Eco-Friendly Inorganic Binders: A Key Alternative for Reducing Harmful Emissions in Molding and Core-Making Technologies
Abstract
1. Introduction
1.1. General Eco-Technological Characteristics of Currently Used Molding and Core Sands
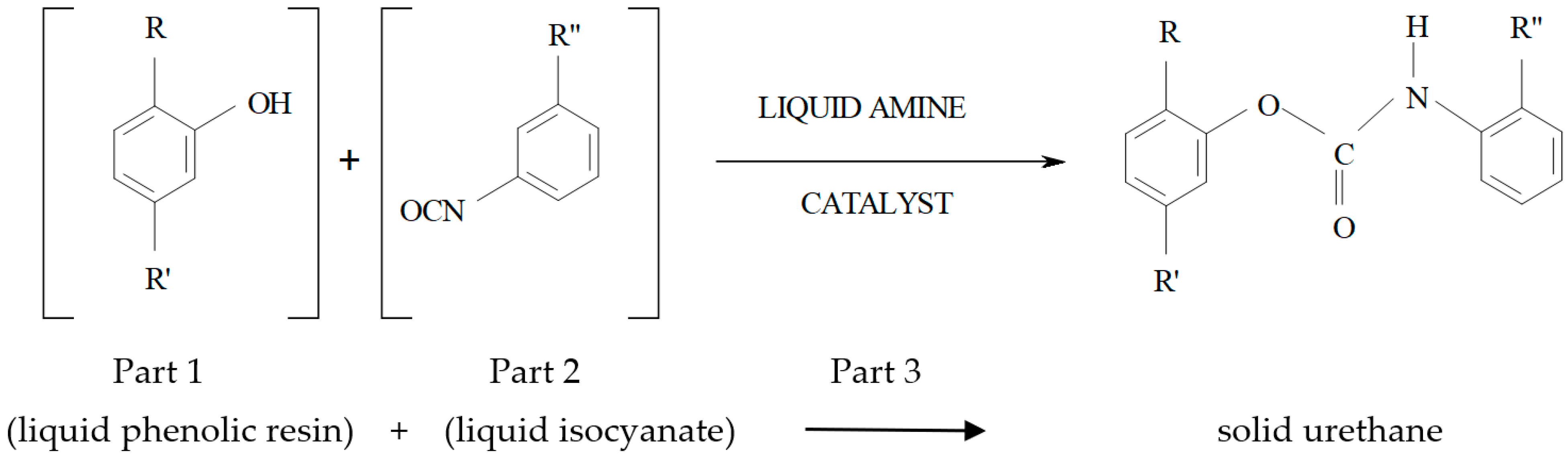
1.2. Mold Sands with Organic Binders
1.3. Mold Sands with Inorganic Binders
2. Results and Discussion
2.1. Volume of Emitted Gases
2.2. Emission of Harmful Gases
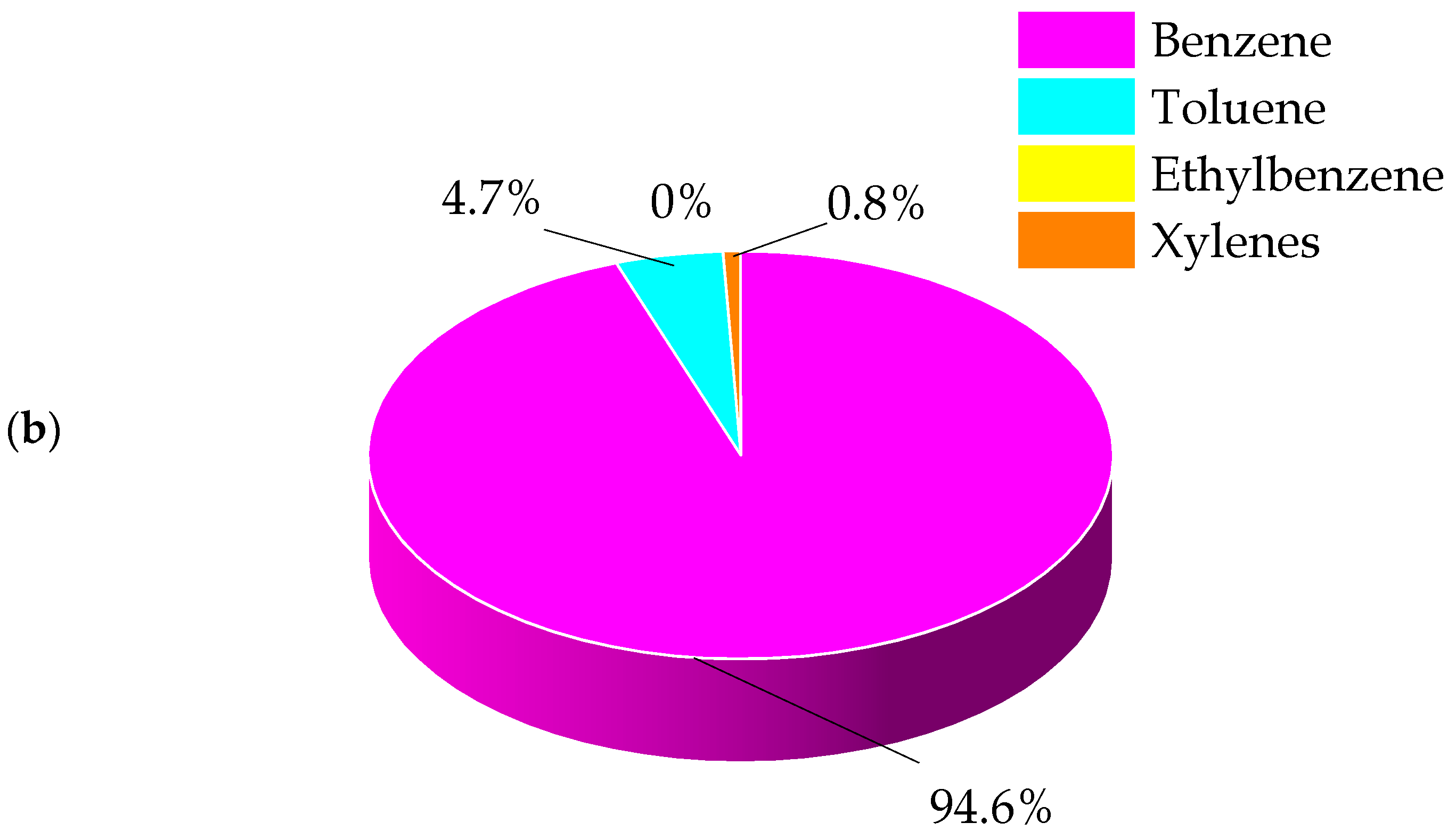
2.2.1. BTEX Emissions
- I red group, code: no. 4, no. 4 + coating—very high content of compounds from the BTEX group.
