In Vivo Immune-Modulatory Activity of Lefamulin in an Influenza Virus A (H1N1) Infection Model in Mice
Abstract
:1. Introduction
2. Results
2.1. Influenza A/H1N1 Infection and Treatment Schedule
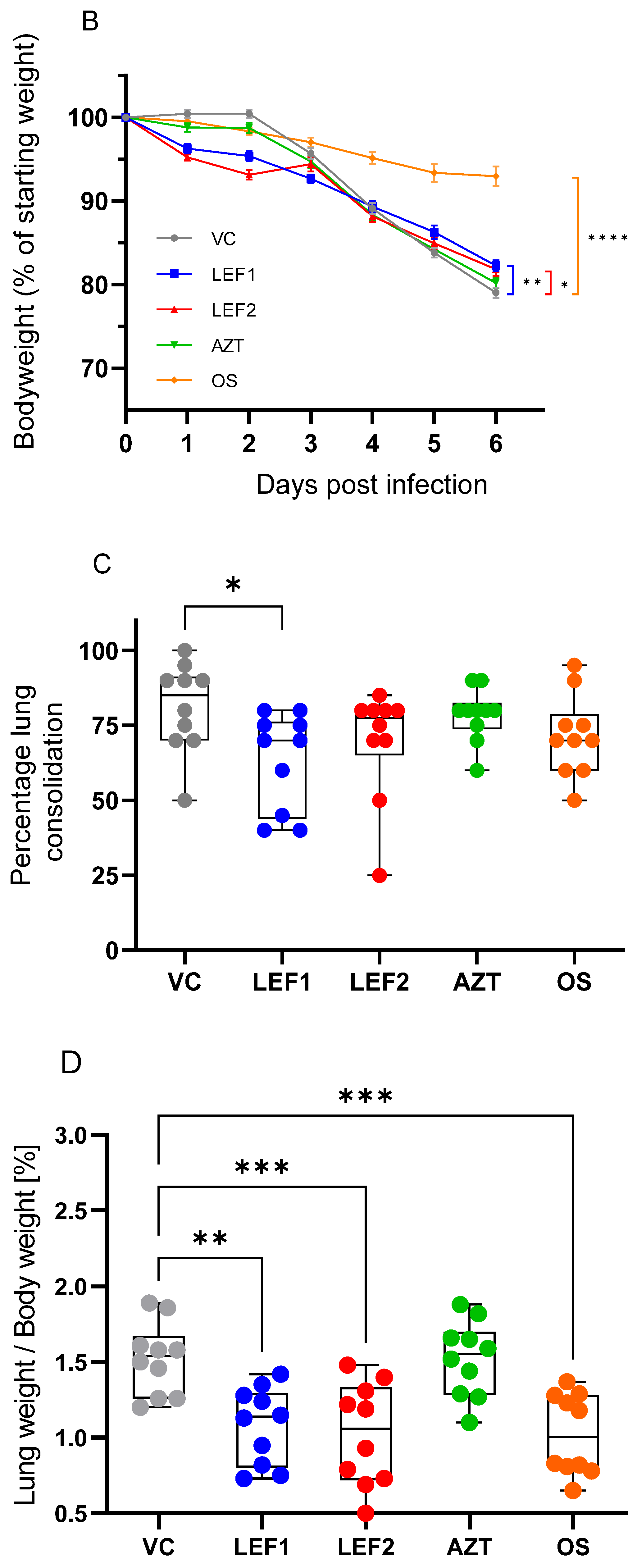
2.2. Survival, Body Weight, Lung Weight, and Lung Consolidation
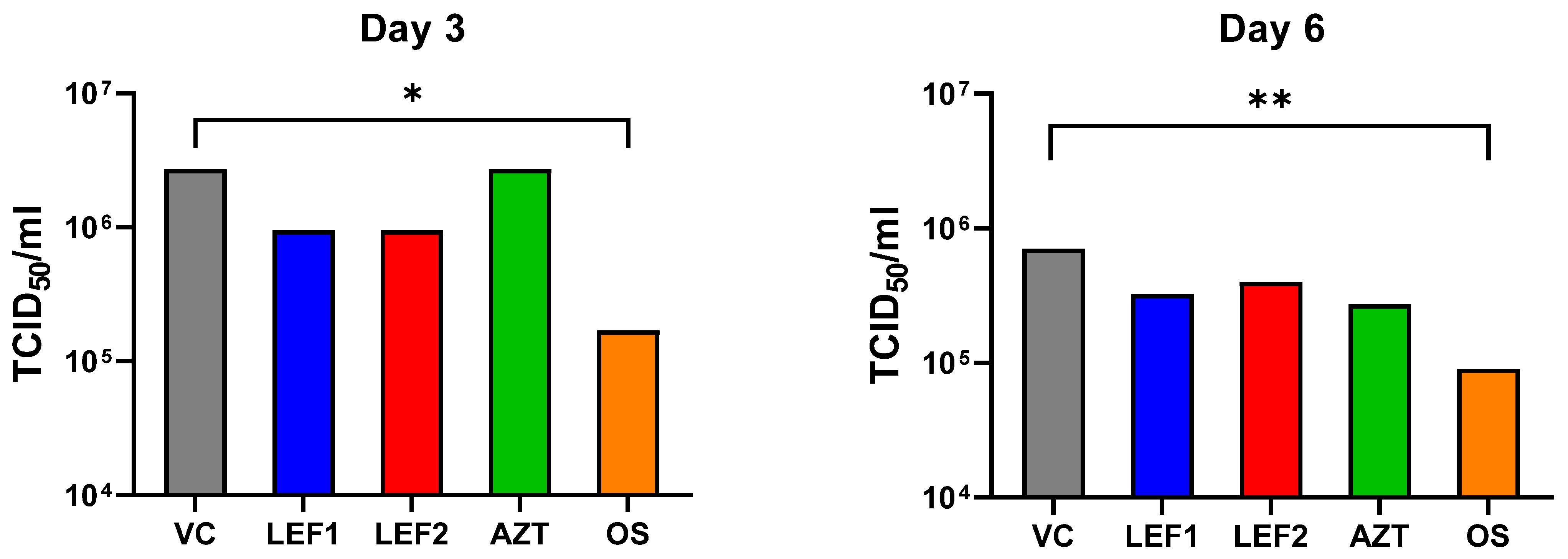
2.3. Lung Viral Titer
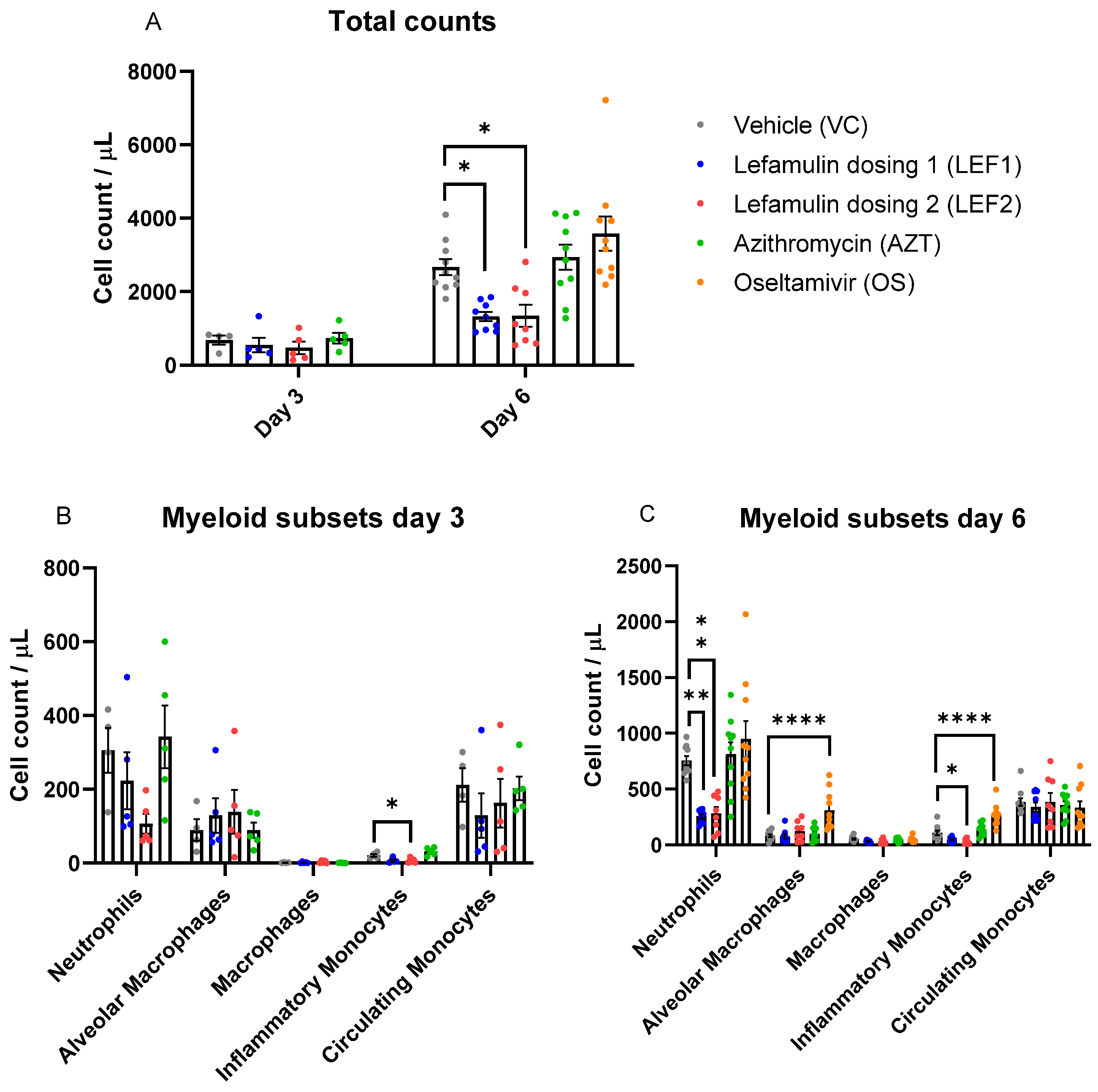
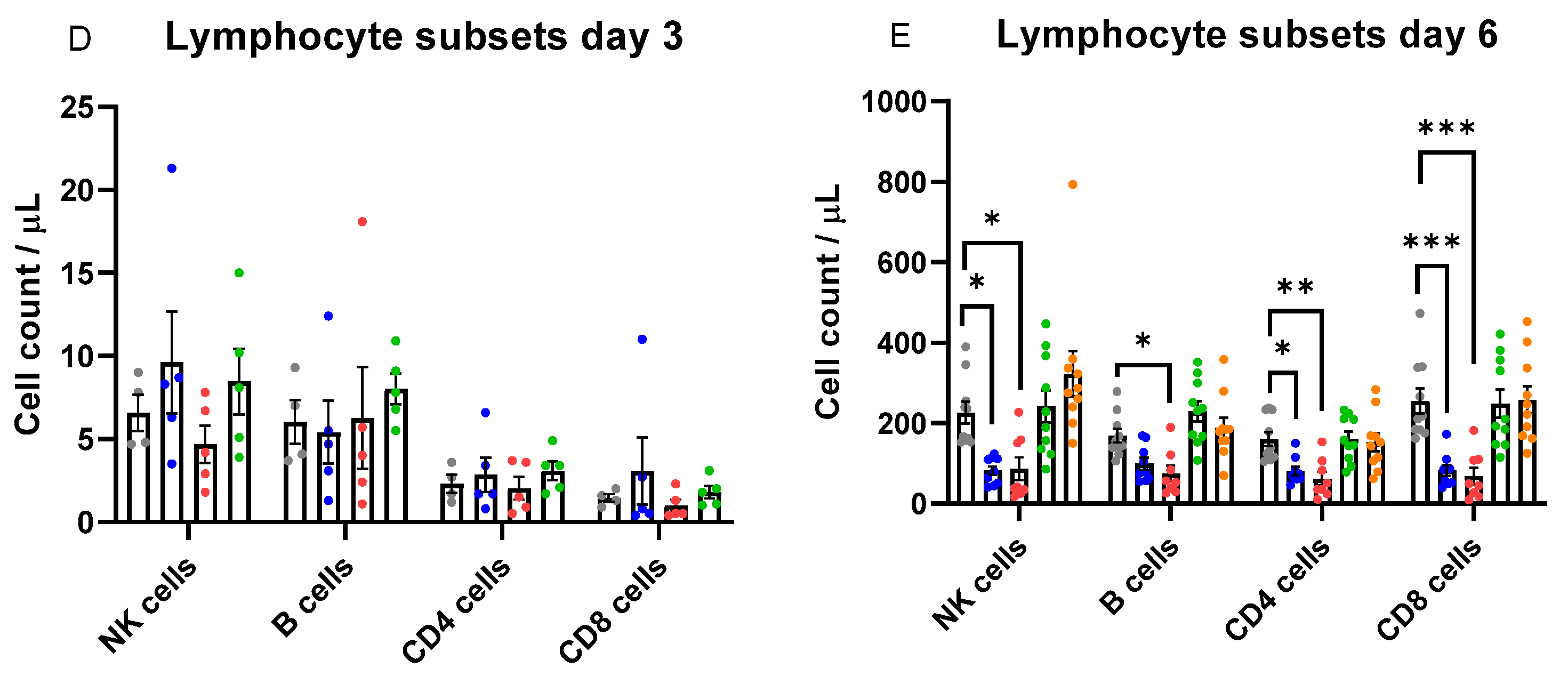
2.4. Immune Cell Infiltration in the Lung
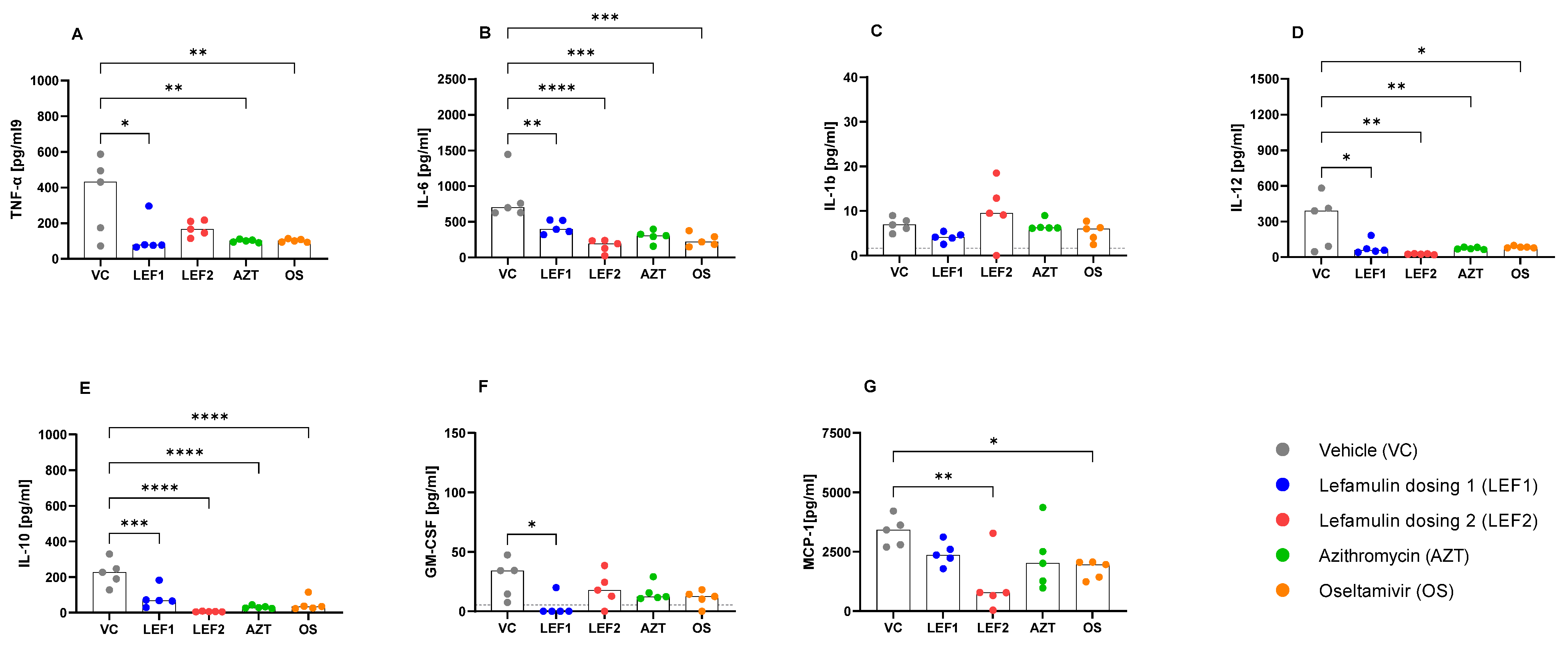
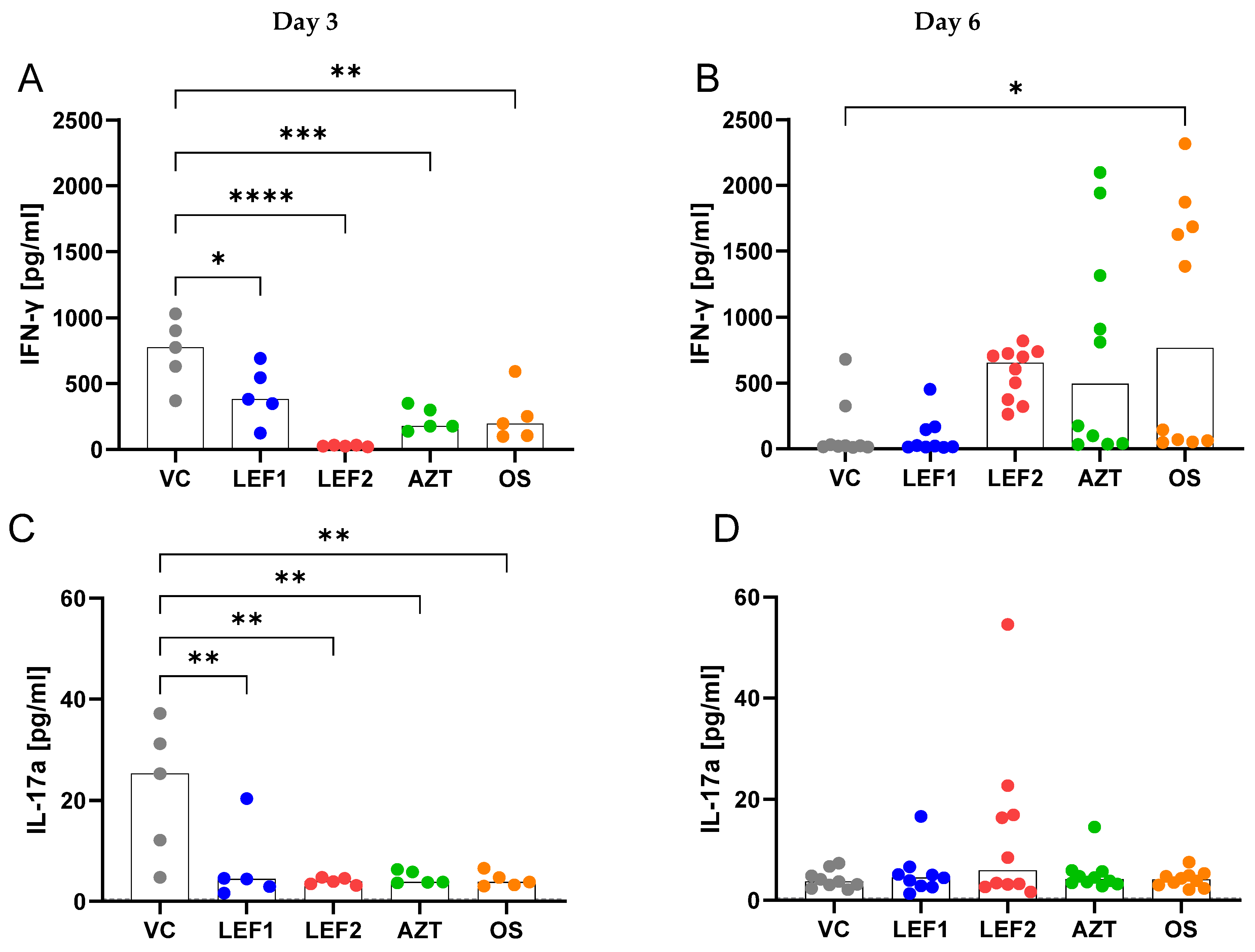
2.5. Cytokines and Chemokine Concentrations in BALF and Plasma
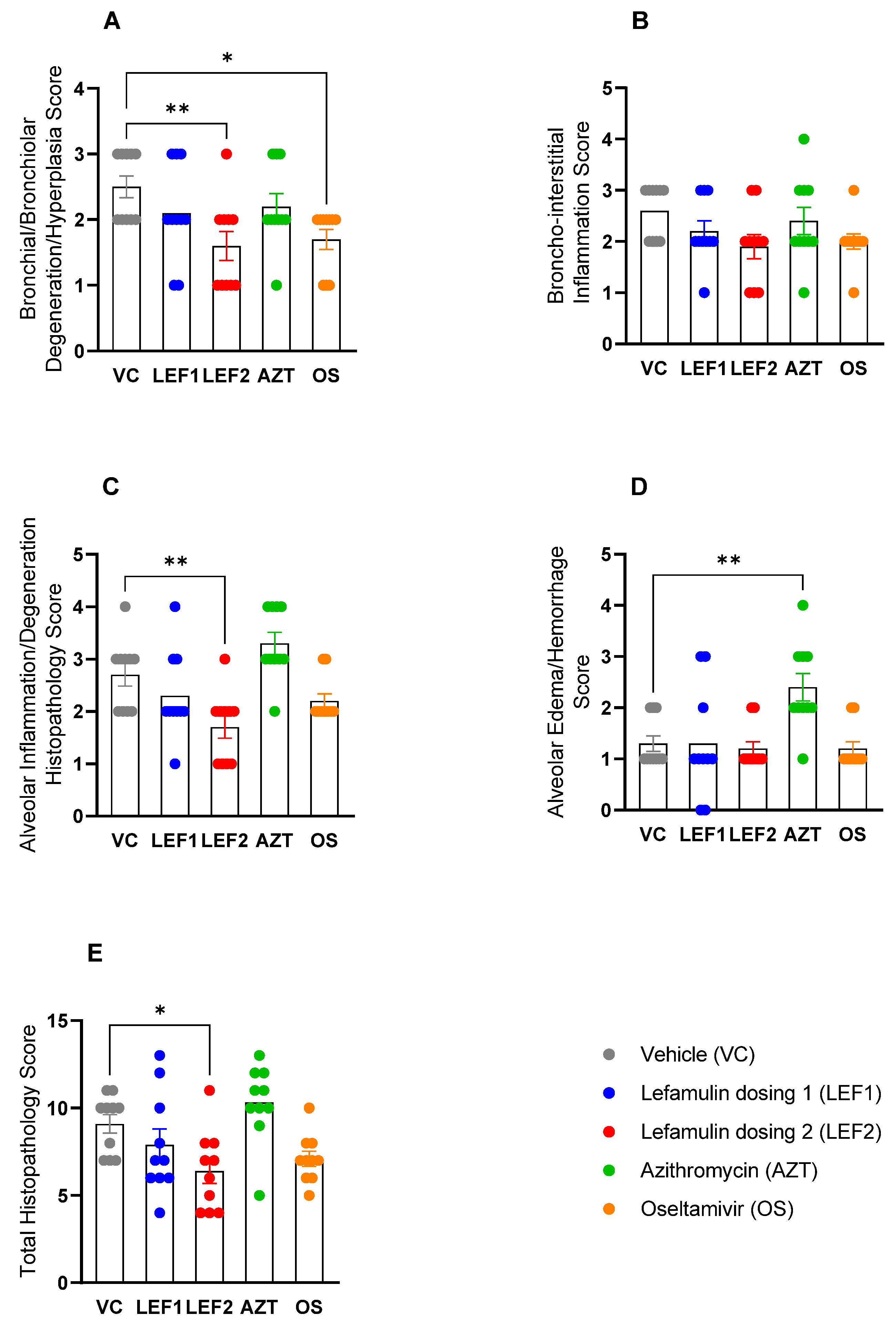
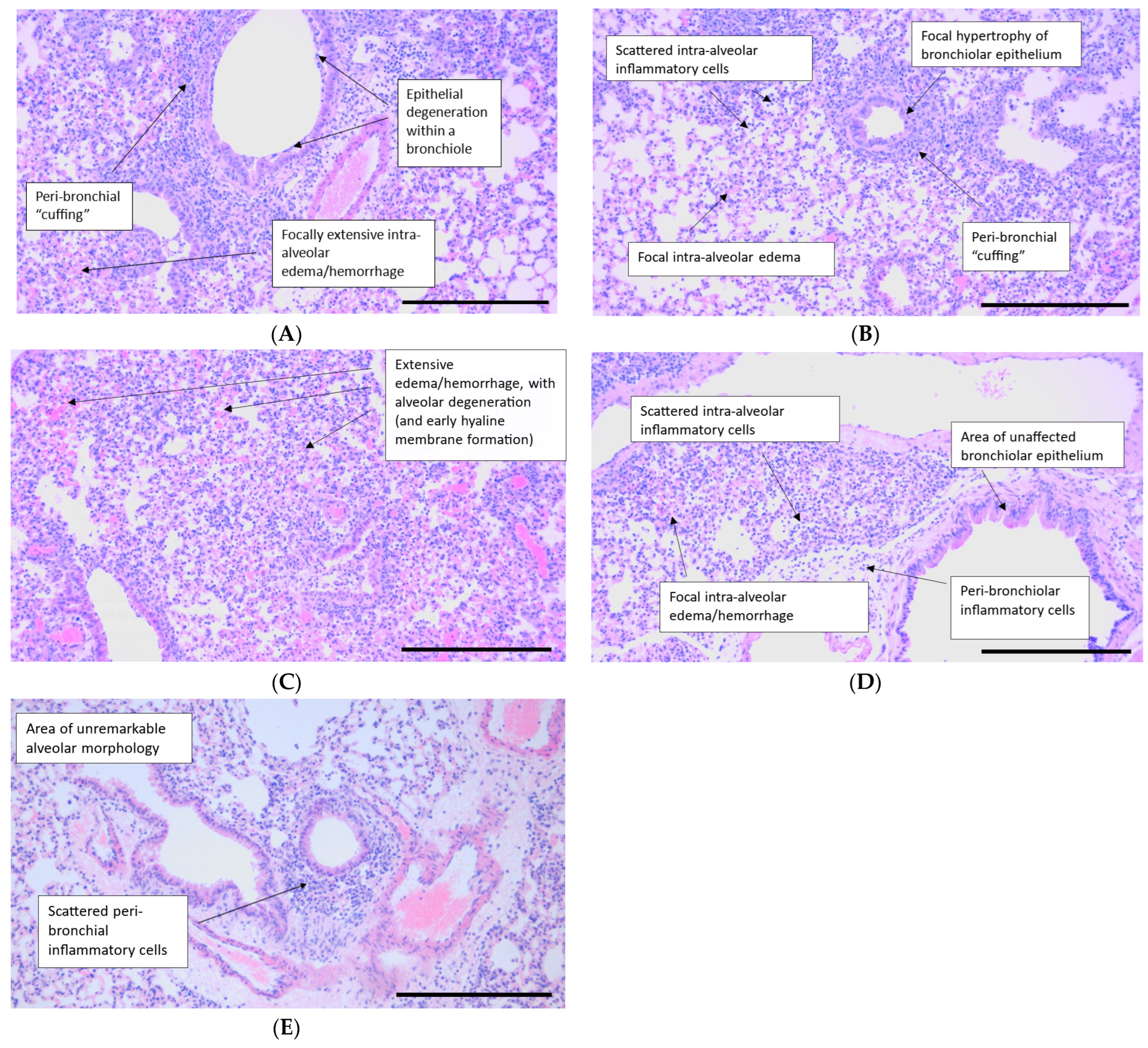
2.6. Histopathology
3. Discussion
4. Materials and Methods
4.1. Test Compounds
4.2. ARDS Model—Experimental Outline and Viral Infection
4.3. Treatment
4.4. Quantification of Viral Load (TCID50 Assay)
