Abstract
The epidemiological burden of liver steatosis associated with metabolic diseases is continuously growing worldwide and in all age classes. This condition generates possible progression of liver damage (i.e., inflammation, fibrosis, cirrhosis, hepatocellular carcinoma) but also independently increases the risk of cardio-metabolic diseases and cancer. In recent years, the terminological evolution from “nonalcoholic fatty liver disease” (NAFLD) to “metabolic dysfunction-associated fatty liver disease” (MAFLD) and, finally, “metabolic dysfunction-associated steatotic liver disease” (MASLD) has been paralleled by increased knowledge of mechanisms linking local (i.e., hepatic) and systemic pathogenic pathways. As a consequence, the need for an appropriate classification of individual phenotypes has been oriented to the investigation of innovative therapeutic tools. Besides the well-known role for lifestyle change, a number of pharmacological approaches have been explored, ranging from antidiabetic drugs to agonists acting on the gut–liver axis and at a systemic level (mainly farnesoid X receptor (FXR) agonists, PPAR agonists, thyroid hormone receptor agonists), anti-fibrotic and anti-inflammatory agents. The intrinsically complex pathophysiological history of MASLD makes the selection of a single effective treatment a major challenge, so far. In this evolving scenario, the cooperation between different stakeholders (including subjects at risk, health professionals, and pharmaceutical industries) could significantly improve the management of disease and the implementation of primary and secondary prevention measures. The high healthcare burden associated with MASLD makes the search for new, effective, and safe drugs a major pressing need, together with an accurate characterization of individual phenotypes. Recent and promising advances indicate that we may soon enter the era of precise and personalized therapy for MASLD/MASH.
1. Introduction
The epidemiological progression of liver steatosis is closely linked to the steady increase in overweight and obesity, adding to a huge social, economic, and healthcare burden globally [1,2]. In fact, the prevalence of obesity has dramatically increased over the past 30–40 years in adults [3,4], children and adolescents [5]. Overweight and obesity, in turn, increase the risk of type 2 diabetes mellitus (T2DM), arterial hypertension, atherosclerosis, cardiovascular diseases [6,7], metabolic syndrome, dyslipidemia, cholesterol cholelithiasis [8], several malignancies [9], increased risk of severe COVID-19 [10], and metabolic dysfunction–associated steatotic liver disease (MASLD), among many other conditions and related complications.
The first definition of liver disease associated with metabolic dysfunction dates back to 1980 when the definition “nonalcoholic steatohepatitis” (NASH) was coined to identify a group of histological features reminiscent of alcohol-associated liver injury among non-drinker individuals [11]. In 1986, nonalcoholic fatty liver disease (NAFLD) was introduced as a term to describe the histological presence of steatosis in at least 5% of hepatocytes [12]. Later, NAFLD was defined as >5.5% liver fat content by magnetic resonance proton spectroscopy in individuals with no or little alcohol consumption (i.e., a daily alcohol intake of ≤20 g in females and ≤30 g in males) [13]. The definition of NAFLD and NASH required the exclusion of other, well-defined causes of chronic liver disease such as viral hepatitis, genetically determined liver diseases (e.g., Wilson disease), and drug-induced or toxic liver injury [11,14,15]. The acronym NAFLD includes a spectrum of hepatic disorders ranging from macrovesicular steatosis (NAFL) with or without mild lobular inflammation [16], to NASH, with hepatocellular injury (ballooning), inflammation, and perisinusoidal fibrosis. Further progress to advanced fibrosis, cirrhosis, and hepatocellular carcinoma is also possible (Figure 1).
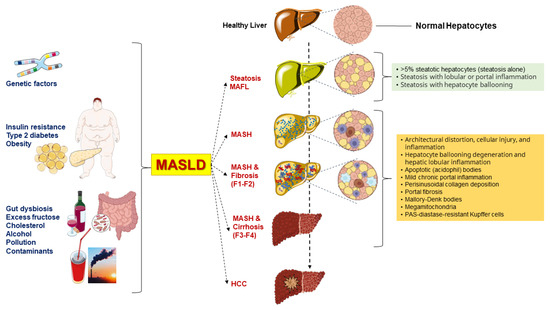
Figure 1.
Genetic background and various metabolic dysfunctions contribute to the advancement of metabolic dysfunction-associated steatotic liver disease (MASLD) [17]. Besides the genetic predisposition, several metabolic dysfunctions, including visceral obesity and type 2 diabetes mellitus (T2DM), are primary risk factors for MASLD progression. Other factors can also contribute to the environmental background and include gut dysbiosis, excess dietary fructose, cholesterol, alcohol intake, environmental pollution, and food contaminants [17,18]. MASLD is characterized by intrahepatic triglyceride accumulation exceeding 5% and follows a complex continuum spectrum of disease [19]. In MAFL, the picture is characterized by steatosis alone, portal inflammation, or hepatocyte ballooning. In MASH, the typical findings include architectural distortion, cellular injury, and inflammation, hepatocyte ballooning degeneration and hepatic lobular inflammation, acidophil apoptotic bodies, mild chronic portal inflammation, perisinusoidal collagen deposition resulting in zone 3 accentuation in a “chicken wire” pattern, portal fibrosis without perisinusoidal or pericellular fibrosis, Mallory-Denk bodies (previously called Mallory bodies or Mallory hyaline), mega-mitochondria, PAS-diastase-resistant Kupffer cells, glycogenated (vacuolated) nuclei in periportal hepatocytes, lobular lipogranulomas, mild hepatic siderosis involving periportal hepatocytes or panacinar reticuloendothelial cells, and macronodular cirrhosis, which is an end-stage result of MASH [15]. About 22% of individuals progress from MASH to cirrhosis, and those with severe cirrhosis may develop hepatocellular carcinoma (HCC). F1: portal fibrosis without septa. F2: portal fibrosis with few septa. F3: numerous septa without cirrhosis. F4: cirrhosis.
In the last 3 years, two panels of scholars from mostly different geographical regions of the world have advocated a change of terminology to move from NAFLD, a diagnosis of exclusion, to more pro-active and metabolically-oriented diagnostic terms. Thus, “metabolic dysfunction-associated fatty liver disease” (MAFLD) was proposed in 2020 [20,21], and “metabolic dysfunction-associated steatotic liver disease” (MASLD) and “metabolic dysfunction-associated steatohepatitis” (MASH) were proposed in 2023 [22]. These changes in the nomenclature of the most commonly observed chronic liver disease, which has been known as NAFLD for the past 40 years, intend to propose a new framework for researchers, practitioners, and patients. The introduction of the acronym MASLD has been made within the construct of steatotic liver disease (SLD) as an umbrella term that facilitates the classification of various liver disorders with abnormal fat accumulation [22]. In addition, with MAFLD removing the potentially stigmatizing term “[non]alcoholic” followed by the purging of “fatty” from the term MASLD, the negative impact of two potentially stigmatizing terms on public perception has now been overcome [22].
The rationale behind these changes has been to improve disease classification, increase disease awareness, and emphasize the link between cardiometabolic risk and all-cause mortality [23]. Epidemiological evidence shows that there is an almost complete overlap of populations when using the terminology MASLD with respect to NAFLD, i.e., ~99% of individuals with NAFLD meet MASLD criteria [24,25,26]. However, it is expected that the latest consensus on the nomenclature change will facilitate the development of personalized therapeutic approaches [27,28]. Furthermore, emerging evidence indicates that the novel criteria for the classification of subjects with steatosis according to clinical phenotype will allow better identification of patients with normal weight and steatosis [29].
We will use in the following paragraphs the terms MASLD and MASH, which have de facto replaced prior terms. However, we are aware that current evidence may not be strong enough to convincingly allow a full translation of data obtained on NAFLD in the last decades, and on MAFLD in the last 4 years, as they pertain to the newest disease definition. We briefly discuss the main clinical and pathogenetic aspects of MASLD. We also discuss the most important and recent advances of pharmacotherapy proposed in the management of subjects with fat over-storage in the liver. In this respect, the terminological change from NAFLD to MAFLD and MASLD has been paralleled, in the last decade, by significant advancement in the knowledge of mechanisms linking the steatotic liver with systemic pathogenic pathways leading to increased cardio-metabolic risk. This, in turn, has generated a number of pharmacological approaches ranging from antidiabetic drugs to agonists acting on the gut–liver axis and at a systemic level (mainly farnesoid X receptor [FXR] agonists, PPAR agonists, thyroid hormone receptor agonists), anti-fibrotic and anti-inflammatory agents.
