Metabolic Response of Peach Fruit to Invasive Brown Marmorated Stink Bug (Halyomorpha halys Stål.)’s Infestation
Abstract
:1. Introduction
2. Results
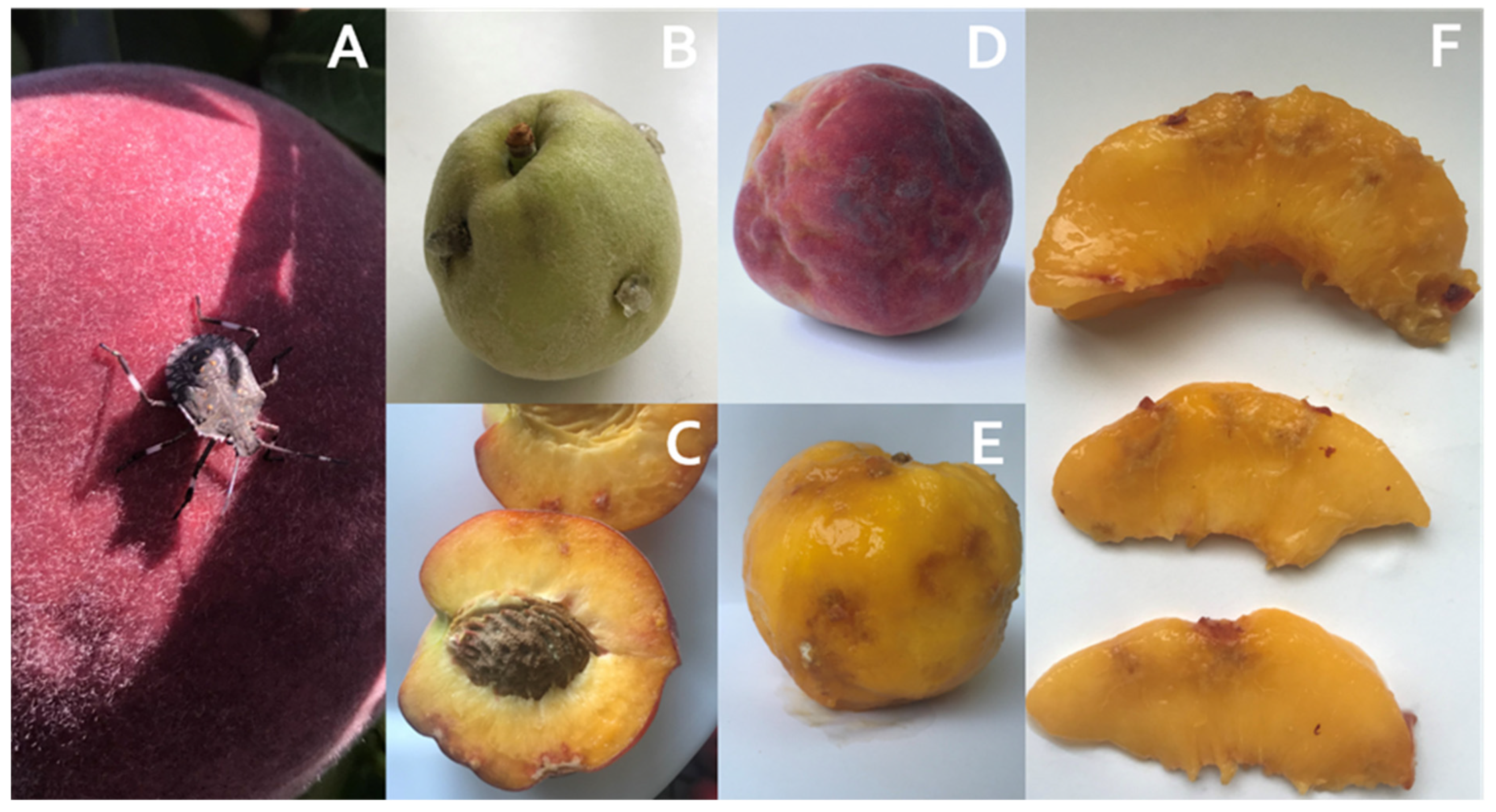
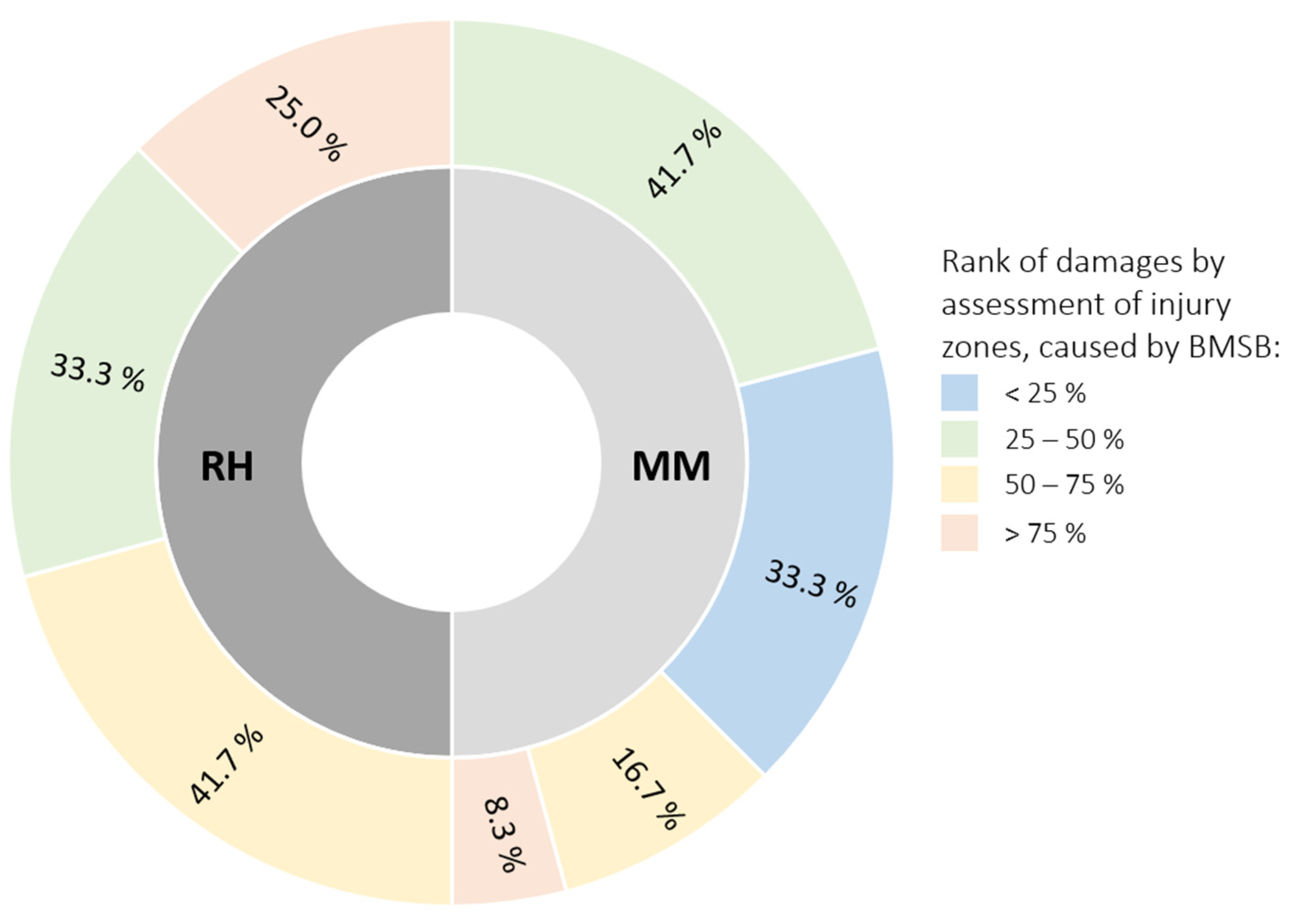
2.1. Visual Appearance of Peaches Damaged by Brown Marmorated Stink Bug (BMSB)
2.2. Effects of BMSB Infestation on Sugar and Organic Acid Content in Peach Fruit
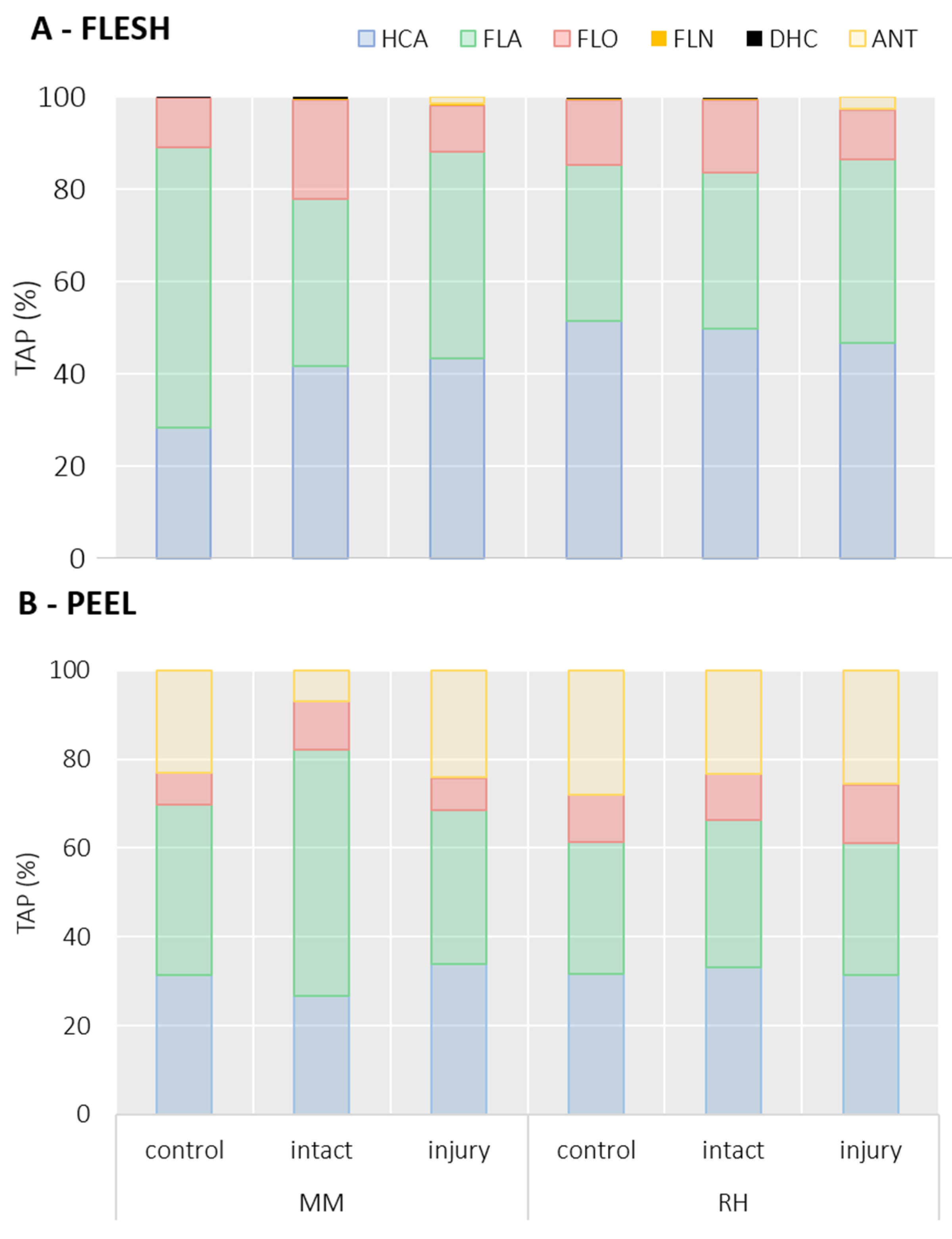
2.3. Effects of BMSB Infestation on Phenolic Content in Peach Pulp
2.4. Effects of BMSB Infestation on Phenolic Content in Peach Peel
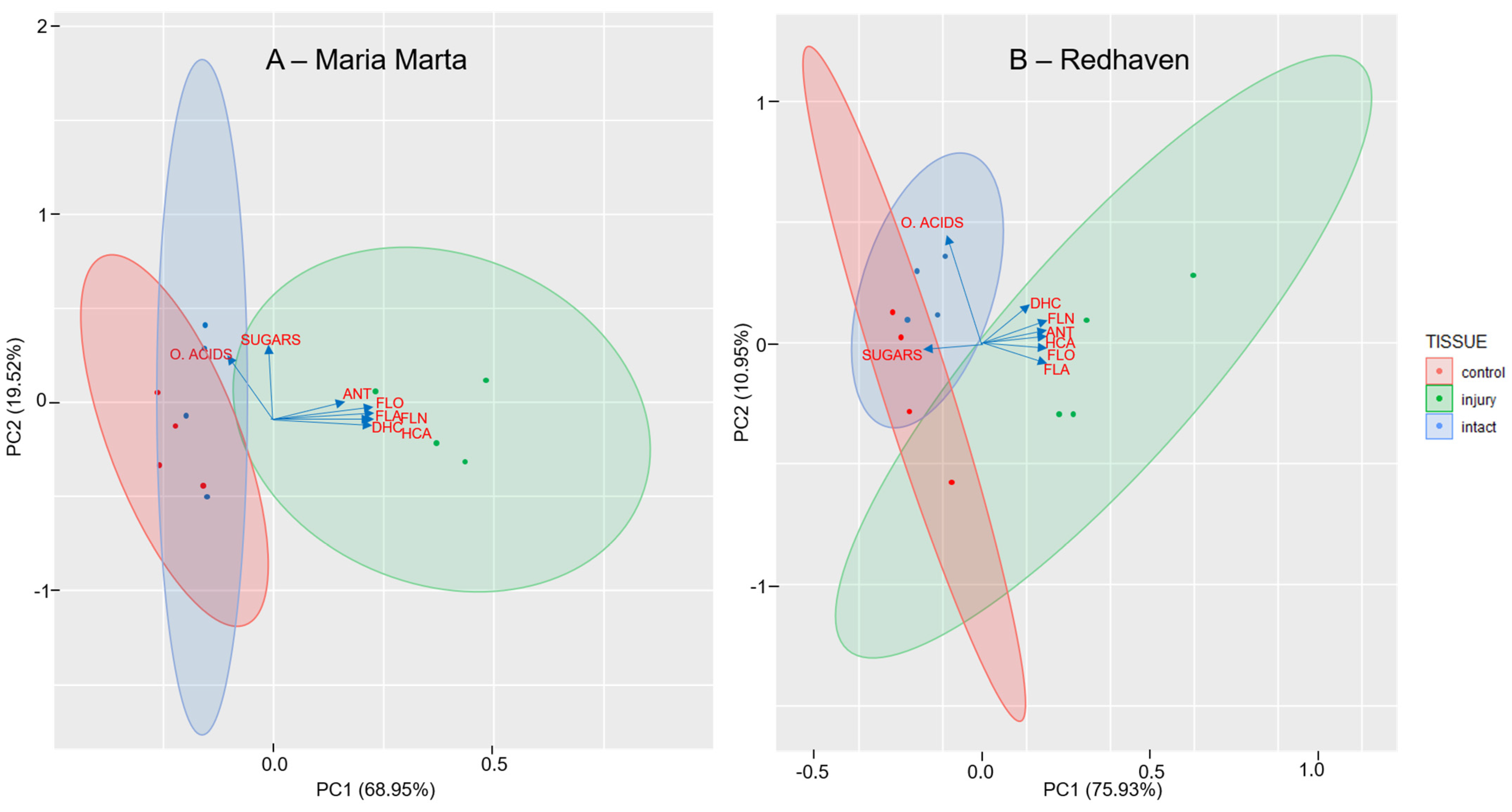
2.5. Principal Component Analysis (PCA) for Peach Flesh
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sampling
4.3. Sugar and Organic Acid Content Analysis
4.4. Analysis of Individual Phenolic Compounds
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergmann, E.J.; Venugopal, P.D.; Martinson, H.M.; Raupp, M.J.; Shrewsbury, P.M. Host Plant Use by the Invasive Halyomorpha halys (Stål.) on Woody Ornamental Trees and Shrubs. PLoS ONE 2016, 11, e0149975. [Google Scholar] [CrossRef] [PubMed]
- Bariselli, M.; Bugiani, R.; Maistrello, L. Distribution and damage caused by Halyomorpha halys in Italy. EPPO Bull. 2016, 46, 332–334. [Google Scholar] [CrossRef]
- Lee, D.-H.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C. Review of the Biology, Ecology, and Management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Hoebeke, E.R.; Carter, M.E. Halyomorpha halys (Stål.) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Wash. 2003, 105, 225–237. [Google Scholar]
- Arnold, K. Halyomorpha halys (Stål., 1855), eine für die europäischen Fauna neu nachgewiesene Wanzenart (Insecta: Heteroptera, Pentatomidae, Pentatominae, Cappaeini). Mitteilungen Thüringer Entomol. 2009, 16, 19. [Google Scholar]
- Rot, M.; Devetak, M.; Carlevaris, B.; Žežlina, J.; Žežlina, I. First record of brown marmorated stink bug (Halyomorpha halys (Stål., 1855)) (Hemiptera: Pentatomidae) in Slovenia. Acta Entomol. Slov. 2018, 26, 5–12. [Google Scholar]
- Schumm, Z.R.; Alston, D.G.; Spears, L.R.; Manlove, K. Impact of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Feeding on Tart Cherry (Rosales: Rosaceae) Quality and Yield in Utah. J. Econ. Entomol. 2020, 113, 2328–2334. [Google Scholar] [CrossRef]
- Zhou, Y.; Giusti, M.M.; Parker, J.; Salamanca, J.; Rodriguez-Saona, C. Frugivory by Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Alters Blueberry Fruit Chemistry and Preference by Conspecifics. Environ. Entomol 2016, 45, 1227–1234. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Hamilton, G.C. Seasonal Occurrence and Impact of Halyomorpha halys (Hemiptera: Pentatomidae) in Tree Fruit. J. Econ. Entomol. 2009, 102, 1133–1140. [Google Scholar] [CrossRef]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, Ecology, and Management of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Injury to apples and peaches at harvest from feeding by Halyomorpha halys (Stål.) (Hemiptera: Pentatomidae) nymphs early and late in the season. Crop Prot. 2016, 89, 58–65. [Google Scholar] [CrossRef]