4.5. Flow Cytometry Analysis of BALF
4.6. Cytokine Analysis (BALF and Plasma)
4.7. Statistics
4.8. Histopathology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.-H.; Yang, Z.-Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef]
- Bacci, M.; Leme, R.; Zing, N.; Murad, N.; Adami, F.; Hinnig, P.; Feder, D.; Chagas, A.; Fonseca, F. IL-6 and TNF-α serum levels are associated with early death in community-acquired pneumonia patients. Braz. J. Med. Biol. Res. 2015, 48, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zuo, X.; Zhang, S.; Ouyang, Z.; Jiang, S.; Wang, F.; Wang, G. The Mechanism behind Influenza Virus Cytokine Storm. Viruses 2021, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Kroeze, E.J.B.V.; Fouchier, R.A.M.; Kuiken, T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014, 14, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Ashbaugh, D.; Bigelow, D.B.; Petty, T.; Levine, B. Acute respiratory distress in adults. Lancet 1967, 2, 319–323. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef]
- Mathur, P.; Kottilil, S. Immunomodulatory therapies for COVID-19. Front. Med. 2022, 9, 921452. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.; Peukert, K.; Putensen, C.; Bode, C. Antibiotics as immunomodulators: A potential pharmacologic approach for ARDS treatment. Eur. Respir. Rev. 2021, 30, 210093. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; A Bonomo, R.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin. Infect. Dis. 2023, 2023, ciad428. [Google Scholar] [CrossRef] [PubMed]
- Kostinov, M.P.; Gainitdinova, V.V.; Kazharova, S.V.; E Vlasenko, A.; Polishchuk, V.B.; Mashilov, K.V. Use of immunomodulatory therapy as part of comprehensive treatment of non-severe community-acquired pneumonia and its long-term results. Drugs Context 