- II blue group, code: no. 3 + coating, 5, 8, 7, 2—small content of compounds from the BTEX group.
- III green group, code: no. 3, 6, 1—trace content of compounds from the BTEX group.
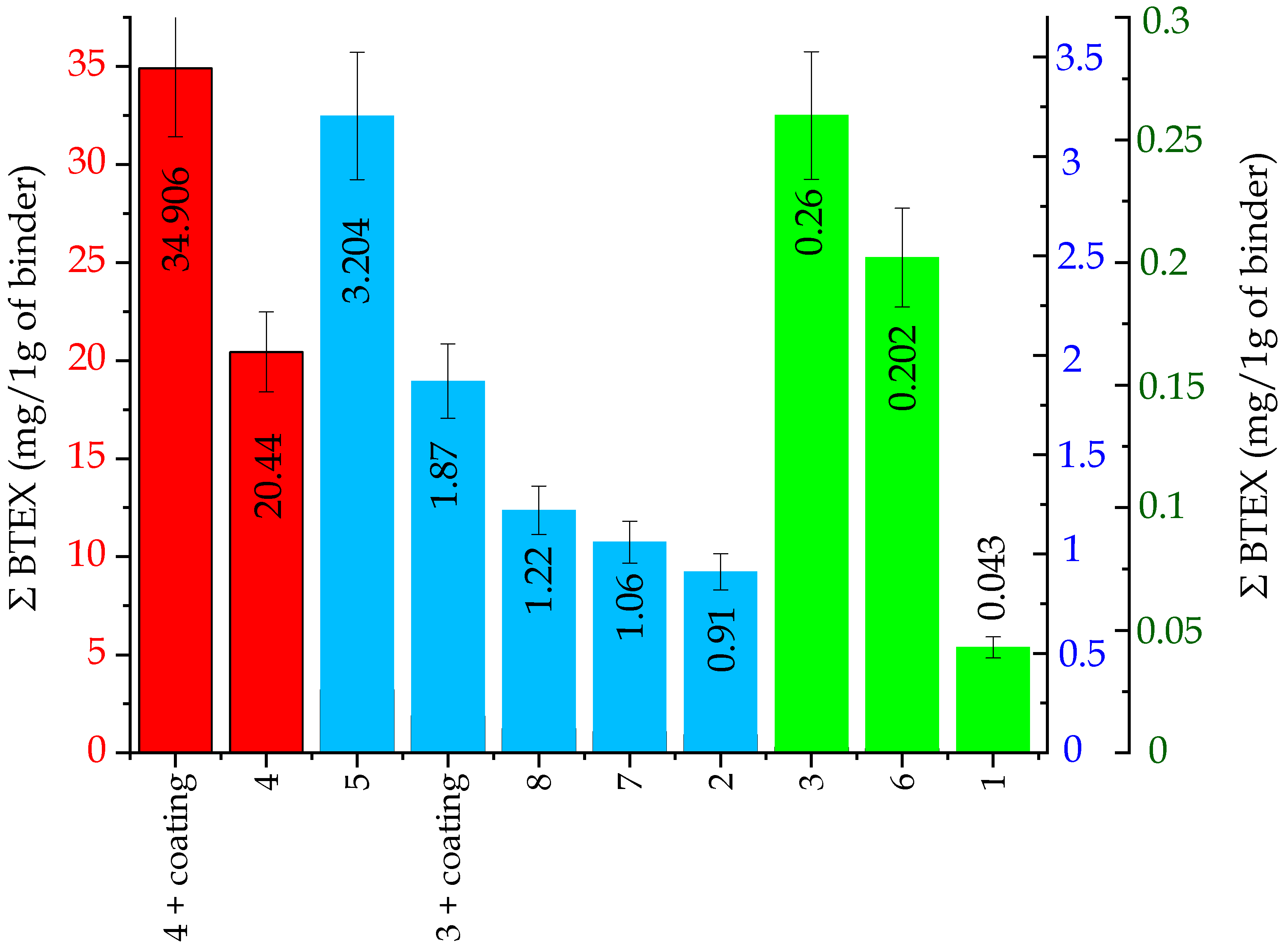
| BTEX | Emission of Individual Substances (mg/per 1 g of a Binder) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molding Sands Code | ||||||||||
| 1 | 2 | 3 | 3 + Coating | 4 | 4 + Coating | 5 | 6 | 7 | 8 | |
| Benzene | 0.042 | 0.881 | 0.256 | 1.793 | 19.338 | 32.286 | 3.163 | 0.199 | 1.038 | 1.203 |
| Toluene | 0.001 | 0.017 | 0.003 | 0.004 | 0.952 | 0.514 | 0.041 | 0.002 | 0.021 | 0.000 |
| Ethylbenzene | < | 0.010 | 0.002 | 0.073 | 0.003 | 1.574 | < | 0.001 | 0.002 | 0.016 |
| Xylenes | < | 0.003 | 0.000 | 0.007 | 0.154 | 0.532 | < | 0.000 | 0.002 | 0.000 |
| Σ BTEX | 0.043 | 0.912 | 0.261 | 1.877 | 20.446 | 34.906 | 3.204 | 0.202 | 1.064 | 1.220 |