- Sagun, S.; Collins, E.; Martin, C.; Nolan, E.J.; Horzempa, J. Alarm Odor Compounds of the Brown Marmorated Stink Bug Exhibit Antibacterial Activity. J. Pharmacogn. Nat. Prod. 2016, 2, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-S.; Hu, C.-C. Irritant contact keratitis caused by the bodily fluids of a brown marmorated stink bug. Taiwan J. Ophthalmol. 2017, 7, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.R.; Polk, D.; Nielsen, A.L. IPM-CPR for peaches: Incorporating behaviorally-based methods to manage Halyomorpha halys and key pests in peach: Behaviorally-based methods to manage H. halys and key pests in peach. Pest. Manag. Sci. 2015, 71, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Damos, P.; Soulopoulou, P.; Thomidis, T. First record and current status of the brown marmorated sting bug Halyomorpha halys damaging peaches and olives in northern Greece. J. Plant Prot. Res. 2020, 60, 323–326. [Google Scholar] [CrossRef]
- Joseph, S.V.; Nita, M.; Leskey, T.C.; Christopher Bergh, J. Temporal Effects on the Incidence and Severity of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Feeding Injury to Peaches and Apples during the Fruiting Period in Virginia. J. Econ. Entomol. 2015, 108, 592–599. [Google Scholar] [CrossRef]
- Desurmont, G.A.; Harvey, J.; Van Dam, N.M.; Cristescu, S.M.; Schiestl, F.P.; Cozzolino, S.; Anderson, P.; Larsson, M.C.; Kindlmann, P.; Danner, H.; et al. Alien interference: Disruption of infochemical networks by invasive insect herbivores: Invasive insects and infochemicals. Plant Cell Environ. 2014, 37, 1854–1865. [Google Scholar] [CrossRef]
- Rondoni, G.; Bertoldi, V.; Malek, R.; Djelouah, K.; Moretti, C.; Buonaurio, R.; Conti, E. Vicia faba plants respond to oviposition by invasive Halyomorpha halys activating direct defences against offspring. J. Pest Sci. 2018, 91, 671–679. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Vet, E.M.L.; Dicke, M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Bandoly, M.; Hilker, M.; Steppuhn, A. Oviposition by Spodoptera exigua on Nicotiana attenuata primes induced plant defence against larval herbivory. Plant J. 2015, 83, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Ode, P.J.; Oliveira-Hofman, C.; Harwood, J.D. Integration of Plant Defense Traits with Biological Control of Arthropod Pests: Challenges and Opportunities. Front. Plant Sci. 2016, 7, 1794. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects; Imperato, F., Ed.; Research Signpost: Trivandrum, India, 2006; Volume 661, ISBN 978-81-308-0034-9. [Google Scholar]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Wiman, N.G.; Parker, J.E.; Rodriguez-Saona, C.; Walton, V.M. Characterizing Damage of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) in Blueberries. J. Econ. Entomol. 2015, 108, 1156–1163. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Singapore, 2020; pp. 517–532. ISBN 9789811548895. [Google Scholar]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and Metabolism of Phenolic Compounds. In Fruit and Vegetable Phytochemicals; Yahia, E.M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 115–124. ISBN 978-1-119-15804-2. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic acid pathway in biosynthesis of phenolic compounds. Plant Physiol. Asp. Phenolic Compd. 2019, 1, 1–15. [Google Scholar]
