G.; Xie, Z.; Zhou, Z. Emerging roles of exosomes in T1DM. Front. Immunol. 2020, 11, 593348. [Google Scholar] [CrossRef]
- Wu, W.-C.; Song, S.-J.; Zhang, Y.; Li, X. Role of extracellular vesicles in autoimmune pathogenesis. Front. Immunol. 2020, 11, 579043. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Tian, Y.; Huang, W.; Tong, N.; Fu, X. Integrative biology of extracellular vesicles in diabetes mellitus and diabetic complications. Theranostics 2022, 12, 1342. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, A.; Kadoi, Y.; Tsuruda, T.; Kim, Y.-S.; Miyoshi, M.; Nomoto, Y.; Nakata, Y.; Miyake, M.; Miyashita, K.; Shimizu, K. Exosomes in ascites from patients with human pancreatic cancer enhance remote metastasis partially through endothelial-mesenchymal transition. Pancreatology 2023, 23, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cai, H.; Deng, R.; Cheng, J.; Shi, Y. Effects of exosomes on tumor immunomodulation and their potential clinical applications. Int. J. Oncol. 2022, 61, 1–14. [Google Scholar] [CrossRef]
- Kurt, F.G.O.; Lasser, S.; Arkhypov, I.; Utikal, J.; Umansky, V. Enhancing immunotherapy response in melanoma: Myeloid-derived suppressor cells as a therapeutic target. J. Clin. Investig. 2023, 133, e170762. [Google Scholar]
- Law, Z.-J.; Khoo, X.H.; Lim, P.T.; Goh, B.H.; Ming, L.C.; Lee, W.-L.; Goh, H.P. Extracellular vesicle-mediated chemoresistance in oral squamous cell carcinoma. Front. Mol. Biosci. 2021, 8, 629888. [Google Scholar] [CrossRef]
- Li, Z.; Fang, R.; Fang, J.; He, S.; Liu, T. Functional implications of Rab27 GTPases in cancer. Cell Commun. Signal. 2018, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- ICH S7A Safety Pharmacology Studies for Human Pharmaceuticals; European Medicines Agency: London, UK, 2000.
- FDA. Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals S6(R1). In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Prague, Czech Republic, 31 October–1 November 2023; FDA: Silver Spring, MD, USA, 2011. [Google Scholar]
- Committee for Human Medicinal Products (CHMP). 2009. Available online: https://www.ema.europa.eu/en/committees/committee-medicinal-products-human-use-chmp (accessed on 25 December 2023).
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.D. Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Decision of the Gdansk Court of Appeal 26 June 2012—Case No. I ACa 320/12. “Bolar Exemption–Poland”; Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. IIC Int. Rev. Intellect. Prop. Compet. Law 2013, 44, 366–368.
- Ramati, Y. Accelerated Access of Advanced Regenerative Therapies: An Industry Perspective. Cell Gene Ther. Insights 2018, 4, 555–561. [Google Scholar] [CrossRef]
- Al-Jipouri, A.; Almurisi, S.H.; Al-Japairai, K.; Bakar, L.M.; Doolaanea, A.A. Liposomes or extracellular vesicles: A comprehensive comparison of both lipid bilayer vesicles for pulmonary drug delivery. Polymers 2023, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Guo, N.; Cai, L.; Wang, M.; Zhu, L.; Li, F.; Jin, L.; Sui, C. Extracellular vesicles from human Fallopian tubal fluid benefit embryo development in vitro. Hum. Reprod. Open 2023, 2023, hoad006. [Google Scholar] [CrossRef] [PubMed]
- Billing, A.M.; Dib, S.S.; Bhagwat, A.M.; da Silva, I.T.; Drummond, R.D.; Hayat, S.; Al-Mismar, R.; Ben-Hamidane, H.; Goswami, N.; Engholm-Keller, K. A Systems-level Characterization of the Differentiation of Human Embryonic Stem Cells into Mesenchymal Stem Cells*[S]. Mol. Cell. Proteom. 2019, 18, 1950–1966. [Google Scholar] [CrossRef]
- de Alcântara-Neto, A.S.; Cuello, C.; Uzbekov, R.; Bauersachs, S.; Mermillod, P.; Almiñana, C. Oviductal extracellular vesicles enhance porcine in vitro embryo development by modulating the embryonic transcriptome. Biomolecules 2022, 12, 1300. [Google Scholar] [CrossRef]
- Avni, D.; Avni, O. Extracellular vesicles: Schistosomal long-range precise weapon to manipulate the immune response. Front. Cell. Infect. Microbiol. 2021, 11, 196. [Google Scholar] [CrossRef]
- Soltani, S.; Mansouri, K.; Emami Aleagha, M.S.; Moasefi, N.; Yavari, N.; Shakouri, S.K.; Notararigo, S.; Shojaeian, A.; Pociot, F.; Yarani, R. Extracellular vesicle therapy for type 1 diabetes. Front. Immunol. 2022, 1574, 865782. [Google Scholar] [CrossRef]
- Bonetto, V.; Grilli, M. Neural stem cell-derived extracellular vesicles: Mini players with key roles in neurogenesis, immunomodulation, neuroprotection and aging. Front. Mol. Biosci. 2023, 10, 1187263. [Google Scholar] [CrossRef] [PubMed]
- Misawa, T.; Hitomi, K.; Miyata, K.; Tanaka, Y.; Fujii, R.; Chiba, M.; Loo, T.M.; Hanyu, A.; Kawasaki, H.; Kato, H. Identification of Novel Senescent Markers in Small Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 2421. [Google Scholar] [CrossRef] [PubMed]
- Loric, S.; Denis, J.A.; Desbene, C.; Sabbah, M.; Conti, M. Extracellular Vesicles in Breast Cancer: From Biology and Function to Clinical Diagnosis and Therapeutic Management. Int. J. Mol. Sci. 2023, 24, 7208. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.D.; Rimmer, M.P. Extracellular vesicles arising from apoptosis: Forms, functions, and applications. J. Pathol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hong, J.; Liang, C.; Li, Y.; Gao, L.; Wu, L.; Yao, R.; Zhang, Y. Endothelial cell-released extracellular vesicles trigger pyroptosis and vascular inflammation to induce atherosclerosis through the delivery of HIF1A-AS2. FASEB J. 2023, 37, e22942. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yang, Y.; Yang, W.; Han, Y.; Huang, L.; Yang, R.; Hu, Z.; Tao, Y.; Liu, L.; Li, Y. Exosomal cargos-mediated metabolic reprogramming in tumor microenvironment. J. Exp. Clin. Cancer Res. 2023, 42, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; Leng, C.; Burenkova, O.; Jang, S.C.; McCoy, C.; Zhang, K.; Dooley, K.; Kasera, S.; Zi, T.; Sisó, S. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci. Adv. 2022, 8, eabj7002. [Google Scholar] [CrossRef]
- Ma, F.; Vayalil, J.; Lee, G.; Wang, Y.; Peng, G. Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J. Immunother. Cancer 2021, 9, e003217. [Google Scholar] [CrossRef]
- Di Bella, M.A. Overview and update on extracellular vesicles: Considerations on exosomes and their application in modern medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Ross, T.J.; Zhang, J. The Microbiome-TIME Axis: A Host of Possibilities. Microorganisms 2023, 11, 288. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-García, N.; Huete-Acevedo, J.; Dromant, M.; Borrás, C. Extracellular vesicles as therapeutic resources in the clinical environment. Int. J. Mol. Sci. 2023, 24, 2344. [Google Scholar] [CrossRef] [PubMed]
- Duong, A.; Parmar, G.; Kirkham, A.M.; Burger, D.; Allan, D.S. Registered clinical trials investigating treatment with cell-derived extracellular vesicles: A scoping review. Cytotherapy 2023, 11, 288. [Google Scholar] [CrossRef]
- Amarasinghe, I.; Phillips, W.; Hill, A.F.; Cheng, L.; Helbig, K.J.; Willms, E.; Monson, E.A. Cellular communication through extracellular vesicles and lipid droplets. J. Extracell. Biol. 2023, 2, e77. [Google Scholar] [CrossRef]
- Ma, Z.-J.; Yang, J.-J.; Lu, Y.-B.; Liu, Z.-Y.; Wang, X.-X. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J. Stem Cells 2020, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, 12283. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 1–21. [Google Scholar] [CrossRef]
- Huang, D.; Rao, D.; Xi, X.; Zhang, Z.; Zhong, T. Application of extracellular vesicles proteins in cancer diagnosis. Front. Cell Dev. Biol. 2022, 10, 1007360. [Google Scholar] [CrossRef]
- Bhatia, R.; Chang, J.; Munoz, J.L.; Walker, N.D. Forging New Therapeutic Targets: Efforts of Tumor Derived Exosomes to Prepare the Pre-Metastatic Niche for Cancer Cell Dissemination and Dormancy. Biomedicines 2023, 11, 1614. [Google Scholar] [CrossRef]
- Gangadaran, P.; Madhyastha, H.; Madhyastha, R.; Rajendran, R.L.; Nakajima, Y.; Watanabe, N.; Velikkakath, A.K.G.; Hong, C.M.; Gopi, R.V.; Muthukalianan, G.K. The emerging role of exosomes in innate immunity, diagnosis and therapy. Front. Immunol. 2023, 13, 1085057. [Google Scholar] [CrossRef]