2. Clinical Manifestations
The clinical course of MASLD is highly variable and poorly predictable, although potentially lethal. The majority of individuals with MASLD will remain asymptomatic and this condition can last until decompensated cirrhosis develops [30]. Early clinical symptoms, if occur, are typically mild and include right upper quadrant pain and fatigue. Usually, ultrasonography reveals a “brighter” steatotic liver, as compared with the right kidney cortex [15]. The overall impression of the sonographer for the presence of macrovesicular hepatic steatosis of any degree has a sensitivity of 60.9% and a specificity of 100%. The sensitivity and specificity increase to 100% and 90% respectively, only if >20% of fat over-storage is present in the liver [31]. We recently showed by an artificial-intelligence-related algorithm, that ultrasonography becomes a reliable method for the evaluation and classification of liver steatosis. In fact, this imaging technique generates acceptable outcomes at least in subjects with liver fat percentage above 7.2%, i.e., very close to the accuracy of nuclear magnetic resonance [32].
Increased levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) point to hepatocellular injury [33] but they can fluctuate over time [15], and might not address the presence of fibrosis [33]. The simple fatty liver (MASL) can be diagnosed non-invasively by imaging. However, a liver biopsy is still necessary to diagnose MASH, a phenotype characterized by the presence of hepatocyte ballooning, lobular inflammation, macrovesicular steatosis, and, very frequently, perisinusoidal fibrosis [15]. These histological features contribute to the construction of the MASLD activity score (NAS), especially useful for quantifying changes during therapeutic trials [34]. A NAS of ≥5 correlates highly with a diagnosis of MASH [35].
In a meta-analysis, rates of fibrosis progression were slower in patients with simple steatosis compared to patients with MASH, i.e., 1 stage of progression over 14.3 years and 7.1 years, respectively [16]. Nevertheless, about one-fifth of patients with MASH rapidly progress towards more aggressive forms of steatohepatitis with significant fibrosis. This progression is promoted by environmental and genetic factors, which remain largely unknown [17,36]. In particular, about 60% of MASLD might progress to MASH featuring inflammatory infiltration and significant fibrosis [37]. From this point, about 22% of patients with MASH-related fibrosis will progress to cirrhosis, and about 2% might progress to hepatocellular carcinoma [19,37].
In addition, MASLD per se puts the patients at risk for several extrahepatic complications, including cardiovascular disease (CVD) and T2DM. Indeed, patients with severe liver fibrosis likely develop subclinical carotid atherosclerosis with cardiovascular diseases, which account for the majority of MASLD-related mortality [38]. Additional extrahepatic diseases include chronic kidney disease, and a few types of extrahepatic cancers [39,40]. Given that the global prevalence of MASLD is about 30%, death rates from extrahepatic cancers in MASLD far exceed those from hepatocellular carcinoma. Of note, the increased extrahepatic cancer risk in MASLD is not dependent on the liver fibrosis stage. Thus. the burden of MAFLD is going to be huge in the near future in terms of access to care, and social costs.
3. Pathogenesis
The pathogenesis of MASLD (and specifically MASH) is complex and results from the interactions of genetic and environmental factors. This interplay leads to systemic metabolic dysfunction involving the liver, with deranged molecular pathways and cell-cell communication between hepatocytes, liver sinusoidal cells, stellate cells, Kupffer cells, and recruited immune cells [17]. Variable combinations of these factors may generate highly different clinical phenotypes among individuals with MASLD. For this reason, finding a single effective treatment has become a major challenge, while the need to adequately characterize subjects with MASLD is also justified for the goal of developing personalized management of the disease.
3.1. Genetic Aspects
Genetic predisposition accounts for the alteration of molecular pathways in liver cells, such as intrahepatic lipolysis, export of triglycerides, mitochondrial oxidation, and glycolysis [41,42,43,44,45,46]. The variant in the “patatin-like phospholipase domain-containing 3” (PNPLA3) gene on chromosome 22 is associated with modifications of retinol metabolism and variable manifestations of MASLD [46,47,48]. Single nucleotide polymorphisms (SNPs) in genes involved in insulin resistance have also been described in individuals with MASLD, including the genes of “ectoenzyme nucleotide pyrophosphate phosphodiesterase 1” (ENPP1 or PC1) and “insulin receptor substrate-1” (IRS1) [49]. The variant in the “transmembrane 6 superfamily member 2” (TM6SF2) gene on chromosome 19 appears to be associated with an impaired lipid transporter and fatty liver disease [50]. SNPs in genes involved in oxidative stress and increases the risk of fibrosis, i.e., “membrane-bound O-acyl-transferase domain-containing 7” (MBOAT7-TMC4) genes, have also been described in patients with MASLD [51]. Additional findings involve genetic variants in the genes of “glucokinase regulatory protein” (GCKR) [52], “solute carrier family 2-member 1” (SLC2A1) [53], and “17-beta hydroxysteroid dehydrogenase 13” (HSD17B13) [54].
3.2. Derangement of Lipid Homeostasis
Metabolic alterations develop at different levels and involve the abnormal expansion of visceral adiposity, an important site of lipid accumulation when subcutaneous adipose tissue capacity is surpassed. This condition is predisposing to increased efflux of free fatty acids (FFA) to the liver. Further steps include the development of insulin resistance with activation of pro-inflammatory cytokines and hormones, and qualitative and/or quantitative changes of gut microbiota, predisposing to “leaky gut” and impaired gut–liver axis [17,55,56,57,58]. The role of diet in increasing the influx of dietary FFA and carbohydrates is also outstanding. Under such systemic changes, the liver becomes the major target organ dysfunction due to modified insulin signaling, lipogenesis, and mitochondrial/microsomal dysfunction [58,59,60].
In MASLD, hepatocellular lipid accumulation, mainly in the form of triglycerides, is the consequence of the imbalance between lipid input and output. Free fatty acids (FFAs) represent the main substrate for triglyceride synthesis by esterification [61]. Upon excessive FFA accumulation in the liver, a cascade of harmful consequences includes lipo-toxicity [62,63,64], mitochondrial and endoplasmic reticulum dysfunction [65], activation of signaling pathways related to metabolism and inflammation [66], and receptor activation which will promote inflammation [67]. Not only FFAs but also de novo lipogenesis (DNL) intermediates, including diacylglycerol, are responsible for further disruption of metabolic homeostasis [68,69]. These steps result in excessive production of reactive oxygen species (ROS), which originate from impaired mitochondrial function [70,71]. At least initially, FFAs are esterified and transported via serum very low-density lipoprotein (VLDL) and/or oxidized with conversion to other substrates. This initial defense mechanism ultimately fails if FFAs excess influx overwhelms the mitochondria’s capacity to metabolize FFAs, leading to increased ROS production. This is the first step that accounts for the potential evolution from simple steatosis to MASH [71,72,73].
FFAs originate from three main sources (Figure 2): (a) spontaneous lipolysis of adipose tissue contributes to about 60% of the total influx of FFAs to the liver; (b) DNL contributes to about 25%; and (c) excessive dietary FFAs will contribute to about 15%. The pathway of lipolysis starts with the production of cyclic adenosine monophosphate (cAMP). Protein kinase A (PKA) is then activated to phosphorylate specific lipases, namely phospho-hormone-sensitive lipase (p-HSL) and phospho-perilipin 1 (p-PLIN1). Insulin inhibits this pathway [74].
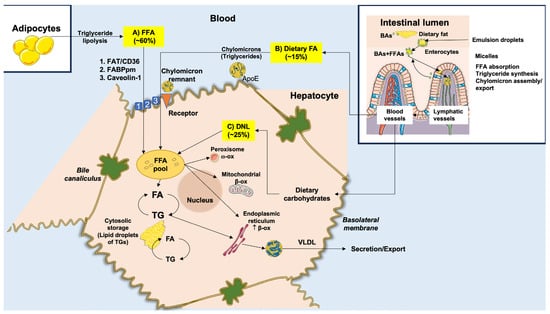
Figure 2.
Origin and metabolism of free fatty acids (FFAs) in the liver. FFAs are supplied to hepatocytes from three major sources. (A) About 60% of the total FFA pool derives from the uptake of circulating FFAs that originate from the lipolysis of triglycerides (TGs) in the adipose tissue. FFAs enter hepatocytes across specific transporters, such as (1) fatty acid translocase/cluster of designation 36 (FAT/CD36) transporter, (2) fatty acid binding protein (FABPpm), and (3) caveolin-1. (B) About 15% of the total FFA pool derives from dietary FFAs. In the intestinal lumen, within enterocytes, FFAs are incorporated into TGs of chylomicrons, following ingestion of fat, with the help of conjugated bile acid (BAs) micelles. Chylomicron remnants are taken up by specific receptors in the hepatocyte with a high affinity for ApoE. (C) About 25% of the total FFA pool originates within the hepatocytes from de novo lipogenesis (DNL), utilizing dietary carbohydrates. The hepatocellular FFA pool can undergo peroxisome ω-oxidation, mitochondrial β-oxidation, endoplasmic reticulum β-oxidation, or re-esterification with glycerol to form TGs. TGs can be stored in lipid droplets in small amounts (<5%) or exported into the circulation as very-low-density lipoproteins (VLDL) which are assembled in the endoplasmic reticulum. Right inlet: stars represent BAs. Adapted from Di Ciaula et al. [41].
