Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers
Abstract
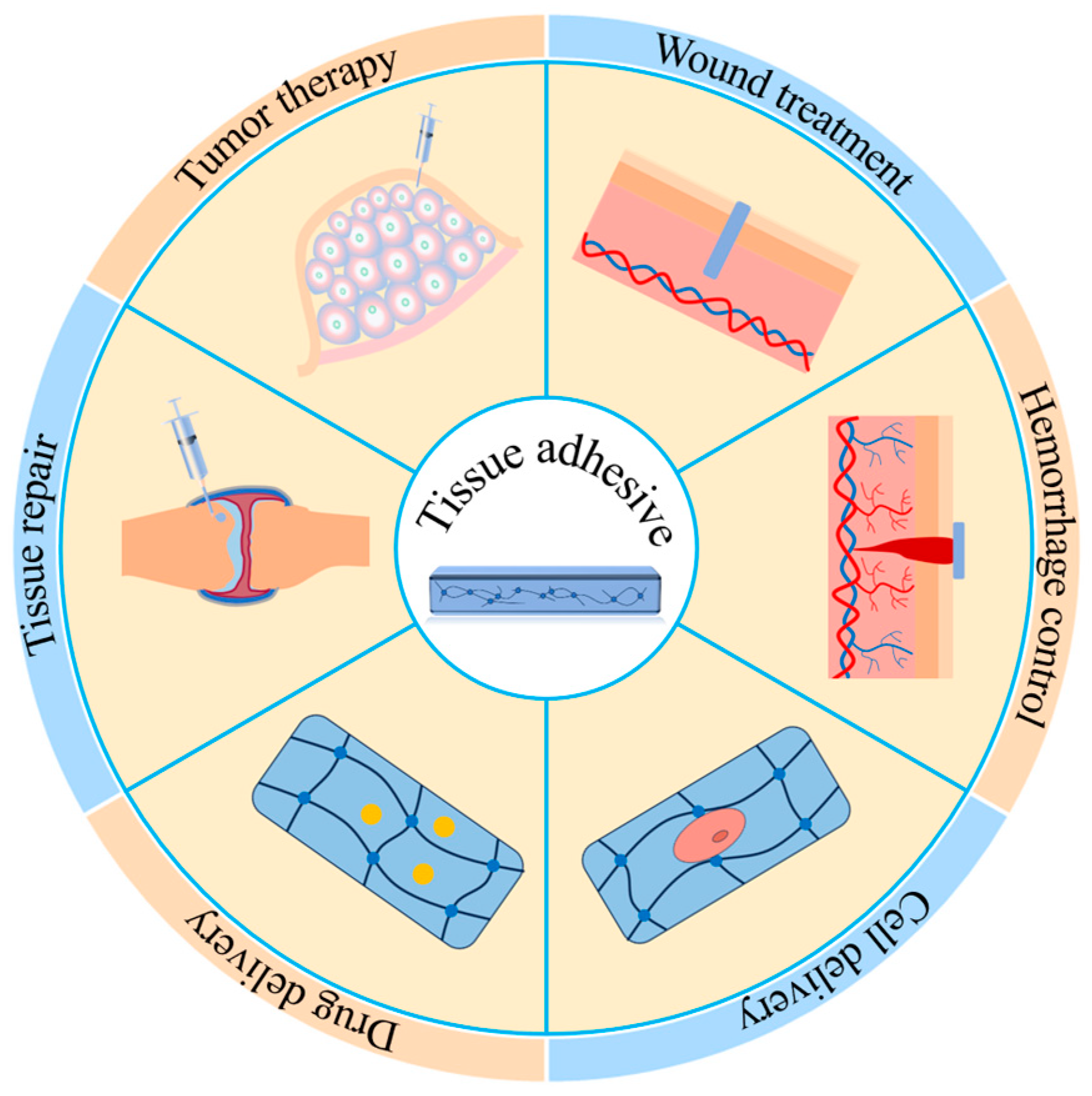
1. Introduction
2. General Design Strategies for Biodegradable Polymer-Based Tissue Adhesives
2.1. Strategies for Improving Interfacial Adhesion
2.2. Strategies for Improving Cohesive Strength
3. Biodegradable Polymers for Tissue Adhesives
3.1. Natural Polymers
3.1.1. Chitosan
3.1.2. Hyaluronic Acid
3.1.3. Gelatin
3.1.4. Chondroitin Sulfate
3.1.5. Starch
3.1.6. Sodium Alginate
3.1.7. Dextran
3.1.8. Pectin
3.1.9. Fibrin Sealants
3.2. Synthetic Polymers
3.2.1. Poly(lactic acid)
3.2.2. Polyurethanes
3.3. Others
4. Challenges and Perspectives of Biodegradable Polymer-Based Tissue Adhesives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; van Goor, H.; van Hest, J.C.M.; Hoogenboom, R. The chemistry of tissue adhesive materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric tissue adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Tauer, N.; Vollkommer, T.; Gosau, M.; Henningsen, A.; Hartjen, P.; Fruh, L.; Beikler, T.; Sturmer, E.K.; Rutkowski, R.; et al. Tissue adhesives in reconstructive and aesthetic surgery-application of silk fibroin-based biomaterials. Int. J. Mol. Sci. 2022, 23, 7687. [Google Scholar] [CrossRef] [PubMed]
- Bal-Ozturk, A.; Cecen, B.; Avci-Adali, M.; Topkaya, S.N.; Alarcin, E.; Yasayan, G.; Ethan, Y.C.; Bulkurcuoglu, B.; Akpek, A.; Avci, H.; et al. Tissue adhesives: From research to clinical translation. Nano Today 2021, 36, 101049. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, Y. Omnipotent tissue adhesive. Sci. Bull. 2020, 65, 428–430. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Z.; Chen, X.; He, C. Stimuli-responsive hydrogel adhesives for wound closure and tissue regeneration. Macromol. Biosci. 2024, 24, e2300379. [Google Scholar] [CrossRef]
- Xu, K.; Wu, X.; Zhang, X.; Xing, M. Bridging wounds: Tissue adhesives’ essential mechanisms, synthesis and characterization, bioinspired adhesives and future perspectives. Burn. Trauma 2022, 10, tkac033. [Google Scholar] [CrossRef]
- Han, G.Y.; Kwack, H.W.; Kim, Y.H.; Je, Y.H.; Kim, H.J.; Cho, C.S. Progress of polysaccharide-based tissue adhesives. Carbohydr. Polym. 2024, 327, 121634. [Google Scholar] [CrossRef]
- Modaresifar, K.; Azizian, S.; Hadjizadeh, A. Nano/biomimetic tissue adhesives development: From research to clinical application. Polym. Rev. 2016, 56, 329–361. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Taboada, G.M.; Yang, K.S.; Pereira, M.J.N.; Liu, S.H.S.; Hu, Y.S.; Karp, J.M.; Artzi, N.; Lee, Y.H. Overcoming the translational barriers of tissue adhesives. Nat. Rev. Mater. 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Bao, Z.; Gao, M.; Sun, Y.; Nian, R.; Xian, M. The recent progress of tissue adhesives in design strategies, adhesive mechanism and applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110796. [Google Scholar] [CrossRef]
- Han, G.Y.; Hwang, S.K.; Cho, K.H.; Kim, H.J.; Cho, C.S. Progress of tissue adhesives based on proteins and synthetic polymers. Biomater. Res. 2023, 27, 57. [Google Scholar] [CrossRef]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Li, S.D.; Chen, N.; Li, X.P.; Li, Y.; Xie, Z.P.; Ma, Z.Y.; Zhao, J.; Hou, X.; Yuan, X.B. Bioinspired double-dynamic-bond crosslinked bioadhesive enables post-wound closure care. Adv. Funct. Mater. 2020, 30, 2000130. [Google Scholar] [CrossRef]
- He, X.Y.; Sun, A.; Li, T.; Qian, Y.J.; Qian, H.; Ling, Y.F.; Zhang, L.H.; Liu, Q.Y.; Peng, T.; Qian, Z. Mussel-inspired antimicrobial gelatin/chitosan tissue adhesive rapidly activated in situ by H2O2/ascorbic acid for infected wound closure. Carbohydr. Polym. 2020, 247, 116692. [Google Scholar] [CrossRef]
- Cui, R.; Chen, F.; Zhao, Y.; Huang, W.; Liu, C. A novel injectable starch-based tissue adhesive for hemostasis. J. Mater. Chem. B 2020, 8, 8282–8293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, Y.; Li, Z.; Chen, J.; Luo, J.; Bai, L.; Huang, H.; Cao, E.; Yin, Z.; Han, Y.; et al. Injectable self-expanding/self-propelling hydrogel adhesive with procoagulant activity and rapid gelation for lethal massive hemorrhage management. Adv. Mater. 2024, 36, e2308701. [Google Scholar] [CrossRef]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef]
- Day, N.B.; Dalhuisen, R.; Loomis, N.E.; Adzema, S.G.; Prakash, J.; Shields Iv, C.W. Tissue-adhesive hydrogel for multimodal drug release to immune cells in skin. Acta Biomater. 2022, 150, 211–220. [Google Scholar] [CrossRef]
- Xue, W.; Shi, W.; Kuss, M.; Kong, Y.; Alimi, O.A.; Wang, H.; DiMaio, D.J.; Yu, C.; Duan, B. A dual-network nerve adhesive with enhanced adhesion strength promotes transected peripheral nerve repair. Adv. Funct. Mater. 2023, 33, 2209971. [Google Scholar] [CrossRef]
- Cui, C.Y.; Wu, T.L.; Chen, X.Y.; Liu, Y.; Li, Y.; Xu, Z.Y.; Fan, C.C.; Liu, W.G. A janus hydrogel wet adhesive for internal tissue repair and anti-postoperative adhesion. Adv. Funct. Mater. 2020, 30, 2005689. [Google Scholar] [CrossRef]
- Wu, D.; Shi, X.; Zhao, F.; Chilengue, S.T.F.; Deng, L.; Dong, A.; Kong, D.; Wang, W.; Zhang, J. An injectable and tumor-specific responsive hydrogel with tissue-adhesive and nanomedicine-releasing abilities for precise locoregional chemotherapy. Acta Biomater. 2019, 96, 123–136. [Google Scholar] [CrossRef]
- Kim, M.K.; Moon, Y.A.; Song, C.K.; Baskaran, R.; Bae, S.; Yang, S.G. Tumor-suppressing miR-141 gene complex-loaded tissue-adhesive glue for the locoregional treatment of hepatocellular carcinoma. Theranostics 2018, 8, 3891–3901. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.L.; Ren, X.Z.; Zhang, J.M.; Lin, Q.; Zhao, Y.L. Supramolecular adhesive hydrogels for tissue engineering applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Ferreira, L.; Sundback, C.; Karp, J.M. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl. Acad. Sci. USA 2008, 105, 2307–2312. [Google Scholar] [CrossRef]