- Fu, M.; Li, J.; Battulga, T.; Li, X.; Xu, M. Biological Functions and Applications of Exosomes in Drug Research. Int. J. Drug Discov. Pharmacol. 2023, 2, 1–9. [Google Scholar] [CrossRef]
- Kang, F.; Yan, Y.; Liu, Y.; Liang, Q.; Xu, Z.; Zhu, W.; Thakur, A. Unraveling the significance of exosomal circRNAs in cancer therapeutic resistance. Front. Pharmacol. 2023, 14, 1093175. [Google Scholar] [CrossRef] [PubMed]
- Mezzasoma, L.; Bellezza, I.; Romani, R.; Talesa, V.N. Extracellular Vesicles and the Inflammasome: An Intricate Network Sustaining Chemoresistance. Front. Oncol. 2022, 1602, 888135. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Yu, Y.; Yang, Z. Progression of Exosome-Mediated Chemotherapy Resistance in Cancer. Oncologie 2022, 24, 247–259. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Redegeld, F.; Asghari, M.H.; Mosaffa, N.; Mortaz, E. Long-chain polyunsaturated omega-3 fatty acids reduce multiple myeloma exosome-mediated suppression of NK cell cytotoxicity. DARU J. Pharm. Sci. 2020, 28, 647–659. [Google Scholar] [CrossRef]
- Aslan, C.; Maralbashi, S.; Kahroba, H.; Asadi, M.; Soltani-Zangbar, M.S.; Javadian, M.; Shanehbandi, D.; Baradaran, B.; Darabi, M.; Kazemi, T. Docosahexaenoic acid(DHA) inhibits pro-angiogenic effects of breast cancer cells via down-regulating cellular and exosomal expression of angiogenic genes and microRNAs. Life Sci. 2020, 258, 118094. [Google Scholar] [CrossRef]
- Ghaffari-Makhmalbaf, P.; Sayyad, M.; Pakravan, K.; Razmara, E.; Bitaraf, A.; Bakhshinejad, B.; Goudarzi, P.; Yousefi, H.; Pournaghshband, M.; Nemati, F. Docosahexaenoic acid reverses the promoting effects of breast tumor cell-derived exosomes on endothelial cell migration and angiogenesis. Life Sci. 2021, 264, 118719. [Google Scholar] [CrossRef]
- Datta, A.; Kim, H.; McGee, L.; Johnson, A.E.; Talwar, S.; Marugan, J.; Southall, N.; Hu, X.; Lal, M.; Mondal, D. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Sci. Rep. 2018, 8, 8161. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Liu, J.; Zhang, G.; Lu, A. Advances in the discovery of exosome inhibitors in cancer. J. Enzym. Inhib. Med. Chem. 2020, 35, 1322–1330. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.-H.; Baek, M.-C. Dissecting exosome inhibitors: Therapeutic insights into small-molecule chemicals against cancer. Exp. Mol. Med. 2022, 54, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Kim, H.; Lal, M.; McGee, L.; Johnson, A.; Moustafa, A.A.; Jones, J.C.; Mondal, D.; Ferrer, M.; Abdel-Mageed, A.B. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 2017, 408, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Asghari, M.H.; Mortaz, E. Inhibiting exosomal MIC-A and MIC-B shedding of cancer cells to overcome immune escape: New insight of approved drugs. DARU J. Pharm. Sci. 2019, 27, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Yamashita, N.; Daimon, T.; Hirose, H.; Yamano, S.; Haratake, N.; Ishikawa, S.; Bhattacharya, A.; Fushimi, A.; Ahmad, R. MUC1-C is a master regulator of MICA/B NKG2D ligand and exosome secretion in human cancer cells. J. Immunother. Cancer 2023, 11, e006238. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Jia, J.; Yao, L.; Li, Z. Crosstalk of exosomal non-coding RNAs in the tumor microenvironment: Novel frontiers. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.J.; Kima, P.E. Current understanding of extracellular vesicle homing/tropism. Zoonoses (Burlingt. Mass.) 2022, 2, 14. [Google Scholar] [CrossRef]
- Ragni, E.; Parolini, O.; Silini, A.R. MSC-Derived Extracellular Vesicles and Secreted Factors as “Cell-Free” Therapeutic Alternatives in Regenerative Medicine. Front. Bioeng. Biotechnol. 2022, 10, 56. [Google Scholar] [CrossRef]
- Yang, G.; Fan, X.; Liu, Y.; Jie, P.; Mazhar, M.; Liu, Y.; Dechsupa, N.; Wang, L. Immunomodulatory Mechanisms and Therapeutic Potential of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2023, 19, 1214–1231. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, T.; He, Z.; Cai, R.; Pang, W. Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte 2021, 10, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Crum, R.J.; Capella-Monsonís, H.; Badylak, S.F.; Hussey, G.S. Extracellular vesicles for regenerative medicine applications. Appl. Sci. 2022, 12, 7472. [Google Scholar] [CrossRef]
- Ju, Y.; Fang, B. Research advances on the mechanism of extracellular vesicles of adipose-derived mesenchymal stem cells in promoting wound angiogenesis. Zhonghua Shao Shang Za Zhi = Zhonghua Shaoshang Zazhi = Chin. J. Burn. 2023, 39, 85–90. [Google Scholar]
- van de Wakker, S.I.; Meijers, F.M.; Sluijter, J.P.G.; Vader, P. Extracellular vesicle heterogeneity and its impact for regenerative medicine applications. Pharmacol. Rev. 2023, 75, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, S.; Yu, Q.; Chen, T.; Liu, D. Application of stem cells in regeneration medicine. MedComm 2023, 4, e291. [Google Scholar] [CrossRef] [PubMed]
- Namjoo, A.R.; Abrbekoh, F.N.; Saghati, S.; Amini, H.; Saadatlou, M.A.E.; Rahbarghazi, R. Tissue engineering modalities in skeletal muscles: Focus on angiogenesis and immunomodulation properties. Stem Cell Res. Ther. 2023, 14, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.-E.; Sim, C.; Chung, Y.; Kim, H.K.; Park, S.; Kim, D.K.; Cho, S.; Lee, S. Skeletal muscle regeneration by the exosomes of adipose tissue-derived mesenchymal stem cells. Curr. Issues Mol. Biol. 2021, 43, 1473–1488. [Google Scholar] [CrossRef]
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Commun. Signal. 2020, 18, 1–16. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Xu, L.; Qian, Y.; Liu, N.; Zhou, C.; Liu, J.; Zhou, L.; Xu, Z.; Jia, R. Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment. Stem Cell Res. Ther. 2022, 13, 238. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Tang, M.; Gao, X.; Liu, W.; Wei, S. Mesenchymal Stem Cell-Derived Exosomes in Cardioprotection: A Novel Application to Prevent Myocardial Injury. Rev. Cardiovasc. Med. 2022, 23, 310. [Google Scholar] [CrossRef]
- Mao, B.; Yuan, W.; Wu, F.; Yan, Y.; Wang, B. Autophagy in hepatic ischemia–reperfusion injury. Cell Death Discov. 2023, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Ahmad, H.; Lin, G.; Carbonneau, M.; Tran, S.D. Mesenchymal Stem Cell-Derived Exosomes in Ophthalmology: A Comprehensive Review. Pharmaceutics 2023, 15, 1167. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, J.; Chen, L. The roles of mesenchymal stem cell-derived exosomes in diabetes mellitus and its related complications. Front. Endocrinol. 2022, 13, 1027686. [Google Scholar] [CrossRef] [PubMed]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Ferrarotti, I.; Perteghella, S.; Di Silvestre, D.; Rossi, R.; Mauri, P.; Grisoli, P.; Barzon, V.; Balderacchi, A.; Corsico, A. Mesenchymal Extracellular Vesicles as Alpha-1-antitrypsin physiological delivery systems for lung regeneration. In Proceedings of the 19th Advanced School in Pharmaceutical Technology, Soverato, Italy, 9–12 September 2019. [Google Scholar]
- Zhang, L.; Song, Y.; Chen, L.; Li, D.; Feng, H.; Lu, Z.; Fan, T.; Chen, Z.; Livingston, M.J.; Geng, Q. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J. Cell. Physiol. 2020, 235, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Xu, W.; Liu, J.; Wang, S.; Jiang, H. The role of autophagy in calcium oxalate kidney stone: A systematic review of the literature. Front. Physiol. 2022, 1981. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Melero, L.; Hernandez, R.M.; Santos-Vizcaino, E.; Igartua, M. Tumour-derived extracellular vesicle based vaccines for melanoma treatment. Drug Deliv. Transl. Res. 2023, 13, 1520–1542. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.; D’Aulerio, R.; Yong, T.; He, M.; Baptista, M.A.; Nylén, S.; Westerberg, L.S. Increased cross-presentation by dendritic cells and enhanced anti-tumour therapy using the Arp2/3 inhibitor CK666. Br. J. Cancer 2023, 128, 982–991. [Google Scholar] [CrossRef]
