Pentoxifylline and Norcantharidin Modify p62 Expression in 2D and 3D Cultures of B16F1 Cells
Abstract
1. Introduction
2. Results
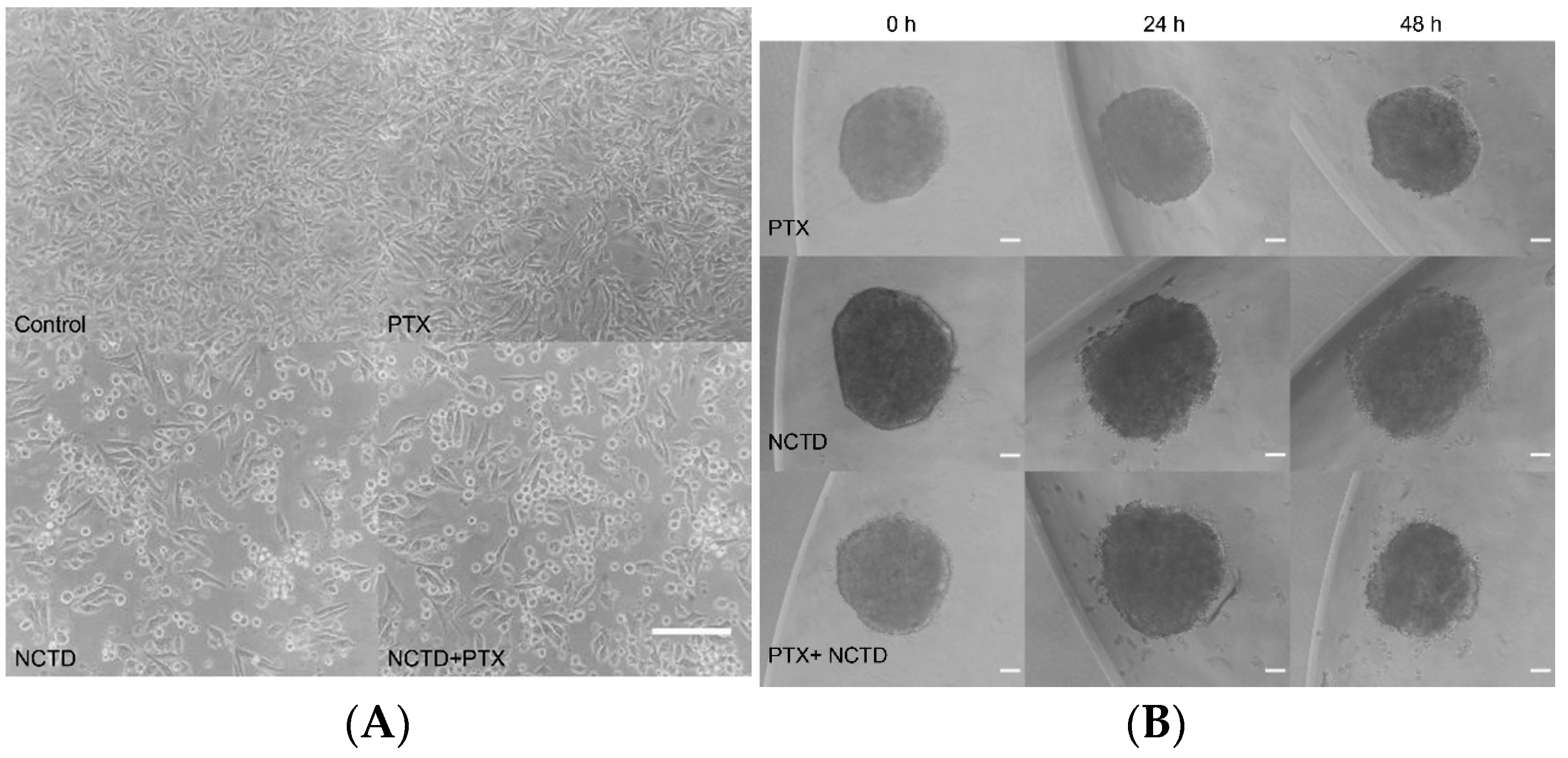
2.1. Two-Dimensional and Three-Dimensional Cell Cultures
2.2. Treatment of 2D and 3D Cell Culture Models with PTX and NCTD
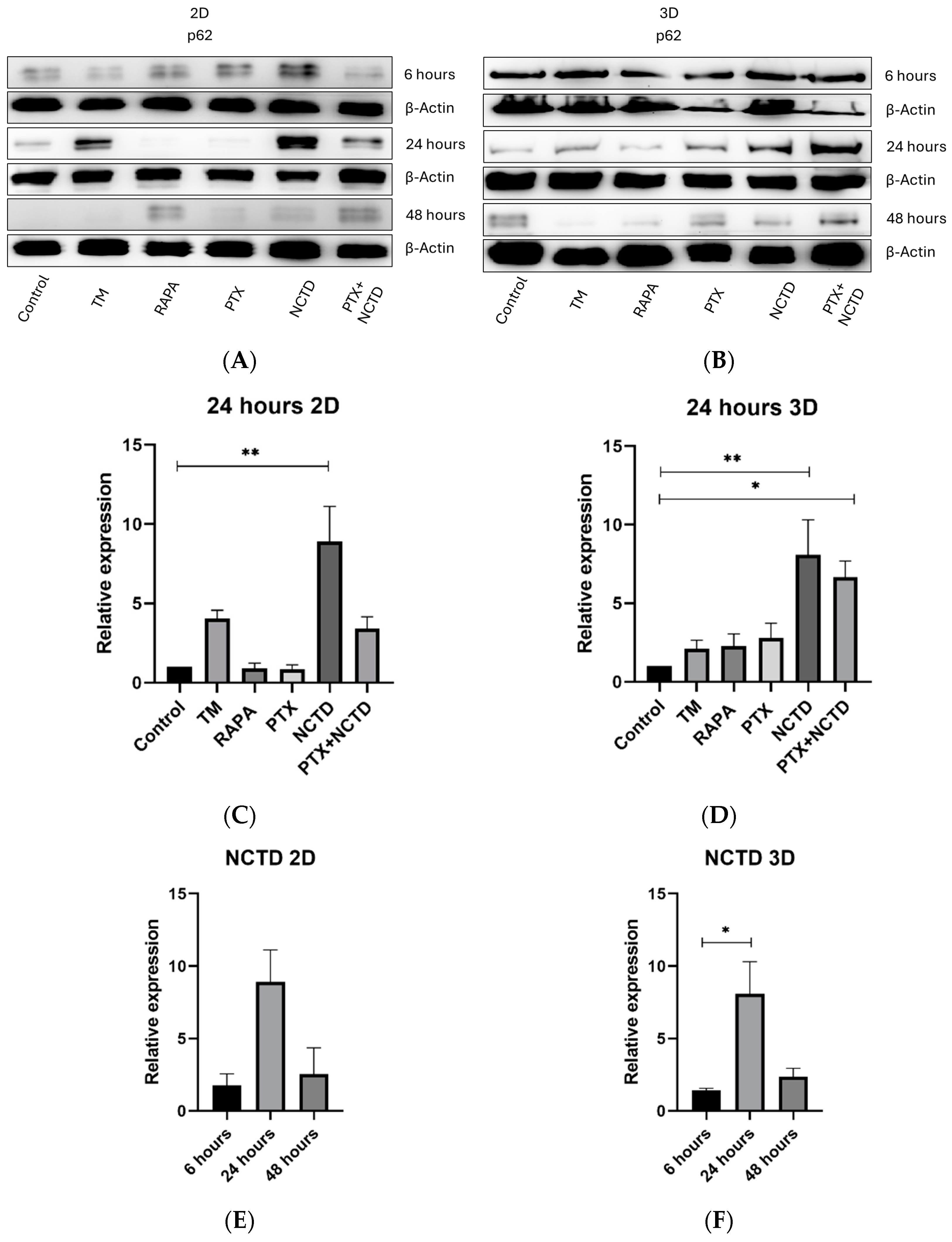
2.3. Autophagy p62 Expression in 2D and 3D Cultures
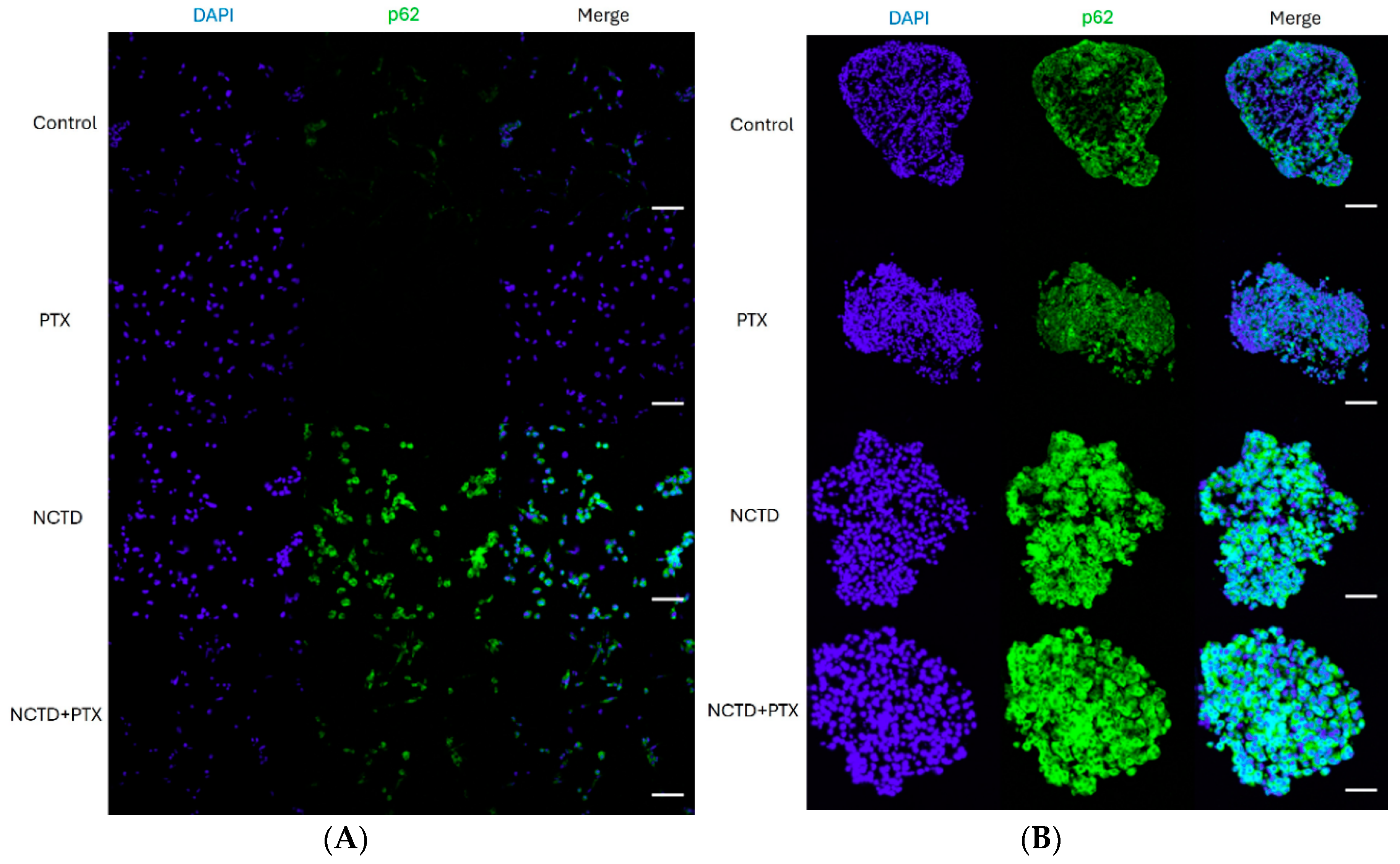
2.4. Expression of p62 in 2D and 3D Cultures by Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. Two-Dimensional (2D) and Three-Dimensional (3D) Cell Culture Models
4.2. Western Blot Analysis
4.3. Slices of the 3D Cultures of the Spheroids
4.4. Immunofluorescence of 2D and 3D Cultures
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhanyamraju, P.K.; Patel, T.N. Melanoma therapeutics: A literature review. J. Biomed. Res. 2022, 36, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Mueller, K.L.; Adams, D.J.; Anandasabapathy, N.; Aplin, A.E. Melanoma models for the next generation of therapies. Cancer Cell. 2021, 39, 610–631. [Google Scholar] [CrossRef] [PubMed]
- Brancato, V.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Could 3D models of cancer enhance drug screening? Biomaterials 2020, 232, 119744. [Google Scholar] [CrossRef] [PubMed]
- Sherman, H.; Gitschier, H.J.; Rossi, A.E. A Novel Three-Dimensional Immune Oncology Model for High-Throughput Testing of Tumoricidal Activity. Front. Immunol. 2018, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Cave, D.D.; Rizzo, R.; Sainz, B., Jr.; Gigli, G.; del Mercato, L.L.; Lonardo, E. The Revolutionary Roads to Study Cell–Cell Interactions in 3D In Vitro Pancreatic Cancer Models. Cancers 2021, 13, 930. [Google Scholar] [CrossRef]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Brier, L.W.; Ge, L.; Stjepanovic, G.; Thelen, A.M.; Hurley, J.H.; Schekman, R. Regulation of LC3 Lipidation by the Autophagy-Specific Class III phosphatidylinositol-3 Kinase Complex. Mol. Biol. Cell. 2019, 30, 1098–1107. [Google Scholar] [CrossRef]
