Comparison of Antimicrobial Properties of Graphene Oxide-Based Materials, Carbon Dots, and Their Combinations Deposited on Cotton Fabrics
Abstract
1. Introduction
2. Results
2.1. Characterisation of Materials
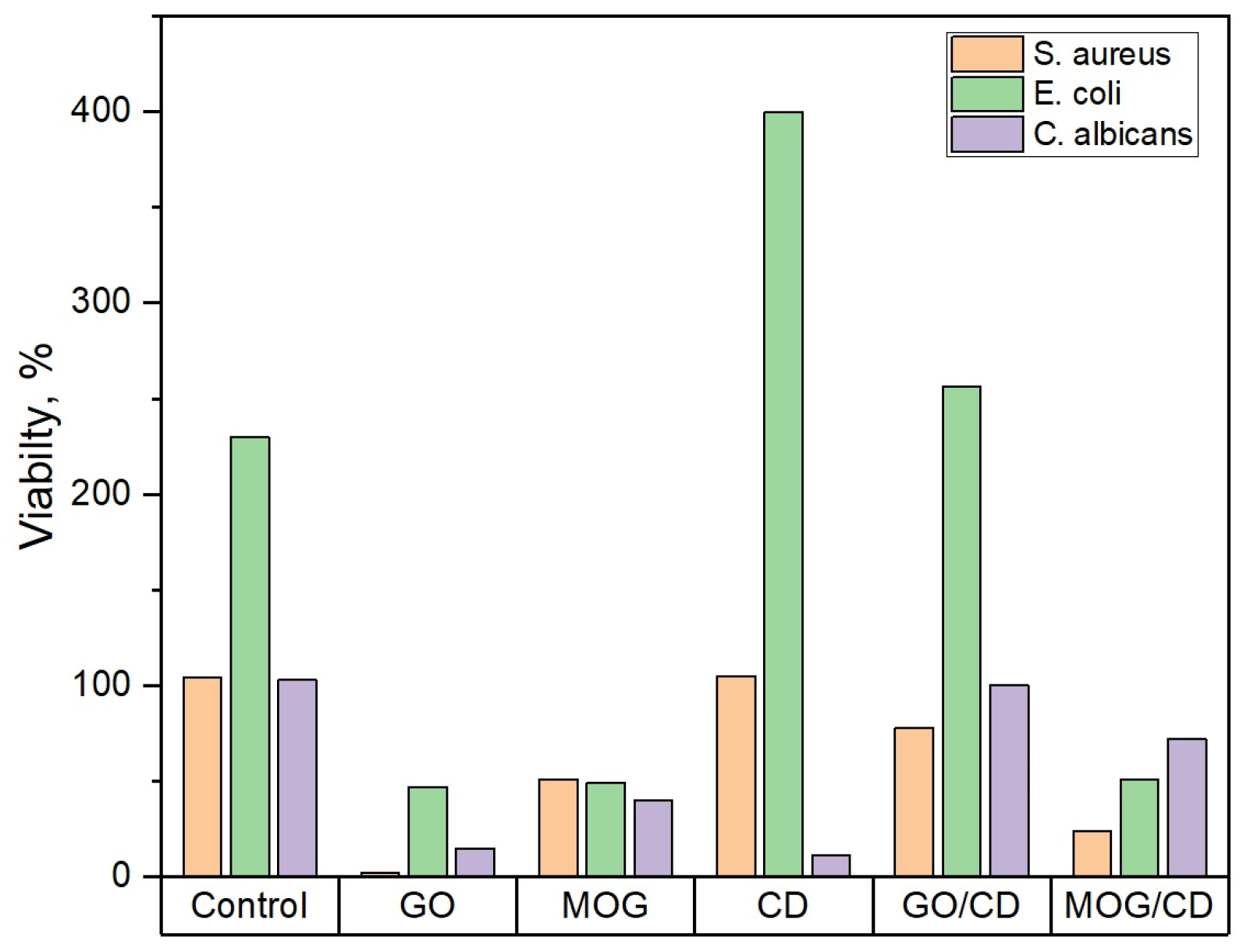
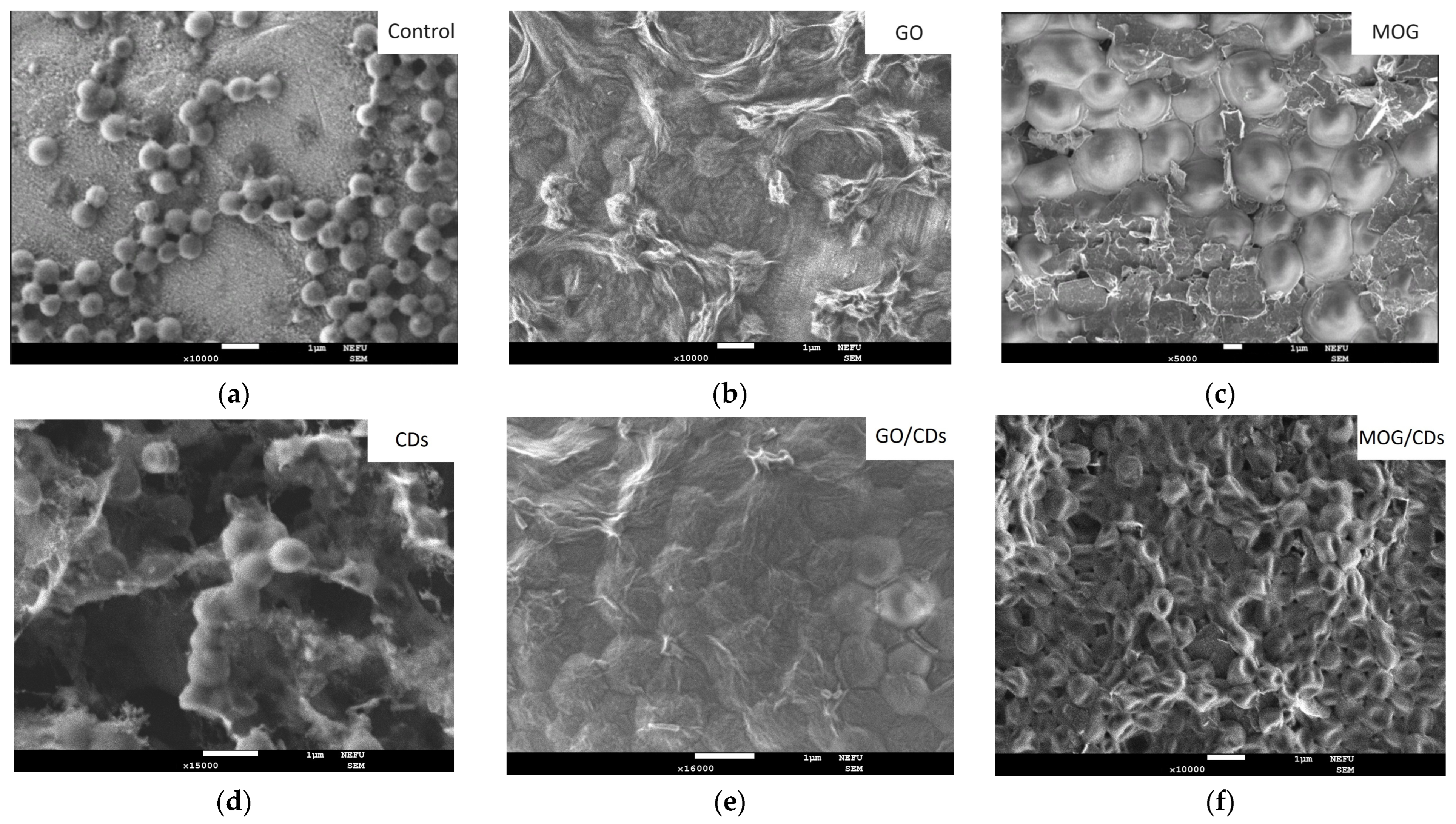
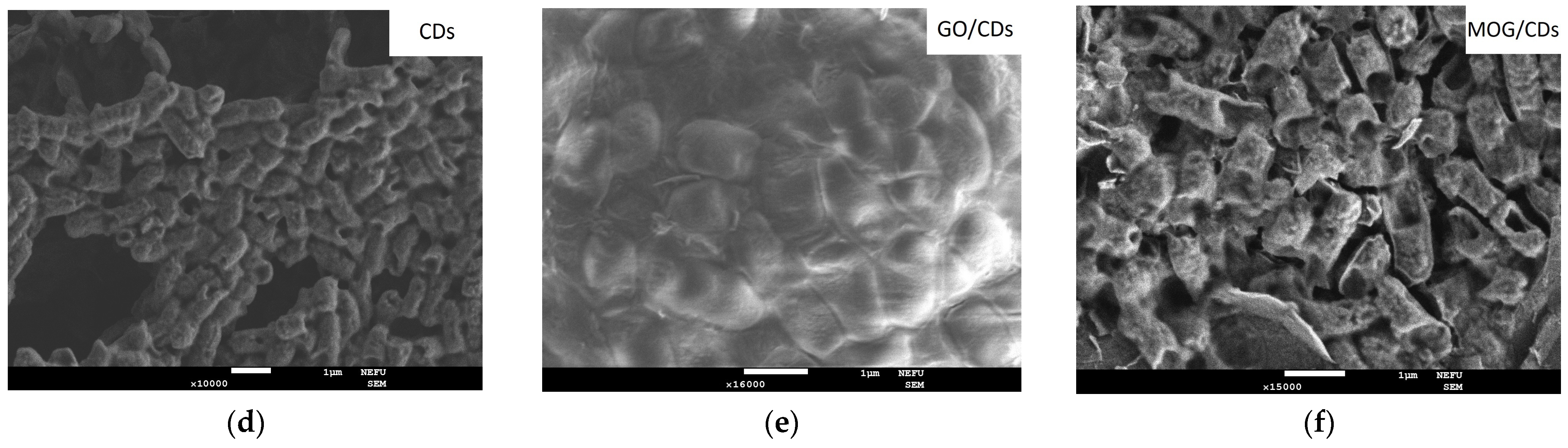
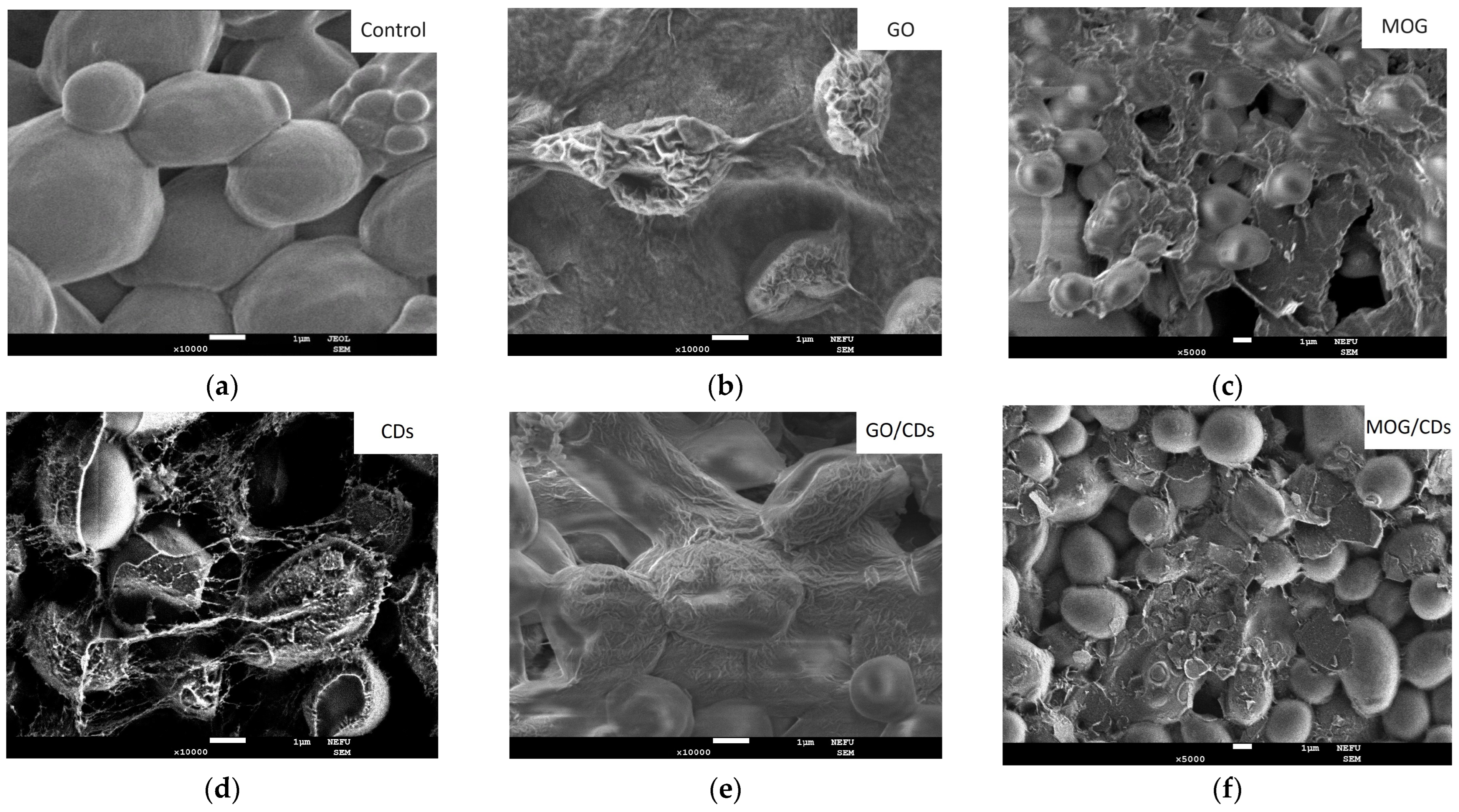
2.2. Comparison of the Antimicrobial Properties of GO, MOG, CDs, and Their Combinations
3. Discussion
4. Materials and Methods
4.1. GO and MOG Synthesis
4.2. Synthesis of CDs
4.3. Preparation of Suspensions and Textiles Decorated with GO, MOG, and CDs
4.4. Research Methods
4.5. Methods for Testing the Antimicrobial Activity of Suspensions of Carbon Nanomaterials and Decorated Textiles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as antibacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents—A minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef]
- Li, P.; Sun, L.; Xue, S.; Qu, D.; An, L.; Wang, X.; Sun, Z. Recent advances of carbon dots as new antimicrobial agents. SmartMat 2022, 3, 226–248. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, W. (Ed.) The chemistry of graphene oxide. In Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications, 1st ed.; Springer: Cham, Switzerland, 2015; pp. 61–95. [Google Scholar]
- Kim, D.Y.; Patel, S.K.; Rasool, K.; Lone, N.; Bhatia, S.K.; Seth, C.S.; Ghodake, G.S. Bioinspired silver nanoparticle-based nanocomposites for effective control of plant pathogens: A review. Sci. Total Environ. 2023, 908, 168318. [Google Scholar] [CrossRef]