| the value of +/− 10% on account | 0.004 | 0.091 | 0.026 | 0.188 | 2.045 | 3.491 | 0.320 | 0.020 | 0.106 | 0.122 |
2.2.2. PAHs Emissions
2.2.3. Emission of Other Harmful Organic Compounds
Phenol Emission
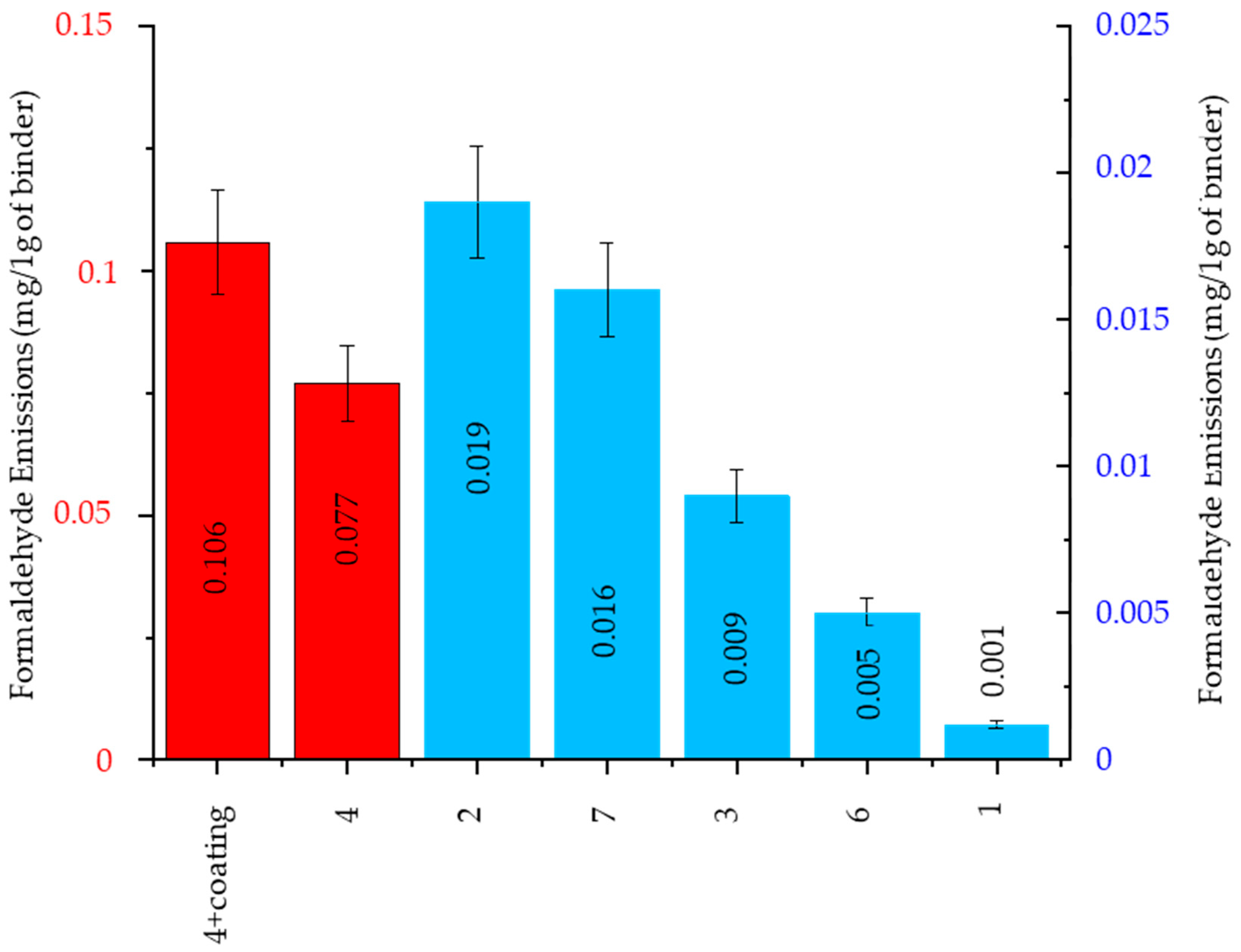
Formaldehyde Emission
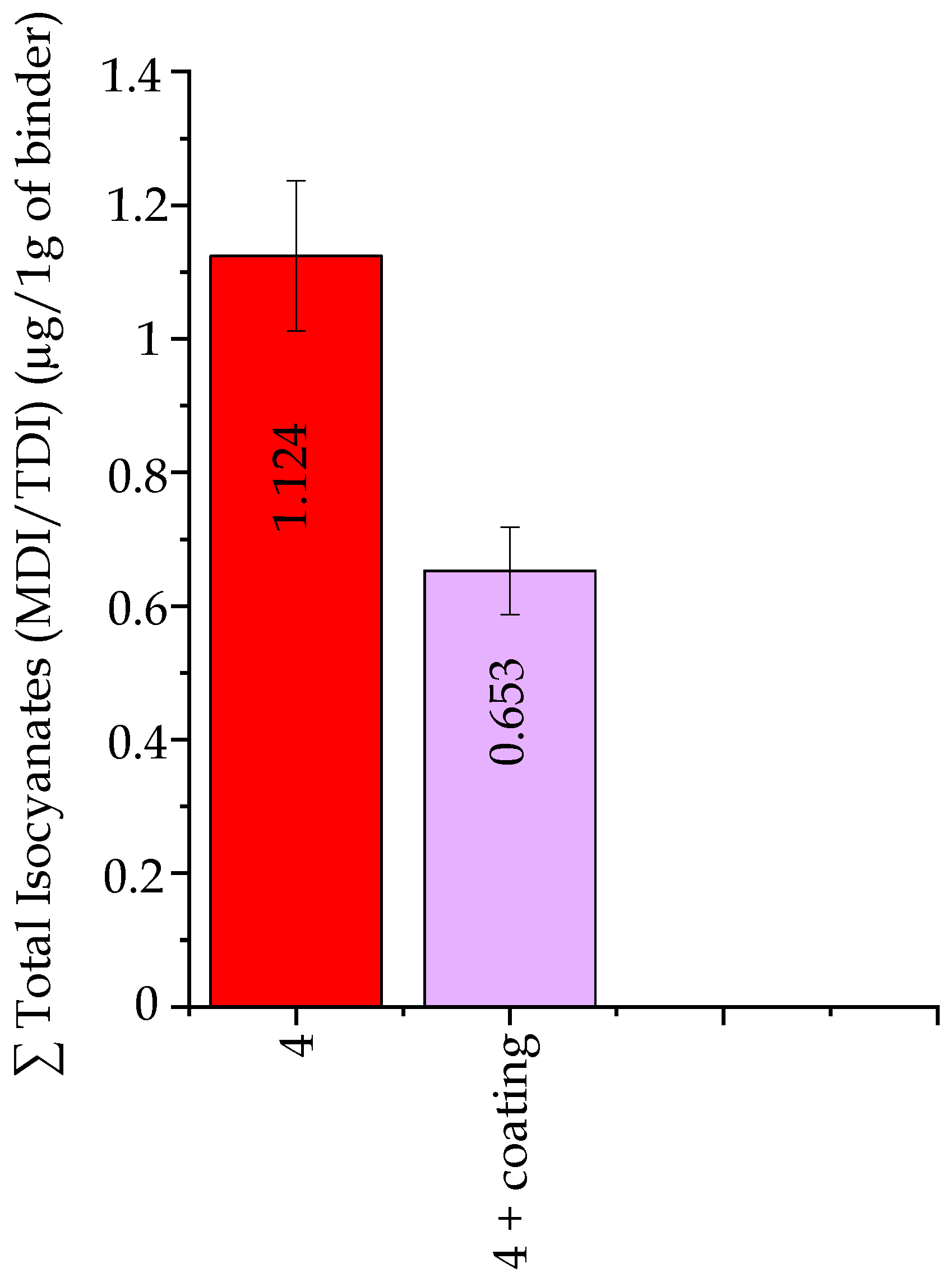
Isocyanates Emission ∑ MDI/TDI
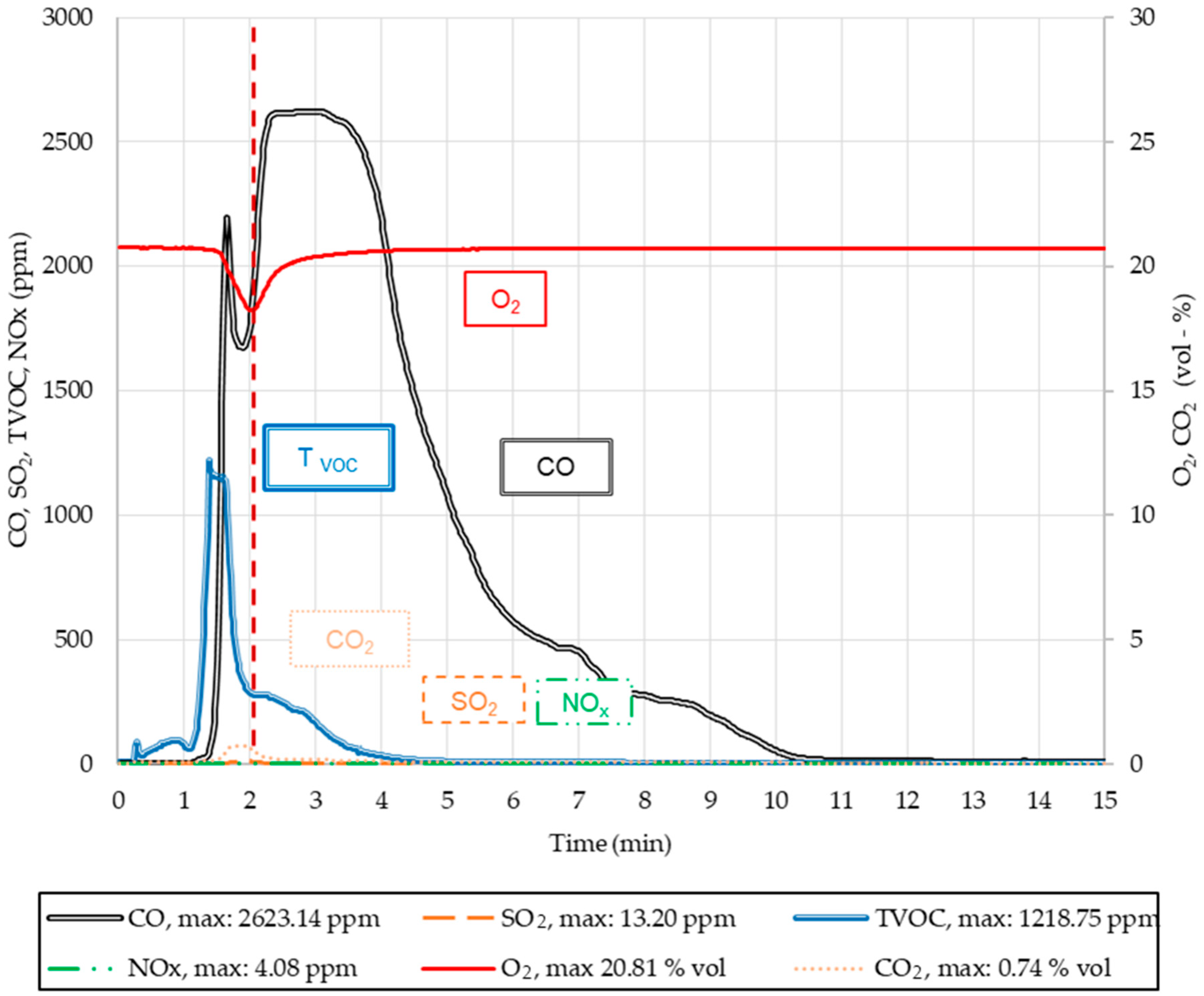
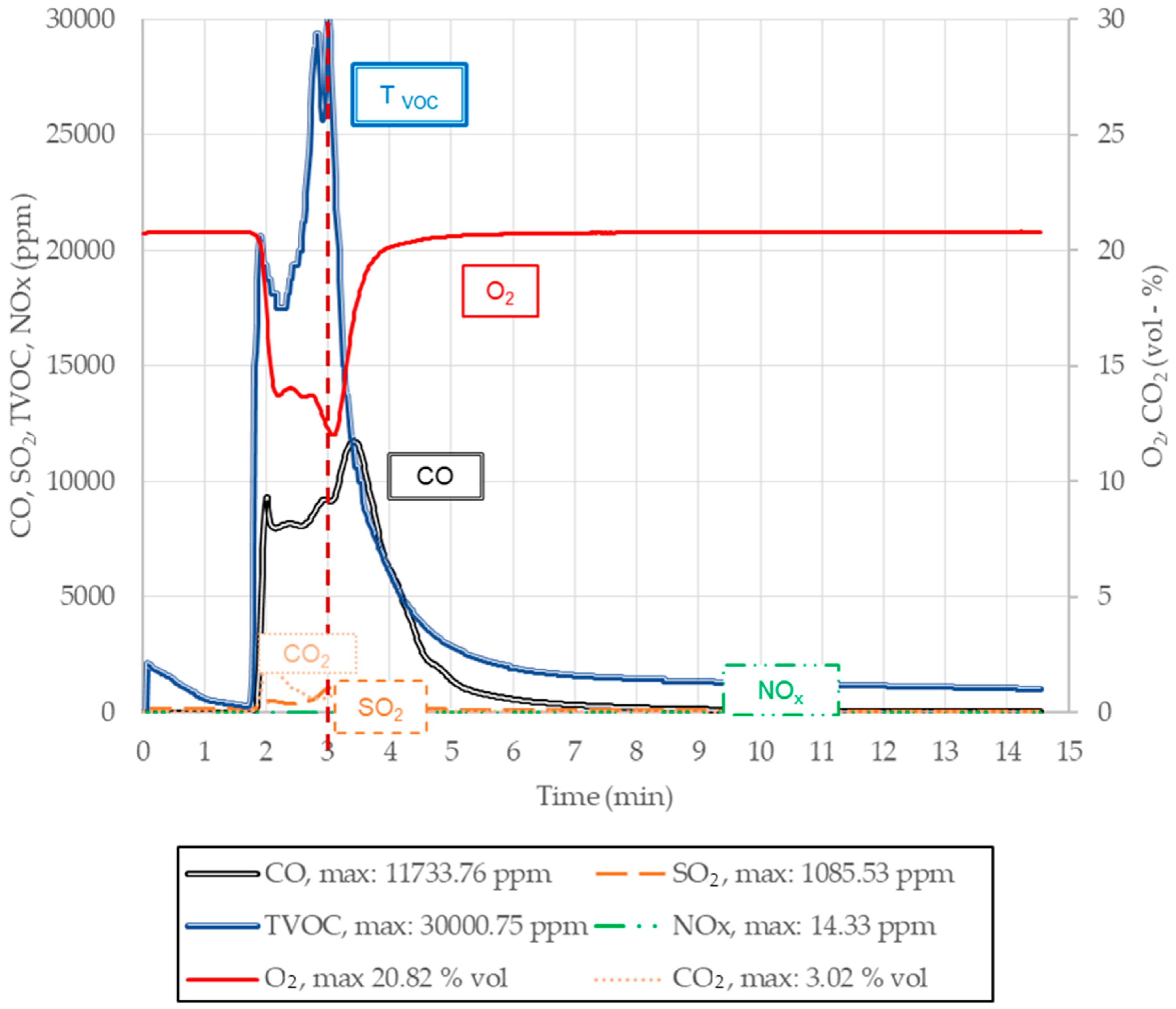
2.2.4. Greenhouse Gases CO, CO2, NOx, SO2, and Tvoc Emissions
| Molding Sand Code | Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O2 (vol %) | CO (ppm) | CO2 (vol %) | NOx (ppm) | SO2 (ppm) | Tvoc (ppm) | |||||||
| !Max Value | $tmax (min) | !Max Value | $tmax (min) | !Max Value | $tmax (min) | !Max Value | $tmax (min) | !Max Value | $tmax (min) | !Max Value | $tmax (min) | |