- Ge, L.P.; Chen, S.X. Recent advances in tissue adhesives for clinical medicine. Polymers 2020, 12, 939. [Google Scholar] [CrossRef]
- Bhagat, V.; Becker, M.L. Degradable adhesives for surgery and tissue engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef]
- Griffith, L.G. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann. N. Y. Acad. Sci. 2002, 961, 83–95. [Google Scholar] [CrossRef]
- Zhang, L.W.; Zhang, Y.J.; Ma, F.S.; Liu, X.Z.; Liu, Y.Z.; Cao, Y.; Pei, R.J. A low-swelling and toughened adhesive hydrogel with anti-microbial and hemostatic capacities for wound healing. J. Mater. Chem. B 2022, 10, 915–926. [Google Scholar] [CrossRef]
- Bian, S.Q.; Hao, L.Z.; Qiu, X.; Wu, J.; Chang, H.; Kuang, G.M.; Zhang, S.; Hu, X.H.; Dai, Y.K.; Zhou, Z.Y.; et al. An injectable rapid-adhesion and anti-swelling adhesive hydrogel for hemostasis and wound sealing. Adv. Funct. Mater. 2022, 32, 2207741. [Google Scholar] [CrossRef]
- Iliev, G.; Hardan, L.; Kassis, C.; Bourgi, R.; Cuevas-Suarez, C.E.; Lukomska-Szymanska, M.; Mancino, D.; Haikel, Y.; Kharouf, N. Shelf life and storage conditions of universal adhesives: A literature review. Polymers 2021, 13, 2708. [Google Scholar] [CrossRef]
- Song, K.C.; Hao, Y.M.; Liu, Y.; Cao, R.F.; Zhang, X.L.; He, S.W.; Wen, J.; Zheng, W.S.; Wang, L.L.; Zhang, Y.J. Preparation of pectin-chitosan hydrogels based on bioadhesive-design micelle to prompt bacterial infection wound healing. Carbohydr. Polym. 2023, 300, 120272. [Google Scholar] [CrossRef]
- Liu, S.D.; Li, D.S.; Wang, Y.; Zhou, G.Q.; Ge, K.; Jiang, L. Adhesive, antibacterial and double crosslinked carboxylated polyvinyl alcohol/chitosan hydrogel to enhance dynamic skin wound healing. Int. J. Biol. Macromol. 2023, 228, 744–753. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloria, M.; Tevis, K.M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K.; Grinstaff, M.W. On-demand dissolution of a dendritic hydrogel-based dressing for second-degree burn wounds through thiol-thioester exchange reaction. Angew. Chem. Int. Ed. 2016, 55, 9984–9987. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.J.; Wang, L.; Liu, J.; Liu, X.W.; Hong, Y.C.; Huang, Y.D.; Ren, S.N. Advances in adhesive hydrogels for tissue engineering. Eur. Polym. J. 2022, 172, 111241. [Google Scholar] [CrossRef]
- Wei, C.Z.; Song, J.L.; Tan, H.Q. A paintable ophthalmic adhesive with customizable properties based on symmetrical/asymmetrical cross-linking. Biomater. Sci. 2021, 9, 7522–7533. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Erdi, M.; Lau, L.W.; Zaki, S.T.; Srinivasan, P.; Balabhadrapatruni, M.; Ayyub, O.B.; Sandler, A.D.; Kofinas, P. Biodegradable, tissue adhesive polyester blends for safe, complete wound healing. ACS Biomater. Sci. Eng. 2021, 7, 3908–3916. [Google Scholar] [CrossRef]
- Fan, C.J.; Fu, J.Y.; Zhu, W.Z.; Wang, D.A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Liu, D.H.; Li, D.J.; Mo, X.M. An injectable double cross-linked hydrogel adhesive inspired by synergistic effects of mussel foot proteins for biomedical application. Colloids Surf. B Biointerfaces 2021, 204, 111782. [Google Scholar] [CrossRef]
- Fan, P.; Dong, Q.; Yang, J.; Chen, Y.; Yang, H.; Gu, S.; Xu, W.; Zhou, Y. Flexible dual-functionalized hyaluronic acid hydrogel adhesives formed in situ for rapid hemostasis. Carbohydr. Polym. 2023, 313, 120854. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, K.; Li, J.; Su, J.; Guan, F.; Li, J. Injectable protocatechuic acid based composite hydrogel with hemostatic and antioxidant properties for skin regeneration. Mater. Des. 2022, 222, 111109. [Google Scholar] [CrossRef]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; de Manzini, N.; Paoletti, S. Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Zhao, X. Bioadhesive technology platforms. Chem. Rev. 2023, 123, 14084–14118. [Google Scholar] [CrossRef] [PubMed]
- Pethrick, R.A. Design and ageing of adhesives for structural adhesive bonding—A review. Proc. Imech. E. Part L J. Mater. Des. Appl. 2015, 229, 349–379. [Google Scholar] [CrossRef]
- Merotto, E.; Pavan, P.G.; Piccoli, M. Three-dimensional bioprinting of naturally derived hydrogels for the production of biomimetic living tissues: Benefits and challenges. Biomedicines 2023, 11, 1742. [Google Scholar] [CrossRef]
- Hoque, M.; Alam, M.; Wang, S.R.; Zaman, J.U.; Rahman, M.S.; Johir, M.A.H.; Tian, L.M.; Choi, J.G.; Ahmed, M.B.; Yoon, M.H. Interaction chemistry of functional groups for natural biopolymer-based hydrogel design. Mater. Sci. Eng. R Rep. 2023, 156, 100758. [Google Scholar] [CrossRef]
- Li, M.; Pan, G.Y.; Zhang, H.L.; Guo, B.L. Hydrogel adhesives for generalized wound treatment: Design and applications. J. Polym. Sci. 2022, 60, 1328–1359. [Google Scholar] [CrossRef]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-modified hyaluronic acid hydrogel adhesives with fast-forming and high tissue adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.W.; Xu, C.Z.; Wang, L.L.; Xia, Y.Z. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Hou, X.; Lin, L.; Li, K.; Jiang, F.; Qiao, D.; Zhang, B.; Xie, F. Towards superior biopolymer gels by enabling interpenetrating network structures: A review on types, applications, and gelation strategies. Adv. Colloid Interface Sci. 2024, 325, 103113. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Z. Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Wang, J.; Niu, J.; Sawada, T.; Shao, Z.; Serizawa, T. A bottom-up synthesis of vinyl-cellulose nanosheets and their nanocomposite hydrogels with enhanced strength. Biomacromolecules 2017, 18, 4196–4205. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, N. Glutathione-responsive multifunctionalizable hydrogels via amine-epoxy “click” chemistry. Eur. Polym. J. 2020, 123, 109441. [Google Scholar] [CrossRef]
