Gut Microbiota Profile Changes in Patients with Inflammatory Bowel Disease and Non-Alcoholic Fatty Liver Disease: A Metagenomic Study
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Gut Microbiota Analysis and Comparisons
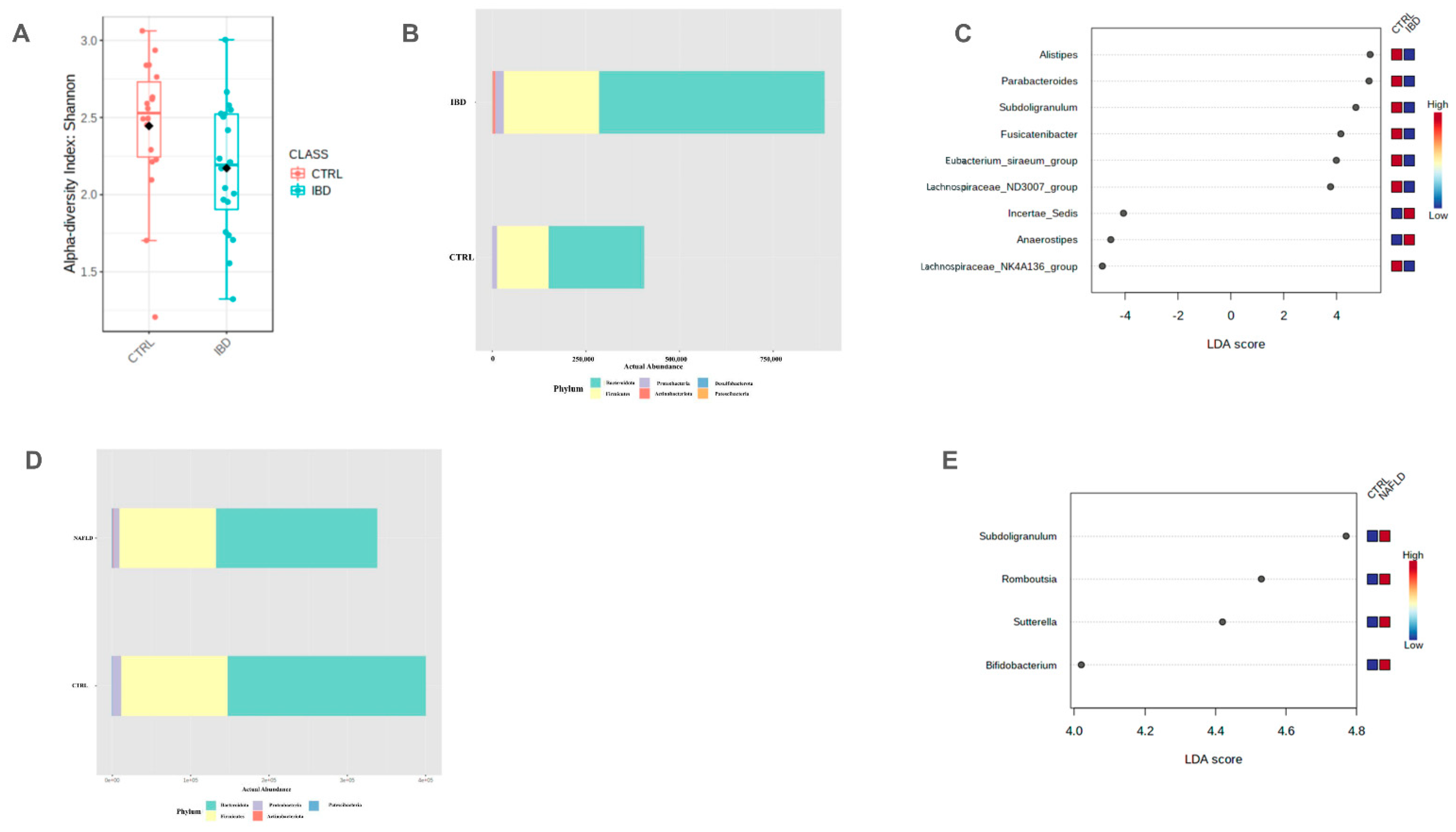
2.2.1. Characteristics of Microbiota Composition in IBD-Only Group
2.2.2. Characteristics of Microbiota Composition in NAFLD-Only Group
2.2.3. Specific Microbiota Signature in the IBD-NAFLD Group
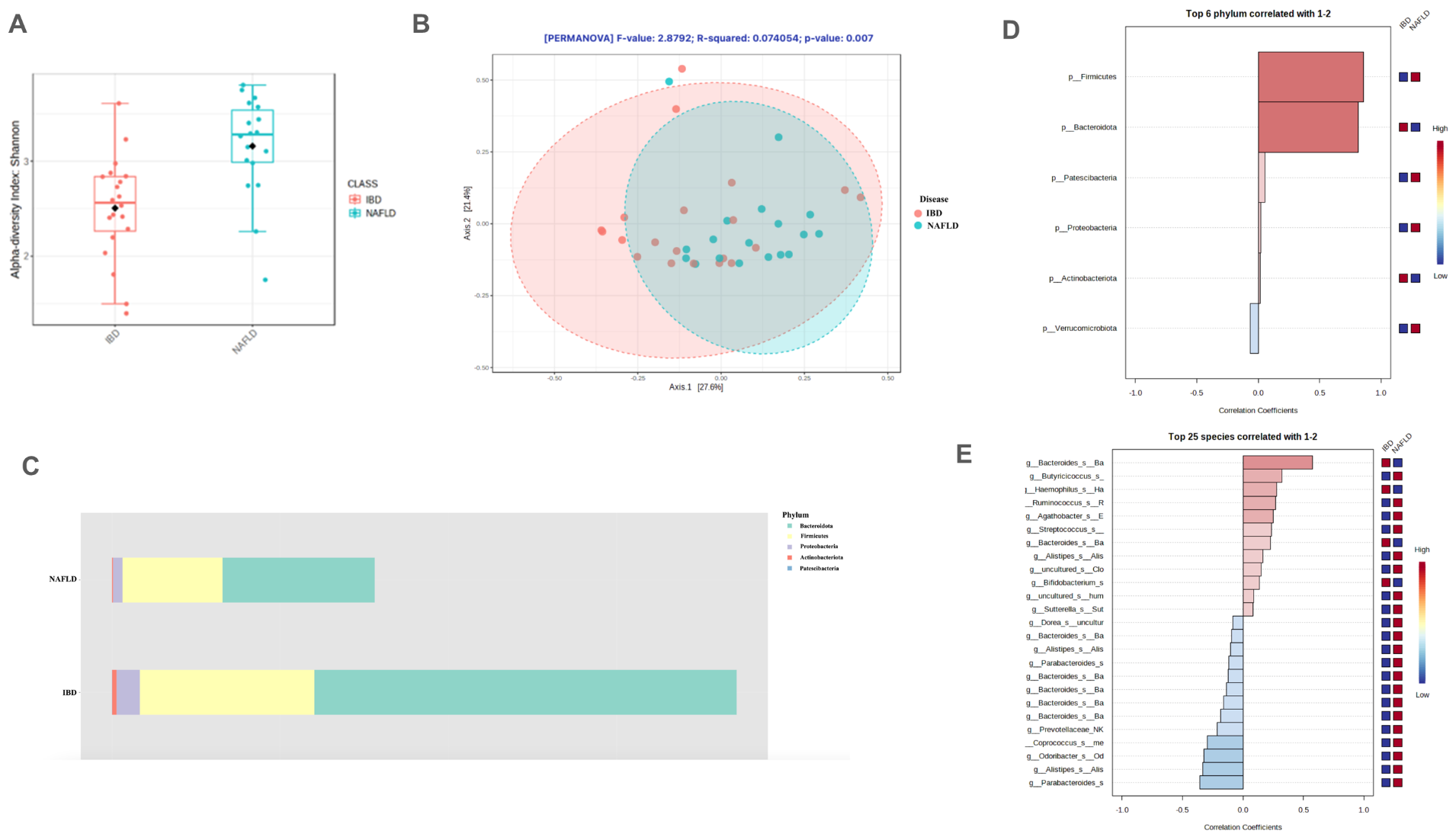
2.2.4. Differences in Microbiota Composition between IBD-Only and NAFLD-Only Groups
2.2.5. Differences in Microbiota Composition between NAFLD-Only and IBD-NAFLD Groups
2.2.6. Differences in Microbiota Composition between IBD-Only and IBD-NAFLD Group
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Study Cohort Recruitment
4.3. Clinical Evaluation and Metabolic Assessment
4.4. Oral Examination
4.5. IBD Patients’ Assessment
4.6. NAFLD Diagnosis
4.7. Next-Generation Sequencing (NGS) of Gut Microbiota
4.8. Sequences Analysis
4.9. Bioinformatics Analysis
4.10. Gut Microbiota Analysis and Comparisons
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert. Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gardet, A.; Xavier, R. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Lo, B.C.; Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.; Reitmeier, S.; Haller, D. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Hyun, C.-K. Molecular and Pathophysiological Links between Metabolic Disorders and Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 9139. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, M.; Jemmieh, K.; Ouda, A.; Haider, M.Z.; Malki, M.I.; Elzouki, A.N. Metabolic-associated fatty liver disease: A selective review of pathogenesis, diagnostic approaches, and therapeutic strategies. Front. Med. 2024, 11, 1291501. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Beheshti Maal, A.; Shahrbaf, M.A.; Sadri, B.; Hossein-Khannazer, N.; Mansournia, M.A.; Vosough, M. Prevalence of hepatobiliary manifestations in inflammatory bowel disease: A GRADE assessed systematic review and meta-analysis on more than 1.7 million patients. J. Crohn’s Colitis 2023, 18, jjad157. