Insect Cell-Expressed Major Ragweed Allergen Amb a 1.01 Exhibits Similar Allergenic Properties to Its Natural Counterpart from Common Ragweed Pollen
Abstract
:1. Introduction
2. Results
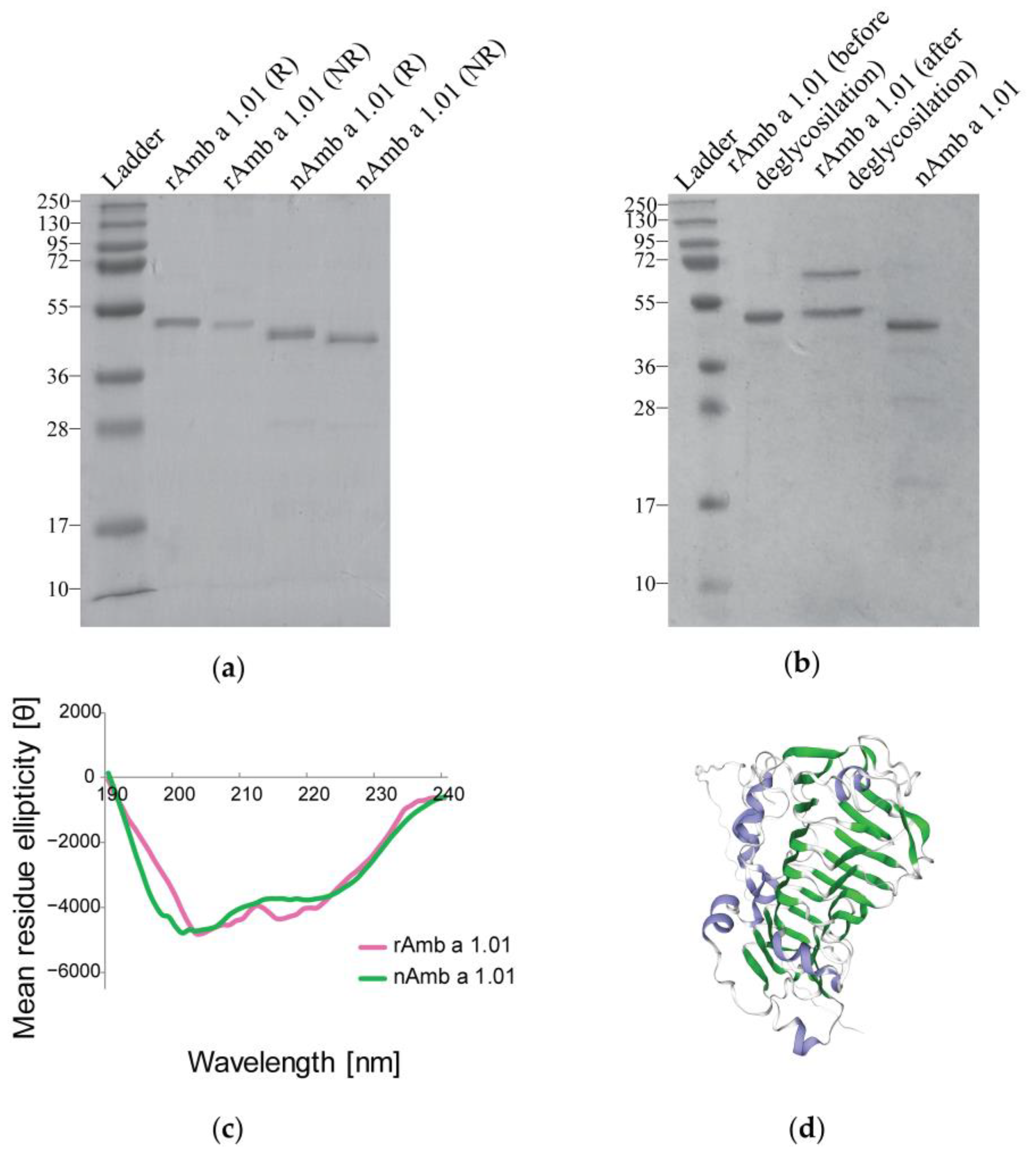
2.1. Characterization of Recombinant Amb a 1.01
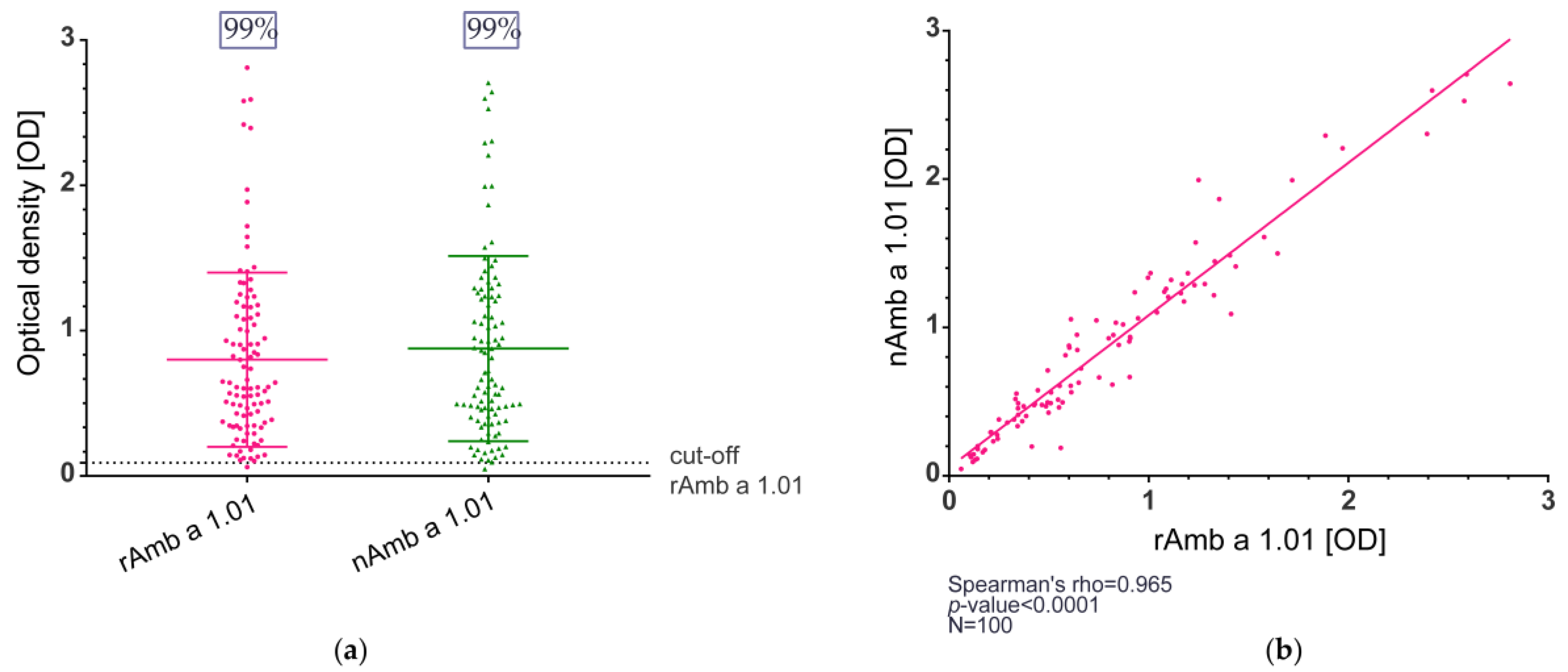
2.2. IgE Reactivity of the Recombinant Amb a 1.01 Allergen
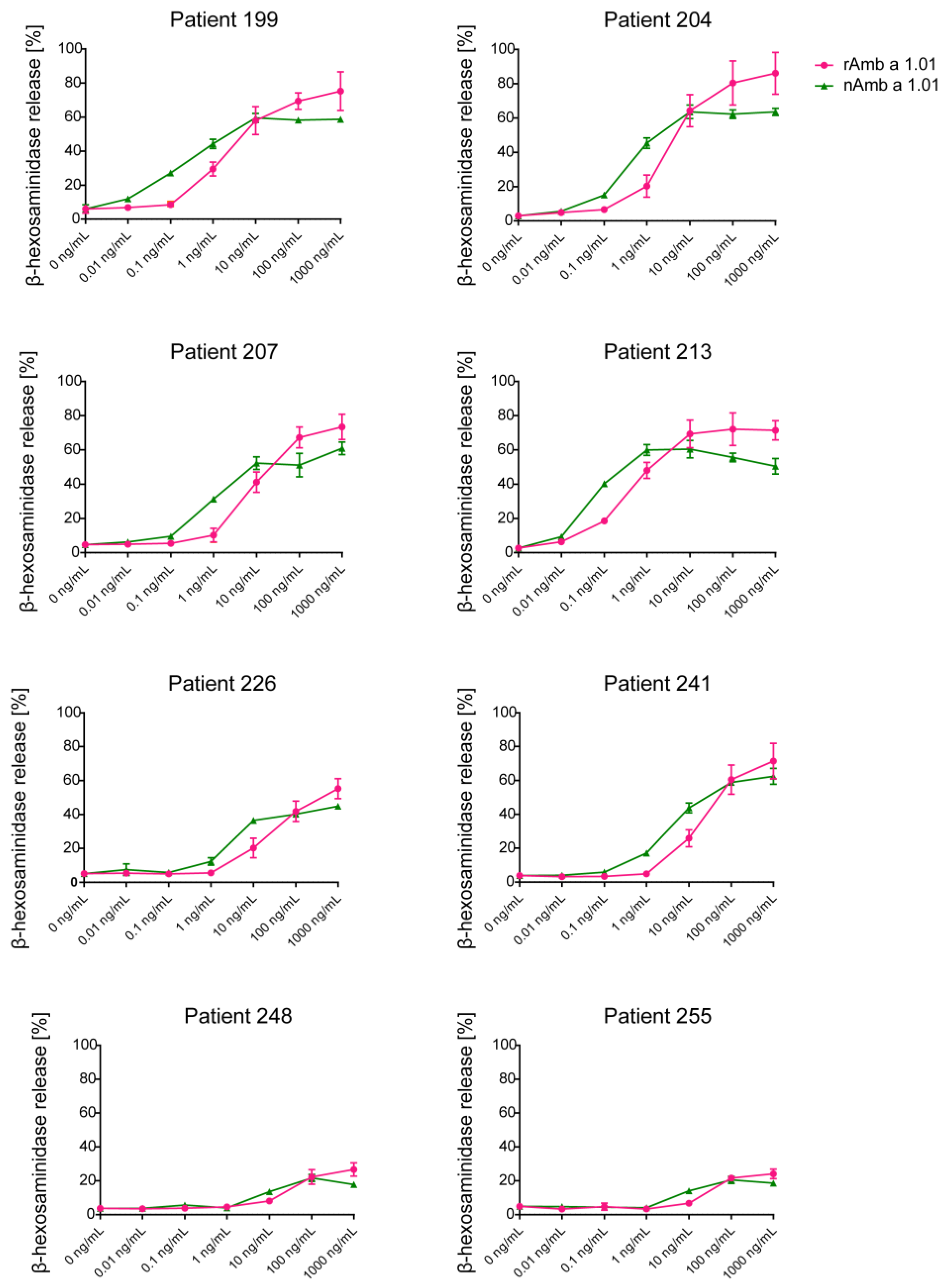
2.3. Allergenic Activity of Recombinant Amb a 1.01
2.4. IgE Inhibition with rAmb a 1.01 and Ragweed Pollen Extract in ImmunoCAP
3. Discussion
4. Materials and Methods
4.1. Patients’ Sera
4.2. Recombinant Amb a 1.01 Allergen Expression
4.3. Isolation and Purification of Recombinant Amb a 1.01
4.4. Natural Amb a 1.01 Isolation and Ragweed Pollen Extract Preparation
4.5. Physicochemical Characterization of the Recombinant Amb a 1.01
4.6. IgE Reactivity Evaluation of the Recombinant Amb a 1.01
4.6.1. IgE Reactivity by ELISA
4.6.2. IgE Reactivity by Immunoblotting
4.7. Allergenic Activity Assessment of the Recombinant Amb a 1.01 Using RBL Mediator Release Assay
4.8. ImmunoCAP IgE Inhibition Experiments
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oswalt, M.L.; Marshall, G.D. Ragweed as an Example of Worldwide Allergen Expansion. Allergy Asthma Clin. Immunol. 2008, 4, 130–135. [Google Scholar] [CrossRef]