- Iglesias-Escudero, M.; Arias-González, N.; Martínez-Cáceres, E. Regulatory cells and the effect of cancer immunotherapy. Mol. Cancer 2023, 22, 26. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—New insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological Consequences of MHC-II Expression by Tumor Cells in CancerBiological Consequences of MHC-II Expression by Tumor Cells. Clin. Cancer Res. 2019, 25, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond just peptide antigens: The complex world of peptide-based cancer vaccines. Front. Immunol. 2021, 12, 696791. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-G.; Sang, Y.-B.; Lee, J.-H.; Chon, H.-J. Combining cancer vaccines with immunotherapy: Establishing a new immunological approach. Int. J. Mol. Sci. 2021, 22, 8035. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, H.; Li, L.; Wang, X.; Yu, Z.; Li, J. Peptide-based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif. 2021, 54, e13025. [Google Scholar] [CrossRef] [PubMed]
- Nin, D.S.; Deng, L.-W. Biology of Cancer-Testis Antigens and Their Therapeutic Implications in Cancer. Cells 2023, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef]
- Naseri, M.; Bozorgmehr, M.; Zöller, M.; Ranaei Pirmardan, E.; Madjd, Z. Tumor-derived exosomes: The next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology 2020, 9, 1779991. [Google Scholar] [CrossRef]
- Raguraman, R.; Bhavsar, D.; Kim, D.; Ren, X.; Sikavitsas, V.; Munshi, A.; Ramesh, R. Tumor-targeted exosomes for delivery of anticancer drugs. Cancer Lett. 2023, 558, 216093. [Google Scholar] [CrossRef]
- Martínez-Santillán, A.; González-Valdez, J. Novel Technologies for Exosome and Exosome-like Nanovesicle Procurement and Enhancement. Biomedicines 2023, 11, 1487. [Google Scholar] [CrossRef]
- Shao, M.; Lopes, D.; Lopes, J.; Yousefiasl, S.; Macário-Soares, A.; Peixoto, D.; Ferreira-Faria, I.; Veiga, F.; Conde, J.; Huang, Y. Exosome membrane-coated nanosystems: Exploring biomedical applications in cancer diagnosis and therapy. Matter 2023, 6, 761–799. [Google Scholar] [CrossRef]
- Gulati, R.; Nandi, D.; Sarkar, K.; Venkataraman, P.; Ramkumar, K.; Ranjan, P.; Janardhanan, R. Exosomes as theranostic targets: Implications for the clinical prognosis of aggressive cancers. Front. Mol. Biosci. 2022, 9, 890768. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Dhar, R.; Mukherjee, S.; Nag, S.; Gorai, S.; Mukerjee, N.; Mukherjee, D.; Vatsa, R.; Chandrakanth Jadhav, M.; Ghosh, A. Exosome-Based Smart Drug Delivery Tool for Cancer Theranostics. ACS Biomater. Sci. Eng. 2023, 9, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Kashanchi, F.; Jafari, R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 1–20. [Google Scholar] [CrossRef]
- Rädler, J.; Gupta, D.; Zickler, A.; Andaloussi, S.E. Exploiting the biogenesis of EVs for bioengineering and therapeutic cargo loading. Mol. Ther. 2023, 31, 1231–1250. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, D.J. Potential for Therapeutic-Loaded Exosomes to Ameliorate the Pathogenic Effects of α-Synuclein in Parkinson’s Disease. Biomedicines 2023, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-H.; Hsieh, S.-C.; Li, T.-H.; Lu, C.-H.; Liao, H.-T.; Shen, C.-Y.; Li, K.-J.; Wu, C.-H.; Kuo, Y.-M.; Tsai, C.-Y. Molecular Basis for Paradoxical Activities of Polymorphonuclear Neutrophils in Inflammation/Anti-Inflammation, Bactericide/Autoimmunity, Pro-Cancer/Anticancer, and Antiviral Infection/SARS-CoV-II-Induced Immunothrombotic Dysregulation. Biomedicines 2022, 10, 773. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, S.; Liu, L.; Dang, P.; Liu, Y.; Sun, Z.; Qiao, B.; Wang, C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 124. [Google Scholar] [CrossRef]
- Hussen, B.M.; Faraj, G.S.H.; Rasul, M.F.; Hidayat, H.J.; Salihi, A.; Baniahmad, A.; Taheri, M.; Ghafouri-Frad, S. Strategies to overcome the main challenges of the use of exosomes as drug carrier for cancer therapy. Cancer Cell Int. 2022, 22, 1–23. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Macion, A.; Wyszyńska, A.; Godlewska, R. Delivery of toxins and effectors by bacterial membrane vesicles. Toxins 2021, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, E.K.; McDowell, A.; Kim, Y.-K. Microbe-derived extracellular vesicles as a smart drug delivery system. Transl. Clin. Pharmacol. 2018, 26, 103. [Google Scholar] [CrossRef] [PubMed]
- Croatti, V.; Parolin, C.; Giordani, B.; Foschi, C.; Fedi, S.; Vitali, B. Lactobacilli extracellular vesicles: Potential postbiotics to support the vaginal microbiota homeostasis. Microb. Cell Factories 2022, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Giv, N.; Basas, A.; Hicks, C.; El-Omar, E.; El-Assaad, F.; Hosseini-Beheshti, E. Bacterial extracellular vesicles and their novel therapeutic applications in health and cancer. Front. Cell. Infect. Microbiol. 2022, 1661, 962216. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus vaccine research and development: The past, present and future, including novel therapeutic strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, E.; Al-Kaabawi, A.; Thune, R.; Simpson, M.R.; Pedersen, S.A.; Cione, E.; Jenmalm, M.C.; Tingö, L. Breast milk microRNAs: Potential players in oral tolerance development. Front. Immunol. 2023, 14, 1154211. [Google Scholar] [CrossRef]
- Kondracka, A.; Gil-Kulik, P.; Kondracki, B.; Frąszczak, K.; Oniszczuk, A.; Rybak-Krzyszkowska, M.; Staniczek, J.; Kwaśniewska, A.; Kocki, J. Occurrence, Role, and Challenges of MicroRNA in Human Breast Milk: A Scoping Review. Biomedicines 2023, 11, 248. [Google Scholar] [CrossRef]
- Mecocci, S.; Trabalza-Marinucci, M.; Cappelli, K. Extracellular vesicles from animal milk: Great potentialities and critical issues. Animals 2022, 12, 3231. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570. [Google Scholar] [CrossRef]
- Jiang, X.; You, L.; Zhang, Z.; Cui, X.; Zhong, H.; Sun, X.; Ji, C.; Chi, X. Biological properties of milk-derived extracellular vesicles and their physiological functions in infant. Front. Cell Dev. Biol. 2021, 9, 693534. [Google Scholar] [CrossRef]
- Mun, D.; Oh, S.; Kim, Y. Perspectives on bovine milk-derived extracellular vesicles for therapeutic applications in gut health. Food Sci. Anim. Resour. 2022, 42, 197. [Google Scholar] [CrossRef] [PubMed]
- Matic, S.; D’Souza, D.H.; Wu, T.; Pangloli, P.; Dia, V.P. Bovine milk exosomes affect proliferation and protect macrophages against cisplatin-induced cytotoxicity. Immunol. Investig. 2020, 49, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Krupova, Z.; Leroux, C.; Péchoux, C.; Bevilacqua, C.; Martin, P. Comparison of goat and cow milk-derived extracellular vesicle miRNomes. Sci. Data 2023, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; El-Magd, M.A.; AlSadrah, S.A. Therapeutic effect of camel milk and its exosomes on MCF7 cells in vitro and in vivo. Integr. Cancer Ther. 2018, 17, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Woodman, P.G.; Futter, C.E. Multivesicular bodies: Co-ordinated progression to maturity. Curr. Opin. Cell Biol. 2008, 20, 408–414. [Google Scholar] [CrossRef] [PubMed]
- O’sullivan, M.J.; Lindsay, A.J. The endosomal recycling pathway—At the crossroads of the cell. Int. J. Mol. Sci. 2020, 21, 6074. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Matsumori, N. Inimitable Impacts of Ceramides on Lipid Rafts Formed in Artificial and Natural Cell Membranes. Membranes 2022, 12, 727. [Google Scholar] [CrossRef]
- Redpath, G.M.; Betzler, V.M.; Rossatti, P.; Rossy, J. Membrane heterogeneity controls cellular endocytic trafficking. Front. Cell Dev. Biol. 2020, 8, 757. [Google Scholar] [CrossRef]
- Xu, M.; Ji, J.; Jin, D.; Wu, Y.; Wu, T.; Lin, R.; Zhu, S.; Jiang, F.; Ji, Y.; Bao, B. The biogenesis and secretion of exosomes and multivesicular bodies(MVBs): Intercellular shuttles and implications in human diseases. Genes Dis. 2022, 10, 1894–1907. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 1–19. [Google Scholar] [CrossRef]