| 1 | 20.80 | 3.0 | 2620 | 3.0 | 0.74 | 1.8 | 4.08 | 2.0 | 13.2 | 2.0 | 1220 | 1.3 |
| 2 | 20.80 | 2.2 | 2540 | 1.85 | 5.19 | 1.0 | 8.63 | 1.0 | 132 | 1.0 | 10,200 | 0.8 |
| 3 | 20.90 | 5.0 | 11,800 | 2.5 | 3.98 | 1.5 | 11.80 | 1.7 | 280 | 1.6 | 14,400 | 1.3 |
| 4 | 20.80 | 5.0 | 11,700 | 3.5 | 3.02 | 2.0 | 14.30 | 3.0 | 1080 | 3.0 | 30,000 | 0.6 |
| 4 + coating | 21.10 | 3.0 | 10,100 | 1.8 | 2.37 | 0.8 | 2.30 | 1.1 | 207 | 1.1 | 22,400 | 1.2 |
| 5 | 20.90 | 4.0 | 2610 | 3.2 | 1.4 | 1.2 | 9.71 | 0.8 | 205 | 0.8 | 11,300 | 0.5 |
| 6 | 20.80 | 4.0 | 2660 | 4.0 | 3.61 | 1.0 | 7.16 | 1.1 | 169 | 1.1 | 8880 | 0.5 |
| 7 | 20.70 | 3.0 | 2560 | 2.1 | 2.90 | 1.5 | 8.57 | 1.8 | 75.7 | 1.8 | 9000 | 1.0 |
| 8 | 21.10 | 2.2 | 10,000 | 3.0 | 1.82 | 1.1 | 1.35 | 1.1 | 171 | 1.1 | 21,900 | 0.6 |
2.2.5. Emissions of Particulate Matter PM 2.5 and PM 10
