The bZIP Transcription Factors in Current Jasmine Genomes: Identification, Characterization, Evolution and Expressions
Abstract
:1. Introduction
2. Results
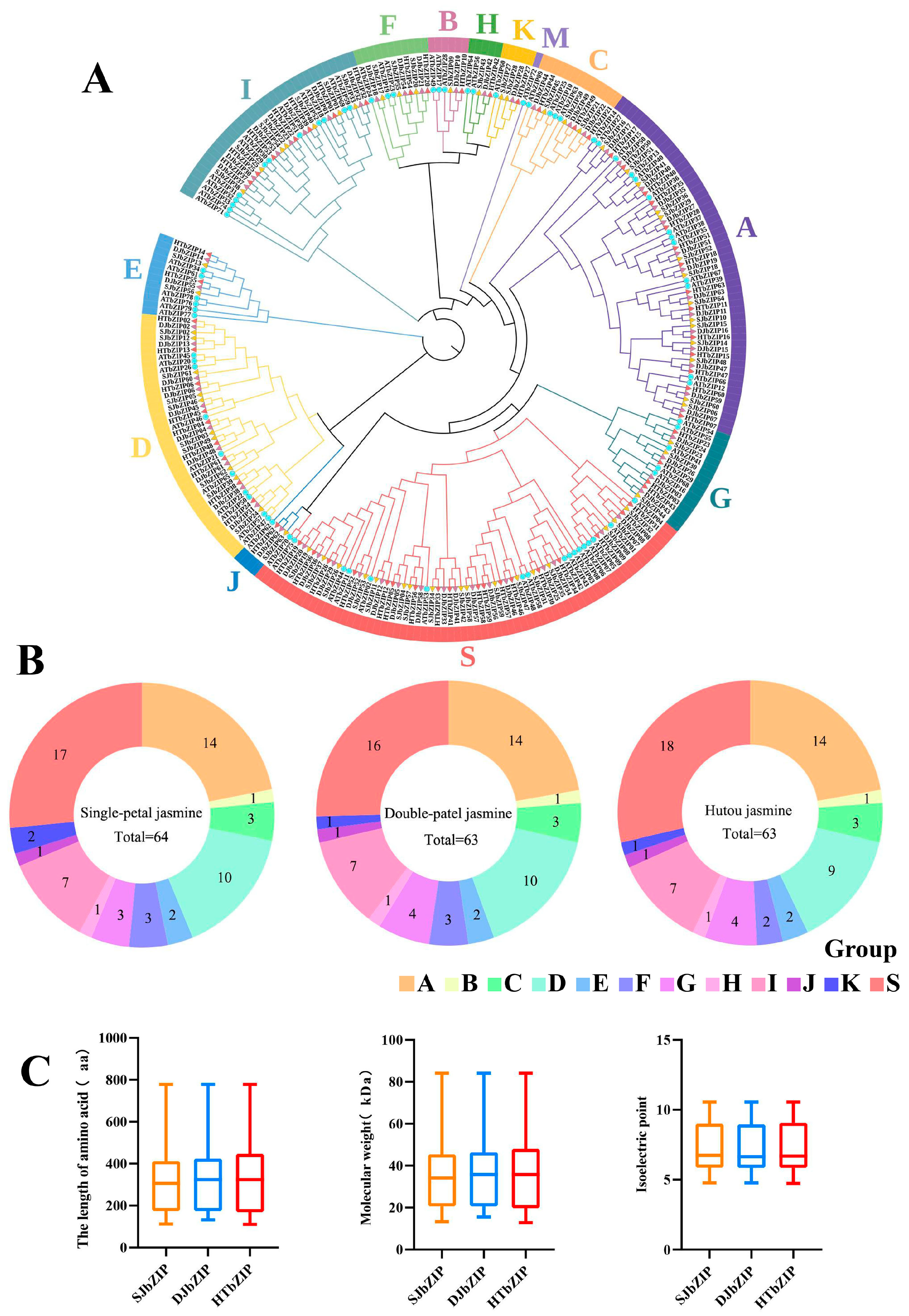
2.1. Gene Identification and Proteins Characteristics of the bZIP Gene Family
2.2. Phylogenetic Tree Analysis of bZIP Genes of Jasminum sambac
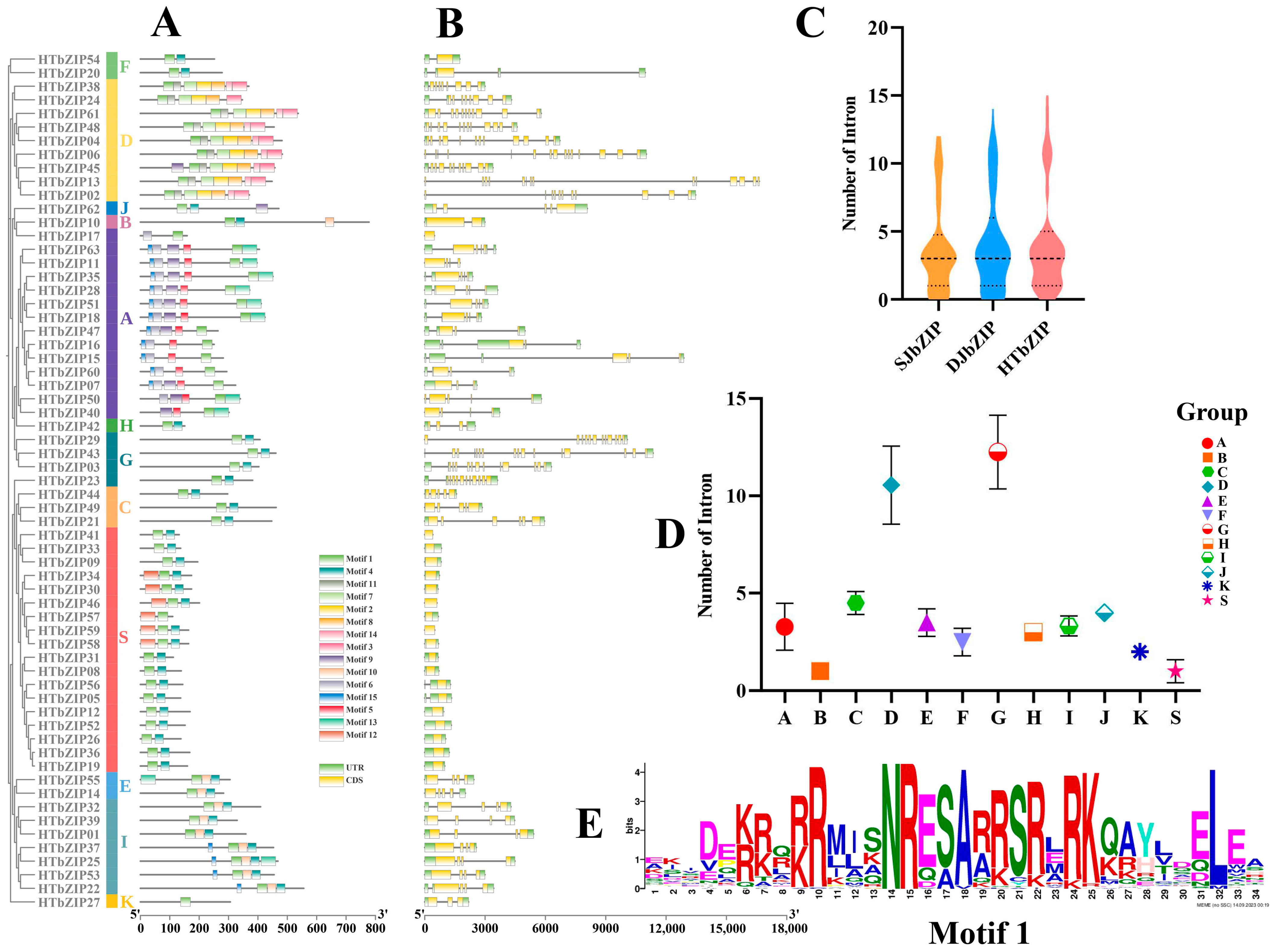
2.3. Analysis of Conserved Domain and bZIP Genes Structure
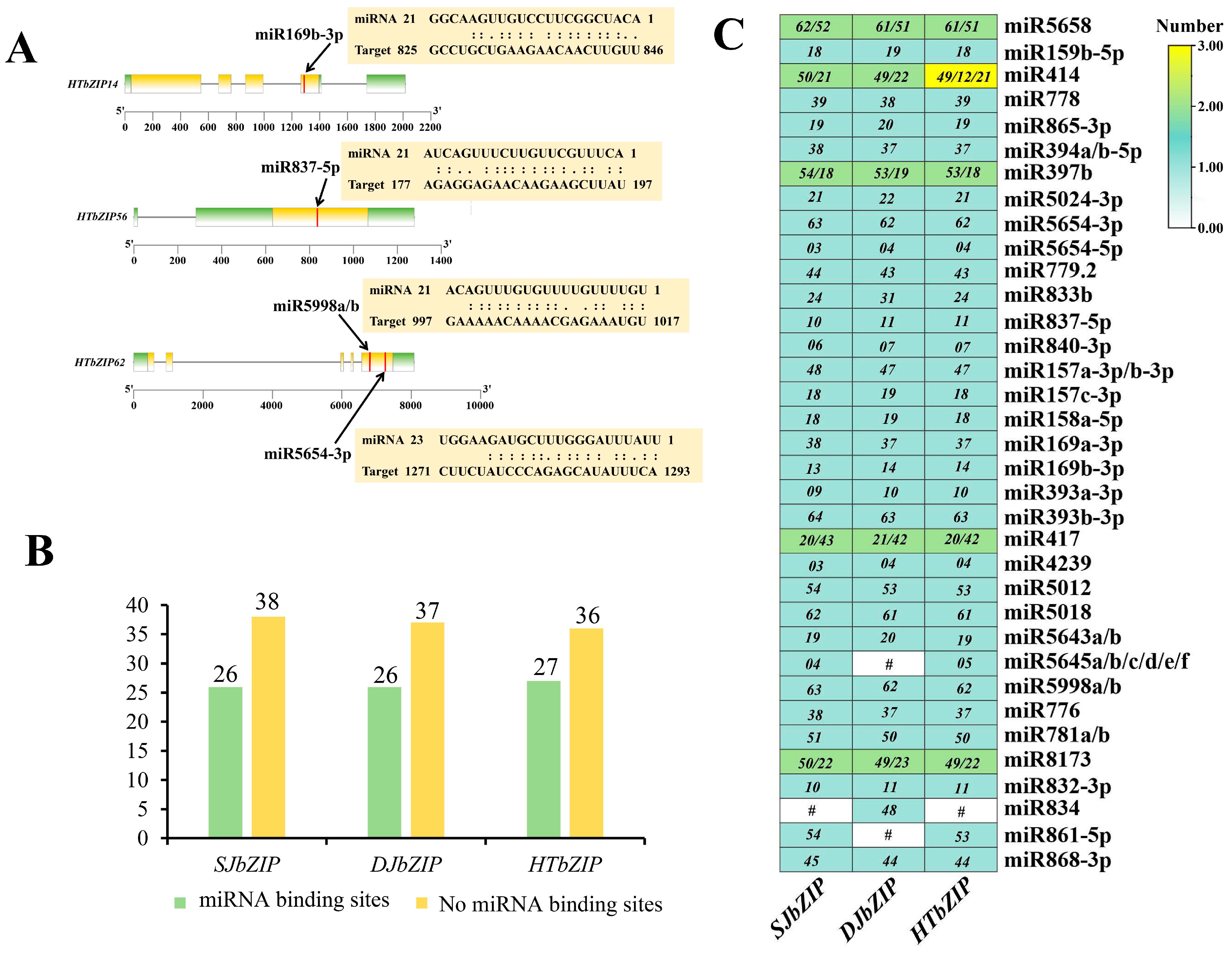
2.4. Target of Specific miRNAs for bZIP Genes
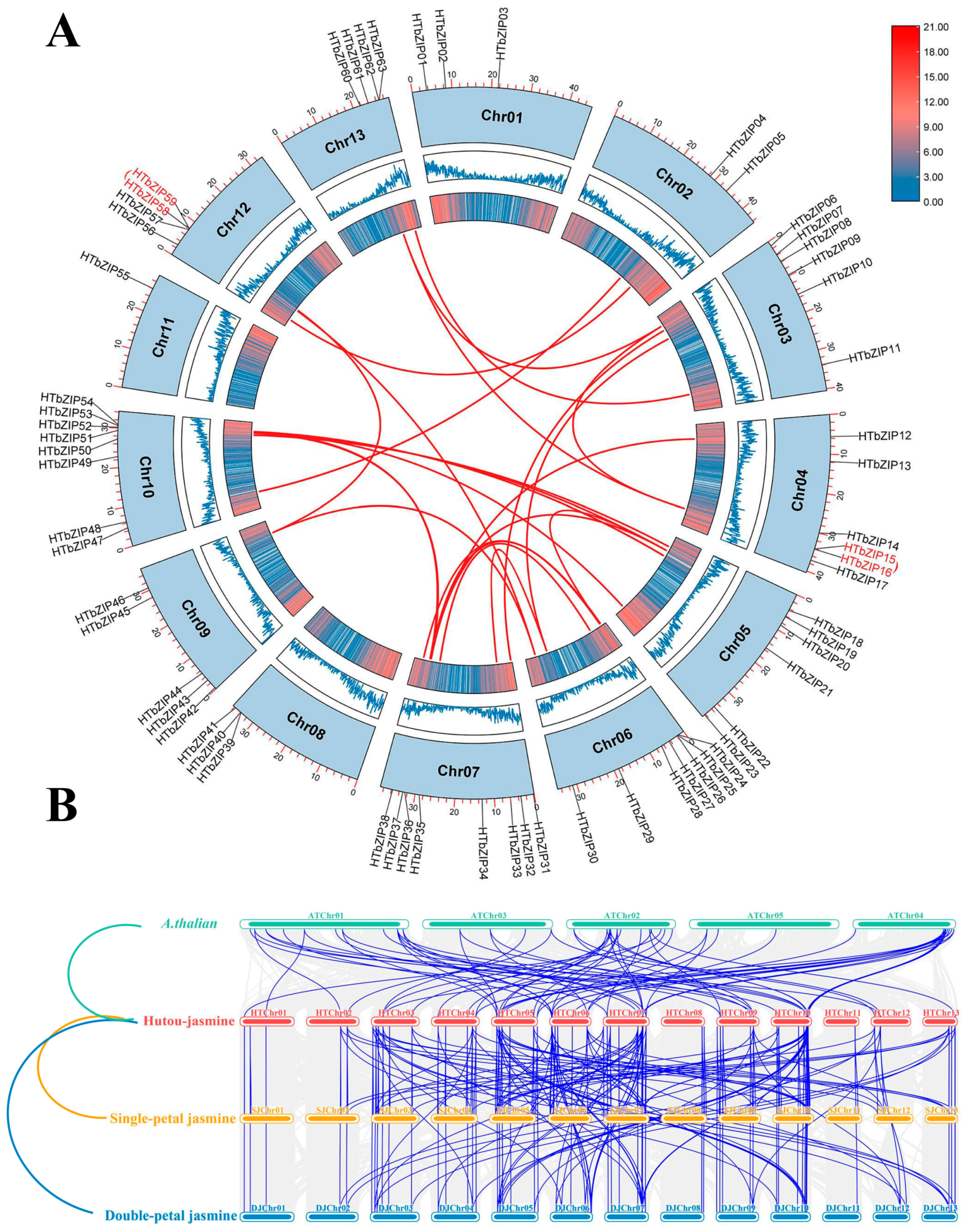
2.5. Synteny Analysis of HTbZIP Genes
2.6. Analysis of Codon Usage Bias of HTbZIP Genes
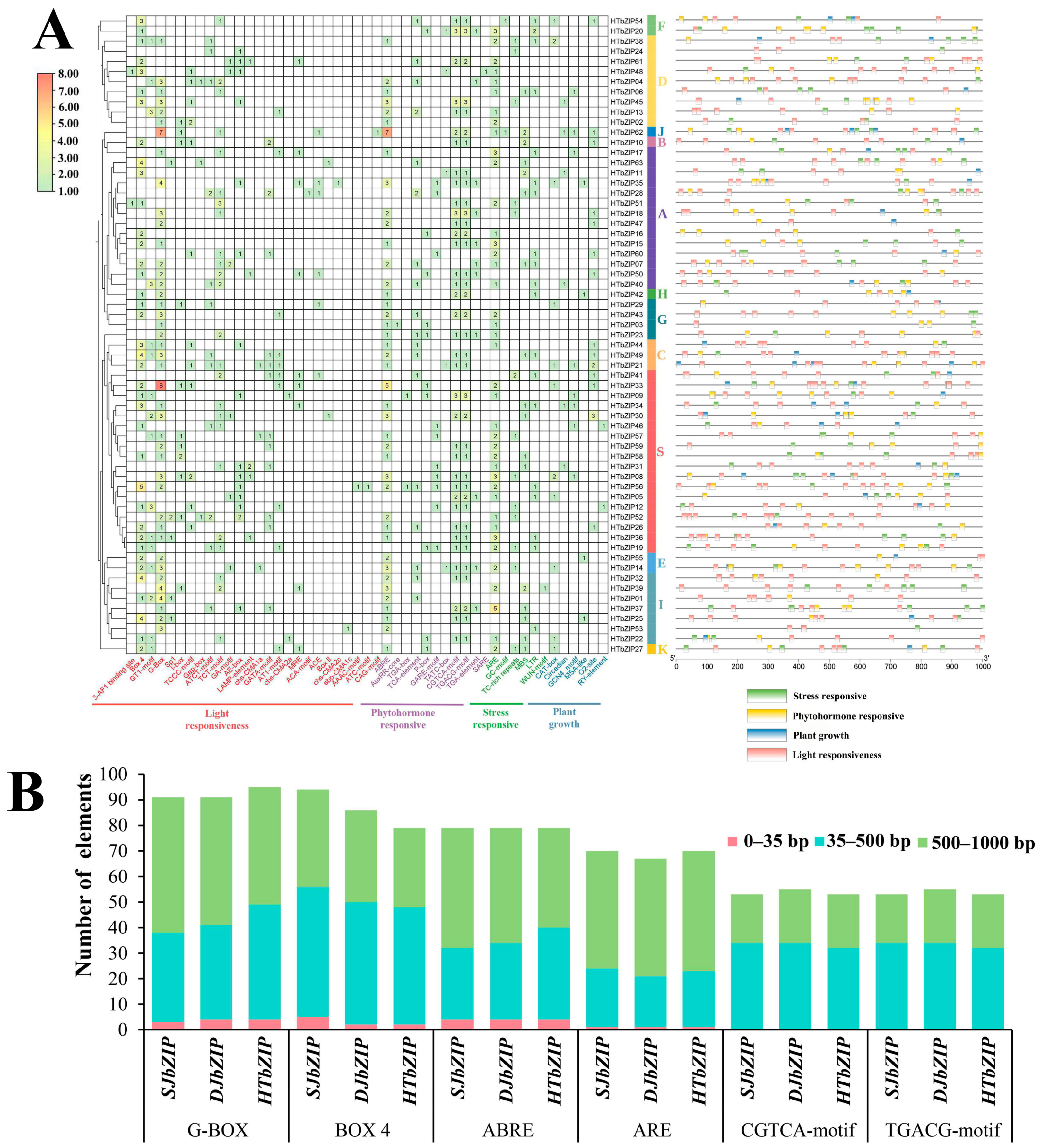
2.7. Prediction of Cis-Acting Elements of Promoter in HTbZIP Genes
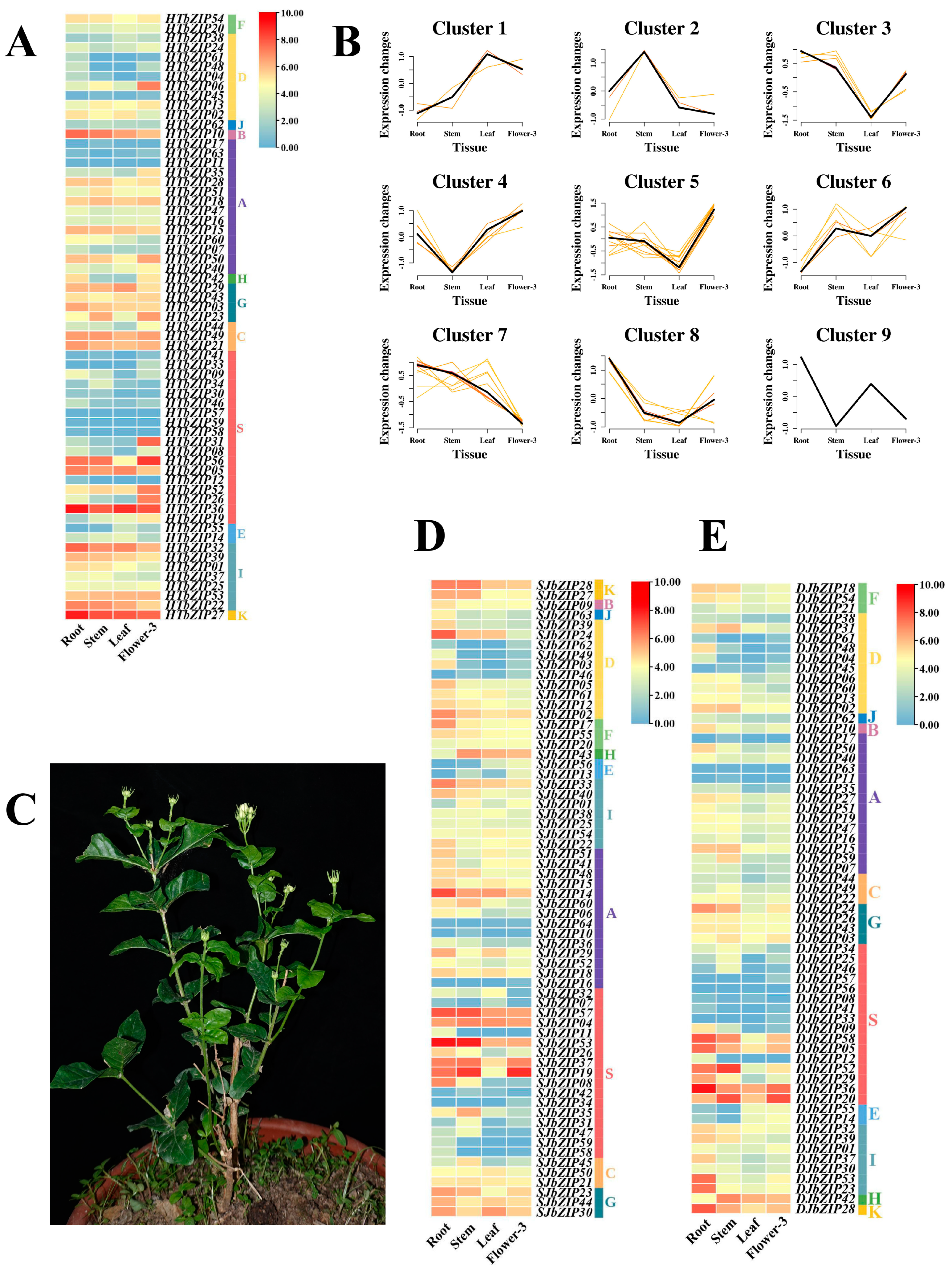
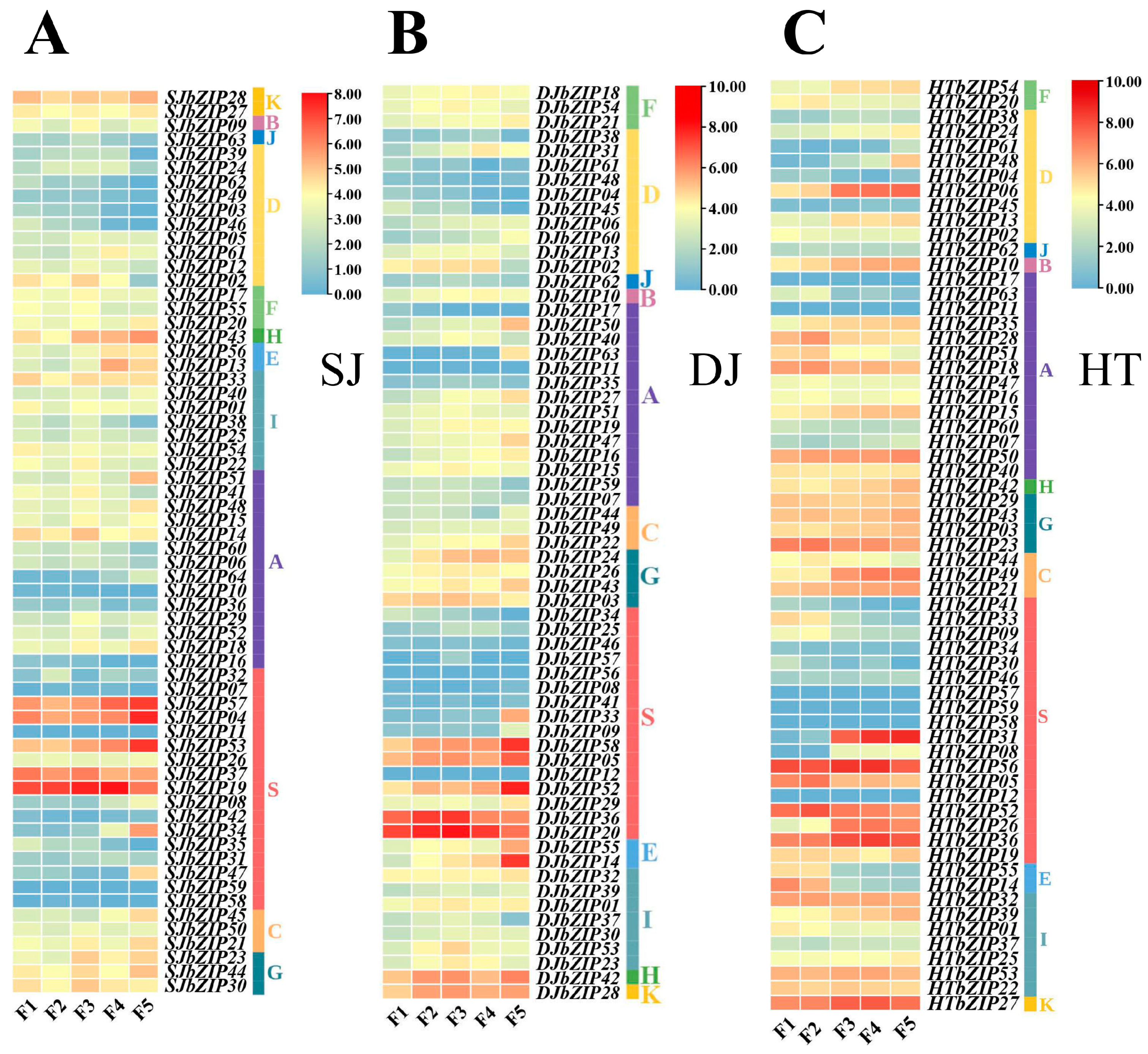
2.8. Expression Profiles of HTbZIP Genes in Different Tissues
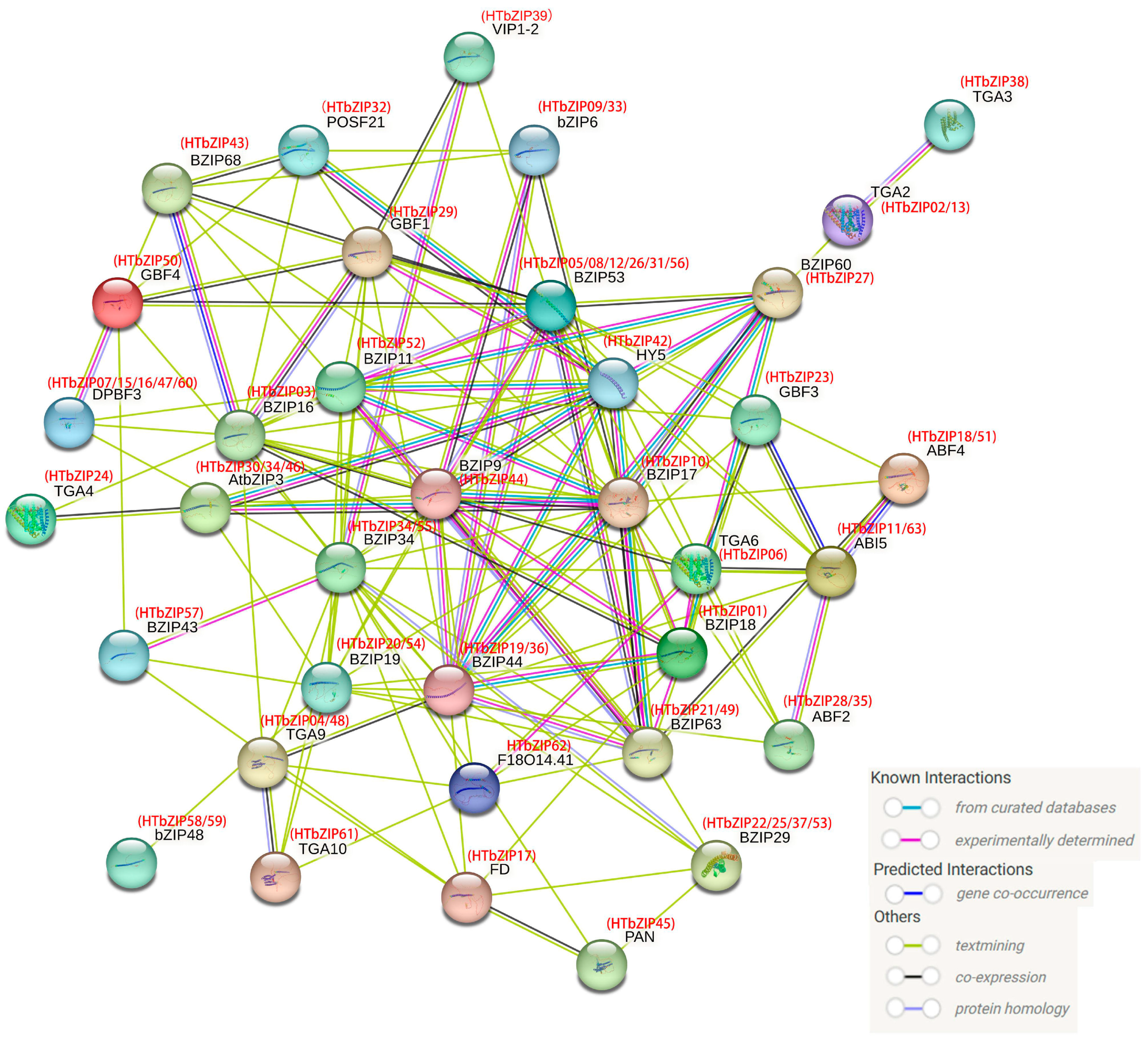
2.9. Analysis of the Protein–Protein Interaction Network of HTbZIPs
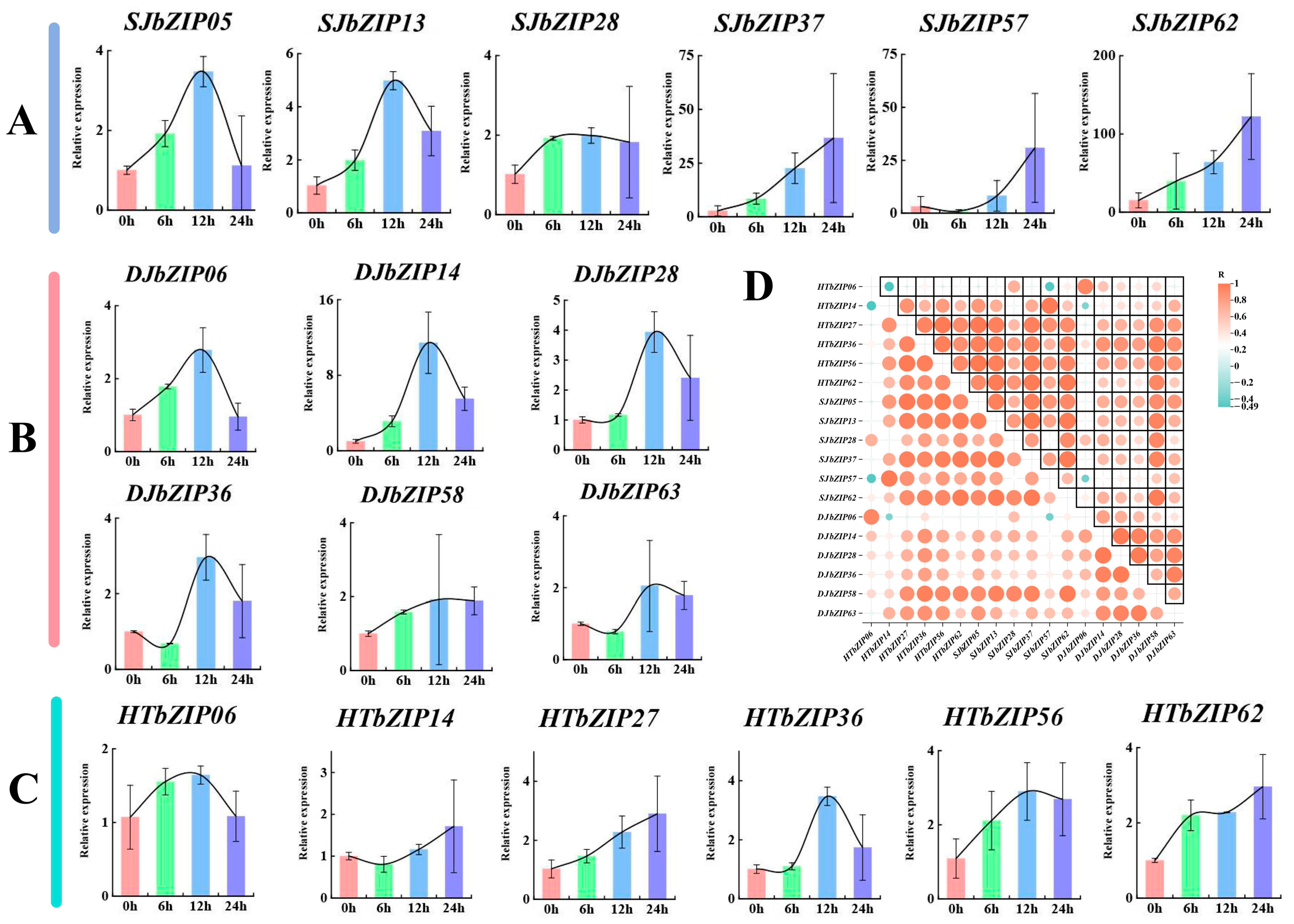
2.10. Response Profiles of HTbZIP Genes with ABA Treatments
3. Discussion
4. Materials and Methods
4.1. Genomics Data and Plant Sources
4.2. The Identification of bZIP Gene Family
4.3. Phylogenetic Tree Construction
4.4. Conservative Motifs, Gene Structure of HTbZIPs
4.5. MiRNA Editing for the bZIP Gene Family
4.6. Synteny Analysis of the bZIP Gene Family
4.7. Analysis of Codon Usage Pattern of HTbZIP Genes
4.8. Cis-Acting Element Analysis of HTbZIP Genes
4.9. Expression Analysis
