Profiling of Early Immune Responses to Vaccination Using THP-1-Derived Dendritic Cells
Abstract
:1. Introduction
2. Results and Discussion
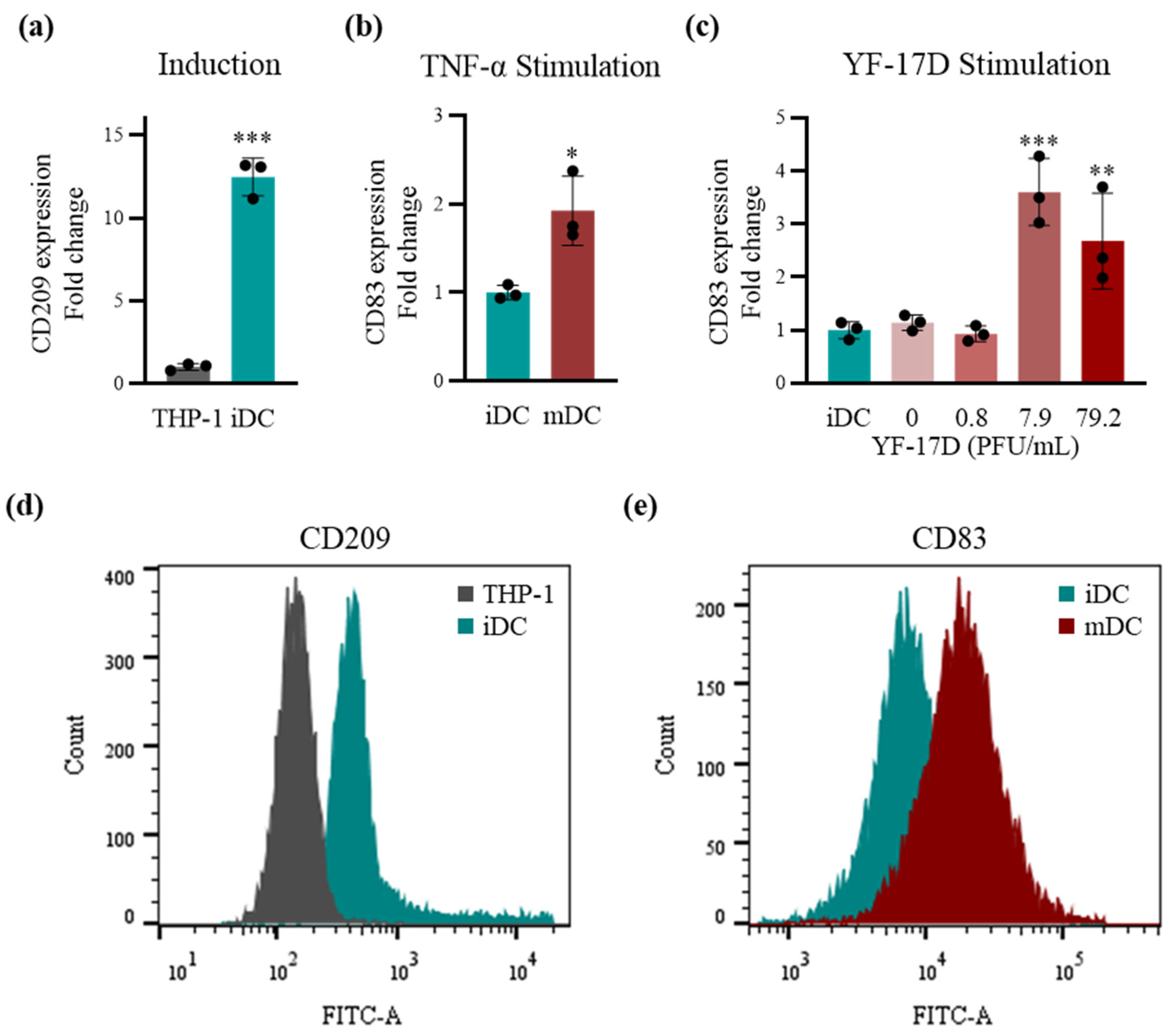
2.1. Stimulation of the TDDCs Using the Optimal Dose of YF-17D In Vitro
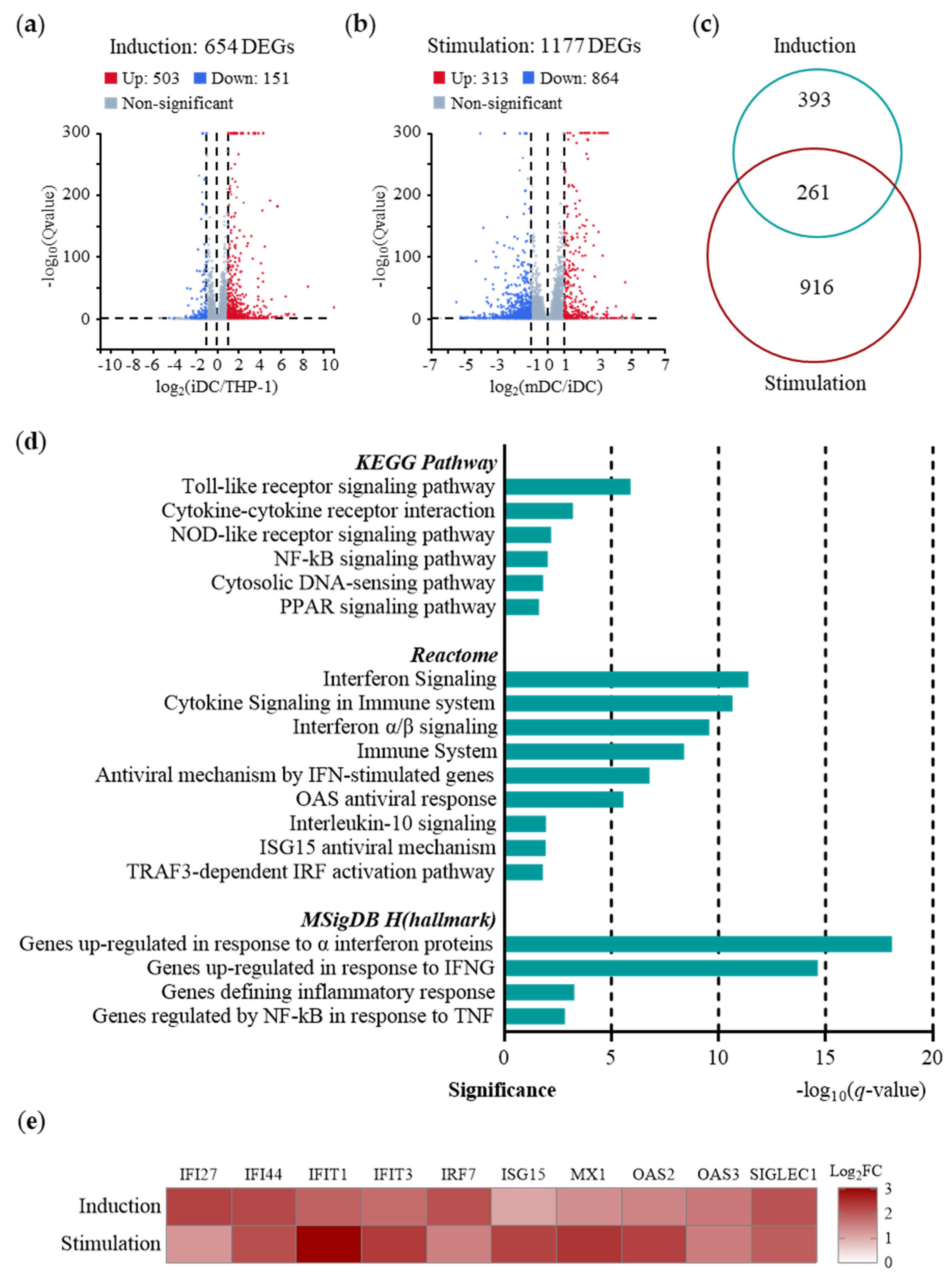
2.2. Predominance of IFN-I-induced Immunity in Early Immune Responses of TDDCs
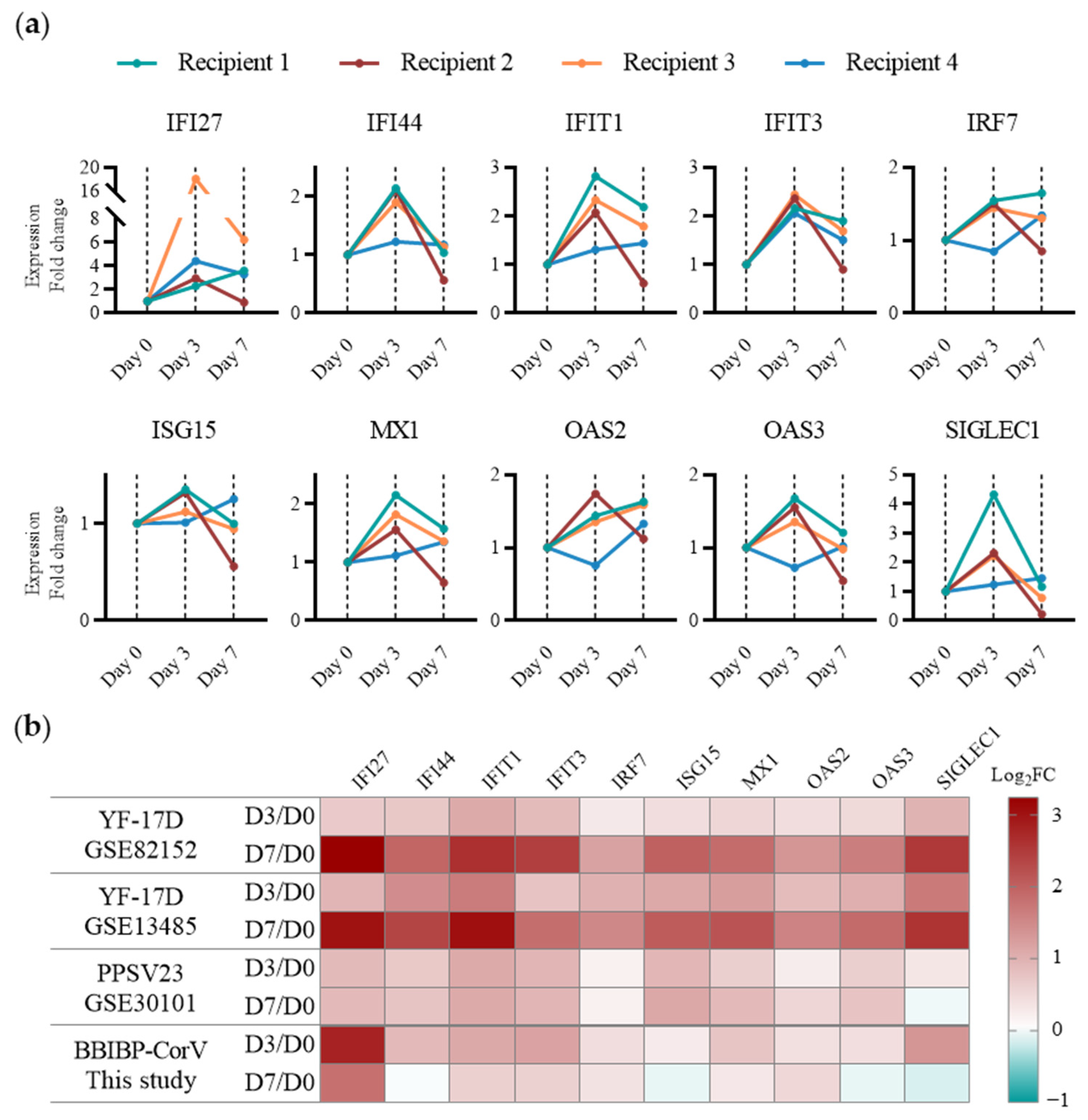
2.3. Application of the TDDC Assessment Approach In Vitro and In Vivo
2.4. Assessment of Early Immune Responses to Various Types of Vaccines of Human PBMCs
3. Materials and Methods
3.1. Materials
3.2. Cell Culture, Induction, and Stimulation
3.3. RNA Preparation and qRT-PCR Testing
3.4. Flow Cytometry
3.5. Transcriptomic Analysis
3.6. Animal Study
3.7. Clinical Study
3.8. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, S.K.; Tripathi, T. One Year Update on the COVID-19 Pandemic: Where Are We Now? Acta Trop. 2021, 214, 105778. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody Responses to SARS-CoV-2 in Patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Shahapur, P.R.; Shahapur, R.; Bagali, S.; Karigoudar, R.; Wavare, D.S.; P, J.; Kandi, V.; Suvvari, T.K.; Mittal, R.J.; Jadhav, M. Breakthrough Infections: Clinical Profile and Outcomes of COVID-19 Vaccinated and Unvaccinated People from a Tertiary Care Hospital. Cureus 2022, 14, e32089. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhao, D.; Zeng, W.; Yang, Y.; Hu, X.; Zhou, P.; Weng, J.; Cheng, L.; Zheng, X.; Jin, T. Decline of SARS-CoV-2-Specific IgG, IgM and IgA in Convalescent COVID-19 Patients within 100 Days after Hospital Discharge. Sci. China Life Sci. 2021, 64, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, E.; Diotti, R.A.; Strollo, M.; Rolla, S.; Ambrosi, A.; Locatelli, M.; Burioni, R.; Mancini, N.; Clementi, M.; Clementi, N. Weak Correlation between Antibody Titers and Neutralizing Activity in Sera from SARS-CoV-2 Infected Subjects. J. Med. Virol. 