- Lürick, A.; Kümmel, D.; Ungermann, C. Multisubunit tethers in membrane fusion. Curr. Biol. 2018, 28, R417–R420. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Galli, T. Role of SNAREs in Unconventional Secretion—Focus on the VAMP7-Dependent Secretion. Front. Cell Dev. Biol. 2022, 10, 884020. [Google Scholar] [CrossRef] [PubMed]
- Pilliod, J.; Desjardins, A.; Pernègre, C.; Jamann, H.; Larochelle, C.; Fon, E.A.; Leclerc, N. Clearance of intracellular tau protein from neuronal cells via VAMP8-induced secretion. J. Biol. Chem. 2020, 295, 17827–17841. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Elgner, F.; Himmelsbach, K.; Akhras, S.; Jiang, B.; Medvedev, R.; Ploen, D.; Hildt, E. Identification of syntaxin 4 as an essential factor for the hepatitis C virus life cycle. Eur. J. Cell Biol. 2017, 96, 542–552. [Google Scholar] [CrossRef]
- Bunz, M.; Ritter, M.; Schindler, M. HCV egress–unconventional secretion of assembled viral particles. Trends Microbiol. 2022, 30, 364–378. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, M.; Stewart, T.; Fernagut, P.-O.; Huang, Y.; Tian, C.; Dehay, B.; Atik, A.; Yang, D.; De Giorgi, F. Reduced oligodendrocyte exosome secretion in multiple system atrophy involves SNARE dysfunction. Brain 2020, 143, 1780–1797. [Google Scholar] [CrossRef]
- Lu, Z.; Luo, W.; Ding, L.; Wang, H.; Li, Y.; wen Yang, B.; Ren, L.; Zheng, Q.; Xie, H.; Wang, R. Exosomes in Genitourinary Cancers: Emerging Mediators of Drug Resistance and Promising Biomarkers. Int. J. Biol. Sci. 2023, 19, 167. [Google Scholar] [CrossRef]
- Very, N.; Yazidi-Belkoura, E. Targeting O-GlcNAcylation to overcome resistance to anti-cancer therapies. Front. Oncol. 2022, 12, 960312. [Google Scholar] [CrossRef]
- Arya, S.B.; Collie, S.P.; Parent, C.A. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2023. [Google Scholar] [CrossRef]
- Fu, X.; Song, J.; Yan, W.; Downs, B.M.; Wang, W.; Li, J. The biological function of tumor-derived extracellular vesicles on metabolism. Cell Commun. Signal. 2023, 21, 150. [Google Scholar] [CrossRef]
- Sun, C.; Wang, P.; Dong, W.; Liu, H.; Sun, J.; Zhao, L. LncRNA PVT1 promotes exosome secretion through YKT6, RAB7, and VAMP3 in pancreatic cancer. Aging (Albany NY) 2020, 12, 10427. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.S.R.; Pavitra, E.; Bandaru, S.S.; Varaprasad, G.L.; Nagaraju, G.P.; Malla, R.R.; Huh, Y.S.; Han, Y.-K. HOTAIR: A potential metastatic, drug-resistant and prognostic regulator of breast cancer. Mol. Cancer 2023, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal. 2023, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Gahan, P.B. MicroRNA shuttle from cell-to-cell by exosomes and its impact in cancer. Non-Coding RNA 2019, 5, 28. [Google Scholar] [CrossRef]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.; Zuhorn, I.S. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Gonda, A.; Kabagwira, J.; Senthil, G.N.; Wall, N.R. Internalization of exosomes through receptor-mediated endocytosis. Mol. Cancer Res. 2019, 17, 337–347. [Google Scholar] [CrossRef]
- Zhao, M.; Nanbo, A.; Sun, L.; Lin, Z. Extracellular vesicles in Epstein-Barr virus’ life cycle and pathogenesis. Microorganisms 2019, 7, 48. [Google Scholar] [CrossRef]
- Lischnig, A.; Bergqvist, M.; Ochiya, T.; Lässer, C. Quantitative proteomics identifies proteins enriched in large and small extracellular vesicles. Mol. Cell. Proteom. 2022, 21, 100273. [Google Scholar] [CrossRef]
- Sung, P.-S.; Hsieh, S.-L. C-type lectins and extracellular vesicles in virus-induced NETosis. J. Biomed. Sci. 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Somiya, M. Where does the cargo go?: Solutions to provide experimental support for the “extracellular vesicle cargo transfer hypothesis”. J. Cell Commun. Signal. 2020, 14, 135–146. [Google Scholar] [CrossRef]
- Ramos-Andrade, I.; Moraes, J.; Brandão-Costa, R.M.; da Silva, S.V.; de Souza, A.; da Silva, C.; Renovato-Martins, M.; Barja-Fidalgo, C. Obese adipose tissue extracellular vesicles raise breast cancer cell malignancy. Endocr.-Relat. Cancer 2020, 27, 571–582. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Ughetto, S.; Mahjoum, S.; Nair, A.V.; Breakefield, X.O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 2022, 39, 110651. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Götz, J. Exosomal and vesicle-free tau seeds—Propagation and convergence in endolysosomal permeabilization. FEBS J. 2022, 289, 6891–6907. [Google Scholar] [CrossRef] [PubMed]
- Oshchepkova, A.; Zenkova, M.; Vlassov, V. Extracellular Vesicles for Therapeutic Nucleic Acid Delivery: Loading Strategies and Challenges. Int. J. Mol. Sci. 2023, 24, 7287. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood–brain barrier: A review. Fluids Barriers CNS 2022, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.-E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-derived extracellular vesicles breach the intact blood–brain barrier via transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef]
- Joshi, B.S.; Zuhorn, I.S. Heparan sulfate proteoglycan-mediated dynamin-dependent transport of neural stem cell exosomes in an in vitro blood–brain barrier model. Eur. J. Neurosci. 2021, 53, 706–719. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Ye, Z.; Xu, J. Engineering Extracellular Vesicles as Delivery Systems in Therapeutic Applications. Adv. Sci. 2023, 10, e2300552. [Google Scholar] [CrossRef]
- Toh, W.S.; Yarani, R.; El Andaloussi, S.; Cho, B.S.; Choi, C.; Corteling, R.; De Fougerolles, A.; Gimona, M.; Herz, J.; Khoury, M. A report on the International Society for Cell & Gene Therapy 2022 Scientific Signature Series,“Therapeutic advances with native and engineered human extracellular vesicles”. Cytotherapy 2023, 25, 810–814. [Google Scholar] [PubMed]
- Bao, C.; Xiang, H.; Chen, Q.; Zhao, Y.; Gao, Q.; Huang, F.; Mao, L. A Review of Labeling Approaches Used in Small Extracellular Vesicles Tracing and Imaging. Int. J. Nanomed. 2023, 18, 4567–4588. [Google Scholar] [CrossRef] [PubMed]
- Verweij, F.J.; Balaj, L.; Boulanger, C.M.; Carter, D.R.; Compeer, E.B.; D’angelo, G.; El Andaloussi, S.; Goetz, J.G.; Gross, J.C.; Hyenne, V. The power of imaging to understand extracellular vesicle biology in vivo. Nat. Methods 2021, 18, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef]
- Dehghani, M.; Gulvin, S.M.; Flax, J.; Gaborski, T.R. Systematic evaluation of PKH labelling on extracellular vesicle size by nanoparticle tracking analysis. Sci. Rep. 2020, 10, 9533. [Google Scholar] [CrossRef]
- Cha, M.; Jeong, S.H.; Bae, S.; Park, J.H.; Baeg, Y.; Han, D.W.; Kim, S.S.; Shin, J.; Park, J.E.; Oh, S.W. Efficient Labeling of Vesicles with Lipophilic Fluorescent Dyes via the Salt-Change Method. Anal. Chem. 2023, 95, 5843–5849. [Google Scholar] [CrossRef]
- Ashique, S.; Anand, K. Radiolabelled Extracellular Vesicles as Imaging Modalities for Precise Targeted Drug Delivery. Pharmaceutics 2023, 15, 1426. [Google Scholar] [CrossRef]
- Kooijmans, S.A.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Recombinant phosphatidylserine-binding nanobodies for targeting of extracellular vesicles to tumor cells: A plug-and-play approach. Nanoscale 2018, 10, 2413–2426. [Google Scholar] [CrossRef]
- Han, C.; Qin, G. Reporter systems for assessments of extracellular vesicle transfer. Front. Cardiovasc. Med. 2022, 9, 922420. [Google Scholar] [CrossRef]
- Chuo, S.T.-Y.; Chien, J.C.-Y.; Lai, C.P.-K. Imaging extracellular vesicles: Current and emerging methods. J. Biomed. Sci. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Cilliers, C.; Liao, J.; Atangcho, L.; Thurber, G.M. Residualization rates of near-infrared dyes for the rational design of molecular imaging agents. Mol. Imaging Biol. 2015, 17, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Gyurkó, I.; Pálóczi, K.; Buzás, E.I.; Horváth, I.; Hegedűs, N.; Máthé, D.; Szigeti, K. Radiolabeling of extracellular vesicles with 99mTc for quantitative in vivo imaging studies. Cancer Biother. Radiopharm. 2016, 31, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Kato, K.; Yamashita, T.; Imai, T.; Saji, H.; Takakura, Y. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J. Pharm. Sci. 2015, 104, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kutchy, N.A.; Ma, R.; Liu, Y.; Buch, S.; Hu, G. Extracellular vesicle-mediated delivery of ultrasmall superparamagnetic iron oxide nanoparticles to mice brain. Front. Pharmacol. 2022, 13, 819516. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: From exosomes to microvesicles. Cardiovasc. Res. 2023, 119, 45–63. [Google Scholar] [CrossRef]