- Yan, G.H.; Chen, G.F.; Peng, Z.Q.; Shen, Z.L.; Tang, X.; Sun, Y.; Zeng, X.H.; Lin, L. The cross-linking mechanism and applications of catechol-metal polymer materials. Adv. Mate. Interfaces 2021, 8, 2100239. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.S.; Hu, X.H.; Chu, L.Q. Recent advances in antimicrobial hydrogels containing metal ions and metals/metal oxide nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, R.; Duan, L.; Ren, X.; Gao, G. Tough, adhesive and conductive polysaccharide hydrogels mediated by ferric solution. Carbohydr. Polym. 2019, 211, 1–10. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Gheybi, H.; Adeli, M. Efficient wound healing by antibacterial property: Advances and trends of hydrogels, hydrogel-metal np composites and photothermal therapy platforms. J. Drug Deliv. Sci. Technol. 2022, 73, 103458. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Qi, C.; Fang, L.; Zhuo, R.; Jiang, X. Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater. 2016, 35, 228–237. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural polymers-based light-induced hydrogels: Promising biomaterials for biomedical applications. Coord. Chem. Rev. 2020, 420, 213432. [Google Scholar] [CrossRef]
- Fonseca, R.G.; De Bon, F.; Pereira, P.; Carvalho, F.M.; Freitas, M.; Tavakoli, M.; Serra, A.C.; Fonseca, A.C.; Coelho, J.F.J. Photo-degradable, tough and highly stretchable hydrogels. Mater. Today Bio. 2022, 15, 100325. [Google Scholar] [CrossRef]
- Vitkova, L.; Musilova, L.; Achbergerova, E.; Kolarik, R.; Mrlik, M.; Korpasova, K.; Mahelova, L.; Capakova, Z.; Mracek, A. Formulation of magneto-responsive hydrogels from dually cross-linked polysaccharides: Synthesis, tuning and evaluation of rheological properties. Int. J. Mol. Sci. 2022, 23, 9633. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef] [PubMed]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Bharti, D.; Sarkar, P.; Anis, A.; Kim, D.; Chalas, R.; Maksymiuk, P.; Stachurski, P.; Jarzebski, M. Selected applications of chitosan composites. Int. J. Mol. Sci. 2021, 22, 10968. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, P.; Tiwari, S. Dynamic imine bond based chitosan smart hydrogel with magnified mechanical strength for controlled drug delivery. Int. J. Biol. Macromol. 2020, 160, 489–495. [Google Scholar] [CrossRef]
- Bhuvanachandra, B.; Sivaramakrishna, D.; Alim, S.; Preethiba, G.; Rambabu, S.; Swamy, M.J.; Podile, A.R. New class of chitosanase from bacillus amyloliquefaciens for the generation of chitooligosaccharides. J. Agric. Food Chem. 2021, 69, 78–87. [Google Scholar] [CrossRef]
- El-Sayed, W.N.; Alkabli, J.; Aloqbi, A.; Elshaarawy, R.F.M. Optimization enzymatic degradation of chitosan into amphiphilic chitooligosaccharides for application in mitigating liver steatosis and cholesterol regulation. Eur. Polym. J. 2021, 153, 110507. [Google Scholar] [CrossRef]
- Rao, K.M.; Narayanan, K.B.; Uthappa, U.T.; Park, P.H.; Choi, I.; Han, S.S. Tissue adhesive, self-healing, biocompatible, hemostasis, and antibacterial properties of fungal-derived carboxymethyl chitosan-polydopamine hydrogels. Pharmaceutics 2022, 14, 1028. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; Karperien, M.; van Blitterswijk, C.A.; Zhong, Z.Y.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Nayal, A.; Malhotra, S.; Koul, V. Dual functionalized chitosan based composite hydrogel for haemostatic efficacy and adhesive property. Carbohydr. Polym. 2020, 247, 116757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Liu, Z.; Shi, Z.; Liu, S.; Wang, X.; Wang, X.; Huang, F. Injectable thermogelling bioadhesive chitosan-based hydrogels for efficient hemostasis. Int. J. Biol. Macromol. 2023, 224, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Cui, B.; Chen, H.; Geng, X.; Geng, X.; Li, Z.; Cao, S.; Shen, J.; Li, J. A chitosan-based self-healing hydrogel for accelerating infected wound healing. Biomater. Sci. 2023, 11, 4226–4237. [Google Scholar] [CrossRef]
- Wang, C.W.; Liu, Y.H.; Yang, X.X.; Li, W.T.; Zhou, X.H.; Ren, Y.; Zhang, C.R.; Yang, H.; Kong, W.Q.; Wang, J.W.; et al. In-situ forming hydrogel incorporated with reactive oxygen species responsive and antibacterial properties for diabetic infected chronic wound healing. Chem. Eng. J. 2022, 450, 138077. [Google Scholar]
- Peng, H.; Li, H.C.; Zhang, X.; Tang, J.Z.; Liang, Y.P.; Qiao, L.P.; Zhu, Y.; Hou, M.M.; Wei, S.M.; Zhang, Z.X.; et al. 3D-exosomes laden multifunctional hydrogel enhances diabetic wound healing via accelerated angiogenesis. Chem. Eng. J. 2023, 475, 146238. [Google Scholar] [CrossRef]
- Graca, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid-based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tan, J.; Zhou, Y.; Guo, Y.; Liao, X.; He, L.; Li, D.; Li, X.; Liu, Y. From crosslinking strategies to biomedical applications of hyaluronic acid-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 231, 123308. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular matrix mimics using hyaluronan-based biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; He, X.; Wang, X.; Wen, Y.; Zeng, L.; Yu, W.; Hu, P.; Chen, H. A multifunctional hydrogel based on nature polysaccharide fabricated by Schiff base reaction. Eur. Polym. J. 2023, 197, 112330. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, A.; Rothley, M.; Schreiber, C.; Moller, S.; Rosswag, S.; Franz, S.; Garvalov, B.K.; Thiele, W.; Spataro, S.; Herskind, C.; et al. Sulfated hyaluronic acid inhibits the hyaluronidase cemip and regulates the ha metabolism, proliferation and differentiation of fibroblasts. Matrix Biol. 2022, 109, 173–191. [Google Scholar] [CrossRef]
