Novel Polyelectrolytes Based on Naphthalene Diimide with Different Counteranions for Cathode Interlayers in Polymer Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
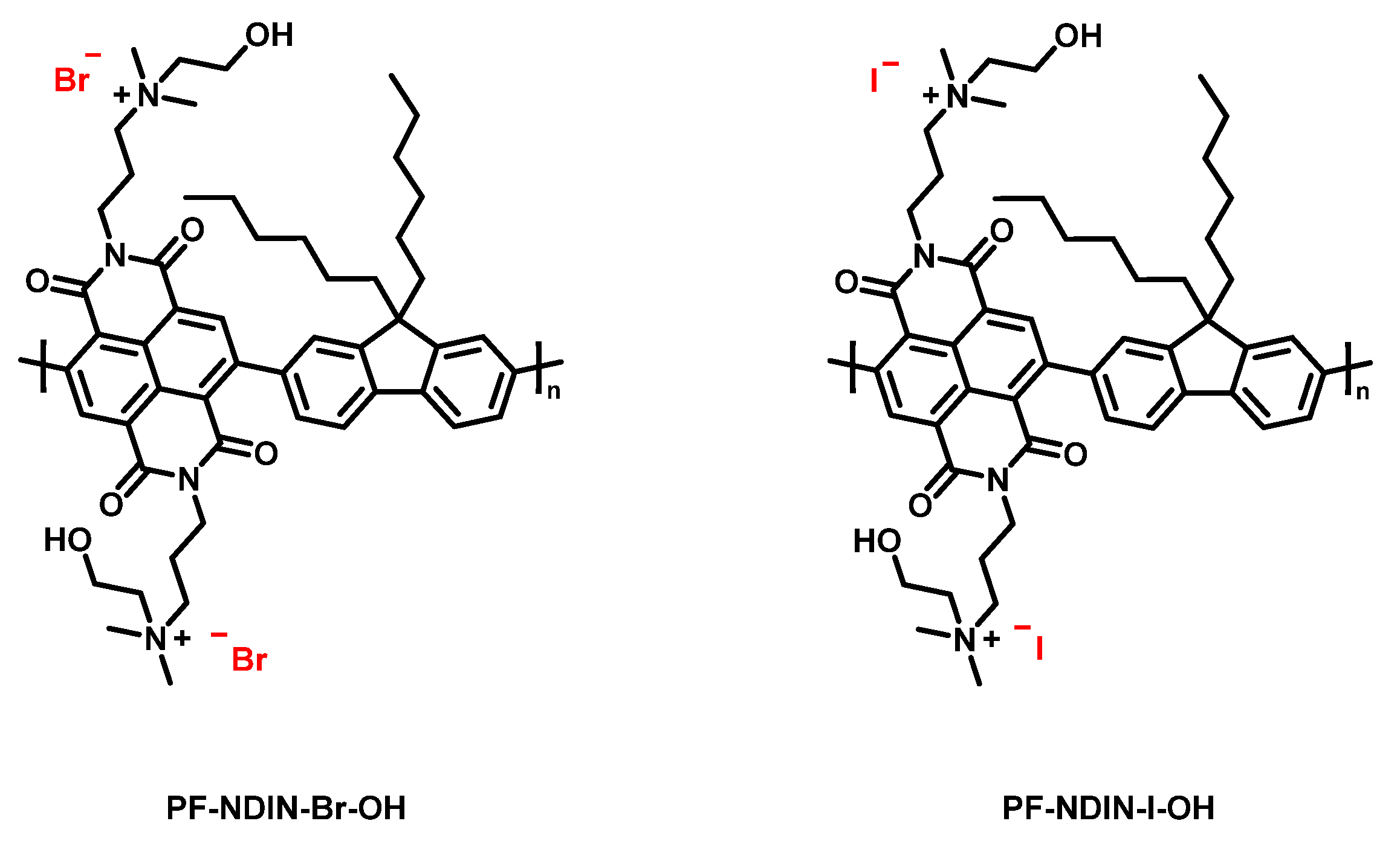
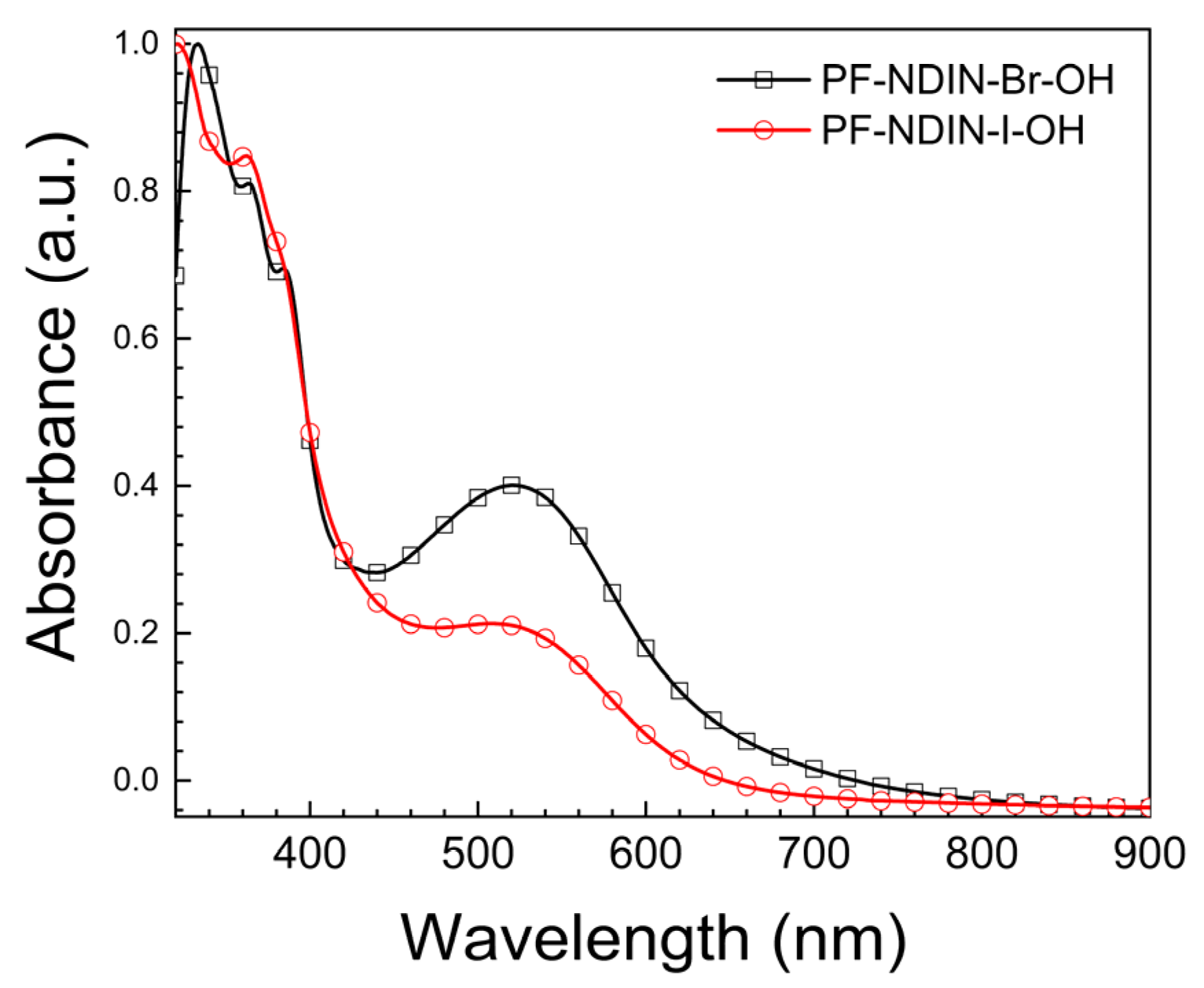
2.1. Synthesis of Materials and the Characterization
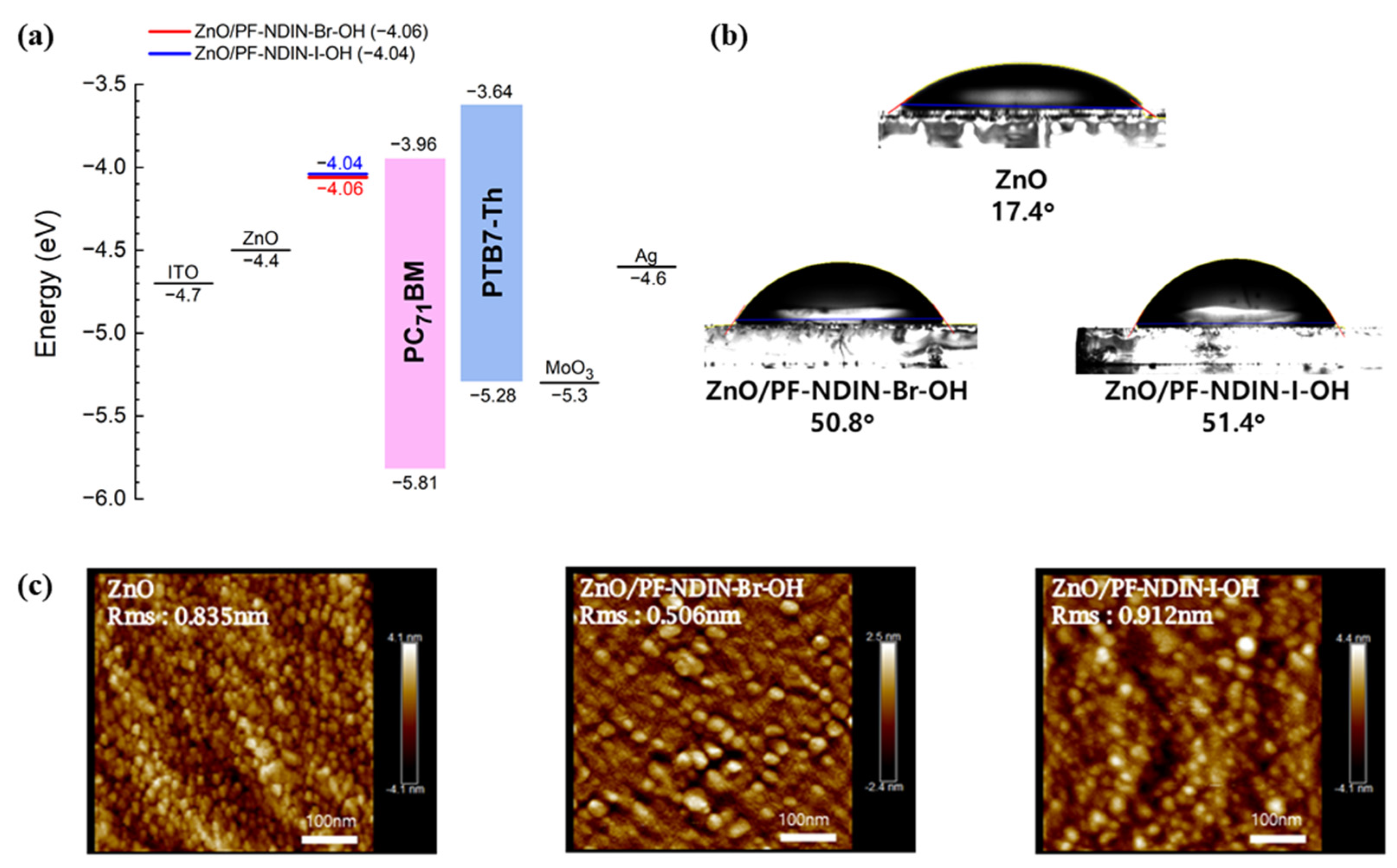
2.2. Investigation of the Surface Characteristics of ZnO/Polyelectrolyte
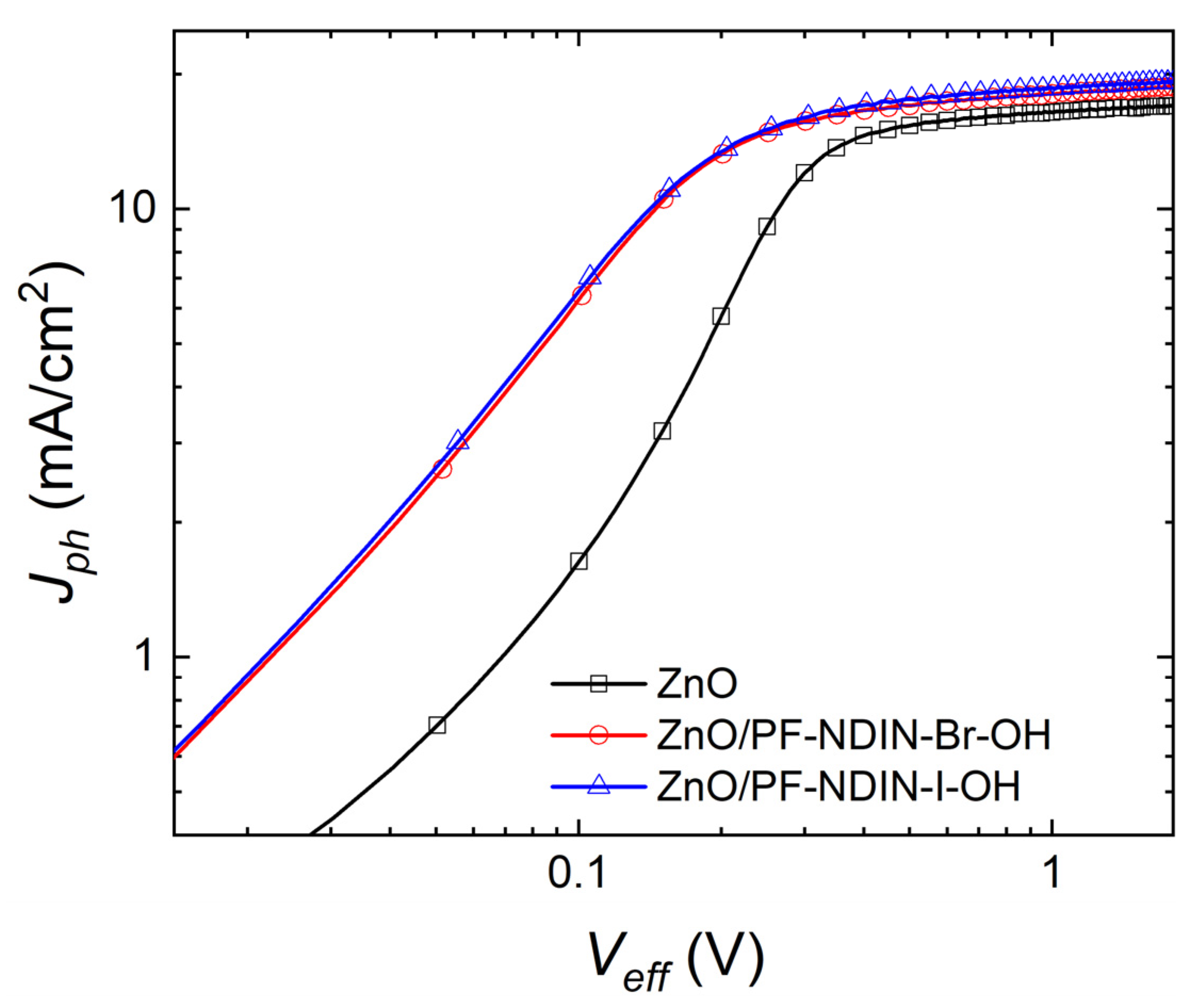
2.3. Photovoltaic Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Compound 1
3.3. Synthesis of Compound 2
3.4. Synthesis of Compound 3
3.5. Synthesis of Compound 4
3.6. Synthesis of PF-NDIN
3.7. The General Synthesis Procedure of PF-NDIN-Br-OH and PF-NDIN-I-OH
3.8. Fabrication of OSCs
3.9. Fabrication of Electron-only Devices
3.10. Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, N.G.; Jeon, S.J.; Kim, Y.H.; Lee, H.S.; Hong, D.H.; Moon, D.K. Interchain Hydrogen-Bonded Conjugated Polymer for Enhancing the Stability of Organic Solar Cells. J. Ind. Eng. Chem. 2022, 112, 76–84. [Google Scholar] [CrossRef]
- Nugraha, D.F.; Son, D.H.; Wardani, R.P.; Lee, S.W.; Whang, D.R.; Kim, J.H.; Chang, D.W. Strategic Structural Evolution for Enhancing the Photovoltaic Performance of Quinoxaline-Based Polymers. J. Ind. Eng. Chem. 2022, 114, 331–337. [Google Scholar] [CrossRef]
- Do, Y.; Park, H.; Gokulnath, T.; Sung, K.; Park, H.Y.; Jin, S.H. Significance of Siloxane Functionalized Side-Chain π-Conjugated Polymer Donor: Optimization of Active Layer Morphology Toward the Stable All-Polymer Solar Cells. Macromol. Res. 2022, 30, 183–189. [Google Scholar] [CrossRef]