4.10. Protein–Protein Interaction Network Analysis of HTbZIPs
4.11. RT-qPCR Assay during the ABA Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Hawary, S.S.; El-Hefnawy, H.M.; Osman, S.M.; El-Raey, M.A.; Mokhtar Ali, F.A. Phenolic profiling of different Jasminum species cultivated in Egypt and their antioxidant activity. Nat. Prod. Res. 2021, 35, 4663–4668. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, X.; Gu, C.; Jia, X.; Liang, L.; Su, J. Identification of pre-fertilization reproductive barriers and the underlying cytological mechanism in crosses among three petal-types of Jasminum sambac and their relevance to phylogenetic relationships. PLoS ONE 2017, 12, e0176026. [Google Scholar] [CrossRef]
- Jing, L.; Bu, C.; Li, C.; Di, Q. Study on genetic Diversity of Phenotypic Traits in 25 Jasminum Germplasm Resources. Chin. J. Trop. Crops 2020, 41, 1762–1769. [Google Scholar] [CrossRef]
- Fu, T.; Guo, C.; Fu, T.; Peng, S.; Lin, X.; Rao, G.; Chen, N.; Zhang, J. Comparison and Analysis of Characteristic Aroma Components of Eight Main Jasmine Teas in Fuzhou. J. Tea Sci. 2020, 40, 656–664. [Google Scholar]
- Liu, H.; Tang, X.; Zhang, N.; Li, S.; Si, H. Role of bZIP Transcription Factors in Plant Salt Stress. Int. J. Mol. Sci. 2023, 24, 7893. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef]
- Izawa, T.; Foster, R.; Chua, N.-H. Plant bZIP Protein DNA Binding Specificity. J. Mol. Biol. 1993, 230, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; He, Y.; Wu, L.; Jia, L.; Wang, L.; Zhiguo, E. Research Progress in the Function of Basic Leucine Zipper (bZIP) Protein Family in Rice. Chin. J. Rice Sci. 2023, 37, 436–448. [Google Scholar]