- Santelices, J.; Ou, M.; Hui, W.W.; Maegawa, G.H.; Edelmann, M.J. Fluorescent labeling of small extracellular vesicles(EVs) isolated from conditioned media. Bio-Protoc. 2022, 12, e4447. [Google Scholar] [CrossRef]
- Kwon, Y.; Park, J. Methods to analyze extracellular vesicles at single particle level. Micro Nano Syst. Lett. 2022, 10, 14. [Google Scholar] [CrossRef]
- Corso, G.; Heusermann, W.; Trojer, D.; Görgens, A.; Steib, E.; Voshol, J.; Graff, A.; Genoud, C.; Lee, Y.; Hean, J. Systematic characterization of extracellular vesicle sorting domains and quantification at the single molecule–single vesicle level by fluorescence correlation spectroscopy and single particle imaging. J. Extracell. Vesicles 2019, 8, 1663043. [Google Scholar] [CrossRef]
- Silva, A.M.; Lázaro-Ibáñez, E.; Gunnarsson, A.; Dhande, A.; Daaboul, G.; Peacock, B.; Osteikoetxea, X.; Salmond, N.; Friis, K.P.; Shatnyeva, O. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J. Extracell. Vesicles 2021, 10, e12130. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Federzoni, E.A.; Wang, X.; Dharmawan, A.; Hu, X.; Wang, H.; Hawley, R.J.; Stevens, S.; Sykes, M. CD47 cross-dressing by extracellular vesicles expressing CD47 inhibits phagocytosis without transmitting cell death signals. eLife 2022, 11, e73677. [Google Scholar] [CrossRef] [PubMed]
- Cowzer, D.; Zameer, M.; Conroy, M.; Kolch, W.; Duffy, A.G. Targeting KRAS in pancreatic cancer. J. Pers. Med. 2022, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, M.; Bryniarski, K.; Nazimek, K. Biodelivery of therapeutic extracellular vesicles: Should mononuclear phagocytes always be feared? Front. Cell Dev. Biol. 2023, 11, 1211833. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Guo, Y.-L.; Tian, J.-W.; Zhang, H.-J.; Li, R.-F.; Gong, P.; Yu, Z.-L. Anti-Tumor Strategies by Harnessing the Phagocytosis of Macrophages. Cancers 2023, 15, 2717. [Google Scholar] [CrossRef] [PubMed]
- Rahimizadeh, P.; Yang, S.; Lim, S.I. Albumin: An emerging opportunity in drug delivery. Biotechnol. Bioprocess Eng. 2020, 25, 985–995. [Google Scholar] [CrossRef]
- Liang, X.; Niu, Z.; Galli, V.; Howe, N.; Zhao, Y.; Wiklander, O.P.; Zheng, W.; Wiklander, R.J.; Corso, G.; Davies, C. Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models. J. Extracell. Vesicles 2022, 11, e12248. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Patras, L.; Ionescu, A.E.; Munteanu, C.; Hajdu, R.; Kosa, A.; Porfire, A.; Licarete, E.; Rauca, V.F.; Sesarman, A.; Luput, L. Trojan horse treatment based on PEG-coated extracellular vesicles to deliver doxorubicin to melanoma in vitro and in vivo. Cancer Biol. Ther. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Gu, W.; Andrews, G.P.; Tian, Y. Recent Clinical Successes in Liposomal Nanomedicines. Int. J. Drug Discov. Pharmacol. 2023, 2, 52–59. [Google Scholar] [CrossRef]
- Han, L.; Zhao, Z.; He, C.; Li, J.; Li, X.; Lu, M. Removing the stumbling block of exosome applications in clinical and translational medicine: Expand production and improve accuracy. Stem Cell Res. Ther. 2023, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Ducrot, C.; Loiseau, S.; Wong, C.; Madec, E.; Volatron, J.; Piffoux, M. Hybrid extracellular vesicles for drug delivery. Cancer Lett. 2023, 558, 216107. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Liposomes for the Treatment of Brain Cancer—A Review. Pharmaceuticals 2023, 16, 1056. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Nagy, N.A.; Castenmiller, C.; Vigario, F.L.; Sparrius, R.; van Capel, T.M.; de Haas, A.M.; van Kooyk, Y.; van Ree, R.; Tas, S.W.; Geijtenbeek, T.B. Uptake kinetics of liposomal formulations of differing charge influences development of in vivo dendritic cell immunotherapy. J. Pharm. Sci. 2022, 111, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Montizaan, D.; Yang, K.; Reker-Smit, C.; Salvati, A. Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa cells. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102300. [Google Scholar] [CrossRef]
- Jyothi, V.G.S.; Bulusu, R.; Rao, B.V.K.; Pranothi, M.; Banda, S.; Bolla, P.K.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022. [Google Scholar]
- Zhao, Y.; Zheng, Y.; Zhu, Y.; Li, H.; Zhu, H.; Liu, T. Docetaxel-loaded M1 macrophage-derived exosomes for a safe and efficient chemoimmunotherapy of breast cancer. J. Nanobiotechnol. 2022, 20, 1–12. [Google Scholar] [CrossRef]
- Ilahibaks, N.F.; Ardisasmita, A.I.; Xie, S.; Gunnarsson, A.; Brealey, J.; Vader, P.; de Jong, O.G.; de Jager, S.; Dekker, N.; Peacock, B. TOP-EVs: Technology of Protein delivery through Extracellular Vesicles is a versatile platform for intracellular protein delivery. J. Control. Release 2023, 355, 579–592. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. An Effective Peptide-Based Platform for Efficient Exosomal Loading and Cellular Delivery of a microRNA. ACS Appl. Mater. Interfaces 2023, 15, 3851–3866. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.G.N.; Kang, J.H.; Kim, W.; Lim, J.; Kang, S.J.; You, J.Y.; Hoang, Q.T.; Kim, W.J.; Rhee, W.J.; Kim, C. Engineered extracellular vesicle-based sonotheranostics for dual stimuli-sensitive drug release and photoacoustic imaging-guided chemo-sonodynamic cancer therapy. Theranostics 2022, 12, 1247. [Google Scholar]
- Zhou, X.; Miao, Y.; Wang, Y.; He, S.; Guo, L.; Mao, J.; Chen, M.; Yang, Y.; Zhang, X.; Gan, Y. Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J. Extracell. Vesicles 2022, 11, e12198. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, L.; Wen, C.; Xia, J.; Zhang, Y.; Liang, Y. Programming assembly of biomimetic exosomes: An emerging theranostic nanomedicine platform. Mater. Today Bio 2023, 22, 100760. [Google Scholar] [CrossRef] [PubMed]
- Filipe, L.; de Sousa, T.; Silva, D.; Santos, M.M.; Ribeiro Carrott, M.; Poeta, P.; Branco, L.C.; Gago, S. In Vitro Antimicrobial Studies of Mesoporous Silica Nanoparticles Comprising Anionic Ciprofloxacin Ionic Liquids and Organic Salts. Pharmaceutics 2023, 15, 1934. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Albero, M.; Martín-Pardillos, A.; Lujan, L.; Sebastian, V.; Santamaria, J.; Martín-Duque, P. Exosomes loaded with ultrasmall Pt nanoparticles: A novel low-toxicity alternative to cisplatin. J. Nanobiotechnol. 2022, 20, 473. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Rakshit, M.; Chua, H.M.; Darwitan, A.; Nguyen, L.T.; Muktabar, A.; Venkatraman, S.; Ng, K.W. Liposome interaction with macrophages and foam cells for atherosclerosis treatment: Effects of size, surface charge and lipid composition. Nanotechnology 2021, 32, 505105. [Google Scholar] [CrossRef]
- Badran, M.M.; Alouny, N.N.; Aldosari, B.N.; Alhusaini, A.M.; Abou El Ela, A.E.S. Transdermal Glipizide Delivery System Based on Chitosan-Coated Deformable Liposomes: Development, Ex Vivo, and In Vivo Studies. Pharmaceutics 2022, 14, 826. [Google Scholar] [CrossRef]
- Mejia, F.; Khan, S.; Omstead, D.T.; Minetos, C.; Bilgicer, B. Identification and optimization of tunable endosomal escape parameters for enhanced efficacy in peptide-targeted prodrug-loaded nanoparticles. Nanoscale 2022, 14, 1226–1240. [Google Scholar] [CrossRef]
- Manoochehri, H.; Jalali, A.; Tanzadehpanah, H.; Taherkhani, A.; Najafi, R. Aptamer-conjugated nanoliposomes containing COL1A1 siRNA sensitize CRC cells to conventional chemotherapeutic drugs. Colloids Surf. B Biointerfaces 2022, 218, 112714. [Google Scholar] [CrossRef]
- Koga, K.; Tagami, T.; Ozeki, T. Gold nanoparticle-coated thermosensitive liposomes for the triggered release of doxorubicin, and photothermal therapy using a near-infrared laser. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127038. [Google Scholar] [CrossRef]