- Bhatt, S.; Punetha, V.D.; Pathak, R.; Punetha, M. Graphene in nanomedicine: A review on nano-bio factors and antibacterial activity. Colloids Surf. B Biointerfaces 2023, 226, 113323. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial properties of graphene oxide nanosheets: Why size matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial mechanisms and effectiveness of graphene and graphene-functionalized biomaterials. A scope review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef]
- Palmieri, V.; Bugli, F.; Lauriola, M.C.; Cacaci, M.; Torelli, R.; Ciasca, G.; Conti, C.; Sanguinetti, M.; Papi, M.; Spirito, M.D. Bacteria meet graphene: Modulation of graphene oxide nanosheet interaction with human pathogens for effective antimicrobial therapy. ACS Biomater. Sci. Eng. 2017, 3, 619–627. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Eaqub, A.M.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene oxide exhibits differential mechanistic action towards Gram-positive and Gram-negative bacteria. Colloids Surf. B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, H.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled surface-mediated antibacterial activity of graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 10, 5731–5736. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Fang, G.; Wu, R.; Zhang, H.; Chai, Z.; Chen, C.; Yin, J.J. Light-enhanced antibacterial activity of graphene oxide, mainly via accelerated electron transfer. Environ. Sci. Technol. 2017, 51, 10154–10161. [Google Scholar] [CrossRef]
- Barbolina, I.; Woods, C.R.; Lozano, N.; Kostarelos, K.; Novoselov, K.S.; Roberts, I.S. Purity of graphene oxide determines its antibacterial activity. 2D Materials 2016, 3, 025025. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Jabari, E.; Jabbari, E. Functionalized carbon-based nanomaterials and quantum dots with antibacterial activity: A review. Expert Rev. Anti-Infect. Ther. 2021, 19, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S-and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Ouadil, B.; Amadine, O.; Essamlali, Y.; Cherkaoui, O.; Zahouily, M. A new route for the preparation of hydrophobic and antibacterial textiles fabrics using Ag-loaded graphene nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123713. [Google Scholar] [CrossRef]
- Molina, J. Graphene-based fabrics and their applications: A review. RSC Adv. 2016, 6, 68261–68291. [Google Scholar] [CrossRef]
- Olborska, A.; Janas-Naze, A.; Kaczmarek, L.; Warga, T.; Halin, D.S.C. Antibacterial effect of graphene and graphene oxide as a potential material for fiber finishes. AUTEX Res. J. 2020, 20, 506–516. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Gan, L.; Long, M. Surface modified graphene oxide based cotton fabric by ion implantation for enhancing antibacterial activity. ACS Sustain. Chem. Eng. 2019, 7, 7686–7692. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, B.; Min, L.; Li, J.; Zhang, Y.; Jiang, H.; Peng, C.; Li, J.; Shi, J.; Huang, Q.; et al. Graphene oxide-based antibacterial cotton fabrics. Adv. Healthc. Mater. 2013, 2, 1259–1266. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Navaneethaiyer, U.; Mohan, R.; Lee, J.; Kim, S.J. Graphene oxide nanostructures modified multifunctional cotton fabrics. Appl. Nanosci. 2012, 2, 119–126. [Google Scholar] [CrossRef]
- Parvez, K.; Li, R.; Puniredd, S.R.; Hernandez, Y.; Hinkel, F.; Wang, S.; Feng, X.; Mullen, K. Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics. ACS Nano 2013, 7, 3598–3606. [Google Scholar] [CrossRef]