- Wang, J.; Ding, F.; Pan, J.; Zhang, S.; Yang, Y.; Huang, X.; Fan, Z.; Li, L.; Wang, Y. Research Progress of bZIP Lineage Transcription Factors in Plant. Chin. J. Trop. Agric. 2019, 39, 39–45. [Google Scholar]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription Factors Interact with ABA through Gene Expression and Signaling Pathways to Mitigate Drought and Salinity Stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, S.H.; Cheong, Y.H.; Yoo, C.M.; Lee, S.I.; Chun, H.J.; Yun, D.J.; Hong, J.C.; Lee, S.Y.; Lim, C.O.; et al. A novel cold-inducible zinc finger protein from soybean, SCOF-1, enhances cold tolerance in transgenic plants. Plant J. Cell Mol. Biol. 2001, 25, 247–259. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Zhang, H.; Wang, X.-C.; Huang, R. Transcriptional Modulation of Ethylene Response Factor Protein JERF3 in the Oxidative Stress Response Enhances Tolerance of Tobacco Seedlings to Salt, Drought, and Freezing. Plant Physiol. 2008, 148, 1953–1963. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef]
- Liu, C.; Ou, S.; Mao, B.; Tang, J.; Wang, W.; Wang, H.; Cao, S.; Schläppi, M.R.; Zhao, B.; Xiao, G.; et al. Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat. Commun. 2018, 9, 3302. [Google Scholar] [CrossRef]
- Cai, W.; Yang, Y.; Wang, W.; Guo, G.; Liu, W.; Bi, C. Overexpression of a wheat (Triticum aestivum L.) bZIP transcription factor gene, TabZIP6, decreased the freezing tolerance of transgenic Arabidopsis seedlings by down-regulating the expression of CBFs. Plant Physiol. Biochem. 2018, 124, 100–111. [Google Scholar] [CrossRef]