2023, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.R.; José, R.J. Current and emerging evidence for immunomodulatory therapy in community-acquired pneumonia. Ann. Res. Hosp. 2017, 1, 33. [Google Scholar] [CrossRef]
- Kournoutou, G.G.; Dinos, G. Azithromycin through the Lens of the COVID-19 Treatment. Antibiotics 2022, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Rosolowski, M.; Oberle, V.; Ahnert, P.; Creutz, P.; Witzenrath, M.; Kiehntopf, M.; Loeffler, M.; Suttorp, N.; Scholz, M. Dynamics of cytokines, immune cell counts and disease severity in patients with community-acquired pneumonia—Unravelling potential causal relationships. Cytokine 2020, 136, 155263. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R.; Fine, J.; Krichevsky, A.; Delude, R.L.; Angus, D.C.; et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007, 167, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Menendez, R.; Reyes, S.; Polverino, E.; Cilloniz, C.; Martinez, A.; Esquinas, C.; Filella, X.; Ramirez, P.; Torres, A. Factors associated with inflammatory cytokine patterns in community-acquired pneumonia. Eur. Respir. J. 2011, 37, 393–399. [Google Scholar] [CrossRef]
- Summers, C.; Singh, N.R.; White, J.F.; Mackenzie, I.M.; Johnston, A.; Solanki, C.; Balan, K.K.; Peters, A.M.; Chilvers, E.R. Pulmonary retention of primed neutrophils: A novel protective host response, which is impaired in the acute respiratory distress syndrome. Thorax 2014, 69, 623–629. [Google Scholar] [CrossRef]
- Kwon, J.W.; Quan, H.; Song, J.; Chung, H.; Jung, D.; Hong, J.J.; Na, Y.R.; Seok, S.H. Liposomal Dexamethasone Reduces A/H1N1 Influenza-Associated Morbidity in Mice. Front. Microbiol. 2022, 13, 845795. [Google Scholar] [CrossRef] [PubMed]
- Endeman, H.; Meijvis, S.C.A.; Rijkers, G.T.; van Velzen-Blad, H.; van Moorsel, C.H.M.; Grutters, J.C.; Biesma, D.H. Systemic cytokine response in patients with community-acquired pneumonia. Eur. Respir. J. 2011, 37, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, S.P.; Estes, L.L.; Steckelberg, J.M. Non-anti-infective effects of antimicrobials and their clinical applications: A review. Mayo. Clin. Proc. 2015, 90, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Welte, T. Azithromycin: The Holy Grail to Prevent Exacerbations in Chronic Respiratory Disease? Am. J. Respir. Crit. Care Med. 2019, 200, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Xenleta. 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xenleta (accessed on 12 May 2024).
- Food and Drug Administration (FDA). XENLETA (Lefamulin) Injection, for Intravenous Use; XENLETA (Lefamulin) Tablets, for Oral Use. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211672s000,211673s000lbl.pdf (accessed on 12 May 2024).