- Kundu, A.; Vadassery, J. Chlorogenic acid-mediated chemical defence of plants against insect herbivores. Plant Biol. J. 2019, 21, 185–189. [Google Scholar] [CrossRef]
- Menin, B.; Comino, C.; Moglia, A.; Dolzhenko, Y.; Portis, E.; Lanteri, S. Identification and mapping of genes related to caffeoylquinic acid synthesis in Cynara cardunculus L. Plant Sci. 2010, 179, 338–347. [Google Scholar] [CrossRef]
- Orazem, P.; Mikulic-Petkovsek, M.; Stampar, F.; Hudina, M. Changes during the last ripening stage in pomological and biochemical parameters of the ‘Redhaven’ peach cultivar grafted on different rootstocks. Sci. Hortic. 2013, 160, 326–334. [Google Scholar] [CrossRef]
- Ivancic, T.; Grohar, M.C.; Jakopic, J.; Veberic, R.; Hudina, M. Effect of Brown Marmorated Stink Bug (Halyomorpha halys Stål.) Infestation on the Phenolic Response and Quality of Olive Fruits (Olea europaea L.). Agronomy 2022, 12, 2200. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signal. Behav. 2011, 6, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, M.; Felton, G.W. Insights into the Saliva of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Pentatomidae). PLoS ONE 2014, 9, e88483. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional Roles of Polyphenols in Plant-Herbivore Interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Hatayama, M.; Isono, Y.; Sato, T.; Watanabe, R.; Yonekura-Sakakibara, K.; Fukuchi-Mizutani, M.; Tanaka, Y.; Kusumi, T.; Nishino, T.; et al. Localization of a flavonoid biosynthetic polyphenol oxidase in vacuoles. Plant J. 2005, 45, 133–143. [Google Scholar] [CrossRef]
- Ryan, A.C. The systemin signaling pathway: Differential activation of plant defensive genes. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 2000, 1477, 112–121. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-0-470-71422-5. [Google Scholar]
- Moctezuma, C.; Hammerbacher, A.; Heil, M.; Gershenzon, J.; Méndez-Alonzo, R.; Oyama, K. Specific Polyphenols and Tannins are Associated with Defense Against Insect Herbivores in the Tropical Oak Quercus oleoides. J. Chem. Ecol. 2014, 40, 458–467. [Google Scholar] [CrossRef]
- Thelen, G.C.; Vivanco, J.M.; Newingham, B.; Good, W.; Bais, H.P.; Landres, P.; Caesar, A.; Callaway, R.M. Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives: Allelopathic exudation by an invasive plant. Ecol. Lett. 2005, 8, 209–217. [Google Scholar] [CrossRef]
- Kumar, P.; Ortiz, E.V.; Garrido, E.; Poveda, K.; Jander, G. Potato tuber herbivory increases resistance to aboveground lepidopteran herbivores. Oecologia 2016, 182, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Mishra, S.; Vadassery, J. Spodoptera litura-mediated chemical defense is differentially modulated in older and younger systemic leaves of Solanum lycopersicum. Planta 2018, 248, 981–997. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Joo, Y.; Kim, S.-G.; Baldwin, I.T. What happens in the pith stays in the pith: Tissue-localized defense responses facilitate chemical niche differentiation between two spatially separated herbivores. Plant J. 2017, 92, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.W.; Duffey, S.S. Protective action of midgut catalase in lepidopteran larvae against oxidative plant defenses. J. Chem. Ecol. 1991, 17, 1715–1732. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Bonsu, M.; Dingha, B.N.; Jackai, L.E.N.; Adjei-Fremah, S.; Worku, M. Evaluation of preference of brown marmorated stink bug, Halyomorpha halys (Stål.) for different colour bell peppers and the role of plant protein. Arthropod.-Plant Interact. 2020, 14, 363–372. [Google Scholar] [CrossRef]