- Nijhawan, A.; Jain, M.; Tyagi, A.K.; Khurana, J.P. Genomic Survey and Gene Expression Analysis of the Basic Leucine Zipper Transcription Factor Family in Rice. Plant Physiol. 2008, 146, 323–324. [Google Scholar] [CrossRef]
- Wei, K.; Chen, J.; Wang, Y.; Chen, Y.; Chen, S.; Lin, Y.; Pan, S.; Zhong, X.; Xie, D. Genome-Wide Analysis of bZIP-Encoding Genes in Maize. DNA Res. 2012, 19, 463–476. [Google Scholar] [CrossRef]
- Liu, J.; Chen, N.; Chen, F.; Cai, B.; Dal Santo, S.; Tornielli, G.B.; Pezzotti, M.; Cheng, Z.-M. Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genom. 2014, 15, 281. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Zhou, J.; Tan, Q.-P.; Fu, X.-L.; Chen, X.-D.; Li, L.; Gao, D.-S. Analysis of Basic Leucine Zipper Genes and Their Expression During Bud Dormancy in Apple (Malus × domestica). Sci. Agric. Sin. 2016, 49, 1325–1345. [Google Scholar]
- Li, D.; Fu, F.; Zhang, H.; Song, F. Genome-wide systematic characterization of the bZIP transcriptional factor family in tomato (Solanum lycopersicum L.). BMC Genom. 2015, 16, 771. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, Q.; Liu, Y.; He, M.; Zhou, Y. Genome-wide Identification and Bioinformatics Analysis of Chrysanthemum indicum bZIP Transcription Factor. Mol. Plant Breed. 2022, 20, 4586–4600. [Google Scholar]