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Gutiérrez-Ramírez, L.; Tejera-Muñoz, A.; Arias, Á.; Lucendo, A.J. Systematic Review and Meta-Analysis: Prevalence of Non-Alcoholic Fatty Liver Disease and Liver Fibrosis in Patients with Inflammatory Bowel Disease. Nutrients 2023, 15, 4507. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; De Bonis, D.; Pagnotta, R.; Cosco, C.; Cosco, V.; Montalcini, T.; Pujia, A.; Doldo, P.; Spagnuolo, R. Ulcerative Colitis as an Independent Risk Factor for Hepatic Steatosis. Gastroenterol. Nurs. 2020, 43, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Spagnuolo, R.; Milano, M.; Brogneri, S.; Morrone, A.; Cosco, C.; Lazzaro, V.; Russo, C.; Ferro, Y.; Pingitore, P.; et al. PNPLA3 148M Carriers with Inflammatory Bowel Diseases Have Higher Susceptibility to Hepatic Steatosis and Higher Liver Enzymes. Inflamm. Bowel Dis. 2016, 22, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Sartini, A.; Gitto, S.; Binda, C.; Sbrancia, M.; Coluccio, C.; Sambri, V.; Fabbri, C. The Other Side of Malnutrition in Inflammatory Bowel Disease (IBD): Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 2772. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, R.; Abenavoli, L.; Corea, A.; Larussa, T.; Mancina, R.M.; Cosco, C.; Luzza, F.; Doldo, P. Multifaceted pathogenesis of liver steatosis in inflammatory bowel disease: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5818–5825. [Google Scholar]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Brusnic, O.; Onișor, D.M. Fecal Microbiota Transplantation in Liver Cirrhosis. Biomedicines 2023, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.A.; Gubatan, J. Gut microbiome–based therapeutics in inflammatory bowel disease. Clin. Transl. Disc. 2023, 3, e182. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Tsou, A.M.; Goettel, J.A.; Bao, B.; Biswas, A.; Kang, Y.H.; Redhu, N.S.; Peng, K.; Putzel, G.G.; Saltzman, J.; Kelly, R.; et al. Utilizing a reductionist model to study host-microbe interactions in intestinal inflammation. Microbiome 2021, 9, 215. [Google Scholar] [CrossRef]