- Nath, S.; Dancourt, J.; Shteyn, V.; Puente, G.; Fong, W.M.; Nag, S.; Bewersdorf, J.; Yamamoto, A.; Antonny, B.; Melia, T.J. Lipidation of the LC3/GABARAP family of autophagy proteins relies upon a membrane curvature-sensing domain in Atg3. Nat. Cell Biol. 2014, 16, 415–424. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Zhao, Y.; Ma, X.; Zhang, K.; He, X.; Wang, Z. Interaction domains of p62: A bridge between p62 and selective autophagy. DNA Cell Biol. 2013, 32, 220–227. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Frampton, J.E.; Brogden, R.N. Pentoxifylline (Oxpentifylline) A Review of its Therapeutic Efficacy in the Management of Peripheral Vascular and Cerebrovascular Disorders. Drugs Aging 1995, 7, 480–503. [Google Scholar] [CrossRef] [PubMed]
- Golunski, G.; Woziwodzka, A.; Piosik, J. Potential Use of Pentoxifylline in Cancer Therapy. Curr. Pharm. Biotechnol. 2018, 19, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Pratibha, D.; Yuvraj, N.; Rajiv, P.G. Pentoxifylline: A Potent Inhibitor of Angiogenesis via Blocking STAT3 Signaling in B16F10 Melanoma. Int. J. Tumor Ther. 2013, 2, 1–9. [Google Scholar]
- Mohammad, Z.K.; Rajiv, P.G. Pentoxifylline Inhibits Melanoma Tumor Growth and Angiogenesis by Targeting STAT3 Signaling Pathway. Biomed. Pharmacother. 2013, 67, 399–405. [Google Scholar]
- Sharma, K.; Ishaq, M.; Sharma, G.; Khan, M.A.; Dutta, R.K.; Majumdar, S. Pentoxifylline triggers autophagy via ER stress response that interferes with Pentoxifylline induced apoptosis in human melanoma cells. Biochem. Pharmacol. 2016, 103, 17–28. [Google Scholar] [CrossRef]
- Wang, G.S. Medical uses of Mylabris in ancient China and recent studies. J. Ethnopharmacol. 1989, 26, 147–162. [Google Scholar] [CrossRef]