- Marković, Z.M.; Matijašević, D.M.; Pavlović, V.B.; Jovanović, S.P.; Holclajtner-Antunović, I.D.; Špitalský, Z.; Mičušik, M.; Dramićanin, M.D.; Milivojević, D.D.; Nikšić, M.P.; et al. Antibacterial potential of electrochemically exfoliated graphene sheets. J. Colloid Interface Sci. 2017, 500, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Yua, W.; Lia, X.; Hea, J.; Chena, Y.; Qia, L.; Yuana, P.; Oua, K.; Liua, F.; Zhoua, Y.; Qinc, X. Graphene oxide-silver nanocomposites embedded nanofiber core-spun yarns for durable antibacterial textiles. J. Colloid Interface Sci. 2020, 584, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Maldonado, S.; Morin, S.; Stevenson, K.J. Structure, composition, and chemical reactivity of carbon nanotubes by selective nitrogen doping. Carbon 2006, 44, 1429–1437. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectrum of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef]
- Asadi Shahi, S.; Roudbar Mohammadi, S.; Roudbary, M.; Delavari, H. A new formulation of graphene oxide/fluconazole compound as a promising agent against Candida albicans. Prog. Biomater. 2019, 8, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cacaci, M.; Squitieri, D.; Palmieri, V.; Torelli, R.; Perini, G.; Campolo, M.; Di Vito, M.; Papi, M.; Posteraro, B.; Sanguinetti, M.; et al. Curcumin-Functionalized Graphene Oxide Strongly Prevents Candida parapsilosis Adhesion and Biofilm Formation. Pharmaceuticals 2023, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Sturabotti, E.; Camilli, A.; Georgian Moldoveanu, V.; Bonincontro, G.; Simonetti, G.; Valletta, A.; Serangeli, I.; Miranda, E.; Amato, F.; Giacomo Marrani, A.; et al. Targeting the Antifungal Activity of Carbon Dots against Candida albicans Biofilm Formation by Tailoring Their Surface Functional Groups. Chem.–A Eur. J. 2024, 30, e202303631. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Yuan, T.; Zhu, J.; Yang, Y. Influence of the iodine content of nitrogen-and iodine-doped carbon dots as a peroxidase mimetic nanozyme exhibiting antifungal activity against C. albicans. Biochem. Eng. J. 2021, 175, 108139. [Google Scholar] [CrossRef]
