Large-Sample Genome-Wide Association Study of Resistance to Retained Placenta in U.S. Holstein Cows
Abstract
:1. Introduction
2. Results and Discussion
2.1. Overview of GWAS Results
2.2. Additive Effects of Chr09
2.3. Additive Effects of Chr23
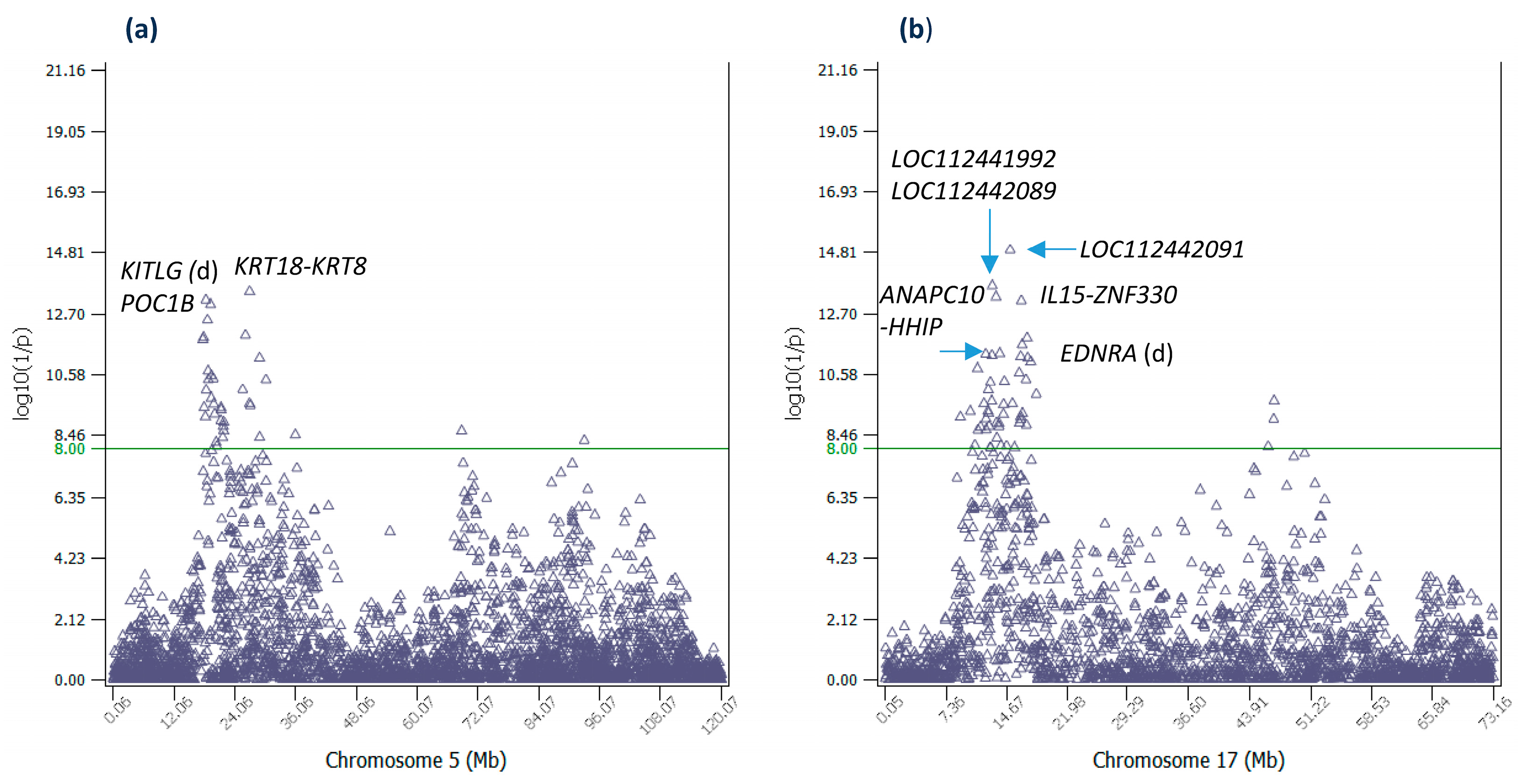
2.4. Additive Effects of Chr05 and Chr17
2.5. Additive Effects of Other Chromosomes
2.6. Gene Ontology Analysis
3. Materials and Methods
3.1. Holstein Population and SNP Data
3.2. GWAS Analysis
3.3. Gene Ontology (GO) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, J.Y. A review on retention of placenta in dairy cattles. Int. J. Vet. Sci. 2016, 5, 200–207. [Google Scholar]
- Amin, Y.A.; Hussein, H.A. Latest update on predictive indicators, risk factors and ‘Omic’technologies research of retained placenta in dairy cattle–A review. Reprod. Domest. Anim. 2022, 57, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Endler, M.; Cnattingius, S.; Granfors, M.; Wikström, A.K. The inherited risk of retained placenta: A population based cohort study. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Freebern, E.; Santos, D.J.; Fang, L.; Jiang, J.; Parker Gaddis, K.L.; Liu, G.E.; VanRaden, P.M.; Maltecca, C.; Cole, J.B.; Ma, L. GWAS and fine-mapping of livability and six disease traits in Holstein cattle. BMC Genom. 2020, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Guarini, A.; Lourenco, D.; Brito, L.; Sargolzaei, M.; Baes, C.F.; Miglior, F.; Misztal, I.; Schenkel, F. Genetics and genomics of reproductive disorders in Canadian Holstein cattle. J. Dairy Sci. 2019, 102, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- CDCB. Resistance to Retained Placenta (RETP). 2018. Available online: https://uscdcb.com/wp-content/uploads/2018/03/CDCB-Reference-Sheet-RETP-03_2018.pdf (accessed on 2 May 2024).
- Nagy, Z.; Jeselsohn, R. ESR1 fusions and therapeutic resistance in metastatic breast cancer. Front. Oncol. 2023, 12, 1037531. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.O.; Ritterhouse, L.L.; Newman, E.T.; Irwin, K.E.; Dawson, M.; Ryan, L.Y.; Spring, L.M.; Rivera, M.N.; Lennerz, J.K.; Dias-Santagata, D. Clinical Implications and Treatment Strategies for ESR1 Fusions in Hormone Receptor-Positive Metastatic Breast Cancer: A Case Series. Oncologist 2023, 28, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, L.; Veeraraghavan, J.; Hu, Y.; Wang, X.; Lee, S.; Tan, Y.; Schiff, R.; Wang, X.-S. Therapeutic role of recurrent ESR1-CCDC170 gene fusions in breast cancer endocrine resistance. Breast Cancer Res. 2020, 22, 84. [Google Scholar] [CrossRef]