- Chen, K.-W.; Marusciac, L.; Tamas, P.T.; Valenta, R.; Panaitescu, C. Ragweed Pollen Allergy: Burden, Characteristics, and Management of an Imported Allergen Source in Europe. Int. Arch. Allergy Immunol. 2018, 176, 163–180. [Google Scholar] [CrossRef]
- Yoon, M.-G.; Kim, M.-A.; Jin, H.-J.; Shin, Y.-S.; Park, H.-S. Identification of IgE Binding Components of Two Major Weed Pollens, Ragweed and Mugwort. Allergy, Asthma Respir. Dis. 2014, 2, 337–343. [Google Scholar] [CrossRef]
- Smith, M.; Cecchi, L.; Skjøth, C.A.; Karrer, G.; Šikoparija, B. Common Ragweed: A Threat to Environmental Health in Europe. Environ. Int. 2013, 61, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Storkey, J.; Stratonovitch, P.; Chapman, D.S.; Vidotto, F.; Semenov, M.A. A Process-Based Approach to Predicting the Effect of Climate Change on the Distribution of an Invasive Allergenic Plant in Europe. PLoS ONE 2014, 9, e88156. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S. Ragweed (Ambrosia) Sensitisation Rates Correlate with the Amount of Inhaled Airborne Pollen. A 14-Year Study in Vienna, Austria. Aerobiologia 2000, 16, 149–153. [Google Scholar] [CrossRef]