Following lipolysis, circulating FFAs are directed to the liver [75]. In MASLD, these mechanisms are upregulated in the adipose tissue and are independent of the presence of diabetes [76,77,78]. If obesity develops in the background of adipocyte hypertrophy and insulin resistance, lipolysis will also increase with excessive transport of FFAs to the liver (Figure 3) [79].
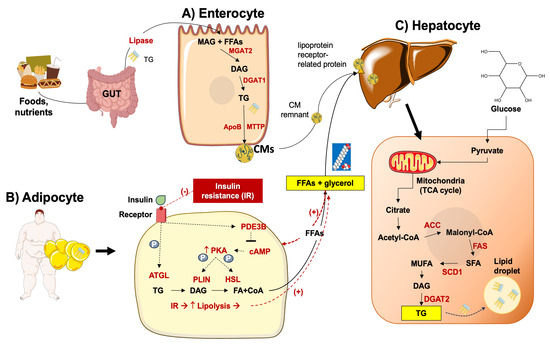
Figure 3.
Lipid metabolism in MASLD. (A) At the gut level, lipase facilitates the breakdown of triacylglycerol (TG) into monoacylglycerol (MAG) and free fatty acids (FFAs) which in the enterocytes are re-synthesized into TG through enzymatic processes mediated by mannoside acetylglucosaminyltransferase (MGAT2) and diglyceride acyltransferase (DGAT1). TGs are transferred to chylomicrons (CMs) via the microsomal triglyceride transfer protein (MTTP), and transported via the lymphatic vessels to the liver, where remnants of CMs are absorbed post-lipolysis [80,81]. (B) In the adipocyte, insulin plays a pivotal role in lipid storage by suppressing lipolysis through the inhibition of adipose triglyceride lipase (ATGL), phosphodiesterase 3B (PDE3B), and hormone-sensitive lipase (HSL) regulated by protein kinase A (PKA) and perilipins (PLINs). However, in insulin-resistant states such as obesity or type 2 diabetes mellitus (T2DM), reduced insulin sensitivity fosters heightened lipolysis, resulting in an increased flux of FFAs to the liver. (C) In the liver, various key enzymes govern the de novo lipogenesis of saturated fatty acids (SFA), monosaturated fatty acids (MUFA), diacylglycerol (DAG), TG, and include including acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD1), and DGAT2. Another important pathway includes the transformation of glucose to pyruvate, which then enters the mitochondrial tricarboxylic acid cycle (TCA), with the production of citrate [82].
DNL utilizes glucose-derived acetyl-CoA subunits [83] condensed with a glycerol backbone [84]. Proteins involved in the transcriptional regulation of DNL are the sterol response element binding protein (SREBP1c) and the carbohydrate response element binding protein (ChREBP) [85,86]. This process develops along with the genetic upregulation and activation of FA synthase (FAS), acetyl-CoA carboxylase (ACC), and stearoyl-CoA desaturase 1 (SCD1). Acetyl-CoA is then transformed into malonyl-CoA via the catalytic activity of ACC [87]. Acyl carrier protein (ACP), a component of the FAS domain, transports malonyl-CoA to the prosthetic phosphopantetheine group of the acyl carrier protein (ACP) [88,89,90], and the elongating FA chain is transported to different catalytic centers at the active site cleft of FAS by its rotation [91,92,93]. This reaction contributes to the elongation of the 16- or 18-carbon FFA chain [94,95], the initial step of triacylglycerol (TG) synthesis. FFAs are incorporated into glycerol-3-phosphate via primary acylation. The result is lysophosphatidic acid (LPA) via glycerol-phosphate acyl transferase (GPAT) [84]. The desaturated acylglycerol-phosphate acyl transferase catalyzes LPA to phosphatidic acid (PA). PA is dephosphorylated by phosphatidic acid phosphorylase (PAP) and diacylglycerol (DG) is produced [96]. The catalytic activity of diacylglycerol acyl-transferase (DGAT) results in DG acylated to TG [80]. The process of DNL increases FFA synthesis and inhibits β-oxidation by the intermediate product malonyl coenzyme [97].
In the gut, the pancreatic lipase transforms the dietary triacylglycerol into FFAs and monoacylglycerol. In the enterocytes, triacylglycerol is resynthesized by monoacylglycerol acyltransferase 2 (MGAT2), and then by DGAT. Triacylglycerol is incorporated into chylomicrons which are secreted into lymphatic vessels. FFAs, after catalysis by lipases, are stored in adipose tissue or utilized by muscle tissue as an energy source. After lipase catalyzation, chylomicron (CM) remnants are absorbed by the liver [98], whereby FFAs form triglycerides and become incorporated into VLDL particles to be exported into the bloodstream (Figure 3) [80,99]. If the absorption of CM remnants increases, the accumulation of lipids in the liver also increases [100,101].
There is evidence that nuclear receptors can be activated or become dysfunctional in MASLD, as found for the bile acid (BA) receptor farnesoid X receptor (FXR) [102,103], the liver X receptor (LXR) [104], the pregnane X receptor (PXR) [105], and the vitamin D receptor (VDR) [106]. Deranged nuclear receptors can contribute to the onset of hepatic inflammation and activation of abnormal inflammatory pathways. In addition, the activation of hepatic stellate cells triggers a fibrogenic response with the production of an extracellular matrix [107].
Dietary factors involving excess consumption of carbohydrates and saturated fat lead to metabolism imbalance at the level of the liver and skeletal muscle. In particular, lipid deposition increases in skeletal muscle, and this step, together with the onset of insulin resistance, leads to increased intramyocellular lipid content and to parallel inhibition of storage of ingested glucose as muscle glycogen [108]. As a consequence, glucose is rerouted to the liver on the background of ongoing insulin resistance, which will further promote DNL. Activated transcription factors are SREBP1c [109] (with increased VLDL production and hypertriglyceridemia), the carbohydrate-responsive element–binding protein (ChREBP) [110], the LXR [111], and the peroxisome proliferator-activated receptor gamma coactivator 1-beta (PPARg coactivator 1-b) [112]. Additional factors involved in the pathogenesis of MASLD include increased levels of endogenous lipoprotein lipase (Lpl) inhibitors, with a decreased clearance ability of circulating triglyceride-enriched lipoproteins by Lpl [113]. Lpl inhibitors include apolipoprotein C3 (ApoC3) [114], angiopoietin-like proteins 3/8 (ANGPTL3/8) complex, and ANGPTL4, among others [115]. These molecular mechanisms collectively promote the hepatocellular uptake of triglycerides.
3.3. Derangement of Carbohydrate Homeostasis
Glucose metabolism is involved in the onset and progression of MASLD/MASH [116,117]. MASLD patients have increased levels of enzymes governing glycolysis which lead to enhanced glycolytic capacity such as hexokinase 2 (HK2) and pyruvate kinase isozyme type M2 (PKM2). One consequence of MASLD is the abnormal accumulation of triglycerides in hepatocytes [118,119]. Upon an increase in glucose transport to the liver, glycolysis will increase. Pyruvate is converted to oxaloacetate and more substrates become available for DNL. Alternatively, pyruvate can be converted to lactate, which decreases the activity of histone deacetylase (HDAC), a step stimulating the DNL pathway [118,120] (Figure 4). Increased levels of lactic acid stimulate the uptake of FFAs by hepatocytes and promote the expression of lipogenic genes [120]. A further step leads to oxidative stress and DNA damage in the stage of steatohepatitis with mitochondrial dysfunction and a deranged TCA cycle [41,121,122]. Insulin resistance, either hepatic or systemic, has negative reflections on MASLD. Hepatic insulin resistance can be triggered by short-term consumption of high-fat diets, and this condition develops in the absence of peripheral insulin resistance [123]. A consequence of insulin resistance is the disinhibition of gluconeogenesis [79], resulting in increased production of glucose [124] and increased DNL. The effects of insulin in promoting DNL involve the activation of the liver X receptor (LXR), with upregulation of Chrebp1 and Srebp1 genes [104]. Insulin also inhibits microsomal triglyceride transport protein (MTTP) and promotes apolipoprotein B (ApoB) degradation, both involved in the regulation of VLDL production. With insulin resistance, the uptake of FFAs increases in the liver, along with decreased phosphorylation of forkhead box transcription factor 1 (FoxO1), with the increase in MTTP [125] and the degradation of ApoB (Figure 4) [126].
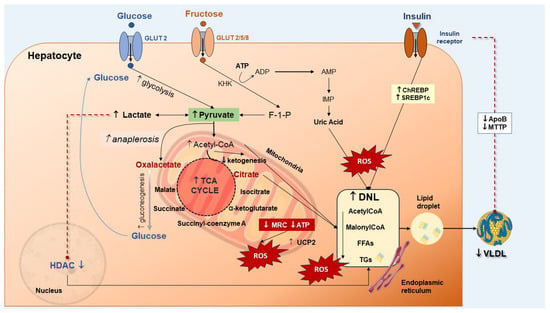
Figure 4.