- Vitale, S.R.; Ruigrok-Ritstier, K.; Timmermans, A.M.; Foekens, R.; Trapman-Jansen, A.M.; Beaufort, C.M.; Vigneri, P.; Sleijfer, S.; Martens, J.W.; Sieuwerts, A.M. The prognostic and predictive value of ESR1 fusion gene transcripts in primary breast cancer. BMC Cancer 2022, 22, 165. [Google Scholar] [CrossRef]
- ESR1 Estrogen Receptor 1. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/2099 (accessed on 2 May 2024).
- Liang, Z.; Prakapenka, D.; VanRaden, P.M.; Jiang, J.; Ma, L.; Da, Y. A Million-cow genome-wide association study of three fertility traits in US Holstein cows. Int. J. Mol. Sci. 2023, 24, 10496. [Google Scholar] [CrossRef]
- SLC17A1 Solute Carrier Family 17 Member 1. Available online: https://www.ncbi.nlm.nih.gov/gene/6568 (accessed on 2 May 2024).
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Lozada, L.G.S. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: Report of a scientific workshop organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Onuchic, L.F.; Furu, L.; Nagasawa, Y.; Hou, X.; Eggermann, T.; Ren, Z.; Bergmann, C.; Senderek, J.; Esquivel, E.; Zeltner, R. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription–factor domains and parallel beta-helix 1 repeats. Am. J. Hum. Genet. 2002, 70, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Senderek, J.; Küpper, F.; Schneider, F.; Dornia, C.; Windelen, E.; Eggermann, T.; Rudnik-Schöneborn, S.; Kirfel, J.; Furu, L. PKHD1 mutations in autosomal recessive polycystic kidney disease (ARPKD). Hum. Mutat. 2004, 23, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Dong, D.; Sun, L.; Che, L.; Li, X.; Liu, Y.; Wang, B. Identification of ACOT13 and PTGER2 as novel candidate genes of autosomal dominant polycystic kidney disease through whole exome sequencing. Eur. J. Med. Res. 2021, 26, 142. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Yamashita, Y.; Yagisawa, T. Copy number variations in BOLA-DQA2, BOLA-DQB, and BOLA-DQA5 show the genomic architecture and haplotype frequency of major histocompatibility complex class II genes in Holstein cows. Hla 2020, 96, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Holling, T.M.; Schooten, E.; van Den Elsen, P.J. Function and regulation of MHC class II molecules in T-lymphocytes: Of mice and men. Hum. Immunol. 2004, 65, 282–290. [Google Scholar] [CrossRef] [PubMed]
- PRL Prolactin. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/5617 (accessed on 2 May 2024).