- Kilic, E.; Kilic, G.; Karadas, G.; Akgul, O.; Aytekin, M.; Sonmez, M.F.; Ozgocmen, S. Serum and tissue levels of hyaluronan in patients with systemic sclerosis. Ann. Rheum. Dis. 2015, 74, 961. [Google Scholar] [CrossRef]
- Juranek, I.; Stern, R.; Soltes, L. Hyaluronan peroxidation is required for normal synovial function: An hypothesis. Med. Hypotheses 2014, 82, 662–666. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and reactive oxygen species signaling-novel cues from the matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef]
- Hao, Y.; He, J.; Ma, X.; Feng, L.; Zhu, M.; Zhai, Y.; Liu, Y.; Ni, P.; Cheng, G. A fully degradable and photocrosslinked polysaccharide-polyphosphate hydrogel for tissue engineering. Carbohydr. Polym. 2019, 225, 115257. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Park, J.D.; Lee, S.; Choi, W.I.; Hwang, Y.; Mori, M.; Yu, J.S.; Lee, D.N. Injectable hyaluronic acid hydrogel encapsulated with si-based nio nanoflower by visible light cross-linking: Its antibacterial applications. Int. J. Biol. Macromol. 2022, 208, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bao, B.; Jiang, F.; Zhang, Y.; Zhou, R.; Lu, Y.; Lin, S.; Lin, Q.; Jiang, X.; Zhu, L. Promoting oral mucosal wound healing with a hydrogel adhesive based on a phototriggered s-nitrosylation coupling reaction. Adv. Mater. 2021, 33, e2105667. [Google Scholar] [CrossRef] [PubMed]
- Flegeau, K.; Toquet, C.; Rethore, G.; d’Arros, C.; Messager, L.; Halgand, B.; Dupont, D.; Autrusseau, F.; Lesoeur, J.; Veziers, J. In situ forming, silanized hyaluronic acid hydrogels with fine control over mechanical properties and in vivo degradation for tissue engineering applications. Adv. Healthc. Mater. 2020, 9, e2000981. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; Huang, C.; Liu, H.; Chen, H.; Shou, J.B.; Cheng, H.W.; Liu, G. Natural hydrogel dressings in wound care: Design, advances, and perspectives. Chin. Chem. Lett. 2023, 109442. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, K.; Zhang, X.; Ma, G.; Zhang, W.; Hu, Z. Facile preparation of polysaccharides-based adhesive hydrogel with antibacterial and antioxidant properties for promoting wound healing. Colloids Surf. B. Biointerfaces 2022, 209, 112208. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Říhová, B. Immunocompatibility and biocompatibility of cell delivery systems. Adv. Drug Del. Rev. 2000, 42, 65–80. [Google Scholar] [CrossRef]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Nishida, K.; Ohki, T.; Kanzaki, M.; Sekine, H.; Shimizu, T.; Okano, T. Cell delivery in regenerative medicine: The cell sheet engineering approach. J. Control. Release 2006, 116, 193–203. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering rhamm and cd44 receptor signaling pathways. Matrix Biol. 2007, 26, 2007. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.H.; Chung, H.M.; et al. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Yazdani, M.; Shahdadfar, A.; Jackson, C.J.; Utheim, T.P. Hyaluronan-based hydrogel scaffolds for limbal stem cell transplantation: A review. Cells 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Wang, L.; Zhang, X.; Heng, B.C.; Wang, D.A.; Ge, Z. Modified hyaluronic acid hydrogels with chemical groups that facilitate adhesion to host tissues enhance cartilage regeneration. Bioact. Mater. 2021, 6, 1689–1698. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Portillo-Lara, R.; Spencer, A.; Yu, W.; Geilich, B.M.; Noshadi, I.; Webster, T.J.; Annabi, N. Engineering adhesive and antimicrobial hyaluronic acid/elastin-like polypeptide hybrid hydrogels for tissue engineering applications. ACS Biomater. Sci. Eng. 2018, 4, 2528–2540. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial nonenzymatic antioxidant mxene nanosheet-anchored injectable hydrogel as a mild photothermal-controlled oxygen release platform for diabetic wound healing. ACS Nano 2022, 16, 7486–7502. [Google Scholar] [CrossRef]
- Mohanto, S.; Narayana, S.; Merai, K.P.; Kumar, J.A.; Bhunia, A.; Hani, U.; Al Fatease, A.; Gowda, B.H.J.; Nag, S.; Ahmed, M.G.; et al. Advancements in gelatin-based hydrogel systems for biomedical applications: A state-of-the-art review. Int. J. Biol. Macromol. 2023, 253, 127143. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.P.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Liu, Y.; Cheong Ng, S.; Yu, J.; Tsai, W.B. Modification and crosslinking of gelatin-based biomaterials as tissue adhesives. Colloids Surf. B. Biointerfaces 2019, 174, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, K.; Yu, K.; Xing, F.; Lai, H.; Zhou, Y.; Xiao, P. Degradable hydrogel adhesives with enhanced tissue adhesion, superior self-healing, cytocompatibility, and antibacterial property. Adv. Healthc. Mater. 2022, 11, e2101504. [Google Scholar] [CrossRef]
- Montazerian, H.; Mitra, S.; Najafabadi, A.H.; Seyedmahmoud, R.; Zheng, Y.T.; Dokmeci, M.R.; Annabi, N.; Khademhosseini, A.; Weiss, P.S. Catechol conjugation for bioadhesion in photo-cross-linkable biomaterials. ACS Mater. Lett. 2023, 5, 1672–1683. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- Fang, X.; Xie, J.; Zhong, L.; Li, J.; Rong, D.; Li, X.; Ouyang, J. Biomimetic gelatin methacrylamide hydrogel scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1070–1080. [Google Scholar] [CrossRef]