- Radhi, A.; Mohamad, D.; Suhaily, F.; Rahman, A.; Abdullah, A.M.; Hasan, H. Mechanism and factors influence of graphene-based nanomaterials antimicrobial activities and application in dentistry. J. Mater. Res. Technol. 2021, 11, 1290–1307. [Google Scholar] [CrossRef]
- Suchodolski, J.; Krasowska, A. Plasma membrane potential of Candida albicans measured by Di-4-ANEPPS fluorescence depends on growth phase and regulatory factors. Microorganisms 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cai, L.; Hu, G.; Mo, L.; Zheng, Y.; Hu, C.; Lei, B.; Zhang, X.; Liu, Y.; Zhuang, J. Red-emissive carbon dots from spinach: Characterization and application in visual detection of time. J. Lumin. 2020, 227, 117534. [Google Scholar] [CrossRef]
- Alexandrov, G.N.; Smagulova, S.A.; Kapitonov, A.N.; Vasil’eva, F.D.; Kurkina, I.I.; Vinokurov, P.V.; Timofeev, V.B.; Antonova, I.V. Thin partially reduced oxide-graphene films: Structural, optical, and electrical properties. Nanotechnol. Russ. 2014, 9, 363–368. [Google Scholar] [CrossRef]
- Vasilieva, F.D.; Kapitonov, A.N.; Yakimchuk, E.A.; Smagulova, S.A.; Antonova, I.V.; Kotin, I.A. Mildly oxidized graphene oxide suspension for printing technologies. Mater. Res. Express 2018, 5, 065608. [Google Scholar] [CrossRef]
- GOST 9733.4-83; Textiles. Test Method of Color Fastness and Washing. USSRs Gosstandard: Moscow, Russia, 1984.
- ISO 20743:2007; Textiles—Determination of Antibacterial Activity of Antibacterial Finished Products (IDT). Federal Agency for Technical Regulation and Metrology: Moscow, Russia, 2014.