- Salma, S.A.; Kim, J.H. Effect of the Side Chain Functionality of the Conjugated Polyelectrolytes as a Cathode Interlayer Material on the Photovoltaic Performances. Macromol. Res. 2022, 30, 146–151. [Google Scholar] [CrossRef]
- Nasrun, R.F.B.; Nisa, Q.A.K.; Salma, S.A.; Kim, J.H. A Simple Approach for Fabrication of Low-Temperature Processible Inverted Organic Solar Cells. J. Ind. Eng. Chem. 2023, 124, 331–339. [Google Scholar] [CrossRef]
- Nugroho, H.S.; Refantero, G.; Septiani, N.L.W.; Iqbal, M.; Marno, S.; Abdullah, H.; Prima, E.C.; Nugraha; Yuliarto, B. A Progress Review on the Modification of CZTS(e)-Based Thin-Film Solar Cells. J. Ind. Eng. Chem. 2022, 105, 83–110. [Google Scholar] [CrossRef]
- Sanap, P.P.; Gupta, S.P.; Kahandal, S.S.; Gunjakar, J.L.; Lokhande, C.D.; Sankapal, B.R.; Said, Z.; Bulakhe, R.N.; Man Kim, J.; Bhalerao, A.B. Exploring Vanadium-Chalcogenides toward Solar Cell Application: A Review. J. Ind. Eng. Chem. 2023, 129, 124–142. [Google Scholar] [CrossRef]
- Liu, M.; Fan, P.; Hu, Q.; Russell, T.P.; Liu, Y. Naphthalene-Diimide-Based Ionenes as Universal Interlayers for Efficient Organic Solar Cells. Angew. Chemie-Int. Ed. 2020, 59, 18131–18135. [Google Scholar] [CrossRef]
- Luo, Z.; Yu, J.; Liu, H.; Liu, T.; Ni, F.; Hu, J.; Zou, Y.; Zeng, A.; Su, C.; Jeng, U.; et al. Heteroheptacene-Based Acceptors with Thieno[3,2-b]Pyrrole Yield High-Performance Polymer Solar Cells. Natl. Sci. Rev. 2022, 9, nwac076. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, T.; Ma, R.; Xiao, Y.; Zhan, L.; Zhang, G.; Sun, H.; Ni, F.; Chai, G.; Wang, J.; et al. Precisely Controlling the Position of Bromine on the End Group Enables Well-Regular Polymer Acceptors for All-Polymer Solar Cells with Efficiencies over 15%. Adv. Mater. 2020, 32, 202005942. [Google Scholar] [CrossRef]