- Takeshita, K.; Mizuno, S.; Mikami, Y.; Sujino, T.; Saigusa, K.; Matsuoka, K.; Naganuma, M.; Sato, T.; Takada, T.; Tsuji, H.; et al. A Single Species of Clostridium Subcluster XIVa Decreased in Ulcerative Colitis Patients. Inflamm. Bowel Dis. 2016, 22, 2802–2810. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Tang, J.; Mo, Q.; Zhong, H.; Zhang, H.; Feng, F. A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: By changing gut barrier. Eur. J. Nutr. 2021, 60, 2317–2330. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Wang, J.; Wu, G.; Long, W.; Xue, Z.; Wang, L.; Zhang, X.; Pang, X.; Zhao, Y.; et al. Accelerated dysbiosis of gut microbiota during aggravation of DSS-induced colitis by a butyrate-producing bacterium. Sci. Rep. 2016, 6, 27572. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Hsu, C.; Singh, S.; Bassirian, S.; Kolar, J.; Faulkner, C.; Sinha, N.; Bettencourt, R.; Gara, N.; Valasek, M.A.; et al. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment. Pharmacol. Ther. 2019, 49, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Fernández, M.; Goikoetxea-Usandizaga, N.; Porras, D.; García-Mediavilla, M.V.; Bravo, M.; Serrano-Maciá, M.; Simón, J.; Delgado, T.C.; Lachiondo-Ortega, S.; Martínez-Flórez, S.; et al. Enhanced mitochondrial activity reshapes a gut microbiota profile that delays NASH progression. Hepatology 2023, 77, 1654–1669. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.P.; Yu, H.C.; Lin, W.Y.; Chang, Y.C. The role of microbiome in the pathogenesis of oral-gut-liver axis between periodontitis and nonalcoholic fatty liver disease. J. Dent. Sci. 2023, 18, 972–975. [Google Scholar] [CrossRef]
- Boicean, A.; Birsan, S.; Ichim, C.; Boeras, I.; Roman-Filip, I.; Blanca, G.; Bacila, C.; Fleaca, R.S.; Dura, H.; Roman-Filip, C. Has-miR-129-5p’s Involvement in Different Disorders, from Digestive Cancer to Neurodegenerative Diseases. Biomedicines 2023, 11, 2058. [Google Scholar] [CrossRef]
- Wang, C.; Gu, Y.; Chu, Q.; Wang, X.; Ding, Y.; Qin, X.; Liu, T.; Wang, S.; Liu, X.; Wang, B.; et al. Gut microbiota and metabolites as predictors of biologics response in inflammatory bowel disease: A comprehensive systematic review. Microbiol. Res. 2024, 282, 127660. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Luo, F.; Li, B.; Li, Z.; Guo, Z.; Chen, Z.; Wu, W.; Hu, M. Gut microbiota and metabolic biomarkers in metabolic dysfunction–associated steatotic liver disease. Hepatol. Commun. 2024, 8, e0310. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.-M.; Ofosu, F.K.; Chelliah, R.; Lee, B.H.; Oh, D.-H. Challenges and Perspective in Integrated Multi-Omics in Gut Microbiota Studies. Biomolecules 2021, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health Surveys. Basic Methods, 5th ed.; World Health Organization: Geneva, Switzerland, 2013; ISBN 9789241548649. [Google Scholar]