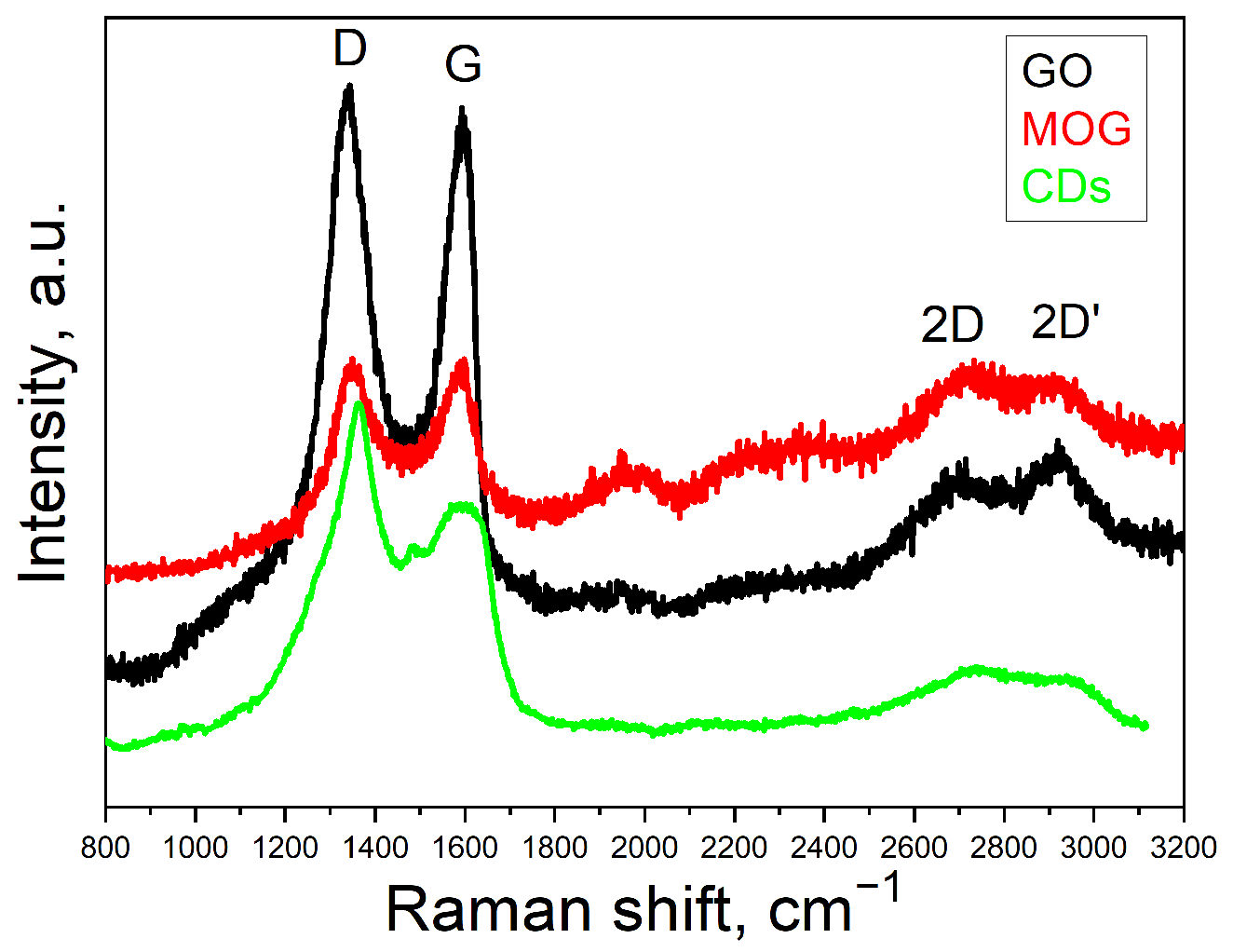
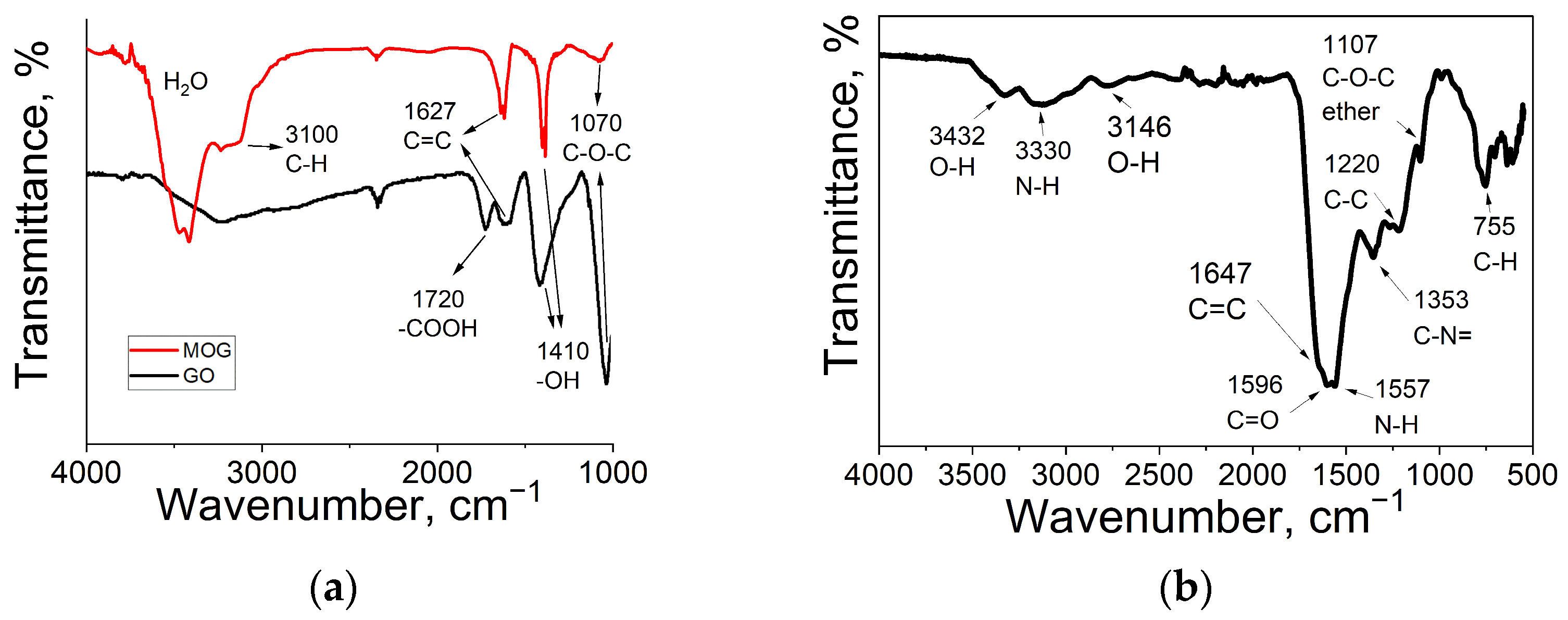
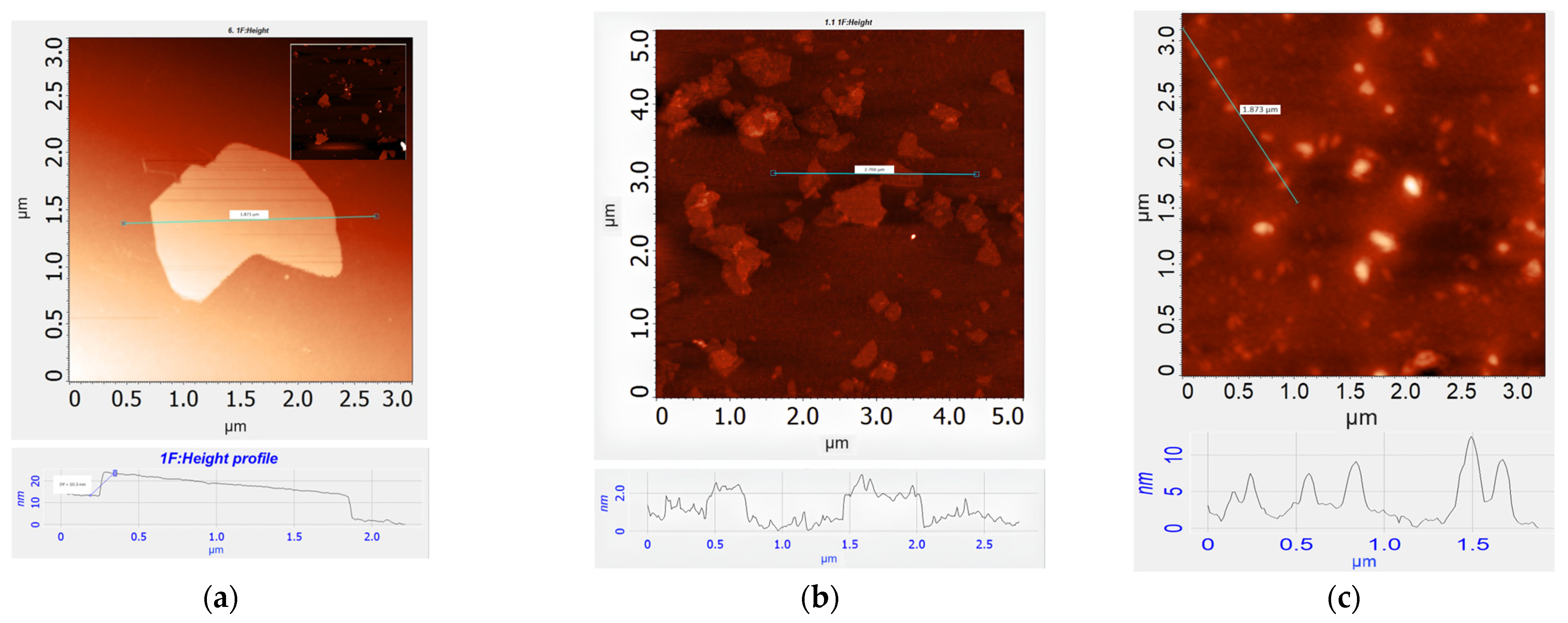

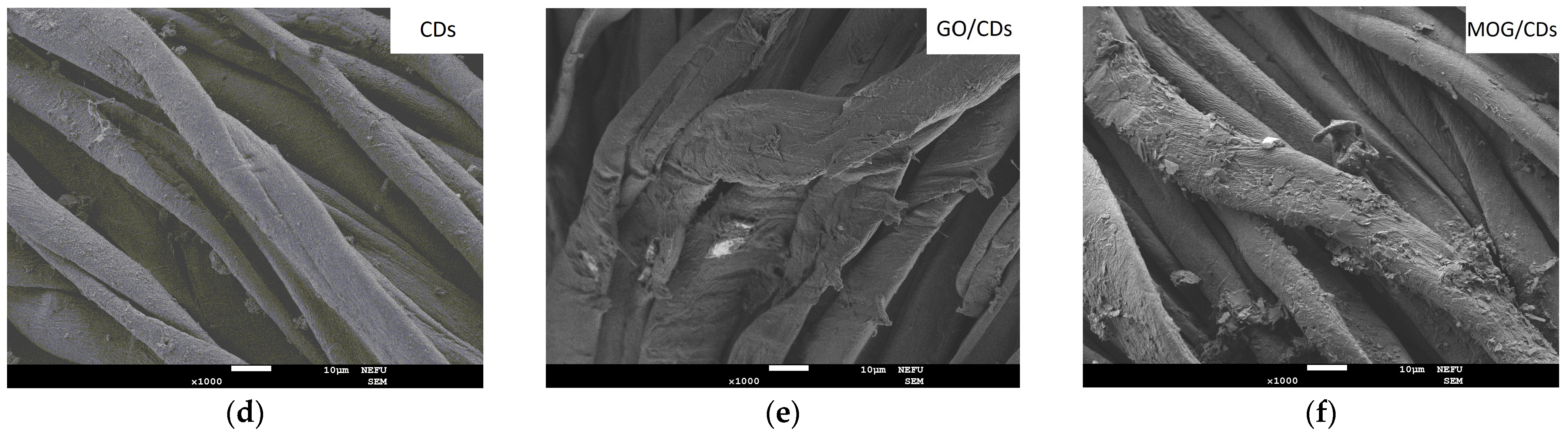


| Element | GO | MOG | CDs |
|---|---|---|---|
| C, % | 58.6 | 93.9 | 37.7 |
| O, % | 41.4 | 6.1 | 33.5 |
| N, % | - | - | 28.8 |
| Microorganism | GO | MOG | CDs | GO/CDs | MOG/CDs |
|---|---|---|---|---|---|
| S. aureus | 2 | 0 | 0 | 1 | 1 |
| E. coli | 2 | 2 | 1 | 1 | 1 |
| C. albicans | 1 | 1 | 0 | 1 | 2 |
| Microorganism | GO | MOG | CDs | GO/CDs | MOG/CDs |
|---|---|---|---|---|---|
| S. aureus | 1 | −1 | 0 | 0 | 1 |
| E. coli | 1 | 1 | −4 | 0 | 0 |
| C. albicans | 1 | 1 | 0 | 1 | −1 |
| Microbial Culture | Cell Vitality, % | ||
|---|---|---|---|
| GO | MOG | CDs | |
| S. aureus | 2 *, 60 [11], 8 [12], 6.3 [14] | 51 *, 0 [33] | 100 *, 0 [41] |