- Tang, H.; Bai, Y.; Zhao, H.; Qin, X.; Hu, Z.; Zhou, C.; Huang, F.; Cao, Y. Interface Engineering for Highly Efficient Organic Solar Cells. Adv. Mater. 2023, 2212236, in press. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Jung, J.G.; Ryu, D.H.; Lee, S.; Song, C.E.; Lim, B.; Jung, Y.J.; Park, J.M.; Hwang, D.H. Thienoquinolinone-Based Acceptor-π-Acceptor-Type Building Block for Polymer Donors in Organic Solar Cells. Macromol. Res. 2023, 31, 25–31. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Kim, H.; Lee, J.; Aryal, U.K.; Reddy, S.S.; Gayathri, R.D.; Gokulnath, T.; Jin, S.H. Side Chain Functionalization of Conjugated Polymer on the Modulation of Photovoltaic Properties of Fullerene and Non-Fullerene Organic Solar Cells. Macromol. Res. 2023, 31, 897–905. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Song, D.; Jee, J.; Gokulnath, T.; Han, S.C.; Jin, S.H.; Lee, J.W. BDT-Based Donor Polymer for Organic Solar Cells to Achieve High Efficiency over 15% for Ternary Organic Solar Cells. Macromol. Res. 2023, 31, 489–497. [Google Scholar] [CrossRef]
- Chau, H.D.; Kataria, M.; Kwon, N.Y.; Park, S.H.; Kim, Y.; Kang, H.; Harit, A.K.; Woo, H.Y.; Yoon, H.J.; Park, S.; et al. Improved Photovoltaic Performance of Ternary All-Polymer Solar Cells by Incorporating a New Y6-Based Polymer Acceptor and PC61BM. Macromol. Res. 2022, 8, 587–596. [Google Scholar] [CrossRef]