- Gacnik, S.; Veberič, R.; Hudina, M.; Marinovic, S.; Halbwirth, H.; Mikulič-Petkovšek, M. Salicylic and Methyl Salicylic Acid Affect Quality and Phenolic Profile of Apple Fruits Three Weeks before the Harvest. Plants 2021, 10, 1807. [Google Scholar] [CrossRef]
- Zupan, A.; Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. Individual phenolic response and peroxidase activity in peel of differently sun-exposed apples in the period favorable for sunburn occurrence. J. Plant Physiol. 2014, 171, 1706–1712. [Google Scholar] [CrossRef]
- Gačnik, S.; Veberič, R.; Hudina, M.; Koron, D.; Mikulič-Petkovšek, M. Salicylate Treatment Affects Fruit Quality and Also Alters the Composition of Metabolites in Strawberries. Horticulturae 2021, 7, 400. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Chemical profile of black currant fruit modified by different degree of infection with black currant leaf spot. Sci. Hortic. 2013, 150, 399–409. [Google Scholar] [CrossRef]
| ‘Maria Marta’ | ‘Redhaven’ | |||||
|---|---|---|---|---|---|---|
| Control | Intact | Injury | Control | Intact | Injury | |
| Sucrose | 92.11 ± 5.60 b | 81.34 ± 2.84 b | 48.92 ± 0.82 a | 72.11 ± 7.46 a | 100.6 ± 14.64 a | 86.14 ± 7.07 a |
| Glucose | 9.57 ± 1.27 a | 9.88 ± 0.33 a | 10.54 ± 0.2 a | 13.02 ± 1.39 a | 12.59 ± 0.25 a | 11.65 ± 0.42 a |
| Fructose | 11.38 ± 0.57 a | 10.88 ± 0.47 a | 12.18 ± 0.31 a | 13.85 ± 1.52 a | 13.14 ± 0.45 a | 13.25 ± 0.4 a |
| Sorbitol | 2.03 ± 0.15 b | 1.64 ± 0.20 b | 0.96 ± 0.02 a | 0.94 ± 0.32 a | 1.20 ± 0.12 a | 0.92 ± 0.30 a |
| Total Sugars | 114.9 ± 9.11 b | 103.7 ± 2.07 b | 72.6 ± 1.09 a | 99.92 ± 5.23 a | 127.5 ± 14.16 a | 112.0 ± 7.52 a |
| Citric | 3.55 ± 0.21 a | 3.69 ± 0.11 a | 3.12 ± 0.17 a | 4.60 ± 1.02 a | 3.73 ± 0.46 a | 3.36 ± 0.17 a |
| Malic | 3.96 ± 0.78 a | 4.95 ± 0.27 a | 3.16 ± 0.40 a | 5.39 ± 0.63 a | 6.38 ± 1.32 a | 4.36 ± 0.40 a |
| Quinic * | 86.92 ± 7.12 a | 100.8 ± 4.39 a | 121.2 ± 55.13 a | 60.06 ± 11.7 a | 113.2 ± 19.95 a | 221.1 ± 9.28 b |
| Shikimic * | 0.21 ± 0.01 a | 0.35 ± 0.05 a | 0.76 ± 0.42 a | 0.35 ± 0.09 a | 0.35 ± 0.05 a | 1.44 ± 0.10 b |
| Fumaric * | 12.51 ± 1.84 a | 10.58 ± 1.07 a | 24.47 ± 9.93 a | 10.97 ± 1.30 a | 11.08 ± 1.22 a | 11.38 ± 0.16 a |
| Total Acids | 7.62 ± 0.89 a | 8.74 ± 0.3 a | 6.43 ± 0.57 a | 10.06 ± 1.6 a | 10.23 ± 1.78 a | 7.95 ± 0.56 a |
| ‘Maria Marta’ | ‘Redhaven’ | |||||
|---|---|---|---|---|---|---|
| Control | Intact | Injured | Control | Intact | Injured | |
| 3-CQA (neochlorogenic acid) | 9.79 ± 3.68 a | 8.97 ± 0.94 a | 23.61 ± 3.63 b | 24.59 ± 5.72 a | 30.7 ± 0.94 a | 75.1 ± 10.15 b |
| Caffeic acid hex 1 | 0.44 ± 0.06 a | 0.14 ± 0.04 a | 1.35 ± 0.29 b | 0.30 ± 0.05 a | 0.40 ± 0.07 a | 3.02 ± 0.47 b |
| Caffeic acid hex 2 | 0.06 ± 0.01 a | 0.04 ± 0.01 a | 0.20 ± 0.03 b | 0.03 ± 0.01 a | 0.06 ± 0.01 a | 0.26 ± 0.04 b |
| p-CoA hex 1 | 0.54 ± 0.07 a | 0.17 ± 0.05 a | 1.65 ± 0.36 b | 0.37 ± 0.07 a | 0.49 ± 0.08 a | 3.70 ± 0.58 b |