- Sharifi, S.; Islam, M.M.; Sharifi, H.; Islam, R.; Koza, D.; Reyes-Ortega, F.; Alba-Molina, D.; Nilsson, P.H.; Dohlman, C.H.; Mollnes, T.E.; et al. Tuning gelatin-based hydrogel towards bioadhesive ocular tissue engineering applications. Bioact. Mater. 2021, 6, 3947–3961. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase b or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Zhang, H.H.; Huang, Y.; Zhong, H.; Qin, P.W.; Cheng, S.B.; Wang, Y.N.; Yang, C.H. Tough and biocompatible hydrogel tissue adhesives entirely based on naturally derived ingredients. ACS Appl. Polym. Mater. 2023, 6, 1141–1151. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Wang, W.; Yan, G.; Zheng, S.; Wang, C.; Li, N.; Tang, H. Facile strategy for gelatin-based hydrogel with multifunctionalities to remodel wound microenvironment and accelerate healing of acute and diabetic wounds. Int. J. Biol. Macromol. 2024, 256, 128372. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.; et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef]
- Jiang, Y.A.; Pan, X.M.; Yao, M.Y.; Han, L.; Zhang, X.; Jia, Z.R.; Weng, J.; Chen, W.X.; Fang, L.M.; Wang, X.L.; et al. Bioinspired adhesive and tumor microenvironment responsive nanomofs assembled 3D-printed scaffold for anti-tumor therapy and bone regeneration. Nano Today 2021, 39, 101182. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive nanocomposite hydrogels based on Gelatin/poly (N-isopropylacrylamide) (PNIPAM) for controlled drug delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Park, H.; Temenoff, J.S.; Holland, T.A.; Tabata, Y.; Mikos, A.G. Delivery of tgf-β1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials 2005, 26, 7095–7103. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, M.; Wang, J.; Zhai, G. Chondroitin sulfate-based nanocarriers for drug/gene delivery. Carbohydr. Polym. 2015, 133, 391–399. [Google Scholar] [CrossRef]
- Shin, J.; Kang, E.H.; Choi, S.; Jeon, E.J.; Cho, J.H.; Kang, D.; Lee, H.; Yun, I.S.; Cho, S.W. Tissue-adhesive chondroitin sulfate hydrogel for cartilage reconstruction. ACS Biomater. Sci. Eng. 2021, 7, 4230–4243. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- Hiraoka, S.; Furuichi, T.; Nishimura, G.; Shibata, S.; Yanagishita, M.; Rimoin, D.L.; Superti-Furga, A.; Nikkels, P.G.; Ogawa, M.; Katsuyama, K.; et al. Nucleotide-sugar transporter slc35d1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat. Med. 2007, 13, 1363–1367. [Google Scholar] [CrossRef]
- Jedrzejas, M.J.; Stern, R. Structures of vertebrate hyaluronidases and their unique enzymatic mechanism of hydrolysis. Annu. Rev. Food Sci. Technol. 2005, 61, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, E.A.; Bryant, S.J. A MMP7-sensitive photoclickable biomimetic hydrogel for msc encapsulation towards engineering human cartilage. J. Biomed. Mater. Res. A 2018, 106, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, F.; Xue, Y.; Peng, Y.; Hu, L.; Kang, X.; Sun, Q.; Ouyang, D.F.; Tang, B.; Lin, L. Chondroitin sulfate zinc with antibacterial properties and anti-inflammatory effects for skin wound healing. Carbohydr. Polym. 2022, 278, 118996. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Kunduru, K.R.; Abtew, E.; Domb, A.J. Polysaccharide-based conjugates for biomedical applications. Bioconjug. Chem. 2015, 26, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J.; Wang, K.; Fang, L.; Chan, C.W.; Zhang, H.; et al. Mussel-inspired tissue-adhesive hydrogel based on the polydopamine-chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015–28026. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.P.; Chen, L.; Jin, Z.Y.; Jiao, A.Q. Research progress of starch-based biodegradable materials: A review. J. Mater. Sci. 2021, 56, 11187–11208. [Google Scholar] [CrossRef]
- Junejo, S.A.; Flanagan, B.M.; Zhang, B.; Dhital, S. Starch structure and nutritional functionality—Past revelations and future prospects. Carbohydr. Polym. 2022, 277, 118837. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Pasting, paste, and gel properties of starch-hydrocolloid combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An overview on starch-based sustainable hydrogels: Potential applications and aspects. J. Polym. Environ. 2021, 30, 19–50. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of starch digestion by alpha-amylase-structural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; Breslin, P.A. Salivary amylase: Digestion and metabolic syndrome. Curr. Diab. Rep. 2016, 16, 102. [Google Scholar] [CrossRef]
- Liu, P.; Ma, L.; Duan, W.; Gao, W.; Fang, Y.; Guo, L.; Yuan, C.; Wu, Z.; Cui, B. Maltogenic amylase: Its structure, molecular modification, and effects on starch and starch-based products. Carbohydr. Polym. 2023, 319, 121183. [Google Scholar] [CrossRef]
- Klip, A.; De Bock, K.; Bilan, P.J.; Richter, E.A. Transcellular barriers to glucose delivery in the body. Annu. Rev. Physiol. 2024, 86, 149–173. [Google Scholar] [CrossRef]
- Dong, D.; Li, J.; Cui, M.; Wang, J.; Zhou, Y.; Luo, L.; Wei, Y.; Ye, L.; Sun, H.; Yao, F. In situ “clickable” zwitterionic starch-based hydrogel for 3D cell encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 4442–4455. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Y.; Wang, Q.; Zhou, X.; Yang, B.; Ji, F.; Dong, D.; Gao, L.; Cui, Y.; Yao, F. Physical cross-linking starch-based zwitterionic hydrogel exhibiting excellent biocompatibility, protein resistance, and biodegradability. ACS Appl. Mater. Interfaces 2016, 8, 15710–15723. [Google Scholar] [CrossRef]
- Mao, Y.X.; Li, P.; Yin, J.W.; Bai, Y.J.; Zhou, H.; Lin, X.; Yang, H.L.; Yang, L. Starch-based adhesive hydrogel with gel-point viscoelastic behavior and its application in wound sealing and hemostasis. J. Mater. Sci. Technol. 2021, 63, 228–235. [Google Scholar] [CrossRef]