- Bianco, A.; Mazzea, S.; Fortunato, L.; Giudice, A.; Papadopoli, R.; Nobile, C.G.A.; Pavia, M. Oral Health Status and the Impact on Oral Health-Related Quality of Life among the Institutionalized Elderly Population: A Cross-Sectional Study in an Area of Southern Italy. Int. J. Environ. Res. Public. Health 2021, 18, 2175. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Lorée, H.; Bastard, C.; Miette, V.; Sandrin, L. Vibration-Guided Transient Elastography: A Novel Fibroscan® Examination with Improved Guidance for Liver Stiffness Measurement. Ultrasound Med. Biol. 2020, 46, 2193–2206. [Google Scholar] [CrossRef]
- Sancandi, M.; De Caro, C.; Cypaite, N.; Marascio, N.; Avagliano, C.; De Marco, C.; Russo, E.; Constanti, A.; Mercer, A. Effects of a probiotic suspension Symprove™ on a rat early-stage Parkinson’s disease model. Front. Aging Neurosci. 2023, 14, 986127. [Google Scholar] [CrossRef] [PubMed]



| Group 1 IBD-NAFLD | Group 2 IBD-Only | Group 3 NAFLD-Only | Group 4 CTRLs | p | |
|---|---|---|---|---|---|
| N | 18 | 20 | 18 | 18 | |
| Age (years) | 45 (37, 53) | 39 (24, 45) | 42 (36, 56) | 33 (23, 49) | 0.03 a |
| Gender male n (%) | 14 (78) | 11 (55) | 13 (72) | 11 (61) | 0.44 |
| BMI (Kg/m2) | 28 (25, 33) | 24 (21, 27) | 28 (26, 30) | 23 (21, 25) | 0.001 b |
| median CAP (dB/m) | 280 (263, 323) | 202 (179, 220) | 284 (255, 316) | 206 (185, 237) | 0.001 c |
| median Stiffness (kPa) | 5 (4, 6) | 5.3 (4, 5) | 4.7 (3, 5) | 4.4 (4, 6) | 0.471 |
| Hb (g/dL) | 13.3 (12, 14) | 13.2 (11, 14) | 13.6 (12, 14) | 14.2 (13, 15) | 0.04 d |
| WBC (×103/μL) | 6.4 (5, 7) | 7.6 (5, 9) | 8.5 (6, 9) | 9.1 (7, 10) | 0.002 e |
| PLTs (×103/μL) | 222 (208, 265) | 207 (179, 259) | 203 (183, 244) | 239 (171, 288) | 0.550 |
| CRP (mg/L) | 3 (3, 4) | 4.3 (3, 8) | 3 (3, 4) | 3 (3, 6) | 0.215 |
| ESR (mm/h) | 6.5 (4, 15) | 9 (5, 15) | 15.5 (3, 22) | 8 (4, 13) | 0.685 |
| AST (UI/L) | 24.5 (17, 49) | 16.5 (13, 27) | 26.5 (19, 37) | 17.5 (15, 22) | 0.005 f |