| E. coli | 47 *, 8 [15], 90 [11], 33.3 [12] | 49 *, ~100 [33] | 100 *, 1 [23], ~100 [24], 0 [41] |
| C. albicans | 15 *, ~20 [42], 28 [43] | 40 * | 12 *, 90 [44], 60 [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evseev, Z.I.; Tarasova, L.A.; Vasilieva, F.D.; Egorova, M.N.; Dmitriev, P.S.; Akhremenko, Y.A.; Smagulova, S.A. Comparison of Antimicrobial Properties of Graphene Oxide-Based Materials, Carbon Dots, and Their Combinations Deposited on Cotton Fabrics. Int. J. Mol. Sci. 2024, 25, 5328. https://doi.org/10.3390/ijms25105328
Evseev ZI, Tarasova LA, Vasilieva FD, Egorova MN, Dmitriev PS, Akhremenko YA, Smagulova SA. Comparison of Antimicrobial Properties of Graphene Oxide-Based Materials, Carbon Dots, and Their Combinations Deposited on Cotton Fabrics. International Journal of Molecular Sciences. 2024; 25(10):5328. https://doi.org/10.3390/ijms25105328
Chicago/Turabian StyleEvseev, Zakhar Ivanovich, Lidia Andreevna Tarasova, Fedora Dmitrievna Vasilieva, Marfa Nikitichna Egorova, Petr Stanislavovich Dmitriev, Yana Aleksandrovna Akhremenko, and Svetlana Afanasyevna Smagulova. 2024. "Comparison of Antimicrobial Properties of Graphene Oxide-Based Materials, Carbon Dots, and Their Combinations Deposited on Cotton Fabrics" International Journal of Molecular Sciences 25, no. 10: 5328. https://doi.org/10.3390/ijms25105328
APA StyleEvseev, Z. I., Tarasova, L. A., Vasilieva, F. D., Egorova, M. N., Dmitriev, P. S., Akhremenko, Y. A., & Smagulova, S. A. (2024). Comparison of Antimicrobial Properties of Graphene Oxide-Based Materials, Carbon Dots, and Their Combinations Deposited on Cotton Fabrics. International Journal of Molecular Sciences, 25(10), 5328. https://doi.org/10.3390/ijms25105328