The interplay between glucose, fructose, insulin, and de novo lipogenesis (DNL) in MASLD. Increased glucose transport into the hepatocyte increases the glycolysis and pyruvate synthesis which contributes to the tricarboxylic acid (TCA) cycle. Increased pyruvate can be converted either to lactate or oxaloacetate via anaplerosis [127]. Conversion of pyruvate to lactate inhibits the histone deacetylase (HDAC) activity, thereby stimulating DNL. Production of oxalacetate is associated with increased gluconeogenesis, glucose production, and DNL. Both oxaloacetate and lactate are enhanced in MASLD [41,128]. Fructose enters the hepatocyte and is rapidly phosphorylated to fructose-1-phosphate (F-1-P) by the ketohexokinase (KHK). Adenosine triphosphate (ATP) hydrolysis to adenosine diphosphate (ADP), to adenosine monophosphate (AMP) and inosine monophosphate (IMP) provides increased uric acid levels which further contributes to DNL. Increased insulin upregulates the liver carbohydrate-responsive element-binding protein (ChREBP) and the sterol regulatory element-binding protein 1 (SREBP-1), which increases DNL with free fatty acids (FFAs) storage as triglycerides (TGs). Insulin also reduces very-low-density lipoprotein (VLDL) production through downregulation of the synthesis of the microsomal triglyceride transfer protein (MTTP) and apolipoprotein B (ApoB) [41]. Production of reactive oxygen species (ROS) which promote inflammation and hepatocellular injury can depend on increased glycolysis and FFA oxidation with acetyl-CoA abundance and enhanced activity of the TCA cycle. At the same time, ketogenesis is reduced. Moreover, the activity of the mitochondrial respiratory chain (MRC) is reduced, increasing the ROS generation. Uncoupling protein (UCP2) expression increases in MASLD. This step is associated with impaired efficiency of ATP synthesis and decreased ATP content [127].
Fructose has profound effects on metabolic homeostasis [129]. It is another player in the pathways involved in MASLD and can worsen hepatic steatosis [130]. At variance with glucose, fructose in the liver is catalyzed by phosphofructokinase, a step that leads to increased substrates for the DNL pathway [130]. There will be a continuous decrease in ATP and phosphate [131,132,133,134] leading to an increase in uric acid, ATP deficiency [135], inhibition of protein synthesis, and increased oxidative stress [134,136]. Fructose is able to stimulate the DNL pathway while inhibiting β-oxidation through ChREBP and SREBP1c, and less FFA consumption [136,137]. These steps worsen steatosis (Figure 4). Additional effects of fructose include the potential for induction of gut dysbiosis, further production of SCFAs, and increase in intestinal permeability, a condition which increases the endotoxin flow to the liver [138,139,140].
3.4. Deranged Bile Acid Homeostasis
The gut microbiota has a role in maintaining systemic homeostasis, and this function is partly dependent on the metabolism of bile acid (BA)-mediated signal transduction together with specific receptors [141]. Disrupted BA metabolism and function contribute to the damage observed in chronic liver diseases [103], which also includes the sequence of steatosis to steatohepatitis, independently of obesity and diabetes [142].
Figure 5 depicts the main pathways regulating BA homeostasis in the entero-hepatic circulation. Inhibiting the ileal/colonic reabsorption of BAs partly interrupts the enterohepatic circulation of BAs and enhances their fecal excretion. This, in turn, prompts the conversion of more cholesterol to BAs, thereby reducing the risk of obesity [143]. Particular relevance has the hormonal function of BAs as ligands for FXR and the membrane-associated G protein-coupled bile acid receptor 1 (GPBAR-1, previously known as Takeda G protein-coupled receptor 5, TGR5) during their absorption in the terminal ileum [102]. In the liver, FXR deactivates the lipogenesis pathway by inhibiting SREBP1c. Furthermore, FXR induces β-oxidation by activating peroxisome proliferator-activated receptor-α (PPARα) and facilitates the clearance of VLDL in plasma, ultimately improving metabolic dysfunction in MASLD [144,145,146]. Furthermore, hepatic FXR promotes the oxidation of FFAs and ketogenesis, a process that relies on fibroblast growth factor 21 (FGF21) [147,148]. In parallel with these events, the activation of intestinal FXR induces intestinal epithelial cells to release FGF15/19 into the liver, effectively diminishing hepatic steatosis and enhancing insulin resistance [149,150,151,152]. The impact of FXR on MASLD remains a topic of debate, due to its widespread distribution in various tissues. Recent findings indicate that global knockout of FXR resulted in improved insulin sensitivity in ob/ob and high-fat diet (HFD) mice. This improvement might be attributed to the notion that prolonged activation of FXR diminishes energy expenditure and exacerbates HFD-induced glucose intolerance (Figure 5) [153,154,155]. In mice with liver-specific FXR knockout, however, this effect was not observed, suggesting a significant contribution by intestinal FXR [156]. Concurrently, it has been shown that elevations in the level of T-β MCA, an intestinal FXR antagonist, improve MASLD by enhancing the synthesis of BAs [157,158,159]. Additionally, the activation of intestinal FXR leads to a reduction in GLP-1 secretion [160]. Therefore, the role of intestinal FXR in preserving metabolic homeostasis requires further validation (Figure 5). Another BA receptor, GPBAR-1, is predominantly expressed in the gallbladder, adipose tissue, intestine, and liver, and is activated primarily by secondary BAs [161]. Once GPBAR-1 is activated in muscles or brown adipose tissue, it stimulates energy consumption, and in the intestine, it increases the secretion of GLP-1 (Figure 5) [103,162,163]. Recent studies also found that GPBAR-1 has beneficial effects on MASLD-related hypothyroidism, regardless of the level of thyroid hormone [164]. Thyroid hormone β receptor (TRβ) regulates the synthesis of BAs by interfering with SHP [165,166] or CYP7A1 directly in the liver [167]. It has also been reported that activation of TRβ reduces systemic lipid content and increases lipid oxidation to improve hepatic lipid homeostasis [168].
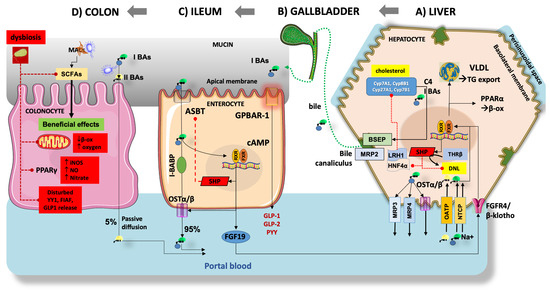
Figure 5.
The interaction between bile acid (BAs) and gut microbiota is shown in the liver, the gallbladder, the terminal ileum, and the colon. Derangement of pathways at several levels can play a role in MASLD (see text for details). (A) Starting from cholesterol in the hepatocyte, the classical pathways use the oxysterol 7α-hydroxylase (CYP7A1), and CYP8B1, resulting in 7α-OH-4-cholesten-3-one (C4) and then “primary” BAs (I BAs) cholic acid (CA) and chenodeoxycholic acid (CDCA). The alternative pathway relies on CYP27A1 and CYP7B1, resulting in small amounts of CDCA [169,170]. Primary BAs are promptly conjugated (symbol O) with taurine and glycine, to increase solubility in bile [102]. The transport of BAs from the hepatocyte includes several pathways. Approximately 5% of BAs are transported to the systemic circulation via the multidrug resistance-associated protein 3 (MRP3), MRP4, and the organic solute transporter (OSTα/β). Basolateral import of BAs is mediated by sodium taurocholate co-transporting polypeptide (NTCP) (Na+-dependent) and organic-anion-transporting polypeptide (OATP) isoforms (Na+-independent). Intracellular BAs contribute to the negative feedback regulation of BA synthesis via the activation of farnesoid X receptor (FXR)-retinoid X receptor (RXR)-dependent pathways. These pathways increase the small heterodimer partner (SHP) expression and inhibit the hepatocyte nuclear factor 4α (HNF4 α) and nuclear receptor liver receptor homolog-1 (LRH1) which, in turn, leads to decreased activity of CYP7A1 and CYP8B1 [171]. Activation of the FXR-SHP pathway also inhibits de novo lipogenesis (DNL), promotes peroxisome proliferator-activated receptor α (PPARα) β-oxidation, and stimulates very-low-density lipoprotein (VLDL) production and TG export [144,145]. The nuclear thyroid hormone receptor β (THRβ) also contributes to DNL and works in concert with the above-mentioned nuclear receptor pathways. Conjugated BAs are secreted in bile canaliculus by the bile salt export pump (BSEP) and multidrug resistance-associated protein 2 (MRP2), and aggregate as micelles and vesicles with secreted cholesterol and phospholipids. (B) Bile enters the gallbladder to be temporarily stored and concentrated during fasting. Upon consumption of a fat-enriched meal, the cholecystokinin release prompts gallbladder contraction and secretion of bile/BAs into the duodenum. (C) In the terminal ileum, approximately 95% of BAs undergo reabsorption by enterocytes through the apical sodium-dependent bile salt transporter (ASBT), transported via the ileal bile acid-binding protein (I-BABP), and subsequently excreted into the portal vein via OSTα/β [172]. In humans, the BA-induced activation of ileal FXR has several consequences, including the activation of SHP with inhibition of ASBT, the RXR-mediated activation of OSTα/β and the fibroblast growth factor 19 (FGF19) production and secretion into the portal blood. Upon reaching the liver, FGF19 binds the liver FGFR4/β-klotho receptor with effects on FXR, with the above-mentioned effects on BA synthesis and DNL [173]. In the ileum, the activation of the membrane BAs receptor G-protein coupled BA receptor-1 (GPBAR-1) increases the cyclic adenosine monophosphate (cAMP) and increases the secretion of glucagon-like peptide-1 (GLP-1), GLP-2 and peptide YY (PYY) leading to a number of systemic metabolic effects. (D) In the colon, small amounts of primary BAs undergo bacterial biotransformation to unconjugated secondary BAs (II BAs) deoxycholic acid (DCA), and lithocholic acid (LCA) which are passively transported back to the liver. Under healthy conditions, undigestible dietary fibers represent the microbiota accessible carbohydrates (MACs). These are fermented by the local microbiota and produce short-chain fatty acids (SCFAs), mainly butyrate, acetate, and propionate. SCFAs are actively transported in the colonocyte to produce local beneficial effects, including anaerobic conditions maintenance through β-oxidation, decreased nitrate production, and balanced metabolic homeostasis in conjunction with peroxisome proliferator-activated receptor gamma (PPARγ). SCFAs also contribute to metabolic stability through the secretion of GLP-1, fasting-induced adipose factor (FIAF), and Yin-Yang 1 (YY1) [174,175]. These mechanisms are highly impaired at the onset and progression of MASLD and gut dysbiosis (red pathways).