- Li, R.-T.; Zhu, Y.-D.; Li, W.-Y.; Hou, Y.-K.; Zou, Y.-M.; Zhao, Y.-H.; Zou, Q.; Zhang, W.-H.; Chen, J.-X. Synergistic photothermal-photodynamic-chemotherapy toward breast cancer based on a liposome-coated core–shell AuNS@ NMOFs nanocomposite encapsulated with gambogic acid. J. Nanobiotechnol. 2022, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pan, D.; Chen, S.; Martikainen, M.-V.; Kårlund, A.; Ke, J.; Pulkkinen, H.; Ruhanen, H.; Roponen, M.; Käkelä, R. Systematic design of cell membrane coating to improve tumor targeting of nanoparticles. Nat. Commun. 2022, 13, 6181. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-H.; Chang, Z.-X.; Huang, C.-Y.F.; Lee, L.J.; Liu, R.-S.; Hsiao, M. Integrated therapy platform of exosomal system: Hybrid inorganic/organic nanoparticles with exosomes for cancer treatment. Nanoscale Horiz. 2022, 7, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Zhao, C.; Wu, S.; Yang, D.; Zhang, C.; Wei, X.; Wei, X.; Su, H.; Liu, H.; Fan, Y. Hydrogel Loaded with VEGF/TFEB-Engineered Extracellular Vesicles for Rescuing Critical Limb Ischemia by a Dual-Pathway Activation Strategy. Adv. Healthc. Mater. 2022, 11, 2100334. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; He, R.; Liang, X.; Roudi, S.; Bost, J.; Coly, P.M.; van Niel, G.; Andaloussi, S.E. Cell-specific targeting of extracellular vesicles though engineering the glycocalyx. J. Extracell. Vesicles 2022, 11, 12290. [Google Scholar] [CrossRef]
- Richter, M.; Vader, P.; Fuhrmann, G. Approaches to surface engineering of extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 173, 416–426. [Google Scholar] [CrossRef]
- Huang, H.; Yi, X.; Wei, Q.; Li, M.; Cai, X.; Lv, Y.; Weng, L.; Mao, Y.; Fan, W.; Zhao, M. Edible and cation-free kiwi fruit derived vesicles mediated EGFR-targeted siRNA delivery to inhibit multidrug resistant lung cancer. J. Nanobiotechnol. 2023, 21, 1–14. [Google Scholar] [CrossRef]
- Villa, A.; Garofalo, M.; Crescenti, D.; Rizzi, N.; Brunialti, E.; Vingiani, A.; Belotti, P.; Sposito, C.; Franzè, S.; Cilurzo, F. Transplantation of autologous extracellular vesicles for cancer-specific targeting. Theranostics 2021, 11, 2034. [Google Scholar] [CrossRef]
- Lázaro-Ibáñez, E.; Faruqu, F.N.; Saleh, A.F.; Silva, A.M.; Tzu-Wen Wang, J.; Rak, J.; Al-Jamal, K.T.; Dekker, N. Selection of fluorescent, bioluminescent, and radioactive tracers to accurately reflect extracellular vesicle biodistribution in vivo. ACS Nano 2021, 15, 3212–3227. [Google Scholar] [CrossRef]
- Skotland, T.; Iversen, T.G.; Llorente, A.; Sandvig, K. Biodistribution, pharmacokinetics and excretion studies of intravenously injected nanoparticles and extracellular vesicles: Possibilities and challenges. Adv. Drug Deliv. Rev. 2022, 186, 114326. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Harrison, E.B.; Zhao, Y.; Kabanov, A.V.; Batrakova, E.V. TPP1 delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease. Adv. Healthc. Mater. 2019, 8, 1801271. [Google Scholar] [CrossRef] [PubMed]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Saraswat, A.; Vartak, R.; Patki, M.; Patel, K. Liposomal formulation: Opportunities, challenges, and industrial applicability. In Multifunctional Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 79–102. [Google Scholar]
- Jensen, G.M.; Hodgson, D.F. Opportunities and challenges in commercial pharmaceutical liposome applications. Adv. Drug Deliv. Rev. 2020, 154, 2–12. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yuan, D.; Wu, Y.; Cao, Y. Pharmacokinetics and pharmacodynamics modeling and simulation systems to support the development and regulation of liposomal drugs. Pharmaceutics 2019, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lu, Y.; Qi, J.; Zhao, W.; Dong, X.; Wu, W. Biomimetic thiamine-and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm. Sin. B 2018, 8, 97–105. [Google Scholar] [CrossRef]
- Arrieta-Molero, J.F.; Aleck, K.; Sinha, M.K.; Brownscheidle, C.M.; Shapiro, L.J.; Sperling, M.A. Orally Administered Liposome-Entrapped Insulin in Diabetic Animals: A Critical Assessment. Horm. Res. Paediatr. 1982, 16, 249–256. [Google Scholar] [CrossRef]
- Song, Z.; Lin, Y.; Zhang, X.; Feng, C.; Lu, Y.; Gao, Y.; Dong, C. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: Enhanced cellular uptake and improved therapeutic effects. Int. J. Nanomed. 2017, 12, 1941–1958. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Xu, X.; Gao, N.; Liu, X. Preparation, characterization and in vivo pharmacokinetic study of PVP-modified oleanolic acid liposomes. Int. J. Pharm. 2017, 517, 1–7. [Google Scholar] [CrossRef]
- Chen, W.-l.; Yuan, Z.-Q.; Liu, Y.; Yang, S.-d.; Zhang, C.-g.; Li, J.-z.; Zhu, W.-j.; Li, F.; Zhou, X.-f.; Lin, Y.-m. Liposomes coated with N-trimethyl chitosan to improve the absorption of harmine in vivo and in vitro. Int. J. Nanomed. 2016, 11, 325–336. [Google Scholar][Green Version]
- Jensen, S.M.; Christensen, C.J.; Petersen, J.M.; Treusch, A.H.; Brandl, M. Liposomes containing lipids from Sulfolobus islandicus withstand intestinal bile salts: An approach for oral drug delivery? Int. J. Pharm. 2015, 493, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, G.M.; Ali, Y.; Boehm, B.O.; Huang, Y.Y.; Venkatraman, S. Layer-by-layer coated nanoliposomes for oral delivery of insulin. Nanoscale 2021, 13, 776–789. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Nan, J.; Yang, L.; Park, H.J.; Li, J. Insulin-loaded liposomes packaged in alginate hydrogels promote the oral bioavailability of insulin. J. Control. Release 2023, 353, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Fernandez-Alonso, N.; Tomas-Zapico, C.; Visioli, F.; Iglesias-Gutierrez, E.; Davalos, A. Breast milk microRNAs harsh journey towards potential effects in infant development and maturation. Lipid encapsulation can help. Pharmacol. Res. 2018, 132, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.R.; Rahimi, E.; Amirkhani, Z.; Salehi, R. Leucine-rich repeat-containing G-protein coupled receptor 5 gene overexpression of the rat small intestinal progenitor cells in response to orally administered grape exosome-like nanovesicles. Adv. Biomed. Res. 2018, 7, 125. [Google Scholar]
- López de las Hazas, M.-C.; del Pozo-Acebo, L.; Hansen, M.S.; Gil-Zamorano, J.; Mantilla-Escalante, D.C.; Gómez-Coronado, D.; Marín, F.; Garcia-Ruiz, A.; Rasmussen, J.T.; Dávalos, A. Dietary bovine milk miRNAs transported in extracellular vesicles are partially stable during GI digestion, are bioavailable and reach target tissues but need a minimum dose to impact on gene expression. Eur. J. Nutr. 2022, 61, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The potential of exosomes from cow milk for oral delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Morishita, M.; Nishikawa, M.; Takakura, Y. Role of extracellular vesicle surface proteins in the pharmacokinetics of extracellular vesicles. Mol. Pharm. 2018, 15, 1073–1080. [Google Scholar] [CrossRef]
- Nolte, M.A.; Nolte, E.N.; Margadant, C. Integrins control vesicular trafficking; new tricks for old dogs. Trends Biochem. Sci. 2021, 46, 124–137. [Google Scholar] [CrossRef]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef] [PubMed]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Sun, B.; Shao, Y.; Jing, A.; Wang, J.; Xiao, Z. Assessing the survival of exogenous plant microRNA in mice. Food Sci. Nutr. 2014, 2, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Mi, F.-L.; Liao, Z.-X.; Hsiao, C.-W.; Sonaje, K.; Chung, M.-F.; Hsu, L.-W.; Sung, H.-W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.-A.; Miinalainen, I.; Rappu, P.; De Geest, B.G. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Liu, X.; Bai, Y.; Wu, R.; Li, X.; Mao, Y.; Zhang, L.; Zheng, Y.; Gong, T. Milk-derived exosomes exhibit versatile effects for improved oral drug delivery. Acta Pharm. Sin. B 2022, 12, 2029–2042. [Google Scholar] [CrossRef]