| ALT (UI/L) | 32 (23, 46) | 15.5 (10, 19) | 33 (24, 44) | 15.5 (11, 20) | 0.001 g |
| GGT (UI/L) | 32 (14, 37) | 17 (10, 34) | 25.5 (21, 35) | 14.5 (9, 23) | 0.002 h |
| ALP (UI/L) | 71.5 (60, 87) | 70 (62, 87) | 67 (62, 81) | 63 (49, 72) | 0.211 |
| Tot. Bilirubine (mg/dL) | 0.5 (0.4, 0.9) | 0.4 (0.2, 0.7) | 0.4 (0.3, 0.9) | 0.6 (0.3, 0.8) | 0.138 |
| Dir. Bilirubine (mg/dL) | 0.2 (0.1, 0.2) | 0.1 (0.1, 0.2) | 0.1 (0.1, 0.2) | 0.2 (0.1, 0.3) | 0.160 |
| Tot. Cholesterol (mg/dL) | 185 (152, 204) | 165 (143, 182) | 199 (181, 207) | 193 (147, 205) | 0.003 i |
| HDL (mg/dL) | 46 (39, 52) | 46 (42, 51) | 42 (36, 48) | 51 (43, 63) | 0.04 l |
| LDL (mg/dL) | 110 (93, 145) | 101 (72, 116) | 129 (117, 143) | 121 (80, 136) | 0.001 m |
| Triglycerides (mg/dL) | 87 (69, 94) | 81 (56, 97) | 113 (83, 135) | 78 (53, 117) | 0.107 |
| Glucose (mg/dL) | 90 (83, 96) | 81 (76, 87) | 88 (81, 93) | 81 (73, 85) | 0.004 n |
| Iron (mcg/dL) | 75 (57, 91) | 80 (38, 91) | 86 (54, 112) | 93 (77, 113) | 0.685 |
| Group 1 IBD-NAFLD | Group 2 IBD-Only | p | |
|---|---|---|---|
| N | 18 | 20 | |
| Age (years) | 45 (37, 53) | 39 (24, 45) | 0.03 |
| Gender n male (%) | 14 (78) | 11 (55) | 0.2 |
| BMI (Kg/m2) | 28 (25, 33) | 24 (21, 27) | 0.003 |
| median CAP (dB/m) | 280 (263, 323) | 202 (179, 220) | 0.000 |
| median Stiffness (kPa) | 5 (4, 6) | 5.3 (4, 5) | 0.965 |
| Disease duration (years) | 13 (5, 17) | 5 (2, 11) | 0.017 |
| MAYO Score | 0.5 (0, 1) | 1 (0, 2) | 0.497 |
| Harvey–Bradshaw | 6.5 (3, 7) | 4.5 (0, 9) | 0.9 |
| Disease activity, years n (%) | 1 (5) | 3 (15) | 0.6 |
| CRP (mg/L) | 3 (3, 4) | 4.3 (3, 8) | 0.067 |
| ESR (mm/h) | 6.5 (4, 15) | 9 (5, 15) | 0.426 |
| Calprotectin (mcg/g) | 36 (22, 99) | 82 (16, 383) | 0.264 |
| Disease Characteristics | |||
| IBD to use n (%) | 0.34 | ||
| Crohn’s Disease n (%) | 6 (33) | 10 (50) | |
| Ulcerative Colitis n (%) | 12 (67) | 10 (50) | |
| Disease extension (CD) n (%) | 0.60 | ||
| Ileitis n (%) | 2 (11) | 2 (10) | |
| Ileo-colitis n (%) | 4 (22) | 8 (40) | |
| Disease extension (UC) | 0.86 | ||
| Proctitis n (%) | 1 (5) | 1 (5) | |
| Left colitis n (%) | 1 (5) | 2 (10) | |
| Pancolitis n (%) | 10 (55) | 7 (35) | |
| Treatment | |||
| Mesalamine y n (%) | 15 (83) | 15 (75) | 0.7 |
| Steroids y n (%) | 0 | 3 (15) | 0.23 |
| Azathioprine y n (%) | 0 | 0 | |