- Bartková-Ščevková, J. The Influence of Temperature, Relative Humidity and Rainfall on the Occurrence of Pollen Allergens (Betula, Poaceae, Ambrosia Artemisiifolia) in the Atmosphere of Bratislava (Slovakia). Int. J. Biometeorol. 2003, 48, 1–5. [Google Scholar] [CrossRef]
- Juhász, M.; Juhász, I.; Gallovich, R.; Radisic, P.; Ianovici, N.; Peternel, R.; Kofol-Seliger, A. Last Years Ragweed Pollen Concentrations in the Southern Part of Carpathian Basin. In Proceedings of the Proceedings: 11th Symposium on Analytical and Environmental Problems, Szeged, Hungary, 21 September 2004. [Google Scholar]
- Šikoparija, B.; Smith, M.; Skjøth, C.A.; Radišić, P.; Milkovska, S.; Šimić, S.; Brandt, J. The Pannonian Plain as a Source of Ambrosia Pollen in the Balkans. Int. J. Biometeorol. 2009, 53, 263–272. [Google Scholar] [CrossRef]
- Peternel, R.; Culig, J.; Srnec, L.; Mitić, B.; Vukusić, I.; Hrga, I. Variation in Ragweed (Ambrosia Artemisiifolia L.) Pollen Concentration in Central Croatia, 2002–2003. Ann. Agric. Environ. Med. 2005, 12, 11–16. [Google Scholar]
- Járai-Komlódi, M. Some Details about Ragweed Airborne Pollen in Hungary. Aerobiologia 2000, 16, 291–294. [Google Scholar] [CrossRef]