| p-CoA hex 2 | 0.03 ± 0.00 a | 0.02 ± 0.00 a | 0.10 ± 0.01 b | 0.02 ± 0.00 a | 0.03 ± 0.01 a | 0.13 ± 0.02 b |
| 3-p-CoQA | 0.47 ± 0.06 a | 0.15 ± 0.05 a | 1.45 ± 0.32 b | 0.32 ± 0.06 a | 0.43 ± 0.07 a | 3.25 ± 0.51 b |
| 5-CQA1 (chlorogenic acid) | 15.2 ± 3.04 a | 16.84 ± 1.27 a | 150.5 ± 36.93 b | 23.17 ± 7.99 a | 31.7 ± 2.17 a | 237.1 ± 30.89 b |
| 5-CQA2 | 9.46 ± 1.05 a | 9.28 ± 1.00 a | 28.04 ± 2.11 b | 12.16 ± 2.1 a | 19.05 ± 4.00 a | 77.25 ± 1.78 b |
| 3-FQA1 | 0.11 ± 0.02 a | 0.13 ± 0.01 a | 1.13 ± 0.28 b | 0.17 ± 0.06 a | 0.24 ± 0.02 a | 1.78 ± 0.23 b |
| 4-CQA | 3.73 ± 0.3 a | 2.21 ± 0.32 a | 6.52 ± 0.59 b | 2.65 ± 0.66 a | 3.60 ± 0.41 a | 10.42 ± 0.28 b |
| 5-p-CoQA 1 | 0.60 ± 0.11 a | 0.15 ± 0.03 b | 0.54 ± 0.08 a | 0.18 ± 0.04 a | 0.21 ± 0.07 a | 0.84 ± 0.04 b |
| 5-p-CoQA 2 | 0.25 ± 0.02 a | 0.05 ± 0.01 b | 0.17 ± 0.03 a | 0.06 ± 0.02 a | 0.07 ± 0.01 a | 0.59 ± 0.20 a |
| diCQA1 | 0.30 ± 0.04 a | 0.62 ± 0.13 a | 3.36 ± 0.84 b | 0.84 ± 0.15 a | 1.50 ± 0.24 a | 3.96 ± 0.32 b |
| diCQA2 | 0.22 ± 0.03 a | 0.46 ± 0.10 a | 2.52 ± 0.63 b | 0.63 ± 0.11 a | 1.13 ± 0.18 a | 2.97 ± 0.24 b |
| diCQA3 | 1.17 ± 0.12 a | 2.75 ± 0.62 b | 3.24 ± 0.14 b | 4.21 ± 0.79 a | 7.08 ± 1.11 a | 17.53 ± 1.62 b |
| Sinapoyl hex | 0.27 ± 0.03 a | 0.32 ± 0.05 a | 1.85 ± 0.25 b | 0.33 ± 0.15 a | 0.45 ± 0.09 a | 2.78 ± 0.32 b |
| HCA 1 | 45.49 ± 8.98 a | 42.63 ± 3.21 a | 228.9 ± 43.52 b | 70.76 ± 17.19 a | 98.25 ± 8.68 a | 444.6 ± 31.21 b |
| Procy dimer 1 | 26.1 ± 5.55 a | 3.76 ± 1.38 a | 65.02 ± 14.71 b | 6.13 ± 1.17 a | 10.31 ± 1.2 a | 111.0 ± 17.22 b |
| procy dimer 2 | 6.40 ± 0.82 a | 2.04 ± 0.62 a | 19.76 ± 4.29 b | 4.40 ± 0.80 a | 5.82 ± 1.00 a | 44.19 ± 6.88 b |
| Procy dimer 3 | 0.99 ± 0.15 a | 0.61 ± 0.11 a | 3.17 ± 0.46 b | 0.53 ± 0.10 a | 0.93 ± 0.19 a | 4.23 ± 0.57 b |
| Procy dimer 4 | 28.03 ± 3.91 a | 10.46 ± 1.13 b | 43.63 ± 6.48 a | 10.42 ± 3.47 a | 14.04 ± 3.05 a | 53.97 ± 5.30 b |
| Catechin | 3.17 ± 0.63 a | 3.52 ± 0.27 a | 31.42 ± 7.71 b | 4.84 ± 1.67 a | 6.62 ± 0.45 a | 49.52 ± 6.45 b |
| Procy trimer 1 | 2.85 ± 0.57 a | 3.16 ± 0.24 a | 28.19 ± 6.92 b | 4.34 ± 1.50 a | 5.94 ± 0.41 a | 44.43 ± 5.79 b |
| Procy trimer 2 | 7.89 ± 0.64 a | 4.68 ± 0.68 a | 13.8 ± 1.25 b | 5.60 ± 1.40 a | 7.61 ± 0.86 a | 22.08 ± 0.59 b |
| Procy trimer 3 | 12.55 ± 2.49 a | 2.98 ± 0.64 b | 12.02 ± 1.28 a | 3.44 ± 1.01 a | 5.51 ± 1.77 a | 19.98 ± 2.75 b |
| Procy tetramer | 9.87 ± 0.80 a | 5.86 ± 0.85 a | 17.25 ± 1.56 b | 7.00 ± 1.75 a | 9.52 ± 1.07 a | 27.59 ± 0.74 b |
| FLA 1 | 97.85 ± 15.42 a | 37.06 ± 5.19 a | 234.3 ± 39.07 b | 46.7 ± 12.03 a | 66.3 ± 9.57 a | 377 ± 35.44 b |
| Roseoside | 6.80 ± 1.03 a | 4.23 ± 0.76 a | 21.84 ± 3.20 b | 3.66 ± 0.70 a | 6.41 ± 1.34 a | 29.12 ± 3.93 b |
| Q-3-rut | 0.06 ± 0.01 a | 0.08 ± 0.03 a | 1.17 ± 0.37 b | 0.13 ± 0.02 a | 0.15 ± 0.04 a | 2.46 ± 0.67 b |
| Q-3-gal | 8.00 ± 2.08 a | 14.49 ± 2.81 ab | 22.26 ± 1.02 b | 12.16 ± 2.92 a | 19.26 ± 3.38 a | 48.86 ± 3.88 b |
| Q-3-glu | 0.68 ± 0.18 a | 1.17 ± 0.24 ab | 1.70 ± 0.17 b | 0.96 ± 0.21 a | 1.71 ± 0.34 a | 4.29 ± 0.26 b |
| Kaem-3-rut | 0.16 ± 0.06 a | 0.40 ± 0.06 a | 1.59 ± 0.27 b | 0.34 ± 0.02 a | 0.57 ± 0.05 a | 5.41 ± 1.28 b |
| Iso-3-rut | 0.86 ± 0.17 ab | 0.68 ± 0.08 b | 1.41 ± 0.16 a | 1.16 ± 0.41 a | 1.78 ± 0.49 a | 3.73 ± 0.36 b |