- An, W.W.; Wang, M.W.; Tashiro, S.; Onodera, S.; Ikejima, T. Norcantharidin induces human melanoma A375-S2 cell apoptosis through mitochondrial and caspase pathways. J. Korean Med. Sci. 2004, 19, 560–566. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Kumar, S.M.; Martin, J.S.; Bing, Z.; Sheng, W.; Bosenberg, M.; Xu, X. Norcantharidin induces melanoma cell apoptosis through activation of TR3 dependent pathway. Cancer Biol. Ther. 2011, 12, 1005–1014. [Google Scholar] [CrossRef]
- Liu, Z.; Li, B.; Cao, M.; Jiang, J. Norcantharidin triggers apoptotic cell death in non-small cell lung cancer via a mitophagy-mediated autophagy pathway. Ann. Transl. Med. 2021, 9, 971. [Google Scholar] [CrossRef]
- Müller, I.; Kulms, D. A 3D Organotypic Melanoma Spheroid Skin Model. J. Vis. Exp. 2018, 135, 57500. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Godinez, J.M.; Gonzalez-Quiroz, J.L.; Cote-Palafox, H.; George, E.; Vergara-Lope Nuñez, J.A.; Villagomez-Olea, G.; Vazquez-Vazquez, F.C.; Lopez-Villegas, E.O.; Leon-Avila, G.; Dominguez-Lopez, M.L.; et al. Primary explants of the postnatal thymus allow the expansion of clonogenic thymic epithelial cells that constitute thymospheres. Stem Cell Res. Ther. 2023, 14, 312. [Google Scholar] [CrossRef]
- Nayak, P.; Bentivoglio, V.; Varani, M.; Signore, A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers 2023, 15, 4846. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, Y.; Tan, L.; Song, X.; Wang, M.; Li, Y.; Cao, Z.; Guo, C. Norcantharidin: Research advances in pharmaceutical activities and derivatives in recent years. Biomed. Pharmacother. 2020, 131, 110755. [Google Scholar] [CrossRef]
- Xiao, W.; Dai, B.; Zhu, Y.; Ye, D. Norcantharidin induces autophagy-related prostate cancer cell death through Beclin-1 upregulation by miR-129-5p suppression. Tumour Biol. 2016, 37, 15643–15648. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, B.; Wang, J.; Zhang, X.; Li, Z.; Dai, L.; Cao, M.; Jiang, J. Norcantharidin Inhibits SK-N-SH Neuroblastoma Cell Growth by Induction of Autophagy and Apoptosis. Technol. Cancer Res. Treat. 2017, 16, 33–44. [Google Scholar] [CrossRef]