- Parada, N.; Romero-Trujillo, A.; Georges, N.; Alcayaga-Miranda, F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J. Adv. Res. 2021, 31, 61–74. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Bardonnet, P.; Faivre, V.; Pugh, W.; Piffaretti, J.; Falson, F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef]
- Aminzadeh, M.A.; Fournier, M.; Akhmerov, A.; Jones-Ungerleider, K.C.; Valle, J.B.; Marbán, E. Casein-enhanced uptake and disease-modifying bioactivity of ingested extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12045. [Google Scholar] [CrossRef]
- Warren, M.R.; Zhang, C.; Vedadghavami, A.; Bokvist, K.; Dhal, P.K.; Bajpayee, A.G. Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA. Biomater. Sci. 2021, 9, 4260–4277. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin barriers in dermal drug delivery: Which barriers have to be overcome and how can we measure them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B: Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.W.; Han, G.; Kim, K.; Yang, Y.; Kim, S.H. Extracellular vesicles as potential theranostic platforms for skin diseases and aging. Pharmaceutics 2021, 13, 760. [Google Scholar] [CrossRef]
- Lu, K.-J.; Wang, W.; Xu, X.-L.; Jin, F.-Y.; Qi, J.; Wang, X.-J.; Kang, X.-Q.; Zhu, M.-L.; Huang, Q.-L.; Yu, C.-H. A dual deformable liposomal ointment functionalized with retinoic acid and epidermal growth factor for enhanced burn wound healing therapy. Biomater. Sci. 2019, 7, 2372–2382. [Google Scholar] [CrossRef]
- Sakdiset, P.; Okada, A.; Todo, H.; Sugibayashi, K. Selection of phospholipids to design liposome preparations with high skin penetration-enhancing effects. J. Drug Deliv. Sci. Technol. 2018, 44, 58–64. [Google Scholar] [CrossRef]
- Kim, Y.-J.; mi Yoo, S.; Park, H.H.; Lim, H.J.; Kim, Y.-L.; Lee, S.; Seo, K.-W.; Kang, K.-S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical application of mesenchymal stem cell exosomes alleviates the imiquimod induced psoriasis-like inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar]
- Zhang, K.; Yu, L.; Li, F.-R.; Li, X.; Wang, Z.; Zou, X.; Zhang, C.; Lv, K.; Zhou, B.; Mitragotri, S. Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging. Int. J. Nanomed. 2020, 15, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Lee, J.; Kim, S.; Choi, C.; Park, T.; Jung, H.; Cho, J.; Kim, S.; Lee, J. Staphylococcus aureus-derived membrane vesicles exacerbate skin inflammation in atopic dermatitis. Clin. Exp. Allergy 2017, 47, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Han, Y.; Sun, Y.-Z.; Jiang, H.-H.; Liu, M.; Qi, R.-Q.; Gao, X.-H. Extracellular vesicles derived from Malassezia furfur stimulate IL-6 production in keratinocytes as demonstrated in in vitro and in vivo models. J. Dermatol. Sci. 2019, 93, 168–175. [Google Scholar] [CrossRef]
- Gu, T.-W.; Wang, M.-Z.; Niu, J.; Chu, Y.; Guo, K.-R.; Peng, L.-H. Outer membrane vesicles derived from E. coli as novel vehicles for transdermal and tumor targeting delivery. Nanoscale 2020, 12, 18965–18977. [Google Scholar] [CrossRef]
- Peng, L.-H.; Wang, M.-Z.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.-T.; Yuan, T.-J.; Jiang, Z.-H.; Gao, J.-Q.; Ning, X.-H. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL–programmed therapy against melanoma. Sci. Adv. 2020, 6, eaba2735. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, e3702518. [Google Scholar] [CrossRef]
- Balouch, M.; Storchmannová, K.I.; Štěpánek, F.E.; Berka, K. Computational Prodrug Design Methodology for Liposome Formulability Enhancement of Small-Molecule APIs. Mol. Pharm. 2023, 20, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- de Jong, O.G.; Kooijmans, S.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.; Sluijter, J.P.; Vader, P.; Schiffelers, R.M. Drug delivery with extracellular vesicles: From imagination to innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular vesicles: A rising star for therapeutics and drug delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Resnier, P.; Mottais, A.; Sibiril, Y.; Le Gall, T.; Montier, T. Challenges and successes using nanomedicines for aerosol delivery to the airways. Curr. Gene Ther. 2016, 16, 34–46. [Google Scholar] [CrossRef]
- Anderson, C.F.; Grimmett, M.E.; Domalewski, C.J.; Cui, H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1586. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W. Pulmonary delivery nanomedicines towards circumventing physiological barriers: Strategies and characterization approaches. Adv. Drug Deliv. Rev. 2022, 185, 114309. [Google Scholar] [CrossRef]
- Rogers, T.D.; Ostrowski, L.E.; Livraghi-Butrico, A.; Button, B.; Grubb, B.R. Mucociliary clearance in mice measured by tracking trans-tracheal fluorescence of nasally aerosolized beads. Sci. Rep. 2018, 8, 14744. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R. A thermostable mRNA vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck Jr, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. New Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Chan, H.-K. Lipid nanoparticles for the inhalation of mRNA. Nat. Biomed. Eng. 2021, 5, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Z.; Dinh, P.-U.C.; Zhu, D.; Popowski, K.D.; Lutz, H.; Hu, S.; Lewis, M.G.; Cook, A.; Andersen, H. Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat. Nanotechnol. 2021, 16, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Popowski, K.D.; de Juan Abad, B.L.; George, A.; Silkstone, D.; Belcher, E.; Chung, J.; Ghodsi, A.; Lutz, H.; Davenport, J.; Flanagan, M. Inhalable exosomes outperform liposomes as mRNA and protein drug carriers to the lung. Extracell. Vesicle 2022, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Popowski, K.D.; Zhu, D.; de Juan Abad, B.L.; Wang, X.; Liu, M.; Lutz, H.; De Naeyer, N.; DeMarco, C.T.; Denny, T.N. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 2022, 6, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.-U.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Popowski, K.D.; Moatti, A.; Scull, G.; Silkstone, D.; Lutz, H.; de Juan Abad, B.L.; George, A.; Belcher, E.; Zhu, D.; Mei, X. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter 2022, 5, 2960–2974. [Google Scholar] [CrossRef]
- Cober, N.D.; Rowe, K.; Deng, Y.; Benavente-Babace, A.; Courtman, D.W.; Godin, M.; Stewart, D.J. Targeting extracellular vesicle delivery to the lungs by microgel encapsulation. J. Extracell. Biol. 2023, 2, e94. [Google Scholar] [CrossRef]
- Kwak, G.; Gololobova, O.; Sharma, N.; Caine, C.; Mazur, M.; Mulka, K.; West, N.E.; Solomon, G.M.; Cutting, G.R.; Witwer, K.W. Extracellular vesicles enhance pulmonary transduction of stably associated adeno-associated virus following intratracheal administration. J. Extracell. Vesicles 2023, 12, 12324. [Google Scholar] [CrossRef]
- Silva, A.K.; Morille, M.; Piffoux, M.; Arumugam, S.; Mauduit, P.; Larghero, J.; Bianchi, A.; Aubertin, K.; Blanc-Brude, O.; Noël, D. Development of extracellular vesicle-based medicinal products: A position paper of the group “Extracellular Vesicle translatiOn to clinicaL perspectiVEs–EVOLVE France”. Adv. Drug Deliv. Rev. 2021, 179, 114001. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today Bio 2022, 18, 100522. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, L.; Mao, Z.; Wang, Z.; Zhang, Z.; Li, M. Bidirectional Communication Between the Brain and Other Organs: The Role of Extracellular Vesicles. Cell. Mol. Neurobiol. 2023, 43, 2675–2696. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhu, Y.; Youngblood, H.A.; Almuntashiri, S.; Jones, T.W.; Wang, X.; Liu, Y.; Somanath, P.R.; Zhang, D. Nebulization of extracellular vesicles: A promising small RNA delivery approach for lung diseases. J. Control. Release 2022, 352, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 187, 109882. [Google Scholar] [CrossRef]
- Murali, V.P.; Holmes, C.A. Biomaterial-based extracellular vesicle delivery for therapeutic applications. Acta Biomater. 2021, 124, 88–107. [Google Scholar] [CrossRef]
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018, 2, 293–303. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Z.; Wei, Q.; Ma, K.; Hu, W.; Huang, Q.; Su, J.; Li, H.; Zhang, C.; Fu, X. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022, 147, 342–355. [Google Scholar] [CrossRef]