- Ianovici, N.; Panaitescu, C.B.; Brudiu, I. Analysis of Airborne Allergenic Pollen Spectrum for 2009 in Timişoara, Romania. Aerobiologia 2013, 29, 95–111. [Google Scholar] [CrossRef]
- Leru, P.M.; Eftimie, A.-M.; Anton, V.F.; Thibaudon, M. Five-Year Data on Pollen Monitoring, Distribution and Health Impact of Allergenic Plants in Bucharest and the Southeastern Region of Romania. Medicina 2019, 55, 140. [Google Scholar] [CrossRef]
- Burbach, G.J.; Heinzerling, L.M.; Edenharter, G.; Bachert, C.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bousquet-Rouanet, L.; Bousquet, P.J.; Bresciani, M.; et al. GA 2 LEN Skin Test Study II: Clinical Relevance of Inhalant Allergen Sensitizations in Europe. Allergy 2009, 64, 1507–1515. [Google Scholar] [CrossRef]
- Buzan, M.-R.; Zbîrcea, L.-E.; Gattinger, P.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Complex IgE Sensitization Patterns in Ragweed Allergic Patients: Implications for Diagnosis and Specific Immunotherapy. Clin. Transl. Allergy 2022, 12, e12179. [Google Scholar] [CrossRef] [PubMed]
- Short Ragweed Allergens. WHO/IUIS Allergen Nomenclature Sub-Committee: Allergen Nomenclature. Available online: https://www.allergen.org/search.php?Species=Ambrosiaartemisiifolia (accessed on 20 January 2024).