| Molding Sand Code | PM 2.5 & % Share | PM 10 & % Share | Total Weight of Dust (mg/1 g of a Binder) |
|---|---|---|---|
| 1 | 19–49.6 | 70.8–100 | 0.484 |
| 2 | 60.7–62.8 | 79.1–91.0 | 0.703 |
| 3 | 30.5–68.9 | 79.2–99.7 | 0.246 |
| 3 + coating | 20.4–46.5 | 53.9–99.5 | 0.160 |
| 4 | 21.6–30.5 | 65.6–89.6 | 7.511 |
| 4 + coating | 5.8–43.7 | 14.2–100 | 5.137 |
| 5 | 20.4–76.3 | 53.9–100 | 1.105 |
| 6 | 28.4–44.6 | 77.8–99.6 | 0.448 |
| 7 | 11.6–40.3 | 70.6–100 | 0.681 |
| 8 | 2.7–57.6 | 2.9–100 | 0.169 |
3. Materials and Methods
3.1. Research Materials
3.2. Methods
3.2.1. Collection of Gas Samples for BTEX, PAHs, Phenol, Formaldehyde, and MDI/TDI Analysis
- Sorption tubes (during the BTEX, phenol, or formaldehyde measurements).
- Polyurethane foam (PUF) (during the PAHs measurement).
- Special quartz filters (during MDI/TDI measurements).