- Adhikary, S.; Duggal, M.K.; Nagendran, S.; Chintamaneni, M.; Tuli, H.S.; Kaur, G. Lefamulin: A New Hope in the Field of Community-Acquired Bacterial Pneumonia. Curr. Pharmacol. Rep. 2022, 8, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Eraikhuemen, N.; Julien, D.; Kelly, A.; Lindsay, T.; Lazaridis, D. Treatment of Community-Acquired Pneumonia: A Focus on Lefamulin. Infect. Dis. Ther. 2021, 10, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Paukner, S.; Goldberg, L.; Alexander, E.; Das, A.F.; Heinrich, S.; Patel, P.; Moran, G.J.; Sandrock, C.; File, T.M.; Vidal, J.E.; et al. Pooled microbiological findings and efficacy outcomes by pathogen in adults with community-acquired bacterial pneumonia from the Lefamulin Evaluation Against Pneumonia (LEAP) 1 and LEAP 2 phase 3 trials of lefamulin versus moxifloxacin. J. Glob. Antimicrob. Resist. 2022, 29, 434–443. [Google Scholar] [CrossRef]
- Paukner, S.; Mariano, D.; Das, A.F.; Moran, G.J.; Sandrock, C.; Waites, K.B.; File, T.M. Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials. Antibiotics 2021, 10, 1489. [Google Scholar] [CrossRef]
- Paukner, S.; E Mendes, R.; Arends, S.J.R.; Gassner, G.; Gelone, S.P.; Sader, H.S. 7-Year (2015–21) longitudinal surveillance of lefamulin in vitro activity against bacterial pathogens collected worldwide from patients with respiratory tract infections including pneumonia and characterization of resistance mechanisms. J. Antimicrob. Chemother. 2024, 79, 360–369. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Deng, C.; Zelenitsky, S.; Lawrence, C.K.; Adam, H.J.; Golden, A.; Berry, L.; Schweizer, F.; Zhanel, M.A.; Irfan, N.; et al. Lefamulin: A Novel Oral and Intravenous Pleuromutilin for the Treatment of Community-Acquired Bacterial Pneumonia. Drugs 2020, 81, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Covvey, J.R.; Guarascio, A.J. Clinical use of lefamulin: A first-in-class semisynthetic pleuromutilin antibiotic. J. Intern. Med. 2022, 291, 51–63. [Google Scholar] [CrossRef]
- Prince, W.T.; Ivezic-Schoenfeld, Z.; Lell, C.; Tack, K.J.; Novak, R.; Obermayr, F.; Talbot, G.H. Phase II clinical study of BC-3781, a pleuromutilin antibiotic, in treatment of patients with acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 2013, 57, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Paukner, S.; Gruss, A.; Jensen, J.S. In vitro activity of lefamulin against sexually transmitted bacterial pathogens. Antimicrob. Agents Chemother. 2018, 62, e02380-17. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Chu, X.; Zhang, X.; Song, K.; Jiang, Y.; Yu, L.; Deng, X. Preventive effects of valnemulin on lipopolysaccharide-induced acute lung injury in mice. Inflammation 2010, 33, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Hafner, M.; Paukner, S.; Wicha, W.W.; Hrvačić, B.; Cedilak, M.; Faraho, I.; Gelone, S.P. Anti-inflammatory activity of lefamulin versus azithromycin and dexamethasone in vivo and in vitro in a lipopolysaccharide-induced lung neutrophilia mouse model. PLoS ONE 2021, 16, e0237659. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Feng, H.; Xiong, H.; Zhang, L.; Song, Y.; Yu, L.; Deng, X. Valnemulin downregulates nitric oxide, prostaglandin E2, and cytokine production via inhibition of NF-kappaB and MAPK activity. Int. Immunopharmacol. 2009, 9, 810–816. [Google Scholar] [CrossRef]
- Andrewes, C.; Laidlaw, P.; Smith, W. The susceptibility of mice to the viruses of human and swine influenza. Lancet 1934, 224, 859–862. [Google Scholar] [CrossRef]
- Sidwell, R.W. The mose model of influenza virus infection. In Handbook of Animal Models of Infection: Experimental Models in Antimicrobial Chemotherapy; Zak, O.S., Sande, M.A., Eds.; Academic Press: London, UK, 1999; Chapter 118; p. 981. [Google Scholar]
- Jia, X.; Liu, B.; Bao, L.; Lv, Q.; Li, F.; Li, H.; An, Y.; Zhang, X.; Cao, B.; Wang, C. Delayed oseltamivir plus sirolimus treatment attenuates H1N1 virus-induced severe lung injury correlated with repressed NLRP3 inflammasome activation and inflammatory cell infiltration. PLoS Pathog. 2018, 14, e1007428. [Google Scholar] [CrossRef]
- Fukushi, M.; Ito, T.; Oka, T.; Kitazawa, T.; Miyoshi-Akiyama, T.; Kirikae, T.; Yamashita, M.; Kudo, K. Serial Histopathological examination of the lungs of mice infected with influenza a virus PR8 strain. PLoS ONE 2011, 6, e21207. [Google Scholar] [CrossRef] [PubMed]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.-A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Wang, I.-H.; Fukuyama, S.; Katsura, H.; Lopes, T.J.d.S.; Neumann, G.; Kawaoka, Y. In vivo imaging of the pathophysiological changes and neutrophil dynamics in influenza virus-infected mouse lungs. Proc. Natl. Acad. Sci. USA 2018, 115, E6622–E6629. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.L. Animal models for the study of influenza pathogenesis and therapy. Antivir. Res. 2009, 82, A110–A122. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Wong, M.-H.; Bailey, K.W.; Sidwell, R.W. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza a (H1N1) and B viruses. Antivir. Chem. Chemother. 2006, 17, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, H.; Fan, L.; Ma, Y.; Sun, Y.; Pu, J.; Yang, J.; Qiao, J.; Ma, G.; Liu, J. Acute respiratory distress syndrome induced by a swine 2009 H1N1 variant in mice. PLoS ONE 2012, 7, e29347. [Google Scholar] [CrossRef] [PubMed]
- Wicha, W.W.; Strickmann, D.B.; Paukner, S. Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae. J. Antimicrob. Chemother. 2019, 74, III11–III18. [Google Scholar] [CrossRef] [PubMed]
- Azoulay-Dupuis, E.; Vallée, E.; Bedos, J.P.; Muffat-Joly, M.; Pocidalo, J.J. Prophylactic and therapeutic activities of azithromycin in a mouse model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 1991, 35, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA) ZITHROMAX (Azithromycin Tablets and Azithromycin for Oral Suspension). 1991. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf (accessed on 12 May 2024).