- Meresa, A.A.; Lee, T.W.; Lee, S.; Kim, F.S.; Park, K. Tetraaryldiamine-Based Electron-Transporting Interlayers for Performance and Stability Enhancement of Organic Solar Cells. J. Ind. Eng. Chem. 2022, 113, 461–467. [Google Scholar] [CrossRef]
- Yip, H.L.; Jen, A.K.Y. Recent Advances in Solution-Processed Interfacial Materials for Efficient and Stable Polymer Solar Cells. Energy Environ. Sci. 2012, 5, 5994–6011. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, N.; Hu, Z.; Wang, Z.; Yang, X.; Huang, F.; Cao, Y. Self-Doped n-Type Small Molecular Electron Transport Materials for High-Performance Organic Solar Cells. Sci. China Chem. 2017, 60, 1136–1144. [Google Scholar] [CrossRef]
- Handoko, S.L.; Jin, H.C.; Whang, D.R.; Putri, S.K.; Kim, J.H.; Chang, D.W. Synthesis of Quinoxaline-Based Polymers with Multiple Electron-Withdrawing Groups for Polymer Solar Cells. J. Ind. Eng. Chem. 2019, 73, 192–197. [Google Scholar] [CrossRef]
- Abdullah; Akhtar, M.S.; Kim, E.B.; Fijahi, L.; Shin, H.S.; Ameen, S. A Symmetric Benzoselenadiazole Based D–A–D Small Molecule for Solution Processed Bulk-Heterojunction Organic Solar Cells. J. Ind. Eng. Chem. 2020, 81, 309–316. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Z.; Zhang, Z.; Han, Y.; Xia, D.; Zhao, C.; Zhang, Y.; Wang, L.; Xiao, C.; You, S.; et al. Stable Radical Based Conjugated Electrolytes as a Cathode Interlayer for Organic Solar Cells with Thickness-Insensitive Fill Factors. J. Mater. Chem. A 2023, 11, 6574–6580. [Google Scholar] [CrossRef]