3.5. Gut Dysbiosis
The gut microbiota is an important player in regulating metabolic homeostasis, and deranged bacterial populations or the onset of dysbiosis can be a predisposing condition for NAFLD [56,57,175]. Indeed, the microbiota profile can differ between obese and lean individuals [55]. The gut microbiota consists of trillion of bacteria that contribute to intestinal barrier protection, selective permeability and immune responses, and maintenance of metabolic balance within the host [176]. Such beneficial effects require the fiber-dependent transformation of gut carbohydrates to SCFAs [177]. Either qualitative and/or quantitative changes of microbiota in response to acute or chronic conditions can evolve as dysbiosis and promote a variety of metabolic disorders [55]. Over-nutrition stands out as a pivotal factor that can influence the composition of the gut microbiota [178]. Chronic low-grade inflammation is a recognized characteristic of MASLD. Inflammatory mediators, including endotoxin, originate from the gut microbiota [179], especially in the context of a high-fat diet, where endotoxin level is increased [180,181]. Recent evidence points to the important role of gut microbiota in MASLD [57,60]. Indeed, a high-fat diet leads to an elevation in certain bacteria, such as Enterobacter cloacae B29, Escherichia coli py102, and Klebsiella pneumoniae A7. These specific bacteria have been identified as contributors to NAFLD progression [182]. Furthermore, at the advanced stage, populations like Proteus and Escherichia coli can increase, whereas the abundance of Firmicutes was notably reduced [183]. Ruminococcaceae and Veillonellaceae have been associated with an increased risk of liver fibrosis [184]. Research has indicated that gut dysbiosis, particularly when dominated by Enterobacteriaceae, Escherichia coli, and Shigella, is linked to the progression of MASLD [185].
A recent study examined gut microbiota in subjects with morbid obesity undergoing bariatric surgery [186]. Subjects with histologically confirmed steatosis/MASH showed microbiota enrichment with Enterobacteriaceae, i.e., ethanol-producing bacteria, Acidaminococcus and Megasphaera, and depletion of Eggerthellaceae and Ruminococcaceae, i.e., SCFAs-producing bacteria. The microbiota patterns changed in subjects with hepatic steatosis, necroinflammatory activity, or fibrosis, mainly in terms of increased Enterobacteriaceae and decreased Ruminococcaceae. Specifically, Escherichia coli was associated with steatosis and necroinflammatory activity, and Escherichia-Shigella was associated with fibrosis and necroinflammatory activity [186].
Several studies have shown an association between metabolic dysfunction and reduced concentrations of bacteria responsible for producing SCFAs, specifically propionate and butyrate [187]. Butyrate has the potential to serve as a substrate, promoting β-oxidation to sustain an anaerobic environment crucial for the microbiota [188]. Butyrate suppresses the expression of nitric oxide synthase through the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ). This leads to a reduction in nitric oxide (NO) which, in turn, hinders the growth of Enterobacteriaceae [189,190]. Butyrate can mitigate inflammatory conditions by activating immune cells, specifically regulatory T cells (Tregs) [191]. Furthermore, SCFAs play a beneficial role in preserving intestinal permeability and enhancing insulin secretion and sensitivity by promoting the increased secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) (Figure 5) [174,192,193]. Gut dysbiosis may increase the severity of MASLD due to a decrease in SCFAs [194]. Particularly, diminished levels of SCFAs are associated with a decreased abundance of Faecalibacterium prausnitzii [195], Akkermansia Muciniphila [195], and Dysosmobacter welbionis [196]. Furthermore, disruption of the gut microbiota inhibits the ability of intestinal epithelial cells to release a lipoprotein lipase inhibitor, the fasting-induced adipose factor (FIAF), thereby leading to elevated levels of FFAs in the liver [175].
4. Therapeutic Management of MASLD
MASLD spectrum of disease is associated with several abnormalities which involve lifestyle, visceral adiposity, skeletal muscle, gut microbiota, and permeability, with dysregulated gut–liver axis [197]. Since the clinical course of MASLD is variable, therapeutic options consist of both general, metabolically-oriented, and liver-specific options (Figure 6). This latter option is especially indicated as the hepatic burden increases in individuals with disease activity testified by increased NAS, presence of NASH, and fibrosis who are at risk of fast progression to cirrhosis and liver-related events [35].
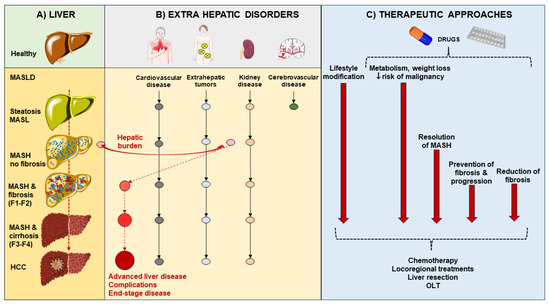
Figure 6.
Potential progression of metabolic dysfunction-associated steatotic liver disease (MASLD) phenotypes in accord with extrahepatic disorders and therapeutic approaches. (A) Starting from the healthy liver, the hepatic burden of MASLD consists of simple steatosis (metabolic dysfunction-associated steatotic liver, MASL), necro-inflammatory status (metabolic dysfunction-associated steatohepatitis, MASH), fibrosis, cirrhosis and hepatocellular carcinoma (HCC). Fibrosis stages F1–F4 are reported. (B) The extrahepatic disorders are depicted, with the main concerns as cardiovascular disease, extrahepatic tumors, and kidney disease. As soon as MASH is demonstrated, the hepatic burden of the disease moves forward and becomes a main concern because of the potential progression to advanced liver disease, complications, and end-stage disease. (C) The mainstay of therapeutic approaches whenever possible consists of early prevention (lifestyle modification) of both hepatic and extrahepatic disorders. At a later stage, the use of precision medicine consists of personalized drugs targeting metabolism, body weight, and risk of malignancy. With MASH, further therapeutic approaches are aimed at the resolution of MASH, prevention of fibrosis and progression, or reduction of fibrosis. With cirrhosis and HCC, specific chemotherapy, locoregional treatments, liver resection and liver transplant (OLT) must be taken into account.
Besides counterbalancing the caloric surplus and physical inactivity (possibly also through bariatric surgery or bariatric endoscopy), individuals with MASLD should be managed by improving the systemic metabolic homeostasis [198], dysregulation of glucose and lipid metabolism [82], and eventually correct the involvement of skeletal muscle (i.e., myosteatosis, sarcopenia, and release of myokines) [199,200], of intestinal dysbiosis [55,57], and leaky gut [60,176]. Systemic- and liver-specific therapy strategies can target liver fat deposition and insulin resistance, oxidative stress, endothelial cell injury, and inflammation. The overall spectrum of available treatments currently available is depicted in Figure 7.