- Singlas, E. Clinical pharmacokinetics of azithromycin. Pathol. Biol. 1995, 43, 505–511. [Google Scholar]
- Parker, J.C.; Townsley, M.I. Evaluation of lung injury in rats and mice. Am. J. Physiol. Cell. Mol. Physiol. 2004, 286, L231–L246. [Google Scholar] [CrossRef]
- Tran, D.H.; Sugamata, R.; Hirose, T.; Suzuki, S.; Noguchi, Y.; Sugawara, A.; Ito, F.; Yamamoto, T.; Kawachi, S.; Akagawa, K.S.; et al. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J. Antibiot. 2019, 72, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Califano, D.; Furuya, Y.; Metzger, D.W. Effects of Influenza on Alveolar Macrophage Viability Are Dependent on Mouse Genetic Strain. J. Immunol. 2018, 201, 134–144. [Google Scholar] [CrossRef]
- Clementi, N.; Ghosh, S.; De Santis, M.; Castelli, M.; Criscuolo, E.; Zanoni, I.; Clementi, M.; Mancini, N. Viral Respiratory Pathogens and Lung Injury. Clin. Microbiol. Rev. 2021, 34, e00103-20. [Google Scholar] [CrossRef]
- Fage, C.; Pizzorno, A.; Rhéaume, C.; Abed, Y.; Boivin, G. The combination of oseltamivir with azithromycin does not show additional benefits over oseltamivir monotherapy in mice infected with influenza A(H1N1)pdm2009 virus. J. Med. Virol. 2017, 89, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- McCormick, T.S.; Hejal, R.B.; Leal, L.O.; Ghannoum, M.A. GM-CSF: Orchestrating the Pulmonary Response to Infection. Front. Pharmacol. 2021, 12, 735443. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.M.; Lee, K.M.-C.; Teijaro, J.R.; Becher, B.; Hamilton, J.A. GM-CSF-based treatments in COVID-19: Reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020, 20, 507–514. [Google Scholar] [CrossRef]
- Dolgachev, V.A.; Yu, B.; Sun, L.; Shanley, T.P.; Raghavendran, K.; Hemmila, M.R. Interleukin 10 overexpression alters survival in the setting of gram-negative pneumonia following lung contusion. Shock 2014, 41, 301–310. [Google Scholar] [CrossRef]
- Liu, C.-H.; Kuo, S.-W.; Ko, W.-J.; Tsai, P.-R.; Wu, S.-W.; Lai, C.-H.; Wang, C.-H.; Chen, Y.-S.; Chen, P.-L.; Liu, T.-T.; et al. Early measurement of IL-10 predicts the outcomes of patients with acute respiratory distress syndrome receiving extracorporeal membrane oxygenation. Sci. Rep. 2017, 7, 1021. [Google Scholar] [CrossRef]
- Antunes, G.; Evans, S.; Lordan, J.; Frew, A. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur. Respir. J. 2002, 20, 990–995. [Google Scholar] [CrossRef]
- Verma, A.K.; Bauer, C.; Palani, S.; Metzger, D.W.; Sun, K. IFN-gamma Drives TNF-alpha Hyperproduction and Lethal Lung Inflammation during Antibiotic Treatment of Postinfluenza Staphylococcus aureus Pneumonia. J. Immunol. 2021, 207, 1371–1376. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]


| Group | Group Size (N) | Dose and Regimen | Daily Dose (mg/kg) | Treatment Days |
|---|---|---|---|---|
| Vehicle control (WFI) | 15 | BID 0 mg/kg SC | 0 | Days −1 to 6 |
| Lefamulin (Regimen 1) | 15 | BID 35 mg/kg SC | 70 | Days −1 to 6 |
| Lefamulin (Regimen 2) | 15 | TID 35 mg/kg SC | 105 | Days −1 to 2 |
| for 4 days followed by | ||||
| BID 70 mg/kg SC a | 140 | Days 3 to 6 | ||
| Azithromycin | 15 | BID 15 mg/kg SC | 30 | Days −1 to 0 |
| BID 15 mg/kg IP b | 30 | Days 1 to 6 | ||
| Oseltamivir | 15 | QD 20 mg/kg PO | 20 | Days −1 to 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paukner, S.; Kimber, S.; Cumper, C.; Rea-Davies, T.; Sueiro Ballesteros, L.; Kirkham, C.; Hargreaves, A.; Gelone, S.P.; Richards, C.; Wicha, W.W. In Vivo Immune-Modulatory Activity of Lefamulin in an Influenza Virus A (H1N1) Infection Model in Mice. Int. J. Mol. Sci. 2024, 25, 5401. https://doi.org/10.3390/ijms25105401
Paukner S, Kimber S, Cumper C, Rea-Davies T, Sueiro Ballesteros L, Kirkham C, Hargreaves A, Gelone SP, Richards C, Wicha WW. In Vivo Immune-Modulatory Activity of Lefamulin in an Influenza Virus A (H1N1) Infection Model in Mice. International Journal of Molecular Sciences. 2024; 25(10):5401. https://doi.org/10.3390/ijms25105401
Chicago/Turabian StylePaukner, Susanne, Sandra Kimber, Charlotte Cumper, Tina Rea-Davies, Lorena Sueiro Ballesteros, Christopher Kirkham, Adam Hargreaves, Steven P. Gelone, Claire Richards, and Wolfgang W. Wicha. 2024. "In Vivo Immune-Modulatory Activity of Lefamulin in an Influenza Virus A (H1N1) Infection Model in Mice" International Journal of Molecular Sciences 25, no. 10: 5401. https://doi.org/10.3390/ijms25105401
APA StylePaukner, S., Kimber, S., Cumper, C., Rea-Davies, T., Sueiro Ballesteros, L., Kirkham, C., Hargreaves, A., Gelone, S. P., Richards, C., & Wicha, W. W. (2024). In Vivo Immune-Modulatory Activity of Lefamulin in an Influenza Virus A (H1N1) Infection Model in Mice. International Journal of Molecular Sciences, 25(10), 5401. https://doi.org/10.3390/ijms25105401