- Peng, Z.; Ye, L.; Ade, H. Understanding, Quantifying, and Controlling the Molecular Ordering of Semiconducting Polymers: From Novices to Experts and Amorphous to Perfect Crystals. Mater. Horiz. 2022, 9, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, B.; Gong, Y.; Deng, J.; Tan, Z. Green-Solvent-Processable Strategies for Achieving Large-Scale Manufacture of Organic Photovoltaics. J. Mater. Chem. A 2019, 7, 22826–22847. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, K.; Zhong, C.; Huang, F.; Cao, Y. Recent Advances in Water/Alcohol-Soluble π-Conjugated Materials: New Materials and Growing Applications in Solar Cells. Chem. Soc. Rev. 2013, 42, 9071–9104. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-Soluble Fluorescent Conjugated Polymers and Their Interactions with Biomacromolecules for Sensitive Biosensors. Chem. Soc. Rev. 2010, 39, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Salma, S.A.; Khoirun Nisa, Q.A.; Nasrun, R.F.B.; Son, D.H.; Kim, J.H. Perylene Diimide Derivatives with Diverse Ionic Functionality as Cathode Interlayer for ZnO-Free Inverted Non-Fullerene Organic Solar Cells. Mater. Today Energy 2023, 35, 101297. [Google Scholar] [CrossRef]
- Nasrun, R.F.B.; Nisa, Q.A.K.; Salma, S.A.; Kim, J.H. Cathode Interlayer Based on Naphthalene Diimide: A Modification Strategy for Zinc-Oxide-Free Inverted Organic Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 21324. [Google Scholar] [CrossRef]
- Salma, S.A.; Nasrun, R.F.B.; Nisa, Q.A.K.; Son, D.H.; Kim, H. The Investigation of the Cathode Buffer Layer Based on Perylene Diimide for the Inverted Organic Solar Cells. Dye Pigments 2023, 217, 111345. [Google Scholar] [CrossRef]