- Prakapenka, D.; Liang, Z.; Da, Y. Genome-wide association study of age at first calving in US Holstein cows. Int. J. Mol. Sci. 2023, 24, 7109. [Google Scholar] [CrossRef] [PubMed]
- KITLG KIT Ligand. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/4254 (accessed on 2 May 2024).
- POC1B POC1 Centriolar Protein B. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/83943 (accessed on 2 May 2024).
- IL15 Interleukin 15. Available online: https://www.ncbi.nlm.nih.gov/gene/3600 (accessed on 2 May 2024).
- ZNF330 Zinc Finger Protein 330. Available online: https://www.ncbi.nlm.nih.gov/gene/27309 (accessed on 2 May 2024).
- SLC1A2 Solute Carrier Family 1 Member 2. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/6506 (accessed on 2 May 2024).
- IL10RA Interleukin 10 Receptor Subunit Alpha. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/3587 (accessed on 2 May 2024).
- SPON1 Spondin 1. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/10418 (accessed on 2 May 2024).
- SS18 SS18 Subunit of BAF Chromatin Remodeling Complex. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/6760 (accessed on 2 May 2024).
- TAF4B TATA-box Binding Protein Associated Factor 4b. The National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/6875 (accessed on 2 May 2024).
- Jiang, J.; Ma, L.; Prakapenka, D.; VanRaden, P.M.; Cole, J.B.; Da, Y. A large-scale genome-wide association study in US Holstein cattle. Front. Genet. 2019, 10, 412. [Google Scholar] [CrossRef]
- RASGRP1 RAS Guanyl Releasing Protein 1. Available online: https://www.ncbi.nlm.nih.gov/gene/10125 (accessed on 2 May 2024).
- LIPC Lipase C, Hepatic Type. Available online: https://www.ncbi.nlm.nih.gov/gene/3990 (accessed on 2 May 2024).
- Ma, L.; Runesha, H.B.; Dvorkin, D.; Garbe, J.; Da, Y. Parallel and serial computing tools for testing single-locus and epistatic SNP effects of quantitative traits in genome-wide association studies. BMC Bioinform. 2008, 9, 315. [Google Scholar] [CrossRef]
- Weeks, N.T.; Luecke, G.R.; Groth, B.M.; Kraeva, M.; Ma, L.; Kramer, L.M.; Koltes, J.E.; Reecy, J.M. High-performance epistasis detection in quantitative trait GWAS. Int. J. High Perform. Comput. Appl. 2016, 32, 1094342016658110. [Google Scholar] [CrossRef]
- Henderson, C. Applications of Linear Models in Animal Breeding; University of Guelph: Guelph, ON, Canada, 1984. [Google Scholar]
- Prakapenka, D.; Liang, Z.; Zaabza, H.B.; VanRaden, P.M.; Van Tassell, C.P.; Da, Y. A million-cow validation of a chromosome 14 region interacting with all chromosomes for fat percentage in US Holstein cows. Int. J. Mol. Sci. 2024, 25, 674. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; London, N.R.; Ma, L.; Dvorkin, D.; Da, Y. Detection of SNP epistasis effects of quantitative traits using an extended Kempthorne model. Physiol. Genom. 2006, 28, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longmans Green: Harlow, UK, 1996. [Google Scholar]