- Jarambasa, T.; Regon, P.; Jyoti, S.Y.; Gupta, D.; Panda, S.K.; Tanti, B. Genome-wide identification and expression analysis of the Pisum sativum (L.) APETALA2/ethylene-responsive factor (AP2/ERF) gene family reveals functions in drought and cold stresses. Genetica 2023, 151, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, J.; Lu, L.; Li, L. Identification and expression analysis of bZIP family in potato. Jiangsu J. Agric. Sci. 2022, 38, 1453–1464. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Yan, Y.; Wei, Y.; Tie, W.; Ding, Z.; Zuo, J.; Peng, M.; Li, K. Genome-wide characterization and analysis of bZIP transcription factor gene family related to abiotic stress in cassava. Sci. Rep. 2016, 6, 22783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Quan, S.; Niu, J.; Guo, C.; Kang, C.; Liu, J.; Yuan, X. Genome-Wide Identification, Classification, Expression and Duplication Analysis of bZIP Family Genes in Juglans regia L. Int. J. Mol. Sci. 2022, 23, 5961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Huang, L.F.; Naylor, M.; Clifford, P. Heterogeneity in codon usages of sobemovirus genes. Arch. Virol. 2005, 150, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, A.; Lamolle, G.; Musto, H. Codon Usage Bias: An Endless Tale. J. Mol. Evol. 2021, 89, 589–593. [Google Scholar] [CrossRef]
- Bufen, S.; Tao, J.; Ling, L.; Yan, L.; Jiajin, C. Climate Suitability Zoning and Assessment of Jasmine in Fujian Province Based on GIS. Meteorol. Sci. Technol. 2022, 50, 885–890. [Google Scholar]