- Yang, R.; Garcia, A.; Korystov, D.; Mikhailovsky, A.; Bazan, G.C.; Nguyen, T.Q. Control of Interchain Contacts, Solid-State Fluorescence Quantum Yield, and Charge Transport of Cationic Conjugated Polyelectrolytes by Choice of Anion. J. Am. Chem. Soc. 2006, 128, 16532–16539. [Google Scholar] [CrossRef]
- Nasrun, R.F.B.; Son, D.H.; Salma, S.A.; Kim, J.H. Effect of Counter Anions and Side-Chain Modification of Conjugated Polymers for Organic Solar Cells as an Interlayer: An in-Depth Investigation of the Diverse Modification of Ionic Functionality. Dye Pigments 2022, 206, 110625. [Google Scholar] [CrossRef]
- Do, T.T.; Hong, H.S.; Ha, Y.E.; Park, J.; Kang, Y.C.; Kim, J.H. Effect of Polyelectrolyte Electron Collection Layer Counteranion on the Properties of Polymer Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Yang, R.; Brzezinski, J.Z.; Walker, B.; Bazan, G.C.; Nguyen, T.Q. Electronic Properties at Gold/Conjugated-Polyelectrolyte Interfaces. Adv. Mater. 2009, 21, 1006–1011. [Google Scholar] [CrossRef]
- Jung, H.S.; Nguyen, T.Q. Electronic Properties of Conjugated Polyelectrolyte Thin Films. J. Am. Chem. Soc. 2008, 130, 10042–10043. [Google Scholar] [CrossRef]
- Garcia, A.; Yang, R.; Jin, Y.; Walker, B.; Nguyen, T.Q. Structure-Function Relationships of Conjugated Polyelectrolyte Electron Injection Layers in Polymer Light Emitting Diodes. Appl. Phys. Lett. 2007, 91, 153502. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, C.; Dong, S.; Jiang, X.F.; Wu, S.; Wu, H.; Yip, H.L.; Huang, F.; Cao, Y. N-Type Water/Alcohol-Soluble Naphthalene Diimide-Based Conjugated Polymers for High-Performance Polymer Solar Cells. J. Am. Chem. Soc. 2016, 138, 2004–2013. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Xu, R.; Zhang, R.; Hu, Z.; Huang, F.; Cao, Y. Finely Tuned Composition in Conjugated Polyelectrolytes for Interfacial Engineering of Efficient Polymer Solar Cells. Small Methods 2018, 2, 1700407. [Google Scholar] [CrossRef]