2021, 93, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Stravalaci, M.; Pagani, I.; Paraboschi, E.M.; Pedotti, M.; Doni, A.; Scavello, F.; Mapelli, S.N.; Sironi, M.; Perucchini, C.; Varani, L.; et al. Recognition and Inhibition of SARS-CoV-2 by Humoral Innate Immunity Pattern Recognition Molecules. Nat. Immunol. 2022, 23, 275–286. [Google Scholar] [CrossRef]
- Corti, D.; Purcell, L.A.; Snell, G.; Veesler, D. Tackling COVID-19 with Neutralizing Monoclonal Antibodies. Cell 2021, 184, 3086–3108. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B. Systems Vaccinology: Probing Humanity’s Diverse Immune Systems with Vaccines. Proc. Natl. Acad. Sci. USA 2014, 111, 12300–12306. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Nakaya, H.I.; Subramaniam, S.; Pulendran, B. Systems Vaccinology: Enabling Rational Vaccine Design with Systems Biological Approaches. Vaccine 2015, 33, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tak-Yin Tsang, O.; et al. Systems Biological Assessment of Immunity to Mild versus Severe COVID-19 Infection in Humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Upreti, S.; Samant, M. A Review on Immunological Responses to SARS-CoV-2 and Various COVID-19 Vaccine Regimens. Pharm. Res. 2022, 39, 2119–2134. [Google Scholar] [CrossRef]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing Immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef]