| SNP | Position | Candidate Gene | Effect | Log10(1/p) | al+ | ae+ | f_al+ | al− | ae− | f_al− |
|---|---|---|---|---|---|---|---|---|---|---|
| rs42026926 | 87610236 | PLEKHG1 (u) | 0.183 | 13.74 | 1 | 0.080 | 0.564 | 2 | −0.103 | 0.436 |
| rs42026914 | 87634273 | PLEKHG1 (u) | 0.158 | 10.75 | 1 | 0.075 | 0.526 | 2 | −0.0833 | 0.474 |
| rs43615609 | 88220932 | MTHFD1L (d) | 0.175 | 11.80 | 1 | 0.062 | 0.644 | 2 | −0.112 | 0.356 |
| rs43615544 | 88254314 | AKAP12 (u) | −0.210 | 15.96 | 2 | 0.062 | 0.706 | 1 | −0.148 | 0.294 |
| rs43612983 | 88482928 | RMND1 | 0.197 | 15.10 | 1 | 0.070 | 0.643 | 2 | −0.127 | 0.357 |
| rs43611719 | 88519048 | LOC112448080 | −0.246 | 21.16 | 2 | 0.071 | 0.71 | 1 | −0.175 | 0.29 |
| rs43611710 | 88529494 | CCDC170 | −0.199 | 15.76 | 2 | 0.070 | 0.646 | 1 | −0.128 | 0.354 |
| rs43611701 | 88540232 | CCDC170 | −0.182 | 14.33 | 2 | 0.081 | 0.556 | 1 | −0.102 | 0.444 |
| rs43610539 | 88598336 | CCDC170 | −0.189 | 13.13 | 2 | 0.057 | 0.699 | 1 | −0.132 | 0.301 |
| rs43608567 | 88684552 | ESR1 | −0.177 | 12.70 | 2 | 0.063 | 0.642 | 1 | −0.114 | 0.358 |
| rs3423297865 | 88722921 | ESR1 | 0.188 | 13.46 | 1 | 0.068 | 0.641 | 2 | −0.12 | 0.359 |
| rs43767108 | 88739977 | ESR1 | 0.188 | 13.48 | 1 | 0.068 | 0.641 | 2 | −0.121 | 0.359 |
| SNP | Position | Candidate Gene | Effect | Log10(1/p) | al+ | ae+ | f_al+ | al− | ae− | f_al− |
|---|---|---|---|---|---|---|---|---|---|---|
| rs110556135 | 23704336 | PKHD1 (u) | 0.224 | 13.33 | 1 | 0.042 | 0.813 | 2 | −0.182 | 0.187 |
| rs43561755 | 23750046 | PKHD1 (u) | −0.216 | 11.75 | 2 | 0.039 | 0.819 | 1 | −0.177 | 0.181 |
| BTA-81662-no-rs | 23890370 | PKHD1 (u) | −0.166 | 11.30 | 2 | 0.062 | 0.629 | 1 | −0.104 | 0.371 |
| rs41670209 | 24060064 | PKHD1 (u) | −0.183 | 11.88 | 2 | 0.052 | 0.718 | 1 | −0.131 | 0.282 |
| rs136181786 | 24252119 | PKHD1 | −0.180 | 11.94 | 2 | 0.058 | 0.68 | 1 | −0.123 | 0.32 |
| rs137762108 | 25024407 | LOC112443711-LOC112443751 | −0.199 | 12.65 | 2 | 0.051 | 0.746 | 1 | −0.148 | 0.254 |
| rs135832378 | 25119540 | GSTA1 (u) | 0.186 | 13.95 | 1 | 0.073 | 0.609 | 2 | −0.113 | 0.391 |
| rs110144575 | 25142236 | GSTA5 | 0.218 | 11.54 | 1 | 0.039 | 0.821 | 2 | −0.179 | 0.179 |
| rs110043199 | 25175265 | LOC112443730 | 0.219 | 12.14 | 1 | 0.039 | 0.82 | 2 | −0.180 | 0.18 |
| rs135146076 | 25491332 | ELOVL5, BOLA-DQA2 | −0.190 | 14.48 | 2 | 0.067 | 0.645 | 1 | −0.123 | 0.355 |
| rs133177329 | 27122907 | ENSBTAG00000048304 | 0.172 | 12.15 | 1 | 0.105 | 0.388 | 2 | −0.067 | 0.612 |
| rs110358203 | 27231641 | PPT2 | −0.192 | 14.51 | 2 | 0.124 | 0.353 | 1 | −0.068 | 0.647 |
| rs110277462 | 28345983 | DHX16 | 0.186 | 13.72 | 1 | 0.121 | 0.351 | 2 | −0.065 | 0.649 |