- Gesevičius, D.; Neels, A.; Jenatsch, S.; Hack, E.; Viani, L.; Athanasopoulos, S.; Nüesch, F.; Heier, J. Increasing Photovoltaic Performance of an Organic Cationic Chromophore by Anion Exchange. Adv. Sci. 2018, 5, 1700496. [Google Scholar] [CrossRef]
- Yip, H.L.; Hau, S.K.; Baek, N.S.; Ma, H.; Jen, A.K.Y. Polymer Solar Cells That Use Self-Assembled-Monolayer-Modified ZnO/Metals as Cathodes. Adv. Mater. 2008, 20, 2376–2382. [Google Scholar] [CrossRef]
- Kang, J.H.; Park, Y.J.; Cha, M.J.; Yi, Y.; Song, A.; Chung, K.B.; Seo, J.H.; Walker, B. Effect of Counter-Ions on the Properties and Performance of Non-Conjugated Polyelectrolyte Interlayers in Solar Cell and Transistor Devices. RSC Adv. 2019, 9, 20670–20676. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Z.G.; Xue, L.; Min, J.; Zhang, J.; Wei, Z.; Li, Y. All-Polymer Solar Cells Based on Absorption-Complementary Polymer Donor and Acceptor with High Power Conversion Efficiency of 8.27%. Adv. Mater. 2016, 28, 1884–1890. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Mihailetchi, V.D.; Xie, H.; Blom, P.W.M. Origin of the Light Intensity Dependence of the Short-Circuit Current of Polymer/Fullerene Solar Cells. Appl. Phys. Lett. 2005, 87, 203502. [Google Scholar] [CrossRef]
- Cowan, S.R.; Roy, A.; Heeger, A.J. Recombination in Polymer-Fullerene Bulk Heterojunction Solar Cells. Phys. Rev. B-Condens. Matter Mater. Phys. 2010, 82, 245207. [Google Scholar] [CrossRef]