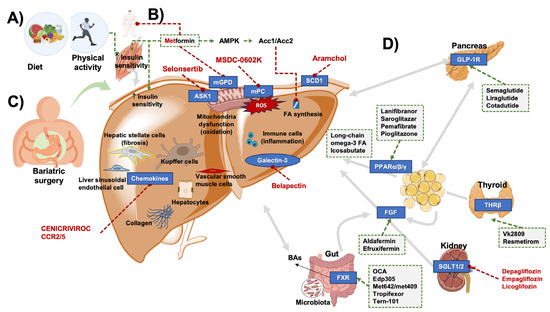
Figure 7.
Potential therapeutic approaches in MASLD. Due to the complex interplay between pathogenesis, pathways, and organs involved, several options are being tested. (A) lifestyle including a healthy, balanced diet and regular physical activity improve insulin sensitivity and liver steatosis. (B) Whenever indicated, metformin can bring beneficial effects. (C) Bariatric surgery can play a role in the subgroup of severe obesity and increased cardiovascular risk. (D) With respect to drugs, effects can target the liver, several organs, and the microbiota, acting on specific pathways (see text for details). Green arrows indicate activation; red lines with dots indicate inhibition; grey arrows indicate interplay between organs. Abbreviations: Acc1/2, acetyl-CoA carboxylase 1, 2; AMPK, AMP-activated protein kinase; ASK1, apoptosis signal-regulating kinase-1; BAs, bile acids; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; mGPD, mitochondrial glycerophosphate dehydrogenase; mitochondrial pyruvate carrier (mPC); PPAR, peroxisome proliferator-activated receptor α/β/γ; ROS, reactive oxygen species; SGLT1/2, sodium-dependent glucose transporters 1,2; SCD1, stearoyl-CoA desaturase-1; THRβ, thyroid hormone receptor β.
4.1. Lifestyle: Diet and Physical Exercise
Increased physical activity generates beneficial effects on metabolic disorders and associated conditions such as liver steatosis, and gallstone disease. These effects involve gut motility, the signaling role of BAs through their enterohepatic circulation, and a beneficial modulation of intestinal microbiota and inflammation [201]. Lifestyle remains the most evidenced approach in MASLD. However, it lacks long-term efficacy [202]. MASH might improve in about 58% of cases when body weight loss is >5% of initial body weight. The success rate is higher (about 90% of cases) if weight loss is >10% of initial body weight [203]. The dietary pattern must be tailored to a hypocaloric, low-fat, low-carbohydrate, or Mediterranean-type diet. Isocaloric diets with high protein content decrease hepatic steatosis and inflammation in T2DM patients [204].
A large systematic review and meta-analysis explored the effects of the Mediterranean diet and calorie restriction in patients with MASLD. Both dietary interventions improved hepatic steatosis and liver stiffness, with a dose-response relationship between the degree of calorie restriction and the beneficial effects in terms of liver function and weight loss [205]. A recent meta-analysis showed that intermittent fasting regimens lasting 2–3 months are generally able to reduce steatosis and ameliorate metabolic homeostasis, improving liver function in patients with MASLD. Evidence, however, is still derived from a limited number of studies, and the impact of diet needs to be further explored [206].
Exercise can reduce hepatic steatosis and improve liver stiffness [207], independently of dietary changes [208]. A moderate-vigorous exercise prevented fatty liver in 233,676 subjects enrolled in a five years follow-up [209]. The best results occurred in individuals exercising over 250 min a week [210]. The amelioration occurs in MASLD if the level of exercise is sufficient, regardless of whether aerobic exercise is performed [211]. Nevertheless, the effects of lifestyle interventions are rather slow, require high compliance in the medium- long-term [203,212], and can be counteracted by a number of factors, including stress and sedentary lifestyle, as reported in a study focusing on people during COVID-19 lockdown [213]. A recent meta-analysis in patients with biopsy-proven NAFLD found that exercise alone is not able to significantly improve NAFLD score or fibrosis, and does not lead to histopathological improvement [214]. In a recent paper exploring the outcome of obese subjects undergoing bariatric endoscopy [215], results obtained after lifestyle modification alone were poor. Despite decreased CAP values obtained by elastography (i.e., decreased extent of steatosis), results showed a modest weight loss (on average 4 Kg), unchanged BMI, and, of note, unmodified grade of liver fibrosis. Furthermore, in patients with fat over-storage, liver steatosis, and insulin resistance, diet, and physical activity had a minor role in the onset of subclinical liver dysfunction (i.e., decreased hepatic extraction efficiency, impaired liver microsomal function), as assessed by (13C)-methacetin breath test [59].
4.2. Bariatric Surgery
To date, there is debate regarding the adaptation of foregut bariatric surgery to MASLD treatment [15]. Bariatric surgery for correction of severe obesity-related comorbidities can include patients with MASLD but is not primarily performed for MASLD [216]. Some improvements in histological features, namely ballooning and lobular inflammation, are reported in about 75% of patients with steatohepatitis [217,218], despite a subgroup of patients being at risk of secondary steatohepatitis and liver fibrosis [219].
In a recent multicenter, open-label, randomized trial, 288 adult individuals with obesity with or without type 2 diabetes, and with histologically confirmed MASH were randomly assigned to lifestyle modification plus best medical care, Roux-en-Y gastric bypass, or sleeve gastrectomy [220]. As a primary endpoint, the authors searched for histological resolution of MASH without worsening of fibrosis at 1-year follow-up. The prevalence of participants meeting the primary endpoint was significantly higher in the Roux-en-Y gastric bypass group (54 [56%]) and sleeve gastrectomy group (55 [57%]) compared with lifestyle modification (15 [16%], intention-to-treat analysis). In the per-protocol analysis (236 [82%] participants who completed the trial), the primary endpoint was met in 54 (70%) of 77 participants in the Roux-en-Y gastric bypass group and 55 (70%) of 79 participants in the sleeve gastrectomy group, compared with 15 (19%) of 80 in the lifestyle modification group. Severe adverse events (6%) occurred in ten participants who had bariatric-metabolic surgery without re-operations. In this study, the authors show that bariatric-metabolic surgery is more effective than lifestyle interventions and optimized medical therapy in the treatment of MASH [220].
A recent meta-analysis involving 19 studies and a total of 911 patients evaluated the outcomes after positioning of intragastric balloon, a promising endoscopic bariatric therapy. Results showed beneficial effects on MASLD activity score, liver volume, and liver steatosis. These results were paralleled by decreased body weight, BMI, glycated hemoglobin, and extent of insulin-resistance [221].
4.3. Pharmacological Therapy
While the impact of lifestyle changes on MASLD is unquestionable, sustained results with weight management through dietary modification and physical exercise alone are notoriously difficult to achieve [222]. In the past decades, an increasing number of molecules targeting many different aspects of MASLD pathogenesis have been developed and tested [41,223,224]. Different biological targets have been identified according to the development and progression of the disease, with special attention to the histologically characterized disease pathophenotypes ranging from steatosis to inflammation to fibrosis.
Among the molecules tested in the last 10 years for the treatment of MASLD/MASH, many have been abandoned before entering clinical trials or following phase 1 and 2 evaluation. Very few drugs have reached the level of phase 3 trial (Table 1). The most promising medications in the field include antidiabetic drugs, FXR agonists, PPAR agonists, and thyroid hormone receptor (THR) agonists (Figure 7). Most recently, the tide in the long quest for MASLD pharmacotherapy seems to be turning as resmetirom has become the first drug to be approved by the FDA in March 2024 for the treatment of MASH with significant (F2 or F3) fibrosis. While the approval of resmetirom as a liver-directed medication is indeed a culmination of many years of research, it is important to note that drug combinations likely represent the way forward in MASLD pharmacotherapy, promising increased efficacy rooted in the complex pathophysiology and reduced rates of adverse events [225].

Table 1.
Phase II, III, and IV clinical trials for (N)MASH treatment.
4.3.1. Antidiabetic Drugs
The use of antidiabetic drugs mainly oriented to counteract insulin-resistance in individuals with a steatotic liver has shown variable outcomes. In three randomized trials in diabetic and prediabetic patients with MASH, pioglitazone induced some histological amelioration by improving MASLD activity score (NAS) and a single inflammatory component of MASH. The effect on liver fibrosis was absent, and worsening of liver fibrosis was not observed [239,240,241].