| rs3423504515 | 28367574 | MRPS18B | 0.193 | 14.78 | 1 | 0.126 | 0.35 | 2 | −0.068 | 0.65 |
| rs3423514031 | 28777609 | LOC785873 | 0.183 | 13.50 | 1 | 0.115 | 0.373 | 2 | −0.068 | 0.627 |
| rs3423494105 | 28783122 | TRIM26 | 0.193 | 12.48 | 1 | 0.051 | 0.737 | 2 | −0.142 | 0.263 |
| rs3423498878 | 31063056 | TRNAF-GAA_18, TRNAI-UAU_6 | −0.165 | 11.75 | 2 | 0.085 | 0.486 | 1 | −0.080 | 0.514 |
| rs109821904 | 32057953 | SLC17A1 | −0.196 | 15.50 | 2 | 0.125 | 0.363 | 1 | −0.071 | 0.637 |
| rs134698463 | 32498379 | CARMIL1 | −0.178 | 13.46 | 2 | 0.086 | 0.514 | 1 | −0.091 | 0.486 |
| rs3423509404 | 33077628 | ACOT13 | −0.177 | 13.65 | 2 | 0.085 | 0.517 | 1 | −0.091 | 0.483 |
| SNP | Chr | Position | Candidate Gene | Effect | Log10(1/p) | al+ | ae+ | f_al+ | al− | ae− | f_al− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs110165899 | 5 | 27005657 | KRT18-KRT8 | 0.184 | 13.51 | 1 | 0.118 | 0.358 | 2 | −0.066 | 0.642 |
| rs41587994 | 5 | 18367350 | KITLG (d) | 0.198 | 13.22 | 1 | 0.147 | 0.258 | 2 | −0.051 | 0.742 |
| rs41603721 | 5 | 19295836 | POC1B | −0.187 | 13.06 | 2 | 0.129 | 0.307 | 1 | −0.057 | 0.693 |
| rs29012239 | 5 | 18679362 | LOC104972350- LOC104972370 | 0.185 | 12.51 | 1 | 0.131 | 0.292 | 2 | −0.054 | 0.708 |
| rs135127542 | 5 | 26178969 | CALCOCO1 (u) | −0.178 | 11.99 | 2 | 0.122 | 0.313 | 1 | −0.056 | 0.687 |
| rs136124246 | 5 | 17909902 | CEP290 | 0.176 | 11.91 | 1 | 0.117 | 0.332 | 2 | −0.058 | 0.668 |
| rs110506590 | 5 | 17780338 | C5H12orf50 (u) | 0.175 | 11.84 | 1 | 0.117 | 0.332 | 2 | −0.058 | 0.668 |
| rs137107793 | 5 | 28970729 | HIGD1C | 0.162 | 11.19 | 1 | 0.070 | 0.564 | 2 | −0.091 | 0.436 |
| rs137455368 | 5 | 18799786 | LOC104972350- LOC104972370 | 0.161 | 10.75 | 1 | 0.101 | 0.373 | 2 | −0.060 | 0.627 |
| rs109747382 | 5 | 19468540 | ATP2B1 (u) | −0.159 | 10.58 | 2 | 0.099 | 0.376 | 1 | −0.060 | 0.624 |
| rs110739449 | 17 | 15056547 | LOC112442091 | −0.187 | 14.94 | 2 | 0.079 | 0.575 | 1 | −0.107 | 0.425 |
| rs135912416 | 17 | 12902838 | LOC112441992- LOC112442089 | −0.180 | 13.70 | 2 | 0.092 | 0.487 | 1 | −0.088 | 0.513 |
| rs137219013 | 17 | 13350634 | ANAPC10-HHIP | 0.176 | 13.31 | 1 | 0.078 | 0.559 | 2 | −0.098 | 0.441 |
| rs109572161 | 17 | 16399921 | IL15-ZNF330 | −0.174 | 13.18 | 2 | 0.079 | 0.546 | 1 | −0.095 | 0.454 |
| rs109486788 | 17 | 17090817 | TBC1D9 | 0.169 | 11.88 | 1 | 0.077 | 0.543 | 2 | −0.092 | 0.457 |
| rs108973145 | 17 | 16489833 | IL15-ZNF330 | −0.164 | 11.64 | 2 | 0.072 | 0.561 | 1 | −0.092 | 0.439 |
| rs137504512 | 17 | 13801294 | TRNAC-GCA_189, TRNAG-UCC_41 | −0.163 | 11.34 | 2 | 0.081 | 0.503 | 1 | −0.082 | 0.497 |
| rs41838712 | 17 | 12116917 | REELD1 | 0.168 | 11.32 | 1 | 0.102 | 0.394 | 2 | −0.066 | 0.606 |
| rs41599601 | 17 | 12866447 | LOC112441992 | 0.160 | 11.28 | 1 | 0.075 | 0.531 | 2 | −0.085 | 0.469 |