| Q-3-xyl | 0.03 ± 0.01 ab | 0.03 ± 0.00 b | 0.05 ± 0.01 a | 0.04 ± 0.02 a | 0.07 ± 0.02 a | 0.14 ± 0.01 b |
| Kaem hex 1 | 0.05 ± 0.01 a | 0.06 ± 0.01 a | 0.32 ± 0.04 b | 0.06 ± 0.03 a | 0.08 ± 0.02 a | 0.49 ± 0.06 b |
| Kaem hex 2 | 0.12 ± 0.05 a | 0.20 ± 0.02 a | 1.02 ± 0.16 b | 0.24 ± 0.07 a | 0.13 ± 0.03 a | 2.51 ± 0.17 b |
| Iso-hex 1 | 0.14 ± 0.05 a | 0.23 ± 0.02 a | 1.18 ± 0.18 b | 0.27 ± 0.08 a | 0.15 ± 0.03 a | 2.89 ± 0.20 b |
| Iso-hex 2 | 0.03 ± 0.00 a | 0.07 ± 0.01 a | 0.39 ± 0.10 b | 0.10 ± 0.02 a | 0.17 ± 0.03 a | 0.46 ± 0.04 b |
| Q-3-arafur | in traces | in traces | in traces | in traces | in traces | in traces |
| Q-3-rham | 0.03 ± 0.00 a | 0.06 ± 0.01 a | 0.32 ± 0.08 b | 0.08 ± 0.01 a | 0.14 ± 0.02 a | 0.38 ± 0.03 b |
| Kaem acetyl hex | in traces | in traces | in traces | in traces | in traces | in traces |
| Q-3-rhamnosyl hex 1 | in traces | in traces | in traces | in traces | in traces | in traces |
| Q-3-rhamnosyl hex 2 | in traces | in traces | in traces | in traces | in traces | in traces |
| Q-3-arapyr | in traces | in traces | in traces | in traces | in traces | in traces |
| FLO 1 | 16.98 ± 3.45 a | 21.72 ± 4.02 a | 53.41 ± 5.17 b | 19.24 ± 4.43 a | 30.65 ± 5.67 a | 101 ± 9.4 b |
| Nar hex 1 | 0.01 ± 0.00 a | 0.02 ± 0.00 a | 0.08 ± 0.01 b | 0.02 ± 0.00 a | 0.03 ± 0.00 a | 0.26 ± 0.06 b |
| Nar hex 2 | 0.04 ± 0.01 a | 0.08 ± 0.02 a | 0.42 ± 0.11 b | 0.11 ± 0.02 a | 0.19 ± 0.03 a | 0.50 ± 0.04 b |
| Nar hex 3 | 0.07 ± 0.01 a | 0.16 ± 0.03 a | 0.85 ± 0.21 b | 0.21 ± 0.04 a | 0.38 ± 0.06 a | 1.00 ± 0.08 b |
| FLN 1 | 0.12 ± 0.02 a | 0.25 ± 0.05 a | 1.35 ± 0.33 b | 0.33 ± 0.06 a | 0.60 ± 0.09 a | 1.76 ± 0.13 b |
| Phloridzin (DHC) 1 | 0.17 ± 0.03 a | 0.43 ± 0.08 b | 0.54 ± 0.03 b | 0.52 ± 0.08 a | 0.76 ± 0.05 a | 2.13 ± 0.09 b |
| Cy-3-glu (ANT) 1 | - | - | 7.09 ± 2.25 | - | - | 23.24 ± 12.38 |
| TAP 2 | 160.6 ± 27.83 a | 102.1 ± 12.28 a | 525.5 ± 87.6 b | 137.6 ± 33.44 a | 196.6 ± 23.82 a | 949.7 ± 73.4 b |
| ‘Maria Marta’ | ‘Redhaven’ | |||||
|---|---|---|---|---|---|---|
| Control | Intact | Intact | Control | Injured | Injured | |
| 3-CQA (neochlorogenic acid) | 153.2 ± 29.99 a | 45.92 ± 6.67 b | 136.9 ± 23.19 a | 528.0 ± 172.4 a | 331.7 ± 44.44 a | 616.8 ± 130.0 a |
| Caffeic acid hex 1 | 0.32 ± 0.05 a | 0.47 ± 0.05 a | 0.28 ± 0.04 a | 0.62 ± 0.12 a | 0.93 ± 0.10 ab | 1.32 ± 0.20 b |
| Caffeic acid hex 2 | 4.95 ± 0.71 a | 7.23 ± 0.55 a | 5.60 ± 0.67 a | 9.39 ± 1.53 a | 12.89 ± 1.08 ab | 18.98 ± 2.73 b |
| Caffeic acid hex 3 | 0.41 ± 0.06 a | 0.60 ± 0.04 a | 0.46 ± 0.05 a | 0.77 ± 0.13 a | 1.06 ± 0.09 ab | 1.56 ± 0.22 b |
| p-CoA hex 1 | 0.35 ± 0.06 a | 0.50 ± 0.05 a | 0.30 ± 0.05 a | 0.66 ± 0.12 a | 1.00 ± 0.1 ab | 1.42 ± 0.22 b |
| p-CoA hex 2 | 0.21 ± 0.03 a | 0.31 ± 0.02 a | 0.24 ± 0.03 a | 0.41 ± 0.07 a | 0.56 ± 0.05 ab | 0.82 ± 0.12 b |
| 3-p-CQA | 0.39 ± 0.07 a | 0.57 ± 0.06 a | 0.34 ± 0.05 a | 0.76 ± 0.14 a | 1.14 ± 0.12 ab | 1.62 ± 0.25 b |
| 5-CQA1 (chlorogenic acid) | 48.88 ± 5.47 a | 53.64 ± 4.24 a | 38.11 ± 7.13 a | 75.18 ± 18.25 a | 102.9 ± 5.03 a | 103.2 ± 12.38 a |
| 5-CQA2 | 16.52 ± 1.96 a | 25.73 ± 1.3 b | 19.48 ± 1.49 ab | 32.33 ± 3.87 a | 54.96 ± 0.68 b | 54.25 ± 4.67 b |