3.2.2. Collection of Gas Samples for CO, CO2, NOx, SO2, and Tvoc Analysis
3.2.3. Collection of Dust Samples for Determination of Particulate Matter PM 2.5 and PM 10
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holtzer, M.; Kmita, A. Mold and Core Sands in Metalcasting: Chemistry and Ecology. Sustainable Development; Springer Nature: Cham, Switzerland, 2020; ISBN 978-3-030-53209-3. [Google Scholar]
- Major-Gabryś, K. Environmentally Friendly Foundry Molding and Core Sands. J. Mater. Eng. Perform. 2019, 28, 3905–3911. [Google Scholar] [CrossRef]
- Anwar, N.; Jalava, K.; Orkas, J. Experimental Study of Inorganic Foundry Sand Binders for Mold and Cast Quality. Int. J. Met. 2023, 17, 1697–1714. [Google Scholar] [CrossRef]
- Lewandowski, J.L. Materials for Foundry Moulds; AKAPIT: Krakow, Poland, 1997; ISBN 9788371080210. (In Polish) [Google Scholar]
- Holtzer, M.; Dańko, R.; Kmita, A.; Drożyński, D.; Kubecki, M.; Skrzyński, M.; Roczniak, A. Environmental impact of the reclaimed sand addition to molding sand with furan and phenol-formaldehyde resin—A comparison. Materials 2020, 13, 4395. [Google Scholar] [CrossRef] [PubMed]
- CAEF—The European Foundry Association—Commission for Economics & Statistics. The European Foundry Industry 2020; CAEF: Düsseldorf, Germany, 2020. [Google Scholar]
- Sawai, H.; Rahman, I.M.M.; Fujita, M.; Jii, N.; Wakabayashi, T.; Begum, Z.A.; Maki, T.; Mizutani, S.; Hasegawa, H. Decontamination of metal-contaminated waste foundry sands using an EDTA–NaOH–NH3 washing solution. Chem. Eng. J. 2016, 296, 199–208. [Google Scholar] [CrossRef]
- Picard, F.; Chaouki, J. Selective extraction of heavy metals from two real calcium-rich contaminated soils by a modified NTA. J. Hazard. Mater. 2016, 318, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Li, Z.; Liu, H.; Chen, J.; Gao, J. Predicting air pollutant emissions of the foundry industry: Based on the electricity big data. Sci. Total Environ. 2024, 917, 170323. [Google Scholar] [CrossRef]
- Yu, B.; Yuan, Z.; Yu, Z.; Xue-song, F. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar] [CrossRef]
- Khodaei, K.; Nassery, H.R.; Asadi, M.M.; Mohammadzadeh, H.; Mahmoodlu, M.G. BTEX biodegradation in contaminated groundwater using a novel strain (Pseudomonas sp. BTEX-30). Int. Biodeterior. Biodegrad. 2017, 116, 234–242. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, Y.; Zhao, X.; Chen, Z.; Zhang, S.; Zhu, Z.; Zhou, Y.; Yang, Y.; Qi, J.; Li, J. Efficient removal of VOCs emission from soil thermal desorption via MnCoOx/Kaolin activating peroxymonosulfate in wet scrubber. Chem. Eng. J. 2024, 480, 148159. [Google Scholar] [CrossRef]
- Khararoodi, M.G.; Zhu, J.; Lee, C.-S.; Niu, J.; Haghighat, F. Dynamic modelling of removal of binary mixtures of VOCs from indoor air through a carbon-based filter. Chem. Eng. J. 2023, 471, 144792. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, X.; Shi, J.; Ren, Y.; Yao, J.; Lin, F.; Qian, J.; Ling, W.; Guan, A.; Wang, W.; et al. Method for evaluating the resource, energy, and environmental impact of the casting fault rectification process in patternless sand castings. Sustain. Mater. Technol. 2023, 35, e00565. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, A.; Yao, J.; Ren, Y.; Zheng, W.; Lin, F.; Shi, J.; Guan, A.; Wang, W. Combination method of multiple molding technologies for reducing energy and carbon emission in the foundry industry. Sustain. Mater. Technol. 2022, 34, e00522. [Google Scholar] [CrossRef]
- Trivedi, J.; Majumdar, D. Memory effect driven emissions of persistent organic pollutants from industrial thermal processes, their implications and management: A review. J. Environ. Manag. 2013, 119, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.; Yang, H.; Chen, H. Ambient BTEX and MTBE in the neighborhoods of different industrial parks in Southern Taiwan. J. Hazard. Mater. 2006, 128, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Filho, W. Risk assessment of chemicals in foundries: The International Chemical Toolkit pilot-project. J. Hazard. Mater. 2006, 136, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Dańko, R.; Kmita, A.; Holtzer, M.; Dańko, J.; Lehmhus, D.; Tapola, S. Development of inorganic binder systems to minimise emissions in ferrous foundries. Sustain. Mater. Technol. 2023, 37, e00666. [Google Scholar] [CrossRef]
- Holtzer, M.; Dańko, R.; Piasny, S.; Kubecki, M.; Drożyński, D.; Roczniak, A.; Skrzyński, M.; Kmita, A. Research on the release of dangerous compounds from the BTEX and PAHs groups in industrial casting conditions. Materials 2021, 14, 2581. [Google Scholar] [CrossRef] [PubMed]
- Kmita, A.; Benko, A.; Roczniak, A.; Holtzer, M. Evaluation of pyrolysis and combustion products from foundry binders: Potential hazards in metal casting. J. Therm. Anal. Calorim. 2020, 140, 2347–2356. [Google Scholar] [CrossRef]
- Holtzer, M.; Kwaśniewska-Królikowska, D.; Bobrowski, A.; Dańko, R.; Grabowska, B.; Żymankowska-Kumon, S.; Solarski, W. Investigations of a harmful components emission from moulding sands with bentonite and lustrous carbon carriers when in contact with liquid metals. Foundry J. Pol. Foundrymen’s Assoc. 2012, 62, 124–132. (In Polish) [Google Scholar]
- Holtzer, M.; Górny, M.; Kmita, A. The influence of motorisation on the climate warming. Int. J. Glob. Warm. 2017, 11, 495–514. [Google Scholar] [CrossRef]
- Zhao, X.; Ning, Z.; Li, Z.; Zou, W.; Li, B.; Huang, Y.; Cao, F.; Sun, J. Evolved gas analysis of PEP-SET sand by TG and FTIR. J. Anal. Appl. Pyrolysis 2017, 127, 490–495. [Google Scholar] [CrossRef]
- Gieniec, A.; Hurkes, N.; Peter, V.; Dieckhues, H. PEP SET—An efficient and environmentally friendly binder system. Foundry Trade J. 2015, 344–345. [Google Scholar]
- Khan, M.M.; Mahajani, S.M.; Jadhav, G.N.; Vishwakarma, R.; Malgaonkar, V.; Mandre, S. Mechanical and thermal methods for reclamation of waste foundry sand. J. Environ. Manag. 2021, 279, 111628. [Google Scholar] [CrossRef] [PubMed]
- Mhamane, D.A.; Rayjadhav, S.B.; Shinde, V.D. Analysis of chemically bonded sand used for molding in foundry. Asian J. Sci. Appl. Technol. 2018, 7, 11–16. [Google Scholar] [CrossRef]
- Pilato, L. Phenolic Resins: A Century of Progress; Springer: Jersey City, NJ, USA, 2010. [Google Scholar]
- Marć, M.; Bielawska, M.; Simeonov, V.; Namieśnik, J.; Zabiegała, B. The effect of anthropogenic activity on BTEX, NO2, SO2, and CO concentrations in urban air of the spa city of Sopot and medium-industrialized city of Tczew located in North Poland. Environ. Res. 2016, 147, 513–524. [Google Scholar] [CrossRef]
- Lochte, K.; Bochm, R. Properties and experience of an inorganic binder. Foundry Trade J. 2006, 180, 28–30. [Google Scholar]
- Green Casting LIFE Project (2022–2026). Towards Zero Emissions in European Ferrous Foundries Using Inorganic Binder Systems. Reference: LIFE21-ENV-FI-GREEN-CASTING-LIFE/101074439|Acronym: LIFE21-ENV-FI-GREEN CASTING LIFE. Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/LIFE21-ENV-FI-GREEN-CASTING-LIFE-101074439/towards-zero-emissions-in-european-ferrous-foundries-using-inorganic-binder-systems (accessed on 13 January 2024).