- Steinman, R.M. The Dendritic Cell System and Its Role in Immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human Dendritic Cell Subsets: An Update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Efficient Presentation of Soluble Antigen by Cultured Human Dendritic Cells Is Maintained by Granulocyte/Macrophage Colony-Stimulating Factor plus Interleukin 4 and Downregulated by Tumor Necrosis Factor Alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef]
- Romani, N.; Gruner, S.; Brang, D.; Kämpgen, E.; Lenz, A.; Trockenbacher, B.; Konwalinka, G.; Fritsch, P.O.; Steinman, R.M.; Schuler, G. Proliferating Dendritic Cell Progenitors in Human Blood. J. Exp. Med. 1994, 180, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.; Matos, I.; Choi, J.H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial Stimulation Fully Differentiates Monocytes to DC-SIGN/CD209(+) Dendritic Cells for Immune T Cell Areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef]
- Johnson, J.S.; De Veaux, N.; Rives, A.W.; Lahaye, X.; Lucas, S.Y.; Perot, B.P.; Luka, M.; Garcia-Paredes, V.; Amon, L.M.; Watters, A.; et al. A Comprehensive Map of the Monocyte-Derived Dendritic Cell Transcriptional Network Engaged upon Innate Sensing of HIV. Cell Rep. 2020, 30, 914–931.e9. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.; Villar, J.; Segura, E. Monocyte Differentiation within Tissues: A Renewed Outlook. Trends Immunol. 2023, 44, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J. Immune Cell Interactions in Tuberculosis. Cell 2022, 185, 4682–4702. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and Macrophage Heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems Biology Approach Predicts Immunogenicity of the Yellow Fever Vaccine in Humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Berges, C.; Naujokat, C.; Tinapp, S.; Wieczorek, H.; Höh, A.; Sadeghi, M.; Opelz, G.; Daniel, V. A Cell Line Model for the Differentiation of Human Dendritic Cells. Biochem. Biophys. Res. Commun. 2005, 333, 896–907. [Google Scholar] [CrossRef]