The pharmacological effects of metformin on liver fat storage and metabolism are still scarcely explored [242] and the use of metformin to improve steatosis and steatohepatitis is controversial, due to contradictory findings [243,244]. A major effect of metformin is to inhibit gluconeogenesis, leading to decreased hepatic glucose output [245,246,247]. The mechanism likely involves the inhibition of mitochondrial glycerophosphate dehydrogenase (mGPD), a specific mitochondrial enzyme. The inhibition decreases the conversion of glycerophosphate to dihydroxyacetone phosphate and prevents glycerol from entering gluconeogenesis [248,249]. In addition, the inhibited mGPD increases the cytoplasmic NADH with decreased conversion of lactate to pyruvate, a step limiting lactate entering the hepatic gluconeogenesis. After a meal, metformin increases insulin-sensitivity and glucose utilization, especially in the skeletal muscle and liver [250]. Via its antilipolytic effect, metformin reduces the concentration of serum FFAs, a substrate for gluconeogenesis [250,251]. This effect involves the Peutz-Jeghers protein (LKB1)-dependent activation of the enzyme AMP-activated protein kinase (AMPK) in hepatocytes [252,253] which explains the AMPK-dependent inhibitory phosphorylation of acetyl-CoA carboxylases Acc1 and Acc2 and suppression of lipogenesis and FAs synthesis in liver and muscle [254,255]. Metformin likely decreases food intake and body weight [256,257].
In patients with new-onset T2DM, metformin generated, during 2 years, an improvement of the hepatic steatosis index but a worsening of liver fibrosis, determined by the FIB-4 index [258]. However, the ability to counteract insulin-resistance, but also to activate AMP-activated protein kinase (AMPK) [259] by inhibiting mitochondrial complex 1, reducing fatty acid synthesis, inducing mitochondrial fatty acid β-oxidation, reducing the production of ROS [260], and positively modulating gut microbiota in presence of metabolic disorders [55] can significantly improve the metabolic homeostasis, in particular in patients with T2DM and a steatotic liver [55,261]. As a result, although the use of metformin alone in treating subjects with liver steatosis is not convincingly supported, so far, a number of ongoing trials oriented to the management of the steatotic liver include the use of metformin together with other anti-diabetic drugs as SGLT-2 inhibitors, pioglitazone, liraglutide, gliclazide, sitagliptin [262]. In a recent Korean retrospective, nonrandomized interventional cohort study in patients with T2DM classified as NAFLD, SGLT2 inhibitors, thiazolidinediones, and DPP-4 inhibitors, each combined with metformin, have been associated with a clinical regression of NAFLD, when compared with the combination metformin- sulfonylureas [263].
Sodium-dependent glucose transporters-2 (SGLT-2) inhibitors function as potent sodium-dependent transporters of glucose after filtration from the kidney [264]. SGLT-2 inhibitors induce glucosuria, decrease glycemia and insulin levels (in particular in patients with T2DM), reduce hepatic de novo lipid synthesis [265], and contribute to weight loss and improved metabolism. This step is partly responsible for the indirect decrease in hepatic lipid accumulation since SGLT-2 is not expressed in the liver [264]. A number of studies have documented the beneficial effects of SGLT-2 inhibitors on the steatotic liver (including liver fibrosis), although the majority of observations are in patients with T2DM or included a limited number of subjects [266]. Among the oral SGLT2 inhibitors, dapagliflozin reduces hepatic lipid accumulation without significant effects on insulin sensitivity [239,267]. A meta-analysis of 7 trials explored by imaging patients with MASLD treated with dapagliflozin 10 mg, compared to placebo or control group. Treatment decreased both ALT and AST but not gamma-glutamyl transferase (GGT) serum levels. The degree of insulin resistance assessed by the homeostatic model assessment of insulin resistance (HOMA-IR) improved. Although levels of total cholesterol increased under dapagliflozin treatment, the safety profile between groups showed no significant difference [268]. The DEAN phase 3 trial (NCT03723252) aims to compare dapagliflozin vs. placebo in patients with histologically confirmed NASH. Primary endpoints are improvement in liver histology score at one year, improvement of MASH, changes in fibrosis score, and metabolic profile such as body weight, hemoglobin A1 (Hb1Ac), or insulin resistance.
Other studies are currently evaluating the efficacy of other SGLT1/2 inhibitors in MASH, e.g., the ELIVATE study (NCT04065841) with licogliflozin alone or combined with the agonist of the BAs receptor FXR tropifexor on fibrosis and/or NAS score in patients with MASH and fibrosis stage 2 or 3.
In patients with T2DM, empagliflozin reduced plasma levels of liver enzymes and decreased hepatic accumulation of lipids. This approach can represent a treatment in patients with T2DM and MASLD [269], and appears to reduce the risk of diabetic ketoacidosis and lower extremity amputation [270].
GLP-1 analogs and other incretins represent additional antidiabetic medications. Glucagon-like peptide 1 (GLP-1) is an endogenous gut hormone (incretin) that promotes insulin production and release, inhibits glucagon secretion indirectly, and, at the same time, reduces appetite. GLP-1 analogs are widely used in the treatment of T2DM. The receptor of GLP-1 (GLP-1R) is not significantly expressed in the liver, although it is widely distributed elsewhere [271]. The general metabolic improvement via GLP-1 includes insulin sensitivity, appetite suppression, and weight loss, and is likely responsible for the improvement in MASLD. In fact, a number of studies documented beneficial effects on the liver linked with the use of GLP-1 analogs, mainly in terms of regression of hepatic steatosis and improvement of NASH. Results, however, are less dramatic in terms of reduced hepatic fibrosis [266]. GLP-1 receptor analogs represent a promising tool primarily in patients with MASLD associated with T2DM and obesity. Seven different GLP-1 receptor agonists have been approved by the US Food and Drug Administration for the treatment of type 2 diabetes (i.e., exenatide, liraglutide, dulaglutide, albiglutide, lixisenatide, semaglutide, and tirzepatide). Among these, three (semaglutide, liraglutide, and tirzepatide) have been also approved for obesity and overweight.
Exenatide, which is one of the first GLP-1R agonists, appears to directly increase hepatocyte uptake of glucose under oral glucose stimulation [228]. Exenatide stimulates the β-oxidation with downregulation of genes related to lipogenesis. This effect is beneficial to MASLD [272,273,274].
The LEAN phase 2 trial [227] reported, in patients with biopsy-confirmed MASH, histological resolution of MASH with liraglutide, another GLP-1 agonist, given at a dose of 1.8 mg daily compared to placebo. Fibrosis progression occurred in 9% and 36% of the liraglutide and placebo groups, respectively. A few side effects included gastrointestinal disorders and local reactions at the administration site. Liraglutide was effective in improving MASLD with 39% efficacy [275].
For the liver, semaglutide was first assessed in a phase 2 clinical trial lasting 72 weeks [226]. The target population was biopsy-proven MASH patients with liver fibrosis stages 1, 2, or 3, randomly assigned to semaglutide 0.1, 0.2, 0.4 mg or placebo. The study fixed as the primary outcome the resolution of MASH and no fibrosis worsening (only stage 2 or 3 fibrosis levels were assessed). The outcome was successful in 40%, 36%, 59%, and 17% of patients with 0.1, 0.2, 0.4 mg or placebo, respectively, meaning p < 0.001 for semaglutide 0.4 mg vs. placebo. The percentage of patients with improved fibrosis staging was not different from placebo (43% with 0.4 mg vs. 33% with placebo). Side effects, such as gastrointestinal and gallbladder disorders and an increase in amylase and lipase, were more common in the semaglutide groups. The ESSENCE phase 3 clinical trial (NCT04822181) is studying the effect of semaglutide on liver fibrosis in non-cirrhotic MASH patients. Results from phase 2 trials have waited for other GLP1R agonists such as cotadutide (NCT04019561), tirzepatide (NCT04166773), or efinopegdutide (NCT04944992).
4.3.2. Statins
Within the wide background of metabolic abnormalities that are associated with MASLD, hyperlipidemia includes triglyceride-rich and cholesterol-rich lipoproteins in the serum. This is a condition pointing to the increased transport of lipids to the liver. Previous small clinical trials found that atorvastatin decreases ALT serum levels while improving hepatic steatosis [276], and that rosuvastatin reduces both ALT and AST serum levels with amelioration of liver fibrosis [277]. Recent clinical trials confirmed that statins can reduce the risk of hepatic steatosis and fibrosis [278] and improve MASLD/MASH expression [279]. More studies are awaited in this field.
4.3.3. Peroxisome Proliferator-Activator Receptor (PPAR) Agonists
The family of PPAR α, γ, and δ receptors is located mainly in the liver, macrophages, and brown adipose tissue. In the liver, PPARs activate fatty acid oxidation, decrease the synthesis of TGs, and increase insulin sensitivity. In previous studies, the agonists of PPARs appear to improve NAFLD [280]. PPAR-γ influences the differentiation of adipocytes, regulates lipid and glucose metabolism, and inhibits inflammation [281]. Thiazolidinediones are potent activators of PPAR-γ and work as antidiabetic agents, in that they improve insulin sensitivity, reduce plasma FFAs, and hepatic lipid accumulation [282]. These molecules also improve fibrosis by inhibiting the activation of hepatic stellate cells [283]. Pioglitazone, a mild PPAR-γ activator, improves liver steatosis, and reduces liver enzymes but does not reduce fibrosis [284]. Its use is limited due to side effects such as weight gain and edema [285,286].