- Kwak, G.; Cheng, J.; Kim, H.; Song, S.; Lee, S.J.; Yang, Y.; Jeong, J.H.; Lee, J.E.; Messersmith, P.B.; Kim, S.H. Sustained Exosome-Guided Macrophage Polarization Using Hydrolytically Degradable PEG Hydrogels for Cutaneous Wound Healing: Identification of Key Proteins and MiRNAs, and Sustained Release Formulation. Small 2022, 18, 2200060. [Google Scholar] [CrossRef]
- Wang, C.; Li, N.; Li, Y.; Hou, S.; Zhang, W.; Meng, Z.; Wang, S.; Jia, Q.; Tan, J.; Wang, R. Engineering a HEK-293T exosome-based delivery platform for efficient tumor-targeting chemotherapy/internal irradiation combination therapy. J. Nanobiotechnol. 2022, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Kis, Z. Stability modelling of mRNA vaccine quality based on temperature monitoring throughout the distribution chain. Pharmaceutics 2022, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Heydarkhan-Hagvall, S.; Tangruksa, B.; González-King Garibotti, H.; Jing, Y.; Maugeri, M.; Kohl, F.; Hultin, L.; Reyahi, A.; Camponeschi, A. Lipid nanoparticles deliver the therapeutic VEGFA mRNA in vitro and in vivo and transform extracellular vesicles for their functional extensions. Adv. Sci. 2023, 10, 2206187. [Google Scholar] [CrossRef] [PubMed]
- Joy, R.; George, J.; John, F. Brief outlook on polymeric nanoparticles, micelles, niosomes, hydrogels and liposomes: Preparative methods and action. ChemistrySelect 2022, 7, e202104045. [Google Scholar] [CrossRef]
- Chabria, Y.; Duffy, G.P.; Lowery, A.J.; Dwyer, R.M. Hydrogels: 3D drug delivery systems for nanoparticles and extracellular vesicles. Biomedicines 2021, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yin, Z.; Wang, X.; Su, J. Exosome-laden hydrogels: A novel cell-free strategy for in-situ bone tissue regeneration. Front. Bioeng. Biotechnol. 2022, 10, 866208. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, H.; Qian, M.; Ji, G.; Wei, Y.; He, J.; Tian, H.; Zhao, Q. MSC-derived sEV-loaded hyaluronan hydrogel promotes scarless skin healing by immunomodulation in a large skin wound model. Biomed. Mater. 2022, 17, 034104. [Google Scholar] [CrossRef]
- Khayambashi, P.; Iyer, J.; Pillai, S.; Upadhyay, A.; Zhang, Y.; Tran, S.D. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int. J. Mol. Sci. 2021, 22, 684. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-Abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Shirakura, T.; Kelson, T.J.; Ray, A.; Malyarenko, A.E.; Kopelman, R. Hydrogel nanoparticles with thermally controlled drug release. ACS Macro Lett. 2014, 3, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bellinger, A.M.; Glettig, D.L.; Barman, R.; Lee, Y.-A.L.; Zhu, J.; Cleveland, C.; Montgomery, V.A.; Gu, L.; Nash, L.D. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater. 2015, 14, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Žakelj, S.; Mravljak, J.; Pajk, S.; Kristl, A.; Schubert, R.; Škalko-Basnet, N. The effect of lipid composition and liposome size on the release properties of liposomes-in-hydrogel. Int. J. Pharm. 2013, 456, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Palac, Z.; Hurler, J.; Škalko-Basnet, N.; Filipović-Grčić, J.; Vanić, Ž. Elastic liposomes-in-vehicle formulations destined for skin therapy: The synergy between type of liposomes and vehicle. Drug Dev. Ind. Pharm. 2015, 41, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Lenzini, S.; Bargi, R.; Chung, G.; Shin, J.-W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 2020, 15, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zeng, Q.; Zhang, Q.; Li, K.; Shi, X.; Liang, F.; Han, D. Temperature/near-infrared light-responsive conductive hydrogels for controlled drug release and real-time monitoring. Nanoscale 2020, 12, 8679–8686. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Lin, W.; Yuan, Z.; Wu, J.; Qian, H.; Xu, L.; Chen, S. Development of ionic strength/pH/enzyme triple-responsive zwitterionic hydrogel of the mixed l-glutamic acid and l-lysine polypeptide for site-specific drug delivery. J. Mater. Chem. B 2017, 5, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, T.; Lee, C.H. Enzyme Responsive Delivery of Anti-Retroviral Peptide via Smart Hydrogel. AAPS PharmSciTech 2022, 23, 234. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, D.; Hui, Q.; Bi, J.; Yu, B.; Huang, Z.; Hu, S.; Wang, Z.; Caranasos, T.; Rossi, J. Injection of ROS-responsive hydrogel loaded with basic fibroblast growth factor into the pericardial cavity for heart repair. Adv. Funct. Mater. 2021, 31, 2004377. [Google Scholar] [CrossRef]
- Kuang, L.; Huang, J.; Liu, Y.; Li, X.; Yuan, Y.; Liu, C. Injectable hydrogel with NIR light-responsive, dual-mode PTH release for osteoregeneration in osteoporosis. Adv. Funct. Mater. 2021, 31, 2105383. [Google Scholar] [CrossRef]
- Kubota, T.; Kurashina, Y.; Zhao, J.; Ando, K.; Onoe, H. Ultrasound-triggered on-demand drug delivery using hydrogel microbeads with release enhancer. Mater. Des. 2021, 203, 109580. [Google Scholar] [CrossRef]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Qiao, Y.; Chu, Y.; Tong, Z.; Zhou, Y.; Zhang, W.; Xie, S.; Hu, J.; Wang, T. Magnetic double-network hydrogels for tissue hyperthermia and drug release. J. Mater. Chem. B 2019, 7, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern wound dressings: Hydrogel dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surgery 2017, 35, 489–494. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, Q.; Jiang, Z.; Liu, J.; Wan, J.; Jin, P.; Lv, Q. Multifunctional silk fibroin methacryloyl microneedle for diabetic wound healing. Small 2022, 18, 2203064. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Liu, Y.; Fan, L.; Gan, J.; Liu, W.; Zhao, Y.; Sun, L. Polydopamine decorated microneedles with Fe-MSC-derived nanovesicles encapsulation for wound healing. Adv. Sci. 2022, 9, 2103317. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K.; Jiang, T.; Li, S.; Chen, J.; Wu, Z.; Li, W.; Tan, R.; Wei, W.; Yang, X. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022, 20, 147. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C. Self-healing injectable hydrogels for tissue regeneration. Chem. Rev. 2022, 123, 834–873. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 22, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, Y.; Chen, C.; Xie, H.; Lu, H.; Hu, J. Local delivery of USC-derived exosomes harboring ANGPTL3 enhances spinal cord functional recovery after injury by promoting angiogenesis. Stem Cell Res. Ther. 2021, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Halevi, O.; Chen, J.; Thangavel, G.; Morris, S.A.; Uliel, T.B.; Tischler, Y.R.; Lee, P.S.; Magdassi, S. Synthesis through 3D printing: Formation of 3D coordination polymers. RSC Adv. 2020, 10, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Born, L.J.; McLoughlin, S.T.; Dutta, D.; Mahadik, B.; Jia, X.; Fisher, J.P.; Jay, S.M. Sustained released of bioactive mesenchymal stromal cell-derived extracellular vesicles from 3D-printed gelatin methacrylate hydrogels. J. Biomed. Mater. Res. Part A 2022, 110, 1190–1198. [Google Scholar] [CrossRef]
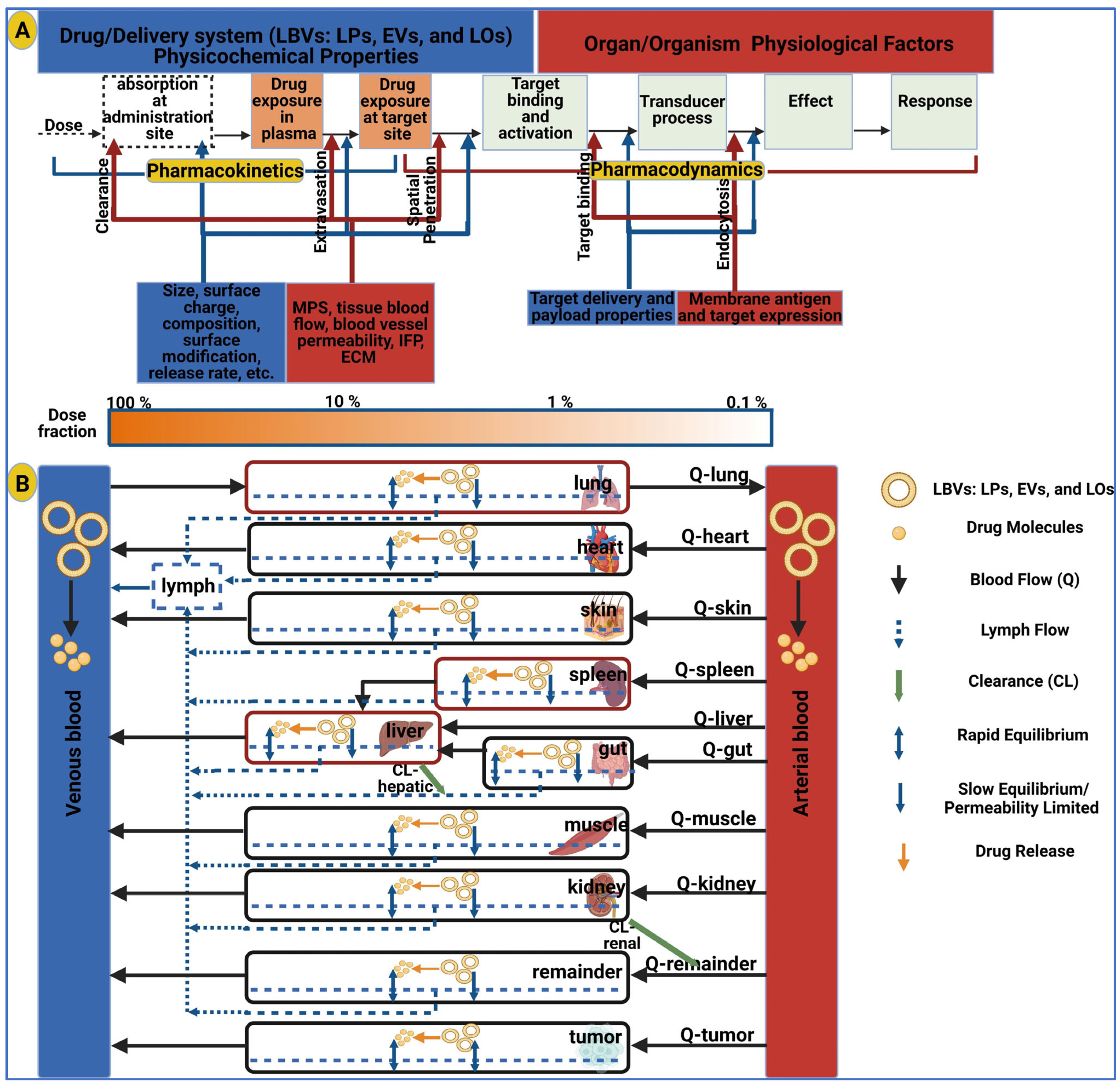
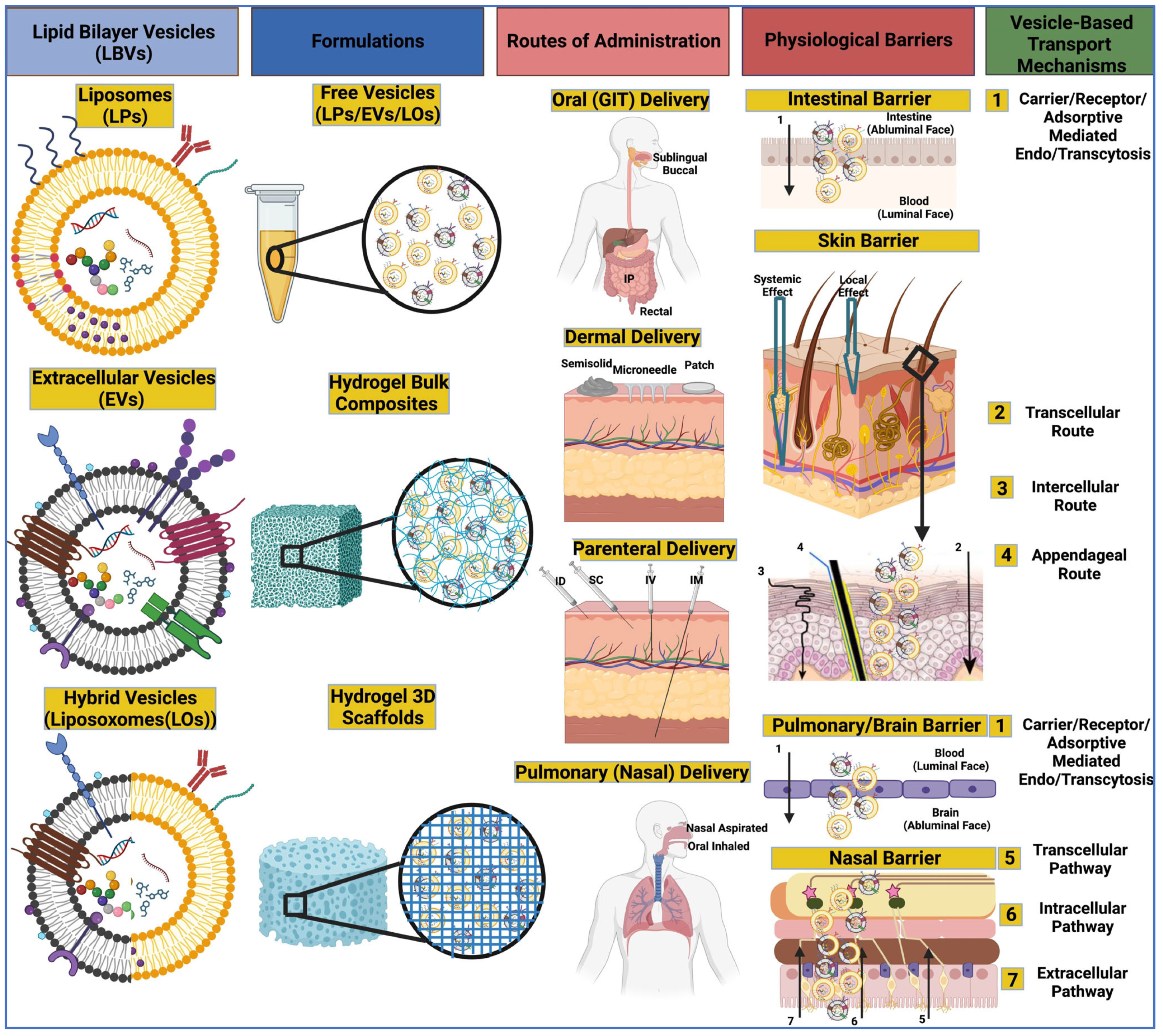
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jipouri, A.; Eritja, À.; Bozic, M. Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery. Int. J. Mol. Sci. 2024, 25, 485. https://doi.org/10.3390/ijms25010485
Al-Jipouri A, Eritja À, Bozic M. Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery. International Journal of Molecular Sciences. 2024; 25(1):485. https://doi.org/10.3390/ijms25010485
Chicago/Turabian StyleAl-Jipouri, Ali, Àuria Eritja, and Milica Bozic. 2024. "Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery" International Journal of Molecular Sciences 25, no. 1: 485. https://doi.org/10.3390/ijms25010485
APA StyleAl-Jipouri, A., Eritja, À., & Bozic, M. (2024). Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery. International Journal of Molecular Sciences, 25(1), 485. https://doi.org/10.3390/ijms25010485