- Iven, T.; Strathmann, A.; Böttner, S.; Zwafink, T.; Heinekamp, T.; Guivarc’h, A.; Roitsch, T.; Dröge-Laser, W. Homo- and heterodimers of tobacco bZIP proteins counteract as positive or negative regulators of transcription during pollen development. Plant J. 2010, 63, 155–166. [Google Scholar] [CrossRef]
- Gibalová, A.; Renák, D.; Matczuk, K.; Dupl’áková, N.; Cháb, D.; Twell, D.; Honys, D. AtbZIP34 is required for Arabidopsis pollen wall patterning and the control of several metabolic pathways in developing pollen. Plant Mol. Biol. 2009, 70, 581–601. [Google Scholar] [CrossRef]
- Weiste, C.; Pedrotti, L.; Selvanayagam, J.; Muralidhara, P.; Fröschel, C.; Novák, O.; Ljung, K.; Hanson, J.; Dröge-Laser, W. The Arabidopsis bZIP11 transcription factor links low-energy signalling to auxin-mediated control of primary root growth. PLoS Genet. 2017, 13, e1006607. [Google Scholar] [CrossRef]
- Ma, J.; Hanssen, M.; Lundgren, K.; Hernández, L.; Delatte, T.; Ehlert, A.; Liu, C.-M.; Schluepmann, H.; Dröge-Laser, W.; Moritz, T.; et al. The sucrose-regulated Arabidopsis transcription factor bZIP11 reprograms metabolism and regulates trehalose metabolism. New Phytol. 2011, 191, 733–745. [Google Scholar] [CrossRef]
- Satoh, R.; Fujita, Y.; Nakashima, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. A novel subgroup of bZIP proteins functions as transcriptional activators in hypoosmolarity-responsive expression of the ProDH gene in Arabidopsis. Plant Cell Physiol. 2004, 45, 309–317. [Google Scholar] [CrossRef]
- Deng, Y.; Humbert, S.; Liu, J.-X.; Srivastava, R.; Rothstein, S.J.; Howell, S.H. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 7247–7252. [Google Scholar] [CrossRef]