| SNP | Chr | Position | Candidate Gene | Effect | Log10(1/p) | al+ | ae+ | f_al+ | al− | ae− | f_al− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs109034709 | 6 | 87316810 | NPFFR2 | −0.152 | 9.53 | 2 | 0.057 | 0.628 | 1 | −0.096 | 0.372 |
| rs110434046 | 6 | 87184768 | GC-NPFFR2 | −0.152 | 9.47 | 2 | 0.056 | 0.628 | 1 | −0.095 | 0.372 |
| rs137664040 | 6 | 86795926 | SLC4A4 | −0.143 | 8.45 | 2 | 0.057 | 0.603 | 1 | −0.086 | 0.397 |
| rs3423224824 | 7 | 8274451 | OR7A5 (d) | −0.174 | 10.18 | 2 | 0.128 | 0.264 | 1 | −0.046 | 0.736 |
| rs109718130 | 10 | 34176744 | RASGRP1 | −0.150 | 8.41 | 2 | 0.047 | 0.689 | 1 | −0.104 | 0.311 |
| rs43626966 | 10 | 51788612 | LIPC | 0.166 | 8.23 | 1 | 0.036 | 0.785 | 2 | −0.130 | 0.215 |
| rs133296429 | 15 | 65887087 | SLC1A2 | 0.163 | 11.46 | 1 | 0.072 | 0.56 | 2 | −0.091 | 0.440 |
| rs110222319 | 15 | 28670668 | IL10RA | 0.200 | 9.88 | 1 | 0.035 | 0.827 | 2 | −0.165 | 0.173 |
| rs133481154 | 15 | 38458720 | SPON1 | 0.198 | 9.83 | 1 | 0.035 | 0.823 | 2 | −0.163 | 0.177 |
| rs110235930 | 19 | 61387218 | ABCA10 | −0.153 | 9.62 | 2 | 0.057 | 0.625 | 1 | −0.096 | 0.375 |
| rs41932313 | 19 | 61518347 | ABCA9 | −0.179 | 9.24 | 2 | 0.038 | 0.788 | 1 | −0.141 | 0.212 |
| rs43772736 | 24 | 30783746 | SS18 (d) | 0.178 | 13.68 | 1 | 0.078 | 0.559 | 2 | −0.099 | 0.441 |
| rs136103342 | 24 | 30564828 | TAF4B | −0.179 | 13.51 | 2 | 0.076 | 0.579 | 1 | −0.104 | 0.421 |
| rs207730478 | 24 | 30578431 | TAF4B | −0.170 | 12.47 | 2 | 0.072 | 0.579 | 1 | −0.099 | 0.421 |
| rs110190049 | 24 | 30486009 | KCTD1-TAF4B | −0.167 | 11.95 | 2 | 0.070 | 0.579 | 1 | −0.097 | 0.421 |
| rs133376988 | 31 | 89494444 | MAGED1-MAGED4B | 0.299 | 10.41 | 1 | 0.022 | 0.927 | 2 | −0.277 | 0.073 |
| rs136268223 | 31 | 89068436 | NUDT11 (d) | −0.184 | 10.36 | 2 | 0.042 | 0.772 | 1 | −0.142 | 0.228 |
| rs137683400 | 31 | 89839370 | LOC100297099 | −0.226 | 9.32 | 2 | 0.027 | 0.883 | 1 | −0.200 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakapenka, D.; Liang, Z.; Zaabza, H.B.; VanRaden, P.M.; Van Tassell, C.P.; Da, Y. Large-Sample Genome-Wide Association Study of Resistance to Retained Placenta in U.S. Holstein Cows. Int. J. Mol. Sci. 2024, 25, 5551. https://doi.org/10.3390/ijms25105551
Prakapenka D, Liang Z, Zaabza HB, VanRaden PM, Van Tassell CP, Da Y. Large-Sample Genome-Wide Association Study of Resistance to Retained Placenta in U.S. Holstein Cows. International Journal of Molecular Sciences. 2024; 25(10):5551. https://doi.org/10.3390/ijms25105551
Chicago/Turabian StylePrakapenka, Dzianis, Zuoxiang Liang, Hafedh B. Zaabza, Paul M. VanRaden, Curtis P. Van Tassell, and Yang Da. 2024. "Large-Sample Genome-Wide Association Study of Resistance to Retained Placenta in U.S. Holstein Cows" International Journal of Molecular Sciences 25, no. 10: 5551. https://doi.org/10.3390/ijms25105551