- Xu, L.; Su, B.; Mo, L.; Zhao, C.; Zhao, Z.; Li, H.; Hu, Z.; Li, J. Norcantharidin Induces Immunogenic Cell Death of Bladder Cancer Cells through Promoting Autophagy in Acidic Culture. Int. J. Mol. Sci. 2022, 23, 3944. [Google Scholar] [CrossRef] [PubMed]
- Pangilinan, C.; Xu, X.; Herlyn, M.; Liang, C. Autophagy Paradox: Strategizing Treatment Modality in Melanoma. Curr. Treat. Options Oncol. 2023, 2, 130–145. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef]
- Li, H.C.; Xia, Z.H.; Chen, Y.F.; Yang, F.; Feng, W.; Cai, H.; Mei, Y.; Jiang, Y.M.; Xu, K.; Feng, D.X. Cantharidin Inhibits the Growth of Triple-Negative Breast Cancer Cells by Suppressing Autophagy and Inducing Apoptosis in Vitro and in Vivo. Cell Physiol. Biochem. 2017, 43, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. Int. J. Mol. Sci. 2021, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Bingel, C.; Koeneke, E.; Ridinger. Three-dimensional tumor cell growth stimulates autophagic flux and recapitulates chemotherapy resistance. Cell Death Dis. 2017, 8, e3013. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Sooro, M.A.; Zhang, P. Autophagic Regulation of p62 is Critical for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 1405. [Google Scholar] [CrossRef] [PubMed]
- Philipson, E.; Engström, C.; Naredi, P.; Bourghardt Fagman, J. High expression of p62/SQSTM1 predicts shorter survival for patients with pancreatic cancer. BMC Cancer 2022, 22, 347. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Karin, M.; Diaz-Meco, M.T. p62 in Cancer: Signaling Adaptor Beyond Autophagy. Cell 2016, 167, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Y.; Ellis, R.A.; Lovat, P.E. Prognostic Impact of Autophagy Biomarkers for Cutaneous Melanoma. Front. Oncol. 2016, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Clissold, S.P. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs 1987, 34, 50–97. [Google Scholar] [CrossRef]