- Li, P.; Zheng, T.; Li, L.; Wang, J.; Cheng, T.; Zhang, Q. Genome-wide investigation of the bZIP transcription factor gene family in Prunus mume: Classification, evolution, expression profile and low-temperature stress responses. Hortic. Plant J. 2022, 8, 230–242. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Liu, Y.; Chai, M.; Zhang, M.; He, Q.; Su, Z.; Priyadarshani, S.V.G.N.; Liu, L.; Dong, G.; Qin, Y. Genome-Wide Analysis, Characterization, and Expression Profile of the Basic Leucine Zipper Transcription Factor Family in Pineapple. Int. J. Genom. 2020, 2020, 3165958. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, P.; Agassin, R.H.; Yao, S.; Wang, D.; Huang, Z.; Zhang, C.; Hao, Q.; Ji, K. Identification, Classification and Characterization of bZIP Transcription Factor Family Members in Pinus massoniana Lamb. Forests 2023, 14, 155. [Google Scholar] [CrossRef]
- Huang, L.-T.; Liu, C.-Y.; Li, L.; Han, X.-S.; Chen, H.-W.; Jiao, C.-H.; Sha, A.-H. Genome-Wide Identification of bZIP Transcription Factors in Faba Bean Based on Transcriptome Analysis and Investigation of Their Function in Drought Response. Plants 2023, 12, 3041. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Yao, H.; Li, T.; Ma, Z.; Wang, X.; Xu, L.; Zhang, Y.; Cai, Y.; Tang, Z. Codon usage pattern of the ancestor of green plants revealed through Rhodophyta. BMC Genom. 2023, 24, 538. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Xiao, M.; Huang, J.; Lou, Y.; Hu, F.; Fu, X.; Li, Y.; He, H.; Cheng, J. An analysis of codon utilization patterns in the chloroplast genomes of three species of Coffea. BMC Genom. Data 2023, 24, 42. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Yang, W.; Li, Z.; Hai, Y.; Duan, B.; Zhang, H.; Yang, X.; Xia, C. Analysis of codon usage patterns in 48 Aconitum species. BMC Genom. 2023, 24, 703. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wan, C.; He, X.; Huang, J.; Lyu, M.; Yuan, Y.; Wu, B. Characterization of Two BAHD Acetyltransferases Highly Expressed in the Flowers of Jasminum sambac (L.) Aiton. Plants 2022, 11, 13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Luo, X.; Shen, M.; Lei, W.; Lin, S.; Lin, Y.; Sun, H.; Ahmad, S.; Wang, G.; Liu, Z.-J. The bZIP Transcription Factors in Current Jasmine Genomes: Identification, Characterization, Evolution and Expressions. Int. J. Mol. Sci. 2024, 25, 488. https://doi.org/10.3390/ijms25010488
Zhao K, Luo X, Shen M, Lei W, Lin S, Lin Y, Sun H, Ahmad S, Wang G, Liu Z-J. The bZIP Transcription Factors in Current Jasmine Genomes: Identification, Characterization, Evolution and Expressions. International Journal of Molecular Sciences. 2024; 25(1):488. https://doi.org/10.3390/ijms25010488
Chicago/Turabian StyleZhao, Kai, Xianmei Luo, Mingli Shen, Wen Lei, Siqing Lin, Yingxuan Lin, Hongyan Sun, Sagheer Ahmad, Guohong Wang, and Zhong-Jian Liu. 2024. "The bZIP Transcription Factors in Current Jasmine Genomes: Identification, Characterization, Evolution and Expressions" International Journal of Molecular Sciences 25, no. 1: 488. https://doi.org/10.3390/ijms25010488