| 3-FQA | 0.67 ± 0.08 a | 0.74 ± 0.06 a | 0.53 ± 0.10 a | 1.04 ± 0.25 a | 1.42 ± 0.07 a | 1.42 ± 0.17 a |
| 4-CQA | 4.25 ± 0.57 a | 4.00 ± 0.34 a | 3.31 ± 0.22 a | 5.06 ± 0.91 a | 3.41 ± 1.93 a | 3.90 ± 2.06 a |
| 4-p-CoQA | 0.67 ± 0.13 a | 0.56 ± 0.11 a | 0.58 ± 0.13 a | 1.43 ± 0.20 a | 1.15 ± 0.27 a | 1.85 ± 0.20 a |
| 5-p-CoQA 1 | 0.80 ± 0.24 a | 1.54 ± 0.49 a | 0.62 ± 0.23 a | 2.12 ± 0.28 a | 2.07 ± 0.32 a | 4.92 ± 1.19 a |
| 5-p-CoQA 2 | 0.95 ± 0.33 a | 0.54 ± 0.14 a | 0.72 ± 0.15 a | 3.41 ± 0.51 a | 2.40 ± 0.24 a | 7.27 ± 1.39 b |
| diCQA1 | 0.12 ± 0.02 a | 0.12 ± 0.01 a | 0.14 ± 0.02 a | 0.31 ± 0.05 a | 0.39 ± 0.04 a | 0.87 ± 0.06 b |
| diCQA2 | In traces | In traces | In traces | In traces | In traces | In traces |
| diCQA3 | In traces | In traces | In traces | In traces | In traces | In traces |
| HCA 1 | 232.7 ± 38.03 a | 142.5 ± 10.82 a | 207.7 ± 32.54 a | 661.5 ± 187.16 a | 518.0 ± 48.7 a | 820.2 ± 148.5 a |
| Procy dimer 1 | 42.16 ± 5.26 a | 29.18 ± 2.21 ab | 25.51 ± 3.55 b | 74.89 ± 9.47 a | 62.41 ± 9.39 a | 111.7 ± 7.12 b |
| Procy dimer 2 | 16.24 ± 2.72 a | 23.41 ± 2.55 a | 14.04 ± 2.15 a | 31.23 ± 5.83 a | 47.04 ± 4.88 ab | 66.58 ± 10.29 b |
| Procy dimer 3 | 1.07 ± 0.15 a | 1.56 ± 0.12 a | 1.21 ± 0.14 a | 2.02 ± 0.33 a | 2.78 ± 0.23 ab | 4.09 ± 0.59 b |
| Procy dimer 4 | 44.09 ± 9.16 a | 55.85 ± 9.05 a | 36.64 ± 6.49 a | 69.97 ± 6.85 a | 73.69 ± 7.04 a | 136.8 ± 20.07 b |
| Procy timer 5 | In traces | In traces | In traces | In traces | In traces | In traces |
| Procy trimer 1 | 19.19 ± 3.22 a | 27.67 ± 3.01 a | 16.59 ± 2.54 a | 36.91 ± 6.89 a | 55.59 ± 5.77 ab | 78.68 ± 12.16 b |
| Procy trimer 2 | 47.37 ± 5.30 a | 51.97 ± 4.11 a | 36.93 ± 6.91 a | 72.85 ± 17.69 a | 99.71 ± 4.87 a | 99.96 ± 11.99 a |
| Procy timer 3 | 16.23 ± 2.19 a | 15.26 ± 1.30 a | 12.65 ± 0.85 a | 19.31 ± 3.48 a | 13.02 ± 7.37 a | 14.88 ± 7.85 a |
| Procy trimer 4 | In traces | In traces | In traces | In traces | In traces | In traces |
| Procy trimer 5 | 16.25 ± 3.69 a | 9.77 ± 3.19 a | 10.97 ± 3.04 a | 20.29 ± 4.54 a | 16.82 ± 2.82 a | 34.72 ± 3.34 b |
| catechin | 56.31 ± 6.30 a | 61.79 ± 4.88 a | 43.91 ± 8.21 a | 86.6 ± 21.03 a | 118.6 ± 5.79 a | 118.8 ± 14.26 a |
| Procy tetramer | 18.10 ± 2.44 a | 17.02 ± 1.45 a | 14.11 ± 0.94 a | 21.54 ± 3.88 a | 14.53 ± 8.23 a | 16.6 ± 8.76 a |
| Epicatechin | 4.33 ± 0.82 a | 3.62 ± 0.71 a | 3.75 ± 0.86 a | 9.33 ± 1.33 a | 7.45 ± 1.74 a | 12.03 ± 1.28 a |
| FLA 1 | 281.3 ± 37.02 a | 297.1 ± 27.27 a | 216.3 ± 27.68 a | 444.9 ± 66.87 a | 511.6 ± 25.53 ab | 694.8 ± 75.23 b |
| Roseoside | 1.29 ± 0.18 a | 1.88 ± 0.14 a | 1.45 ± 0.17 a | 2.44 ± 0.40 a | 3.35 ± 0.28 ab | 4.93 ± 0.71 b |
| Q-3-rut | 3.58 ± 0.63 a | 8.05 ± 0.93 b | 4.58 ± 1.75 ab | 21.22 ± 4.10 a | 21.2 ± 3.51 a | 58.79 ± 11.89 b |
| Q-3-gal | 5.00 ± 0.35 a | 6.80 ± 0.49 a | 11.15 ± 1.39 b | 26.92 ± 8.05 a | 38.14 ± 2.35 a | 60.74 ± 15.59 a |
| Q-3-glu | 4.39 ± 0.24 a | 3.75 ± 0.33 a | 6.39 ± 1.87 a | 38.62 ± 8.46 a | 24.82 ± 4.56 a | 86.67 ± 25.64 a |
| Kaem-3-rut | 5.28 ± 0.73 a | 5.26 ± 0.39 a | 2.96 ± 0.43 b | 9.87 ± 2.01 a | 11.93 ± 1.46 ab | 18.5 ± 1.99 b |
| Q-3-arafur | 0.91 ± 0.17 a | 2.72 ± 0.21 b | 1.17 ± 0.36 a | 7.77 ± 1.02 a | 7.31 ± 0.15 a | 14.95 ± 2.43 b |
| Q-3-xyl | 0.20 ± 0.02 ab | 0.23 ± 0.01 b | 0.13 ± 0.03 a | 0.36 ± 0.05 a | 0.44 ± 0.05 ab | 0.65 ± 0.07 b |