- Dańko, R.; Holtzer, M.; Kmita, A.; Lehmhus, D.; Tapola, S. Reduction of emission in ferrous foundries by using inorganic binder systems. In Proceedings of the 74th World Foundry Congress, Busan, Republic of Korea, 16–21 October 2022. [Google Scholar]
- EU. Report on the Implementation of the Project: Inorganic Binder System to Minimize Emissions, Improve Indoor Air Quality, Purify and Reuse of Contaminated Foundry Sand. Green Foundry LIFE Project (LIFE17 ENV/FI/000173); EU: Tampere, Finland, 2022. [Google Scholar]
- Holtzer, M.; Dańko, J.; Lewandowski, J.L.; Solarski, W.; Dańko, R.; Grabowska, B.; Bobrowski, A.; Żymankowska-Kumon, S.; Sroczyński, A.; Różycki, A.; et al. Station for Research of the Volume and Harmfulness of Gases Compounds from the Materials Used in Foundry and Metallurgical Processes. Polish Patent PL 224705 B1, 31 January 2017. [Google Scholar]
- Dańko, R. Criteria for an advanced assessment of quality of moulding sands with organic binders and reclamation process products. China Founrdy 2013, 10, 181–186. [Google Scholar]
- PN-EN 14789:2017-04; Emissions from Stationary Sources—Determination of Oxygen Volume Concentration—Standard Reference Method: Paramagnetism. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN 14792:2017-04; Emissions from Stationary Sources—Determination of Mass Concentration of Nitrogen Oxides—Standard Reference Method: Chemiluminescence. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN 15058:2017-04; Emissions from Stationary Sources—Determination of Carbon Monoxide Mass Concentration—Standard Reference Method: Non-Dispersive Infrared Spectrometry. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-ISO 10396:2001; Emissions from Stationary Sources—Sampling for Automatic Measurement of Gaseous Component Concentrations. Polish Committee for Standardization: Warsaw, Poland, 2001.
- PN-EN 12619:2013-05; Emissions from Stationary Sources—Determination of Mass Concentration of Total Gaseous Organic Carbon—Continuous Measurement Method with Flame Ionization Detection. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Cheng, Y.-H.; Chao, Y.-C.; Wu, C.-H.; Tsai, C.-J.; Uang, S.-N.; Shih, T.-S. Measurements of ultrafine particle concentrations and size distribution in an iron foundry. J. Hazard. Mater. 2008, 158, 124–130. [Google Scholar] [CrossRef]
- ISO 13320:2009; Particle Size Analysis—Laser Diffraction Methods. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 14488:2007; Particulate Materials—Sampling and Sample Splitting for the Determination of Particulate Properties. International Organization for Standardization: Geneva, Switzerland, 2007.

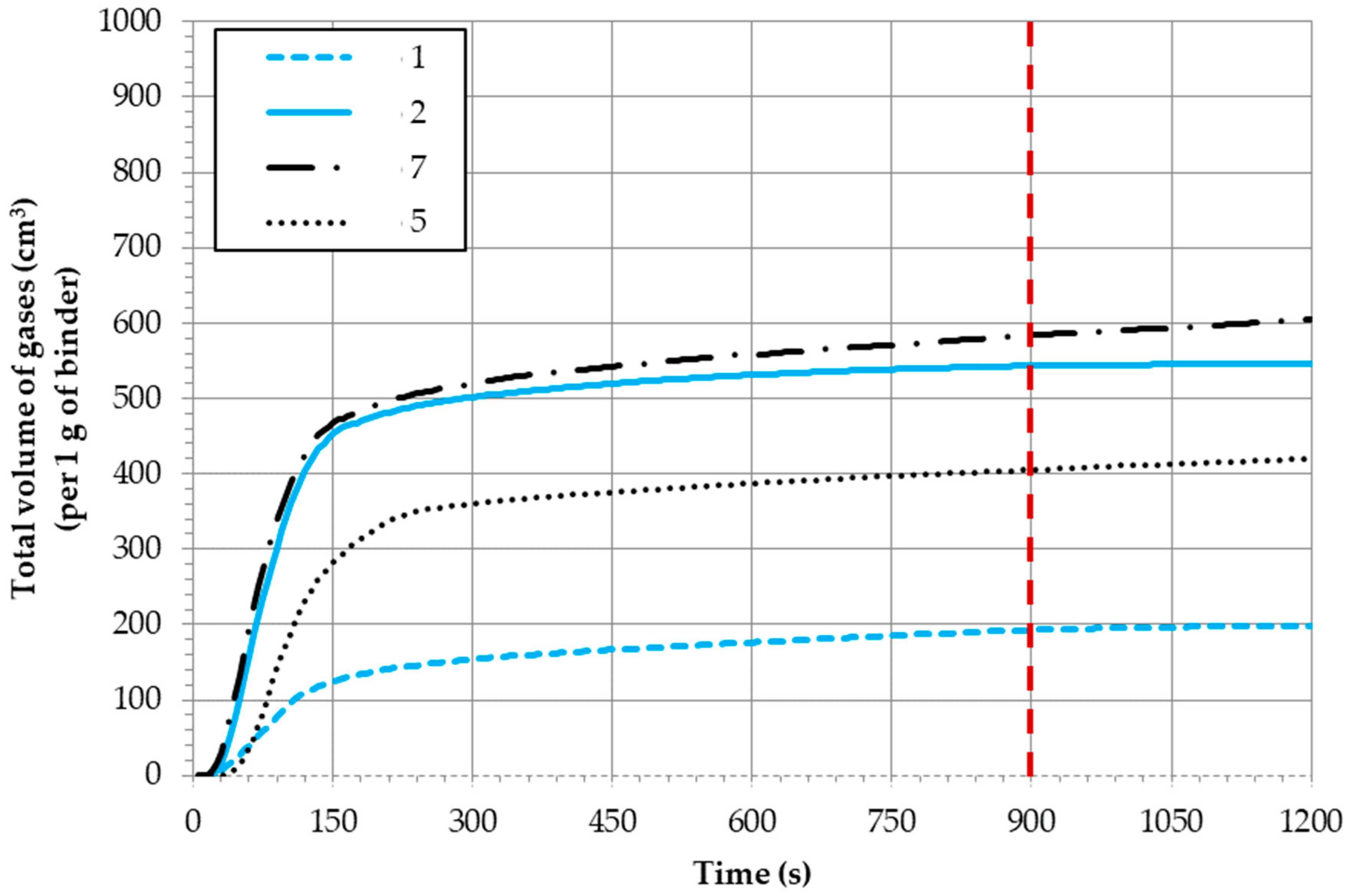









| Molding Sand Code | ^ V900 s (cm3/1 g Binder) | Max. Gas Release Rate (cm3/s) (per 1 g of Binder) | ^^ Tmax. (s) |
|---|---|---|---|
| 1 | 192.41 | 1.55 | 50–95 |
| 2 | 543.01 | 5.59 | 60 |
| 3 | 508.51 | 5.71 | 60 |
| 3 + coating | 375.16 | 5.96 | 35 |
| 4 | 855.16 | 9.74 | 55 |
| 4 + coating | 855.83 | 10.05 | 65 |
| 5 | 405.55 | 4.75 | 80 |
| 6 | 537.07 | 7.78 | 55 |
| 7 | 583.97 | 6.42 | 55 |
| 8 | 341.70 | 3.70 | 10 |
| PAHs | Emission of Individual Substances (µg/1 g of Binder) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molding Sand Code | ||||||||||
| 1 | 2 | 3 | 3 + Coating | 4 | 4 + Coating | 5 | 6 | 7 | 8 | |
| Naphthalene | 21.541 | 63.878 | 22.465 | 93.307 | 375.127 | 1866.723 | 121.096 | 19.251 | 67.072 | 70.438 |
| Acenaphthene | 0.204 | 0.328 | 0.459 | 0.631 | 0.918 | 9.344 | 1.416 | 0.318 | 0.273 | 0.303 |
| Fluorene | 0.250 | 0.564 | 0.801 | 1.879 | 9.988 | 50.097 | 0.907 | 0.375 | 0.463 | 0.499 |
| Phenanthrene | 1.979 | 3.271 | 3.195 | 4.428 | 56.612 | 571.015 | 1.458 | 1.398 | 3.246 | 2.655 |