- Dua, P.; Gude, R.P. Antiproliferative and antiproteolytic activity of pentoxifylline in cultures of B16F10 melanoma cells. Cancer Chemother. Pharmacol. 2006, 58, 195–202. [Google Scholar] [CrossRef]
- Kamran, M.Z.; Gude, R.P. Preclinical evaluation of the antimetastatic efficacy of Pentoxifylline on A375 human melanoma cell line. Biomed. Pharmacother. 2012, 66, 617–626. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Quiroz, J.L.; Ocampo-Godínez, J.M.; Hernández-González, V.N.; Lezama, R.A.; Reyes-Maldonado, E.; Vega-López, A.; Domínguez-López, M.L. Pentoxifylline and Norcantharidin Modify p62 Expression in 2D and 3D Cultures of B16F1 Cells. Int. J. Mol. Sci. 2024, 25, 5140. https://doi.org/10.3390/ijms25105140
González-Quiroz JL, Ocampo-Godínez JM, Hernández-González VN, Lezama RA, Reyes-Maldonado E, Vega-López A, Domínguez-López ML. Pentoxifylline and Norcantharidin Modify p62 Expression in 2D and 3D Cultures of B16F1 Cells. International Journal of Molecular Sciences. 2024; 25(10):5140. https://doi.org/10.3390/ijms25105140
Chicago/Turabian StyleGonzález-Quiroz, José Luis, Juan Moisés Ocampo-Godínez, Victoria Noemi Hernández-González, Ruth Angélica Lezama, Elba Reyes-Maldonado, Armando Vega-López, and María Lilia Domínguez-López. 2024. "Pentoxifylline and Norcantharidin Modify p62 Expression in 2D and 3D Cultures of B16F1 Cells" International Journal of Molecular Sciences 25, no. 10: 5140. https://doi.org/10.3390/ijms25105140
APA StyleGonzález-Quiroz, J. L., Ocampo-Godínez, J. M., Hernández-González, V. N., Lezama, R. A., Reyes-Maldonado, E., Vega-López, A., & Domínguez-López, M. L. (2024). Pentoxifylline and Norcantharidin Modify p62 Expression in 2D and 3D Cultures of B16F1 Cells. International Journal of Molecular Sciences, 25(10), 5140. https://doi.org/10.3390/ijms25105140