| Iso-3-rut | 16.01 ± 1.62 ab | 17.76 ± 0.97 b | 10.42 ± 2.06 a | 27.99 ± 4.2 a | 34.19 ± 3.93 ab | 51.24 ± 5.6 b |
| Q-3-glucuronide | 0.02 ± 0.00 ab | 0.03 ± 0.00 b | 0.02 ± 0.00 a | 0.04 ± 0.01 a | 0.05 ± 0.01 ab | 0.07 ± 0.01 b |
| Kaem hex 1 | 9.34 ± 1.04 a | 6.28 ± 0.47 b | 4.17 ± 0.60 b | 7.74 ± 2.13 a | 5.06 ± 1.19 a | 4.64 ± 0.99 a |
| Kaem hex 2 | 0.79 ± 0.22 a | 2.09 ± 0.25 b | 1.59 ± 0.26 ab | 13.31 ± 1.84 a | 7.39 ± 0.89 a | 25.96 ± 4.28 b |
| Q-3-arapyr | 0.08 ± 0.02 a | 0.07 ± 0.01 a | 0.10 ± 0.01 a | 0.18 ± 0.03 a | 0.19 ± 0.00 a | 0.32 ± 0.04 b |
| Iso hex 1 | 0.29 ± 0.08 a | 0.77 ± 0.09 b | 0.58 ± 0.09 ab | 4.90 ± 0.68 a | 2.72 ± 0.33 a | 9.55 ± 1.58 b |
| Iso hex 2 | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.08 ± 0.01 a | 0.18 ± 0.03 a | 0.23 ± 0.02 a | 0.51 ± 0.03 b |
| Q-3-rha | 0.24 ± 0.05 a | 0.24 ± 0.02 a | 0.28 ± 0.04 a | 0.62 ± 0.1 a | 0.76 ± 0.07 a | 1.71 ± 0.11 b |
| Kaem acetylhex | 0.51 ± 0.13 a | 0.36 ± 0.07 a | 0.40 ± 0.09 a | 0.69 ± 0.17 a | 1.09 ± 0.06 a | 2.57 ± 0.39 b |
| Iso acetylhex | 0.31 ± 0.02 a | 0.36 ± 0.05 a | 0.37 ± 0.11 a | 0.59 ± 0.18 a | 0.96 ± 0.07 ab | 2.00 ± 0.50 b |
| Q-rhamnosyl hex 1 | 0.11 ± 0.03 a | 0.13 ± 0.02 a | 0.15 ± 0.04 a | 0.57 ± 0.13 a | 0.48 ± 0.03 a | 1.14 ± 0.12 b |
| Q-rhamnosyl hex 2 | 0.17 ± 0.03 a | 0.31 ± 0.05 a | 0.26 ± 0.08 a | 1.85 ± 0.28 a | 1.30 ± 0.10 a | 3.46 ± 0.35 b |
| Q-rhamnosyl hex 3 | In traces | In traces | In traces | In traces | In traces | In traces |
| FLO 1 | 48.61 ± 4.61 a | 57.6 ± 3.37 a | 46.25 ± 8.2 a | 165.8 ± 28.58 a | 161.6 ± 15.5 a | 348.4 ± 69.23 b |
| Nar hex 1 (FLN) 1 | 0.01 ± 0.00 a | 0.01 ± 00.0 a | 0.01 ± 00.0 a | 0.02 ± 0.00 a | 0.03 ± 0.00 a | 0.06 ± 0.00 b |
| Nar hex 2 | In traces | In traces | In traces | In traces | In traces | In traces |
| Phloridzin (DHC) 1 | 0.29 ± 0.08 a | 0.30 ± 0.03 a | 0.46 ± 0.07 a | 0.57 ± 0.19 a | 1.32 ± 0.12 ab | 1.50 ± 0.30 b |
| Cy-3-glu | 147.1 ± 30.54 b | 31.37 ± 5.9 a | 130.6 ± 23.48 b | 345.8 ± 49.29 a | 312.7 ± 47.12 a | 463.9 ± 33.98 a |
| Cy-3-rut | 26.48 ± 5.50 b | 5.65 ± 1.06 a | 23.5 ± 4.23 b | 62.24 ± 8.87 a | 56.29 ± 8.48 a | 83.50 ± 6.12 a |
| ANT 1 | 173.6 ± 36.04 b | 37.02 ± 6.96 a | 154.1 ± 27.7 b | 408.0 ± 58.16 a | 369.0 ± 55.6 a | 547.4 ± 34.73 a |
| TAP 2 | 736.6 ± 100.5 a | 534.5 ± 45.08 a | 624.7 ± 92.98 a | 1479 ± 271.2 | 1562 ± 137.2 | 2140 ± 126.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacnik, S.; Rusjan, D.; Mikulic-Petkovsek, M. Metabolic Response of Peach Fruit to Invasive Brown Marmorated Stink Bug (Halyomorpha halys Stål.)’s Infestation. Int. J. Mol. Sci. 2024, 25, 606. https://doi.org/10.3390/ijms25010606
Gacnik S, Rusjan D, Mikulic-Petkovsek M. Metabolic Response of Peach Fruit to Invasive Brown Marmorated Stink Bug (Halyomorpha halys Stål.)’s Infestation. International Journal of Molecular Sciences. 2024; 25(1):606. https://doi.org/10.3390/ijms25010606
Chicago/Turabian StyleGacnik, Sasa, Denis Rusjan, and Maja Mikulic-Petkovsek. 2024. "Metabolic Response of Peach Fruit to Invasive Brown Marmorated Stink Bug (Halyomorpha halys Stål.)’s Infestation" International Journal of Molecular Sciences 25, no. 1: 606. https://doi.org/10.3390/ijms25010606