| Anthracene | 5.354 | 3.555 | 3.678 | 6.209 | 29.453 | 175.056 | 8.804 | 4.103 | 4.359 | 2.630 |
| Fluoranthene | 0.445 | 2.103 | 0.243 | < | 131.342 | 249.146 | 1.735 | 0.266 | 2.396 | < |
| Pyrene | 0.339 | 3.371 | 0.304 | < | 106.345 | 191.701 | 4.213 | 0.265 | 3.343 | 0.683 |
| ** Benzo[a]anthracene | 0.057 | 0.617 | 1.233 | 0.219 | 16.296 | 61.852 | 0.833 | 0.048 | 1.697 | 0.068 |
| ** Chrysene | 0.767 | 0.403 | 7.230 | 0.459 | 7.692 | 63.392 | 0.911 | 0.584 | 7.631 | 0.903 |
| ** Benzo[b]fluoranthene | 0.096 | 1.322 | 0.109 | 1.102 | 30.961 | 64.230 | 7.439 | 0.082 | 1.754 | 0.204 |
| ** Benzo[k]fluoranthene | 0.016 | 0.531 | 0.030 | 0.339 | 12.055 | 28.627 | 8.927 | 0.021 | 0.594 | 0.053 |
| ** Benzo(a)pyrene | 1.054 | 1.500 | 1.029 | 1.819 | 34.132 | 64.511 | 30.129 | 1.514 | 2.180 | 0.838 |
| ** Dibenz[a,h]anthracene | 0.031 | 0.763 | 0.075 | 0.477 | 18.577 | 24.514 | 2.083 | 0.031 | 0.921 | 0.094 |
| ** Benzo[g,h,i]perylene | 0.249 | 2.159 | 0.480 | 2.707 | 40.888 | 34.531 | 23.433 | 0.141 | 2.542 | 0.580 |
| ** Indeno [1,2,3c,d]pyrene | 0.084 | 1.562 | 0.159 | 1.611 | 34.289 | 40.499 | 3.583 | 0.049 | 1.886 | 0.275 |
| Σ PAHs | 32.464 | 85.927 | 41.490 | 115.189 | 904.675 | 3495.238 | 216.967 | 28.449 | 100.358 | 80.223 |
| the value of +/− 20% on account | 6.493 | 17.185 | 8.298 | 23.038 | 180.935 | 699.048 | 43.393 | 5.690 | 20.072 | 16.045 |
| Molding Sand Code | Technology of Molding Sand | Type of Hardened Agent | Type of Protective Coating |
|---|---|---|---|
| 1 | Mold sand with inorganic binder (geopolymer) | warm box technology | - |
| 2 | Mold sand with inorganic binder (geopolymer) | CO2 | - |
| 3 | Mold sand with inorganic binder (silicate) | ester (no-bake technology) | - |
| 3 + coating | water-based coating type A | ||
| * 4 | Mold sand with organic binder | amine (PUNB no-bake technology) | - |
| * 4 + coating | water-based coating type A | ||
| 5 | Mold sand with inorganic binder (silicate) | warm box technology | - |
| 6 | Mold sand with inorganic binder (silicate) | ester (no-bake technology) | - |
| 7 | Mold sand with inorganic binder (silicate) | CO2 | - |
| 8 | Mold sand with inorganic binder (silicate) | ester (no-bake technology) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmita, A.; Dańko, R.; Holtzer, M.; Dańko, J.; Drożyński, D.; Skrzyński, M.; Roczniak, A.; Gruszka, D.R.; Jakubski, J.; Tapola, S. Eco-Friendly Inorganic Binders: A Key Alternative for Reducing Harmful Emissions in Molding and Core-Making Technologies. Int. J. Mol. Sci. 2024, 25, 5496. https://doi.org/10.3390/ijms25105496
Kmita A, Dańko R, Holtzer M, Dańko J, Drożyński D, Skrzyński M, Roczniak A, Gruszka DR, Jakubski J, Tapola S. Eco-Friendly Inorganic Binders: A Key Alternative for Reducing Harmful Emissions in Molding and Core-Making Technologies. International Journal of Molecular Sciences. 2024; 25(10):5496. https://doi.org/10.3390/ijms25105496
Chicago/Turabian StyleKmita, Angelika, Rafał Dańko, Mariusz Holtzer, Józef Dańko, Dariusz Drożyński, Mateusz Skrzyński, Agnieszka Roczniak, Daniel Robert Gruszka, Jarosław Jakubski, and Sara Tapola. 2024. "Eco-Friendly Inorganic Binders: A Key Alternative for Reducing Harmful Emissions in Molding and Core-Making Technologies" International Journal of Molecular Sciences 25, no. 10: 5496. https://doi.org/10.3390/ijms25105496
APA StyleKmita, A., Dańko, R., Holtzer, M., Dańko, J., Drożyński, D., Skrzyński, M., Roczniak, A., Gruszka, D. R., Jakubski, J., & Tapola, S. (2024). Eco-Friendly Inorganic Binders: A Key Alternative for Reducing Harmful Emissions in Molding and Core-Making Technologies. International Journal of Molecular Sciences, 25(10), 5496. https://doi.org/10.3390/ijms25105496