- King, T.P.; Norman, P.S.; Connell, J.T. Isolation and Characterization of Allergens from Ragweed Pollen. II *. Biochemistry 1964, 3, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Zbîrcea, L.-E.; Buzan, M.-R.; Grijincu, M.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Relationship between IgE Levels Specific for Ragweed Pollen Extract, Amb a 1 and Cross-Reactive Allergen Molecules. Int. J. Mol. Sci. 2023, 24, 4040. [Google Scholar] [CrossRef] [PubMed]
- Gadermaier, G.; Hauser, M.; Ferreira, F. Allergens of Weed Pollen: An Overview on Recombinant and Natural Molecules. Methods 2014, 66, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Würtzen, P.A.; Hoof, I.; Christensen, L.H.; Váczy, Z.; Henmar, H.; Salamanca, G.; Lundegaard, C.; Lund, L.; Kráľova, T.; Brooks, E.G.; et al. Diverse and Highly Cross-reactive T-cell Responses in Ragweed Allergic Patients Independent of Geographical Region. Allergy 2020, 75, 137–147. [Google Scholar] [CrossRef]
- Wolf, M.; Twaroch, T.E.; Huber, S.; Reithofer, M.; Steiner, M.; Aglas, L.; Hauser, M.; Aloisi, I.; Asam, C.; Hofer, H.; et al. Amb a 1 Isoforms: Unequal Siblings with Distinct Immunological Features. Allergy 2017, 72, 1874–1882. [Google Scholar] [CrossRef]
- UniProt Entry for Ragweed Pollen Allergen Amb a 1.01, P27759. Available online: https://www.uniprot.org/uniprotkb/P27759/entry (accessed on 20 January 2024).
- King, T.P.; Alagón, A.; Kochoumian, L.; Kuan, J.; Sobotka, A.K.; Lichtenstein, L.M. Limited Proteolysis of Antigens E and K from Ragweed Pollen. Arch. Biochem. Biophys. 1981, 212, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bordas-Le Floch, V.; Groeme, R.; Chabre, H.; Baron-Bodo, V.; Nony, E.; Mascarell, L.; Moingeon, P. New Insights into Ragweed Pollen Allergens. Curr. Allergy Asthma Rep. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- King, T.P.; Norman, P.S.; Tao, N. Chemical Modifications of the Major Allergen of Ragweed Pollen, Antigen E. Immunochemistry 1974, 11, 83–92. [Google Scholar] [CrossRef]
- Wopfner, N.; Jahn-Schmid, B.; Schmidt, G.; Christ, T.; Hubinger, G.; Briza, P.; Radauer, C.; Bohle, B.; Vogel, L.; Ebner, C.; et al. The Alpha and Beta Subchain of Amb a 1, the Major Ragweed-Pollen Allergen Show Divergent Reactivity at the IgE and T-Cell Level. Mol. Immunol. 2009, 46, 2090–2097. [Google Scholar] [CrossRef]
- Amb a 1 Isoallergens. WHO/IUIS Allergen Nomenclature Sub-Committee: Allergen Nomenclature. Available online: https://www.allergen.org/viewallergen.php?aid=32 (accessed on 20 January 2024).
- Radauer, C.; Nandy, A.; Ferreira, F.; Goodman, R.E.; Larsen, J.N.; Lidholm, J.; Pomés, A.; Raulf-Heimsoth, M.; Rozynek, P.; Thomas, W.R.; et al. Update of the WHO/IUIS Allergen Nomenclature Database Based on Analysis of Allergen Sequences. Allergy 2014, 69, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Pomés, A.; Davies, J.M.; Gadermaier, G.; Hilger, C.; Holzhauser, T.; Lidholm, J.; Lopata, A.L.; Mueller, G.A.; Nandy, A.; Radauer, C.; et al. WHO/IUIS Allergen Nomenclature: Providing a Common Language. Mol. Immunol. 2018, 100, 3–13. [Google Scholar] [CrossRef]
- Bouley, J.; Groeme, R.; Le Mignon, M.; Jain, K.; Chabre, H.; Bordas-Le Floch, V.; Couret, M.-N.; Bussières, L.; Lautrette, A.; Naveau, M.; et al. Identification of the Cysteine Protease Amb a 11 as a Novel Major Allergen from Short Ragweed. J. Allergy Clin. Immunol. 2015, 136, 1055–1064. [Google Scholar] [CrossRef]
- Nandy, A.; Augustin, S.; Mitulski, L.; Cromwell, O. Isoallergen Analysis of Pectate Lyases (Amb a 1 and Amb a 2) from Commercial Short Ragweed Pollen. J. Allergy Clin. Immunol. 2011, 127, AB168. [Google Scholar] [CrossRef]