- Zhuang, P.; Greenberg, Z.; He, M. Biologically enhanced starch bio-ink for promoting 3D cell growth. Adv. Mater. Technol. 2021, 6, 2100551. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubarak, N.M.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A critical review on the synthesis of natural sodium alginate based composite materials: An innovative biological polymer for biomedical delivery applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Cai, Y.; Lu, Q.; Guo, X.; Wang, S.; Qiao, J.; Jiang, L. Salt-tolerant superoleophobicity on alginate gel surfaces inspired by seaweed (saccharina japonica). Adv. Mater. 2015, 27, 4162–4168. [Google Scholar] [CrossRef]
- Gao, C.M.; Liu, M.Z.; Chen, J.; Zhang, X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym. Degrad. Stabil. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Cao, B.; Wang, C.; Guo, P.; Zhang, Q.; Wang, C.; Sun, H.; Wen, H.; Chen, X.; Wang, Y.; Wang, Y.; et al. Photo-crosslinked enhanced double-network hydrogels based on modified gelatin and oxidized sodium alginate for diabetic wound healing. Int. J. Biol. Macromol. 2023, 245, 125528. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Liu, C.; Lu, Z.; Li, M.; Hurren, C.; Wang, D. Photopolymerized multifunctional sodium alginate-based hydrogel for antibacterial and coagulation dressings. Int. J. Biol. Macromol. 2024, 260, 129428. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surf. B. Biointerfaces 2020, 192, 111041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kong, L.; Farhadi, F.; Xia, W.; Chang, J.; He, Y.; Li, H. An injectable continuous stratified structurally and functionally biomimetic construct for enhancing osteochondral regeneration. Biomaterials 2019, 192, 149–158. [Google Scholar] [CrossRef]
- Yao, B.; Ni, C.; Xiong, C.; Zhu, C.; Huang, B. Hydrophobic modification of sodium alginate and its application in drug controlled release. Bioprocess Biosyst. Eng. 2010, 33, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lin, M.; Li, P.; Huang, X.; Tian, K.; Li, C. Local anesthetic effects of lidocaine-loaded carboxymethyl chitosan cross-linked with sodium alginate hydrogels for drug delivery system, cell adhesion, and pain management. J. Drug Deliv. Sci. Technol. 2023, 79, 104007. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; da Cunha, M.A.; Barbosa, A.M.; Dekker, R.F.; Malfatti, C.R. Biological activities of derivatized D-glucans: A review. Int. J. Biol. Macromol. 2015, 72, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E. Dextran: Sources, structures, and properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Mejia, S.M.V.; de Francisco, A.; Bohrer, B. A comprehensive review on cereal beta-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020, 60, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, Y.; Li, D.; Mo, X.; Pan, J. Hofmeister effect-enhanced gelatin/oxidized dextran hydrogels with improved mechanical properties and biocompatibility for wound healing. Acta Biomater. 2022, 151, 235–253. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, H.; Wang, H.; Zaldivar-Silva, D.; Aguero, L.; Liu, Y.; Zhang, Z.; Yin, Y.; Qiu, B.; Zhao, J.; et al. An injectable anti-microbial and adhesive hydrogel for the effective noncompressible visceral hemostasis and wound repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112422. [Google Scholar] [CrossRef]
- Yang, R.; Xue, W.; Liao, H.; Wu, F.; Guo, H.; Zhang, W.; Wang, P.; Tan, X.; Xu, H.; Chi, B. Injectable polylysine and dextran hydrogels with robust antibacterial and ros-scavenging activity for wound healing. Int. J. Biol. Macromol. 2022, 223, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, S.; Du, X.; Li, W.; Wang, Q.; He, D.; Yuan, J. Natural polymer-derived photocurable bioadhesive hydrogels for sutureless keratoplasty. Bioact. Mater. 2022, 8, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nan, K.; Zhang, Y.; Song, K.; Geng, Z.; Shang, D.; Guan, X.; Fan, L. A novel injectable hydrogel prepared from phenylboronic acid modified gelatin and oxidized-dextran for bone tissue engineering. Int. J. Biol. Macromol. 2024, 261, 129666. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zhang, K.; Cui, L.; Liang, J.; Li, J.; Guan, F. Injectable gelatin/oxidized dextran hydrogel loaded with apocynin for skin tissue regeneration. Biomater. Adv. 2022, 133, 112604. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.F.; Yuan, L.; Guo, C.A.; Geng, X.H.; Fei, T.; Fan, W.S.; Li, S.; Yuan, H.F.; Yan, Z.Q.; Mo, X.M. Fabrication of modified dextran-gelatin in situ forming hydrogel and application in cartilage tissue engineering. J. Mater. Chem. B 2014, 2, 8346–8360. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Shi, T.; Zhao, P.; An, K.; Lin, C.; Liu, H. Injectable dextran hydrogels fabricated by metal-free click chemistry for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, L.; Chen, Y. Direct 3D printing of thermosensitive aop127-oxidized dextran hydrogel with dual dynamic crosslinking and high toughness. Carbohydr. Polym. 2022, 291, 119616. [Google Scholar] [CrossRef] [PubMed]
- Musilova, L.; Achbergerova, E.; Vitkova, L.; Kolarik, R.; Martinkova, M.; Minarik, A.; Mracek, A.; Humpolicek, P.; Pecha, J. Cross-linked gelatine by modified dextran as a potential bioink prepared by a simple and non-toxic process. Polymers 2022, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, K.; Xian, Y.; He, G.; Pan, Z.; Wang, H.; Zhang, C.; Wu, D. A choline phosphoryl-conjugated chitosan/oxidized dextran injectable self-healing hydrogel for improved hemostatic efficacy. Biomacromolecules 2023, 24, 690–703. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Wang, S.; Dong, C.; Zhang, X.; Zhang, R.; Yang, L. Dextran and peptide-based ph-sensitive hydrogel boosts healing process in multidrug-resistant bacteria-infected wounds. Carbohydr. Polym. 2022, 278, 118994. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millan, E.; Balandran-Quintana, R.; Lopez-Franco, Y.; Rascon-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.L.; Chen, J.F.; Zhang, S.G. The effect of degree of esterification of pectin on the interaction between pectin and wheat gluten protein. Food Hydrocoll. 2022, 136, 108272. [Google Scholar] [CrossRef]