- Peng, W.; Lin, Y.; Jeong, S.Y.; Firdaus, Y.; Genene, Z.; Nikitaras, A.; Tsetseris, L.; Woo, H.Y.; Zhu, W.; Anthopoulos, T.D.; et al. Using Two Compatible Donor Polymers Boosts the Efficiency of Ternary Organic Solar Cells to 17.7%. Chem. Mater 2021, 33, 19. [Google Scholar] [CrossRef]


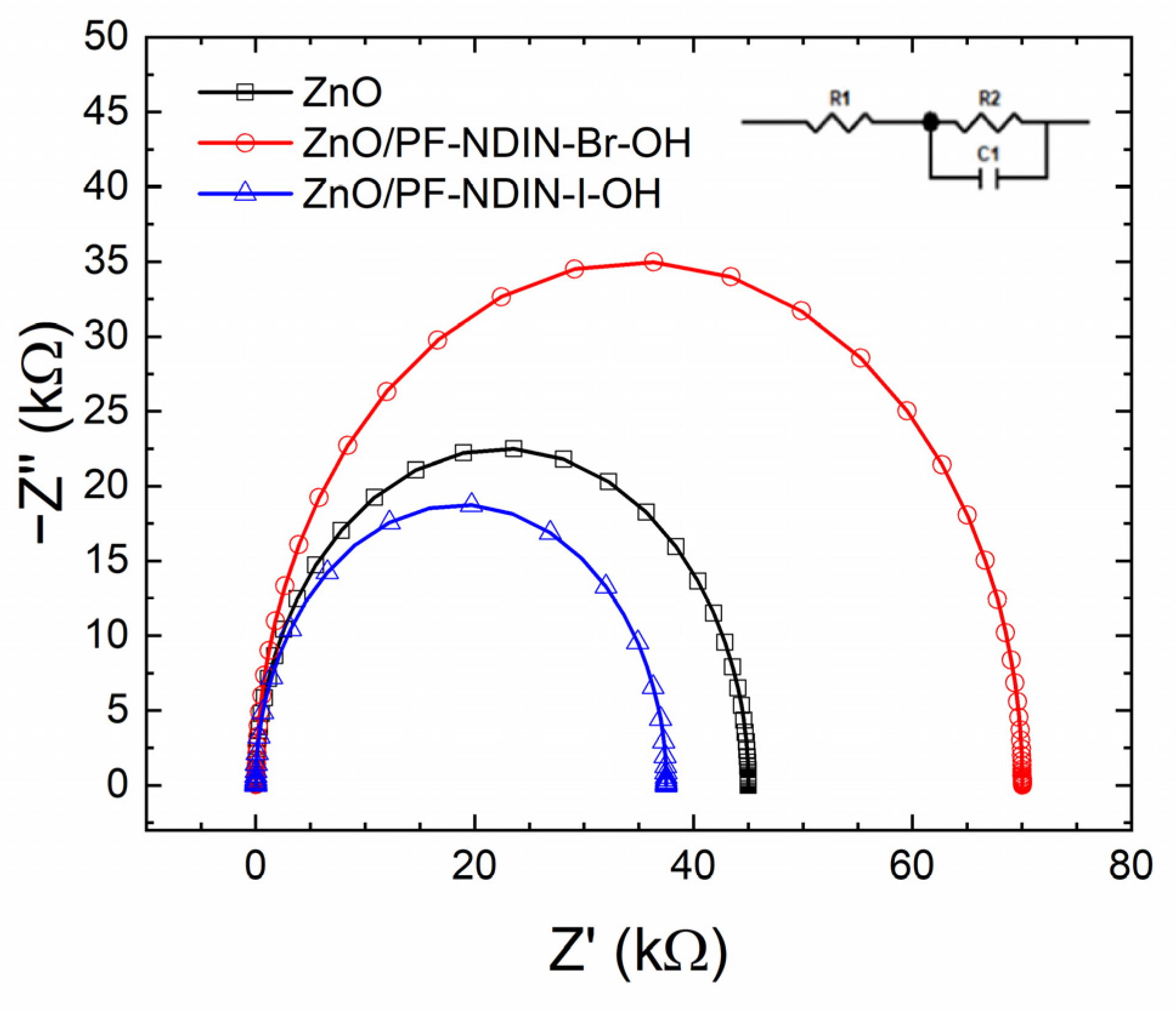

| Interlayer | (mA/cm2) | (V) | (%) | PCE (%) | (Ω cm2) | (kΩ cm2) |
|---|---|---|---|---|---|---|
| ZnO | 17.1 (16.9 ± 0.4) | 0.79 (0.79 ± 0.00) | 66.3 (65.9 ± 0.6) | 8.96 (8.77 ± 0.27) | 2.01 | 0.77 |
| ZnO/PF-NDIN-Br-OH | 17.9 (17.8 ± 0.4) | 0.79 (0.79 ± 0.00) | 67.7 (67.7 ± 0.8) | 9.51 (9.43 ± 0.15) | 1.37 | 1.58 |
| ZnO/PF-NDIN-I-OH | 18.5 (18.4 ± 0.1) | 0.79 (0.79 ± 0.00) | 65.7 (65.7 ± 0.3) | 9.59 (9.55 ± 0.03) | 1.28 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrun, R.F.B.; Son, D.H.; Kim, J.H. Novel Polyelectrolytes Based on Naphthalene Diimide with Different Counteranions for Cathode Interlayers in Polymer Solar Cells. Int. J. Mol. Sci. 2024, 25, 522. https://doi.org/10.3390/ijms25010522
Nasrun RFB, Son DH, Kim JH. Novel Polyelectrolytes Based on Naphthalene Diimide with Different Counteranions for Cathode Interlayers in Polymer Solar Cells. International Journal of Molecular Sciences. 2024; 25(1):522. https://doi.org/10.3390/ijms25010522
Chicago/Turabian StyleNasrun, Rahmatia Fitri Binti, Dong Hwan Son, and Joo Hyun Kim. 2024. "Novel Polyelectrolytes Based on Naphthalene Diimide with Different Counteranions for Cathode Interlayers in Polymer Solar Cells" International Journal of Molecular Sciences 25, no. 1: 522. https://doi.org/10.3390/ijms25010522