- Grosse-Kathoefer, S.; Aglas, L.; Ferreira, F.; Pointner, L. What Inhalant Allergens Can Do and Not Do?—The Cooperation of Allergens and Their Source in Th2 Polarization and Allergic Sensitization. Allergo J. Int. 2023, 32, 258–268. [Google Scholar] [CrossRef]
- Zimmer, J.; Bridgewater, J.; Ferreira, F.; van Ree, R.; Rabin, R.L.; Vieths, S. The History, Present and Future of Allergen Standardization in the United States and Europe. Front. Immunol. 2021, 12, 725831. [Google Scholar] [CrossRef]
- Valenta, R.; Karaulov, A.; Niederberger, V.; Zhernov, Y.; Elisyutina, O.; Campana, R.; Focke-Tejkl, M.; Curin, M.; Namazova-Baranova, L.; Wang, J.-Y.; et al. Allergen Extracts for In Vivo Diagnosis and Treatment of Allergy: Is There a Future? J. Allergy Clin. Immunol. Pract. 2018, 6, 1845–1855.e2. [Google Scholar] [CrossRef]
- Van der Veen, M.; Mulder, M.; Witteman, A.; Van Ree, R.; Aalberse, R.; Jansen, H.; Van der Zee, J. False-Positive Skin Prick Test Responses to Commercially Available Dog Dander Extracts Caused by Contamination with House Dust Mite (Dermatophagoides pteronyssinus) Allergens. J. Allergy Clin. Immunol. 1996, 98, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Curin, M.; Garib, V.; Valenta, R. Single Recombinant and Purified Major Allergens and Peptides: How They Are Made and How They Change Allergy Diagnosis and Treatment. Ann. Allergy. Asthma Immunol. 2017, 119, 201–209. [Google Scholar] [CrossRef]
- Curin, M.; Khaitov, M.; Karaulov, A.; Namazova-Baranova, L.; Campana, R.; Garib, V.; Valenta, R. Next-Generation of Allergen-Specific Immunotherapies: Molecular Approaches. Curr. Allergy Asthma Rep. 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Tscheppe, A.; Breiteneder, H. Recombinant Allergens in Structural Biology, Diagnosis, and Immunotherapy. Int. Arch. Allergy Immunol. 2017, 172, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Dorofeeva, Y.; Shilovskiy, I.; Tulaeva, I.; Focke-Tejkl, M.; Flicker, S.; Kudlay, D.; Khaitov, M.; Karsonova, A.; Riabova, K.; Karaulov, A.; et al. Past, Present, and Future of Allergen Immunotherapy Vaccines. Allergy 2021, 76, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Shamji, M.H. Allergen Immunotherapy: Past, Present and Future. Nat. Rev. Immunol. 2023, 23, 317–328. [Google Scholar] [CrossRef]
- Wopfner, N.; Bauer, R.; Thalhamer, J.; Ferreira, F.; Chapman, M. Natural and Recombinant Amb a 1: Immunologic Analysis of IgE and Monoclonal Antibody Epitopes. J. Allergy Clin. Immunol. 2007, 119, S106. [Google Scholar] [CrossRef]
- Gadermaier, G.; Wopfner, N.; Wallner, M.; Egger, M.; Didierlaurent, A.; Regl, G.; Aberger, F.; Lang, R.; Ferreira, F.; Hawranek, T. Array-Based Profiling of Ragweed and Mugwort Pollen Allergens. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- SWISS-MODEL Tool. Available online: https://swissmodel.expasy.org/ (accessed on 22 December 2023).
- Panaitescu, C.; Haidar, L.; Buzan, M.R.; Grijincu, M.; Spanu, D.E.; Cojanu, C.; Laculiceanu, A.; Bumbacea, R.; Agache, I. Precision Medicine in the Allergy Clinic: The Application of Component Resolved Diagnosis. Expert Rev. Clin. Immunol. 2022, 18, 145–162. [Google Scholar] [CrossRef]
- Haidar, L.; Tamas, T.-P.; Stolz, F.; Petrisor Patrascu, R.F.; Chen, K.-W.; Panaitescu, C. Symptom Patterns and Comparison of Diagnostic Methods in Ragweed Pollen Allergy. Exp. Ther. Med. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Oeder, S.; Alessandrini, F.; Wirz, O.F.; Braun, A.; Wimmer, M.; Frank, U.; Hauser, M.; Durner, J.; Ferreira, F.; Ernst, D.; et al. Pollen-Derived Nonallergenic Substances Enhance Th2-Induced IgE Production in B Cells. Allergy 2015, 70, 1450–1460. [Google Scholar] [CrossRef]