- An, H.; Yang, Y.; Zhou, Z.; Bo, Y.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Pectin-based injectable and biodegradable self-healing hydrogels for enhanced synergistic anticancer therapy. Acta Biomater. 2021, 131, 149–161. [Google Scholar] [CrossRef]
- Pereira, R.F.; Barrias, C.C.; Bartolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef]
- Wang, J.H.; Tsai, C.W.; Tsai, N.Y.; Chiang, C.Y.; Lin, R.S.; Pereira, R.F.; Li, Y.E. An injectable, dual crosslinkable hybrid pectin methacrylate (pecma)/gelatin methacryloyl (gelma) hydrogel for skin hemostasis applications. Int. J. Biol. Macromol. 2021, 185, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ni, Y.; Liu, B.; Zhou, T.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y. Self-crosslinking and injectable hyaluronic acid/rgd-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr. Polym. 2017, 166, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Thakur, A.; Kadumudi, F.B.; Pierchala, M.K.; Cordova, J.A.V.; Shahbazi, M.A.; Mehrali, M.; Pennisi, C.P.; Orive, G.; Gaharwar, A.K.; et al. Pectin methacrylate (PEMA) and gelatin-based hydrogels for cell delivery: Converting waste materials into biomaterials. ACS Appl. Mater. Interfaces 2019, 11, 12283–12297. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.S.; Buyuksungur, S.; Hasirci, N.; Tezcaner, A. pH responsive release of curcumin from photocrosslinked pectin/gelatin hydrogel wound dressings. Biomater. Adv. 2022, 134, 112717. [Google Scholar] [CrossRef]
- Simaan-Yameen, H.; Bar-Am, O.; Saar, G.; Seliktar, D. Methacrylated fibrinogen hydrogels for 3D cell culture and delivery. Acta Biomater. 2023, 164, 94–110. [Google Scholar] [CrossRef]
- Chen, J.; Xu, M.; Wang, L.; Li, T.; Li, Z.; Wang, T.; Li, P. Converting lysozyme to hydrogel: A multifunctional wound dressing that is more than antibacterial. Colloids Surf. B. Biointerfaces 2022, 219, 112854. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yin, W.; Wang, H.; Gao, J.; Gu, Y.; Wang, W.; Liu, C.; Pan, G.; Li, B. Dynamic proteinaceous hydrogel enables in-situ recruitment of endogenous TGF-β1 and stem cells for cartilage regeneration. Adv. Funct. Mater. 2024, 2403055. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly developed techniques on polycondensation, ring-opening polymerization and polymer modification: Focus on poly(lactic acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and biological application of polylactic acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sust. Energ. Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.W.; Wang, X.Z. New advances in the biodegradation of poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Gesine Schliecker, C.S. Stefan Fuchs, Thomas Kissel, Characterization of a homologous series of d,l-lactic acid oligomers; a mechanistic study on the degradation kinetics in vitro. Biomaterials 2003, 24, 3835–3844. [Google Scholar] [CrossRef]
- Jang, W.; Mun, S.J.; Kim, S.Y.; Bong, K.W. Controlled growth factor delivery via a degradable poly(lactic acid) hydrogel microcarrier synthesized using degassed micromolding lithography. Colloids Surf. B. Biointerfaces 2023, 222, 113088. [Google Scholar] [CrossRef]
- Pasini, C.; Pandini, S.; Re, F.; Ferroni, M.; Borsani, E.; Russo, D.; Sartore, L. New poly(lactic acid)-hydrogel core-shell scaffolds highly support mscs’ viability, proliferation and osteogenic differentiation. Polymers 2023, 15, 4631. [Google Scholar] [CrossRef]
- Fu, S.Z.; Li, Z.; Fan, J.M.; Meng, X.H.; Shi, K.; Qu, Y.; Yang, L.L.; Wu, J.B.; Fan, J.; Luot, F.; et al. Biodegradable and thermosensitive monomethoxy poly(ethylene glycol)-poly(lactic acid) hydrogel as a barrier for prevention of post-operative abdominal adhesion. J. Biomed. Nanotechnol. 2014, 10, 427–435. [Google Scholar] [CrossRef]
- Schneider, M.; Gunter, C.; Taubert, A. Co-deposition of a hydrogel/calcium phosphate hybrid layer on 3d printed poly(lactic acid) scaffolds via dip coating: Towards automated biomaterials fabrication. Polymers 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Grindy, S.; Gil, D.; Suhardi, J.; Fan, Y.; Moore, K.; Hugard, S.; Leape, C.; Randolph, M.; Asik, M.D.; Muratoglu, O.; et al. Hydrogel device for analgesic drugs with in-situ loading and polymerization. J. Control. Release 2023, 361, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. Rsc Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Wendels, S.; Averous, L. Biobased polyurethanes for biomedical applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef] [PubMed]
- Magnin, A.; Pollet, E.; Phalip, V.; Averous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, K.C.; He, Y.Y.; Du, B.H.; Hong, J.H.; Yin, H.; Lu, D.; Luo, F.; Li, Z.; Li, J.H.; et al. Tough and biodegradable polyurethane-curcumin composited hydrogel with antioxidant, antibacterial and antitumor properties. Mater. Sci. Eng. C 2021, 121, 111820. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ou, Y.; Wang, X.; Yuan, L.; He, N.; Li, Z.; Luo, F.; Li, J.; Tan, H. A biodegradable injectable fluorescent polyurethane-oxidized dextran hydrogel for non-invasive monitoring. J. Mater. Chem. B 2023, 11, 8506–8518. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Wang, Y.; Zheng, Y.; Xie, Y.; Zhang, H.; Chen, J.; Hussain, M.I.; Meng, H.; Peng, J. A novel bioactive polyurethane with controlled degradation and l-arg release used as strong adhesive tissue patch for hemostasis and promoting wound healing. Bioact. Mater. 2022, 17, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Ferrao, R.; Marta, D.; Vilaca, A.; Lino, M.; Rondao, T.; Ji, J.; Paiva, A.; Ferreira, L. Antimicrobial peptide-tether dressing able to enhance wound healing by tissue contact. ACS Appl. Mater. Interfaces 2022, 14, 24213–24228. [Google Scholar] [CrossRef]