| Biologics y n (%) | 7 (39) | 11 (55) | 0.35 |
| Score | Gingival Index, GI | Plaque Index, PI |
|---|---|---|
| 0 | normal gingiva | absence of microbial plaque |
| 1 | mild inflammation (i.e., slight change in color, slight edema, no bleeding on probing) | thin film of microbial plaque along the free gingival margin |
| 2 | moderate inflammation (i.e., redness, edema, and glazing, or bleeding on probing) | moderate accumulation with plaque in the sulcus |
| 3 | severe inflammation (i.e., marked redness and edema, tendency toward spontaneous bleeding, ulceration) | large amount of plaque in sulcus or pocket along the free gingiva margin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Caro, C.; Spagnuolo, R.; Quirino, A.; Mazza, E.; Carrabetta, F.; Maurotti, S.; Cosco, C.; Bennardo, F.; Roberti, R.; Russo, E.; et al. Gut Microbiota Profile Changes in Patients with Inflammatory Bowel Disease and Non-Alcoholic Fatty Liver Disease: A Metagenomic Study. Int. J. Mol. Sci. 2024, 25, 5453. https://doi.org/10.3390/ijms25105453
De Caro C, Spagnuolo R, Quirino A, Mazza E, Carrabetta F, Maurotti S, Cosco C, Bennardo F, Roberti R, Russo E, et al. Gut Microbiota Profile Changes in Patients with Inflammatory Bowel Disease and Non-Alcoholic Fatty Liver Disease: A Metagenomic Study. International Journal of Molecular Sciences. 2024; 25(10):5453. https://doi.org/10.3390/ijms25105453
Chicago/Turabian StyleDe Caro, Carmen, Rocco Spagnuolo, Angela Quirino, Elisa Mazza, Federico Carrabetta, Samantha Maurotti, Cristina Cosco, Francesco Bennardo, Roberta Roberti, Emilio Russo, and et al. 2024. "Gut Microbiota Profile Changes in Patients with Inflammatory Bowel Disease and Non-Alcoholic Fatty Liver Disease: A Metagenomic Study" International Journal of Molecular Sciences 25, no. 10: 5453. https://doi.org/10.3390/ijms25105453
APA StyleDe Caro, C., Spagnuolo, R., Quirino, A., Mazza, E., Carrabetta, F., Maurotti, S., Cosco, C., Bennardo, F., Roberti, R., Russo, E., Giudice, A., Pujia, A., Doldo, P., Matera, G., & Marascio, N. (2024). Gut Microbiota Profile Changes in Patients with Inflammatory Bowel Disease and Non-Alcoholic Fatty Liver Disease: A Metagenomic Study. International Journal of Molecular Sciences, 25(10), 5453. https://doi.org/10.3390/ijms25105453