- Blanchard, G.C.; Gardner, R. The Characterization of Some of the Antigens and Allergens in Ragweed Pollen. Ann. Allergy 1977, 39, 253–262. [Google Scholar]
- Wopfner, N.; Bauer, R.; Thalhamer, J.; Ferreira, F.; Chapman, M. Immunologic Analysis of Monoclonal and Immunoglobulin E Antibody Epitopes on Natural and Recombinant Amb a 1. Clin. Exp. Allergy 2008, 38, 219–226. [Google Scholar] [CrossRef]
- Grijincu, M.; Huțu, I.; Weber, M.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Physicochemical and Immunological Characterization of Amb a 12, a Novel Ragweed (Ambrosia artemisiifolia) Pollen Allergen. Mol. Immunol. 2023, 157, 18–29. [Google Scholar] [CrossRef]
- Tamaș, T.-P.; Buzan, M.-R.; Zbîrcea, L.-E.; Cotarcă, M.-D.; Grijincu, M.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Ragweed Major Allergen Amb a 11 Recombinant Production and Clinical Implications. Biomolecules 2023, 13, 182. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, L.; Teng, F.; Wang, N.; Zhou, Y.; Zhang, C.; Yang, L. Expression of Recombinant Allergen, Der f 1, Der f 2 and Der f 4 Using Baculovirus-Insect Cell Systems. Arch. Med. Sci. 2018, 14, 1348–1354. [Google Scholar] [CrossRef]
- Soldatova, L.N.; Crameri, R.; Gmachl, M.; Kemeny, D.M.; Schmidt, M.; Weber, M.; Mueller, U.R. Superior Biologic Activity of the Recombinant Bee Venom Allergen Hyaluronidase Expressed in Baculovirus-Infected Insect Cells as Compared with Escherichia Coli. J. Allergy Clin. Immunol. 1998, 101, 691–698. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of Recombinant Proteins by Microbes and Higher Organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Carson, M.; Johnson, D.H.; McDonald, H.; Brouillette, C.; DeLucas, L.J. His-Tag Impact on Structure. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 295–301. [Google Scholar] [CrossRef]
- Chant, A.; Kraemer-Pecore, C.M.; Watkin, R.; Kneale, G.G. Attachment of a Histidine Tag to the Minimal Zinc Finger Protein of the Aspergillus Nidulans Gene Regulatory Protein AreA Causes a Conformational Change at the DNA-Binding Site. Protein Expr. Purif. 2005, 39, 152–159. [Google Scholar] [CrossRef]
- Khan, F.; Legler, P.M.; Mease, R.M.; Duncan, E.H.; Bergmann-Leitner, E.S.; Angov, E. Histidine Affinity Tags Affect MSP1 42 Structural Stability and Immunodominance in Mice. Biotechnol. J. 2012, 7, 133–147. [Google Scholar] [CrossRef] [PubMed]
- ProtParam Tool. Available online: https://web.expasy.org/protparam/ (accessed on 22 December 2023).
- Wopfner, N.; Gadermaier, G.; Egger, M.; Asero, R.; Ebner, C.; Jahn-Schmid, B.; Ferreira, F. The Spectrum of Allergens in Ragweed and Mugwort Pollen. Int. Arch. Allergy Immunol. 2005, 138, 337–346. [Google Scholar] [CrossRef]
- Wolf, M.; Aglas, L.; Twaroch, T.E.; Steiner, M.; Huber, S.; Hauser, M.; Hofer, H.; Parigiani, M.A.; Ebner, C.; Bohle, B.; et al. Endolysosomal Protease Susceptibility of Amb a 1 as a Determinant of Allergenicity. J. Allergy Clin. Immunol. 2018, 141, 1488–1491.e5. [Google Scholar] [CrossRef] [PubMed]
- Wachholz, P.A.; Dearman, R.J.; Kimber, I. Detection of Allergen-Specific IgE Antibody Responses. J. Immunotoxicol. 2005, 1, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Gieras, A.; Focke-Tejkl, M.; Ball, T.; Verdino, P.; Hartl, A.; Thalhamer, J.; Valenta, R. Molecular Determinants of Allergen-Induced Effector Cell Degranulation. J. Allergy Clin. Immunol. 2007, 119, 384–390. [Google Scholar] [CrossRef]
- Gieras, A.; Linhart, B.; Roux, K.H.; Dutta, M.; Khodoun, M.; Zafred, D.; Cabauatan, C.R.; Lupinek, C.; Weber, M.; Focke-Tejkl, M. IgE Epitope Proximity Determines Immune Complex Shape and Effector Cell Activation Capacity. J. Allergy Clin. Immunol. 2016, 137, 1557–1565. [Google Scholar] [CrossRef]