- Hitzler, M.; Bergert, A.; Luch, A.; Peiser, M. Evaluation of Selected Biomarkers for the Detection of Chemical Sensitization in Human Skin: A Comparative Study Applying THP-1, MUTZ-3 and Primary Dendritic Cells in Culture. Toxicol. Vitr. 2013, 27, 1659–1669. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Zou, Z.; Tao, A.; Ai, Y. Activation Profile of THP-1 Derived Dendritic Cells Stimulated by Allergen Mal f 1 beyond Its IgE-Binding Ability. Int. Immunopharmacol. 2018, 62, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lechmann, M.; Berchtold, S.; Steinkasserer, A.; Hauber, J. CD83 on Dendritic Cells: More than Just a Marker for Maturation. Trends Immunol. 2002, 23, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Tedder, T.F. CD14+ Blood Monocytes Can Differentiate into Functionally Mature CD83+ Dendritic Cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2588–2592. [Google Scholar] [CrossRef]
- Barrett, A.D.T.; Monath, T.P.; Barban, V.; Niedrig, M.; Teuwen, D.E. 17D Yellow Fever Vaccines: New Insights. Vaccine 2007, 25, 2758–2765. [Google Scholar] [CrossRef]
- Hou, J.; Wang, S.; Jia, M.; Li, D.; Liu, Y.; Li, Z.; Zhu, H.; Xu, H.; Sun, M.; Lu, L.; et al. A Systems Vaccinology Approach Reveals Temporal Transcriptomic Changes of Immune Responses to the Yellow Fever 17D Vaccine. J. Immunol. 2017, 199, 1476–1489. [Google Scholar] [CrossRef]
- Pulendran, B. Learning Immunology from the Yellow Fever Vaccine: Innate Immunity to Systems Vaccinology. Nat. Rev. Immunol. 2009, 9, 741–747. [Google Scholar] [CrossRef]
- Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The Reactome Pathway Knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Taniguchi, T. IRFs: Master Regulators of Signalling by Toll-like Receptors and Cytosolic Pattern-Recognition Receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Perng, Y.-C.; Lenschow, D.J. ISG15 in Antiviral Immunity and Beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ewbank, J.J. Signaling in the Innate Immune Response. WormBook 2018, 2018, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Hartmann, R.; Ablasser, A.; Hopfner, K.-P. OAS Proteins and cGAS: Unifying Concepts in Sensing and Responding to Cytosolic Nucleic Acids. Nat. Rev. Immunol. 2014, 14, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Wetzel, J.L.; Ramachandran, S.; Ogino, T.; Stohlman, S.A.; Bergmann, C.C.; Diamond, M.S.; Virgin, H.W.; Sen, G.C. Interferon-Induced Ifit2/ISG54 Protects Mice from Lethal VSV Neuropathogenesis. PLoS Pathog. 2012, 8, e1002712. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S. IFIT1: A Dual Sensor and Effector Molecule That Detects Non-2′-O Methylated Viral RNA and Inhibits Its Translation. Cytokine Growth Factor. Rev. 2014, 25, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Chen, W.; Wei, B.; Shan, Y.-F.; Wang, C. IFN-Induced TPR Protein IFIT3 Potentiates Antiviral Signaling by Bridging MAVS and TBK1. J. Immunol. 2011, 187, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Cheriyath, V.; Leaman, D.W.; Borden, E.C. Emerging Roles of FAM14 Family Members (G1P3/ISG 6–16 and ISG12/IFI27) in Innate Immunity and Cancer. J. Interferon Cytokine Res. 2011, 31, 173–181. [Google Scholar] [CrossRef]
- Power, D.; Santoso, N.; Dieringer, M.; Yu, J.; Huang, H.; Simpson, S.; Seth, I.; Miao, H.; Zhu, J. IFI44 Suppresses HIV-1 LTR Promoter Activity and Facilitates Its Latency. Virology 2015, 481, 142–150. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, J.; Zhou, Y.; Yang, Y.; Xie, B.; Cao, X. Siglec1 Suppresses Antiviral Innate Immune Response by Inducing TBK1 Degradation via the Ubiquitin Ligase TRIM27. Cell Res. 2015, 25, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Wu, K.; Werner, A.P.; Koch, M.; Choi, A.; Narayanan, E.; Stewart-Jones, G.B.E.; Colpitts, T.; Bennett, H.; Boyoglu-Barnum, S.; Shi, W.; et al. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. N. Engl. J. Med. 2021, 384, 1468–1470. [Google Scholar] [CrossRef]
- Obermoser, G.; Presnell, S.; Domico, K.; Xu, H.; Wang, Y.; Anguiano, E.; Thompson-Snipes, L.; Ranganathan, R.; Zeitner, B.; Bjork, A.; et al. Systems Scale Interactive Exploration Reveals Quantitative and Qualitative Differences in Response to Influenza and Pneumococcal Vaccines. Immunity 2013, 38, 831–844. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Li, P.; Wang, M.; Wu, F.; Han, S.; Ma, L. Profiling of Early Immune Responses to Vaccination Using THP-1-Derived Dendritic Cells. Int. J. Mol. Sci. 2024, 25, 5509. https://doi.org/10.3390/ijms25105509
Ye L, Li P, Wang M, Wu F, Han S, Ma L. Profiling of Early Immune Responses to Vaccination Using THP-1-Derived Dendritic Cells. International Journal of Molecular Sciences. 2024; 25(10):5509. https://doi.org/10.3390/ijms25105509
Chicago/Turabian StyleYe, Lei, Ping Li, Mingzhe Wang, Feng Wu, Sanyang Han, and Lan Ma. 2024. "Profiling of Early Immune Responses to Vaccination Using THP-1-Derived Dendritic Cells" International Journal of Molecular Sciences 25, no. 10: 5509. https://doi.org/10.3390/ijms25105509
APA StyleYe, L., Li, P., Wang, M., Wu, F., Han, S., & Ma, L. (2024). Profiling of Early Immune Responses to Vaccination Using THP-1-Derived Dendritic Cells. International Journal of Molecular Sciences, 25(10), 5509. https://doi.org/10.3390/ijms25105509