- Cheng, Q.P.; Hsu, S.H. A self-healing hydrogel and injectable cryogel of gelatin methacryloyl-polyurethane double network for 3D printing. Acta Biomater. 2023, 164, 124–138. [Google Scholar] [CrossRef]
- Polo Fonseca, L.; Trinca, R.B.; Felisberti, M.I. Amphiphilic polyurethane hydrogels as smart carriers for acidic hydrophobic drugs. Int. J. Pharm. 2018, 546, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Boffito, M.; Abrami, M.; Grassi, M.; Zoso, A.; Chiono, V.; Ciardelli, G. Dual stimuli-responsive polyurethane-based hydrogels as smart drug delivery carriers for the advanced treatment of chronic skin wounds. Bioact. Mater. 2021, 6, 3013–3024. [Google Scholar] [CrossRef]
- Werkmeister, J.A.; Adhikari, R.; White, J.F.; Tebb, T.A.; Le, T.P.; Taing, H.C.; Mayadunne, R.; Gunatillake, P.A.; Danon, S.J.; Ramshaw, J.A. Biodegradable and injectable cure-on-demand polyurethane scaffolds for regeneration of articular cartilage. Acta Biomater. 2010, 6, 3471–3481. [Google Scholar] [CrossRef] [PubMed]
- Thielemans, M.L.W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly(ε-caprolactone): A potential polymer for biodegradable food packaging applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Salehi, A.O.M.; Keshel, S.H.; Sefat, F.; Tayebi, L. Use of polycaprolactone in corneal tissue engineering: A review. Mater. Today Commun. 2021, 27, 102402. [Google Scholar] [CrossRef]
- Deng, H.; Dong, A.; Song, J.; Chen, X. Injectable thermosensitive hydrogel systems based on functional peg/pcl block polymer for local drug delivery. J. Control Release 2019, 297, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Koh, W.G. Composite hydrogel of methacrylated hyaluronic acid and fragmented polycaprolactone nanofiber for osteogenic differentiation of adipose-derived stem cells. Pharmaceutics 2020, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of poly (lactic-co-glycolic acid) and progress of poly (lactic-co-glycolic acid)-based biodegradable materials in biomedical research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, B. Degradable polymeric bio(nano)materials and their biomedical applications: A comprehensive overview and recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Zolnik B, S.; Burgess D, J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhou, M.; Dong, W.; Zhao, S.; Wang, Y.; Yao, J.; Liu, Z.; Han, H.; Sun, D.; Zhang, M. A bi-layered scaffold of a poly(lactic-co-glycolic acid) nanofiber mat and an alginate-gelatin hydrogel for wound healing. J. Mater. Chem. B 2021, 9, 7492–7505. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; He, J.; Bai, L.; Huang, Y.; Chen, B.; Li, Z.; Xu, M.; Liu, Q.; Wang, S.; Wen, N.; et al. Injectable bioadhesive photocrosslinkable hydrogels with sustained release of kartogenin to promote chondrogenic differentiation and partial-thickness cartilage defects repair. Adv. Healthc. Mater. 2024, 13, e2303255. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart hydrogel-based dvdms/bfgf nanohybrids for antibacterial phototherapy with multiple damaging sites and accelerated wound healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiță, D.; Moldovan, H.; Robu, A.; Bița, A.I.; Grosu, E.; Antoniac, A.; Corneschi, I.; Antoniac, I.; Bodog, A.D.; Băcilă, C.I. Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances. Int. J. Mol. Sci. 2023, 24, 10540. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable polymers-an overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Moss, J.A.; Mark, S.S.; Hixon, S.; Ashley, F.T.; Chang, J.Y.; Janda, K.D. Solid-phase synthesis and kinetic characterization of fluorogenic enzyme-degradable hydrogel cross-linkers. Biomacromolecules 2006, 7, 1011–1016. [Google Scholar] [CrossRef]







| Main Component | Natural or Synthetic | Trade Name |
|---|---|---|
| Chitosan | Natural | Polysaccharide HemCon Bandage Pro |
| Alginate | Natural | Seal-V |
| Hyaluronic acid | Natural | Seprafilm Adhesion Barrier |
| Gelatin | Natural | LifeSeal GRF Biological Glue |
| Collagen | Natural | Angio-Seal |
| Human fibrinogen | Natural | Tisseel Evicel Hemaseel Crosseal VISTASEAL CryoSeal Fibrin Sealant System |
| Polyurethane | Synthetic | MAR-CUTIS (Flix) |
| Polymers | Advantages | Drawbacks |
|---|---|---|
| Natural polymers | High biocompatibility in most cases; Suitable biodegradability; Non-toxic degradation products. | Relatively weak mechanical properties; Restricted sources; High production costs. |
| Synthetic polymers | Low cost; Easy to produce massively; Adjustable mechanical properties. | Lack of biological cues; Relatively poor biodegradability in most cases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Dou, W.; Zeng, X.; Chen, X.; Gao, Y.; Liu, H.; Li, S. Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers. Int. J. Mol. Sci. 2024, 25, 5249. https://doi.org/10.3390/ijms25105249
Zhu S, Dou W, Zeng X, Chen X, Gao Y, Liu H, Li S. Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers. International Journal of Molecular Sciences. 2024; 25(10):5249. https://doi.org/10.3390/ijms25105249
Chicago/Turabian StyleZhu, Shuzhuang, Wenguang Dou, Xiaojun Zeng, Xingchao Chen, Yonglin Gao, Hongliang Liu, and Sidi Li. 2024. "Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers" International Journal of Molecular Sciences 25, no. 10: 5249. https://doi.org/10.3390/ijms25105249
APA StyleZhu, S., Dou, W., Zeng, X., Chen, X., Gao, Y., Liu, H., & Li, S. (2024). Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers. International Journal of Molecular Sciences, 25(10), 5249. https://doi.org/10.3390/ijms25105249