- Hjort, C.; Schiøtz, P.O.; Ohlin, M.; Würtzen, P.A.; Christensen, L.H.; Hoffmann, H.J. The Number and Affinity of Productive IgE Pairs Determine Allergen Activation of Mast Cells. J. Allergy Clin. Immunol. 2017, 140, 1167–1170. [Google Scholar] [CrossRef]
- Pichler, U.; Hauser, M.; Wolf, M.; Bernardi, M.L.; Gadermaier, G.; Weiss, R.; Ebner, C.; Yokoi, H.; Takai, T.; Didierlaurent, A.; et al. Pectate Lyase Pollen Allergens: Sensitization Profiles and Cross-Reactivity Pattern. PLoS ONE 2015, 10, e0120038. [Google Scholar] [CrossRef]
- Egger, C.; Focke, M.; Bircher, A.J.; Scherer, K.; Mothes-Luksch, N.; Horak, F.; Valenta, R. The Allergen Profile of Beech and Oak Pollen. Clin. Exp. Allergy 2008, 38, 1688–1696. [Google Scholar] [CrossRef]
- Campana, R.; Vrtala, S.; Maderegger, B.; Dall’Antonia, Y.; Zafred, D.; Blatt, K.; Herrmann, H.; Focke-Tejkl, M.; Swoboda, I.; Scheiblhofer, S.; et al. Altered IgE Epitope Presentation: A Model for Hypoallergenic Activity Revealed for Bet v 1 Trimer. Mol. Immunol. 2011, 48, 431–441. [Google Scholar] [CrossRef]
- Resch, Y.; Blatt, K.; Malkus, U.; Fercher, C.; Swoboda, I.; Focke-Tejkl, M.; Chen, K.-W.; Seiberler, S.; Mittermann, I.; Lupinek, C.; et al. Molecular, Structural and Immunological Characterization of Der p 18, a Chitinase-Like House Dust Mite Allergen. PLoS ONE 2016, 11, e0160641. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, N.; Woody, R.W. Estimation of Protein Secondary Structure from Circular Dichroism Spectra: Comparison of CONTIN, SELCON, and CDSSTR Methods with an Expanded Reference Set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated Comparative Protein Structure Modeling with SWISS-MODEL and Swiss-PdbViewer: A Historical Perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef]
- Nakamura, R.; Uchida, Y.; Higuchi, M.; Nakamura, R.; Tsuge, I.; Urisu, A.; Teshima, R. A Convenient and Sensitive Allergy Test: IgE Crosslinking-induced Luciferase Expression in Cultured Mast Cells. Allergy 2010, 65, 1266–1273. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzan, M.-R.; Grijincu, M.; Zbîrcea, L.-E.; Haidar, L.; Tamaș, T.-P.; Cotarcă, M.-D.; Tănasie, G.; Weber, M.; Babaev, E.; Stolz, F.; et al. Insect Cell-Expressed Major Ragweed Allergen Amb a 1.01 Exhibits Similar Allergenic Properties to Its Natural Counterpart from Common Ragweed Pollen. Int. J. Mol. Sci. 2024, 25, 5175. https://doi.org/10.3390/ijms25105175
Buzan M-R, Grijincu M, Zbîrcea L-E, Haidar L, Tamaș T-P, Cotarcă M-D, Tănasie G, Weber M, Babaev E, Stolz F, et al. Insect Cell-Expressed Major Ragweed Allergen Amb a 1.01 Exhibits Similar Allergenic Properties to Its Natural Counterpart from Common Ragweed Pollen. International Journal of Molecular Sciences. 2024; 25(10):5175. https://doi.org/10.3390/ijms25105175
Chicago/Turabian StyleBuzan, Maria-Roxana, Manuela Grijincu, Lauriana-Eunice Zbîrcea, Laura Haidar, Tudor-Paul Tamaș, Monica-Daniela Cotarcă, Gabriela Tănasie, Milena Weber, Elijahu Babaev, Frank Stolz, and et al. 2024. "Insect Cell-Expressed Major Ragweed Allergen Amb a 1.01 Exhibits Similar Allergenic Properties to Its Natural Counterpart from Common Ragweed Pollen" International Journal of Molecular Sciences 25, no. 10: 5175. https://doi.org/10.3390/ijms25105175
APA StyleBuzan, M. -R., Grijincu, M., Zbîrcea, L. -E., Haidar, L., Tamaș, T. -P., Cotarcă, M. -D., Tănasie, G., Weber, M., Babaev, E., Stolz, F., Valenta, R., Păunescu, V., Panaitescu, C., & Chen, K. -W. (2024). Insect Cell-Expressed Major Ragweed Allergen Amb a 1.01 Exhibits Similar Allergenic Properties to Its Natural Counterpart from Common Ragweed Pollen. International Journal of Molecular Sciences, 25(10), 5175. https://doi.org/10.3390/ijms25105175