Pemafibrate is a PPARα agonist tested in MASH patients screened by MRI and ALT elevation [229]. Although the percentage change in liver fat content by MRI at week 24 was only −5.3% vs. −4.2% in controls, liver stiffness by MRI significantly decreased at week 48 and maintained at week 72 (treatment difference −6.2%).
Saroglitazar acts as a dual PPARα/γ agonist, first approved in India for the treatment of patients with T2DM and hypertriglyceridemia [287]. Saroglitazar was tested in MASLD patients diagnosed according to imaging (ultrasound, CT scan, or MRI), liver biopsy (MASH or simple steatosis), and biomarkers, i.e., ALT > 1.5 UNL (16 weeks Phase 3 trial EVIDENCES II) [235]. Saroglitazar 4 mg significantly reduced ALT levels (−45.8% vs. 3.4% treatment vs. placebo, respectively). MASH histology also significantly improved, showing decreased liver fat content (−19.7% vs. 4.1% with placebo). The safety and tolerability of saroglitazar were further assessed. Diarrhea and cough were the most frequent adverse effects, as reported in the phase 2 study EVIDENCES IV [288].
Elafibranor, a dual PPARα/δ agonist, reduced hepatic lipid accumulation, and improved inflammation and fibrosis [289]. Patients with obesity treated with elafibranor showed decreased liver enzymes and improved insulin sensitivity [290]. Elafibranor, however, failed to meet histological endpoints (i.e., NASH resolution, without worsening of fibrosis) and the secondary end-point (i.e., fibrosis improvement at least one stage) [291].
The pan-PPAR agonist lanifibranor can decrease the accumulation of hepatic lipids, liver enzyme levels, and biomarkers of plasma inflammation. In a 2b clinical trial, the drug improved liver fibrosis. Gastrointestinal side effects and weight gain were observed [292]. Volunteers are being recruited in a phase III trial. Lanifibranor is used in patients with non-cirrhotic, biopsy-confirmed highly active (stages 0–3) MASH (Phase 2b trial NATIVE). When assessing the histological resolution of MASH, the dose of 1200 mg was better than 800 mg or placebo (49% and 39%, respectively, vs. 22%). Similar results were evident for histological improvement of fibrosis (48% and 34%, respectively, vs. 29%), or both (35% and 25%, respectively, vs. 9%) [228]. Nausea, peripheral edema, anemia, diarrhea, and weight gain were the main side effects seen more frequently with lanifibranor than with placebo.
4.3.4. FXR Agonists
FXR plays a key role in the pathways involved in BAs, glucose and lipid homeostasis [293], and regulation of inflammation [102,294,295,296,297]. A complex pathway involves the sequence governing BA synthesis. In particular, luminal BAs are re-absorbed into the ileal enterocyte and interact with the nuclear FXR with upregulation of fibroblast growth factor (FGF)-19 expression. In the process of enterohepatic circulation of BAs, FGF-19 binds the specific hepatic FGFR4/β-Klotho receptor and inhibits the expression of CYP7A1, the rate-limiting enzyme of BA synthesis [28,102]. This multilevel pathway is responsible for a protective effect against the toxic accumulation of BAs in the liver and in bile canaliculi. In addition, liver FXR activation ameliorates glucose tolerance due to reduced hepatic gluconeogenesis and increased synthesis of glycogen [298]. FXR activation reduces hepatic fat accumulation via SHP expression and CYP7A1 activity [299].
The semi-synthetic BA obeticholic acid (OCA), the analog of the primary BA chenodeoxycholic acid, has been tested due to a potent agonistic effect on FRX and possible beneficial metabolic effects on hepatic lipid and glucose metabolism. However, in a large phase 3 randomized, placebo-controlled trial designed to test the long-term effects of OCA on MASH and fibrosis in patients with stage 1–3 fibrosis, OCA treatment did not meet the endpoint related to MASH resolution. A dose-dependent pruritus was reported requiring treatment discontinuation in <10% of the cases and about 50% of patients in the OCA group developed hypercholesterolemia requiring newly prescribed statins [300].
Other synthetic FXR agonists are being tested and might produce fewer adverse events than OCA. MET642 is included in a phase 2 clinical trial enrolling NASH patients (NCT0477396). MET409 alone, in a phase 1b trial, reduced liver fat content, and currently is associated with empagliflozin in a phase 2b trial. The non-BA tropifexor is another highly potent FXR agonist tested in the FLIGHT FXR phase 2 clinical trial (NCT02855164) in MASH patients with stage 1–3 fibrosis. In an initial analysis, tropifexor safely reduced hepatic fat, liver transaminases, and body weight, as compared to placebo [301].
The nonsteroidal FXR agonist cilofexor 30 mg decreased liver steatosis and reduced the content of primary BAs without significant changes in liver fibrosis in a phase 2 trial. With 100 mg, however, moderate to severe pruritus occurred [233]. Acting at different therapeutic levels might be another therapeutic option, since in a phase 2 trial testing cilofexor combined with the acetyl-CoA carboxylase (ACC) inhibitor firsocostat and the antidiabetic GLP-1 RAs semaglutide improved liver steatosis and liver biochemistry [302].
The potential therapeutic role of ACC inhibitors in MASLD must be considered. Firsocostat reduces lipid accumulation while improving liver fibrosis. The effect on de novo lipogenesis is involved and a study achieved 12 weeks of intervention. The risk of hypertriglyceridemia is increased [303]. PF-05221304 is a potent and reversible dual ACC1/2 inhibitor. In a 16-week phase 2 clinical trial, a dose of 10 mg daily decreased lipid accumulation in the liver by up to 65%. However, serum TGs increased in 8% of subjects [304].
Other FXR agonists are being tested as well. EDP305, in a phase 2 randomized, double-blind, placebo-controlled, dose-ranging trial (ARGON-1), reduced liver fat content and decreased ALT in non-cirrhotic biopsy-proven MASH patients treated during a 12-week period [305]. Mild pruritus and changes in lipid parameters occurred. EDP305 is being tested in NASH patients with stage 2–3 fibrosis in the phase 2b study ARGON-2 (NCT04378010). TERN-101 is another nonsteroidal FXR agonist tested in the LIFT phase 2 trial. This treatment decreased ALT and GGT levels in patients with stage 1–3 liver fibrosis after a 12-week course. TERN-101 beneficially affected inflammation and fibrosis measured by a non-invasive composite marker including MRI (cT1) and MRI-PDFF [306].
Fibroblast growth factor (FGF) analogs can also play a role in the therapy of MASLD. The FGF superfamily includes FGF19 and FGF21, which have beneficial effects on glucose and lipid metabolism. In animal models, the administration of FGF19 and FGF21 improves insulin sensitivity, lipid levels, and liver steatosis while ameliorating body weight and fat mass. A potential explanation is the inhibition of SREBP1 and the reduced expression of genes involved in TG synthesis [307].
Aldafermin (NGM282) is a 190-amino-acid peptide and an engineered analog of recombinant human FGF19 with a 95.4% homology. Aldafermin can inhibit BAs synthesis and regulate metabolic homeostasis. In a 24-week phase 2b trial, aldafermin decreased hepatic lipid accumulation by 7.7% but liver fibrosis did not improve among patients with MASH-related stage 2 or 3 fibrosis [308]. Aldafermin was well tolerated in another phase 2b trial, without a significant dose-dependent response in fibrosis [309].
FGF21 secretion is dependent on starvation, nutritional stress, a high-fat diet, or a nutritional restriction diet [280,281]. In addition, FGF21 can modulate obesity and hepatic metabolic homeostasis via increased energy consumption and insulin sensitivity [282]. The ultimate mechanism accounting for the hepatoprotective effect of FGF21 is still unknown [283]. In a phase II trial, the FGF21 analog PEGylated pegbelfermin (PGBF), given subcutaneously for 16 weeks, decreased hepatic lipid accumulation in NAFLD patients. However, 16% of patients developed adverse effects, such as nausea [284]. The trial testing the effect of PGBF on fibrosis in MASLD needs to report data [286].
B1344 is a long-acting PEGylated FGF21 analog that significantly reduced hepatic steatosis, inflammation, and fibrosis in cynomolgus monkeys with MASLD undergoing liver biopsies [285].
Another FGF21 analog, efruxifermin, in a phase 2 trial reduced liver fat content, improved liver function tests, fibrosis and inflammation markers, and NAS score in patients with liver fibrosis stage 1–3. The fibrosis stage improved by at least 1 point in about 50% of the patients and resolution of MASH was seen in about 30%. The safety profile was favorable [232].
4.3.5. Thyroid Hormone Receptor Beta (TR-β) Agonists
TR-β is also known as nuclear receptor subfamily 1, group A, member 2 (NR1A2), a nuclear receptor protein encoded by the THRB gene in humans. The protein is a sensor for triiodothyronine, improves hepatic regulation of lipid metabolism, plays a role in insulin sensitivity, promotes liver regeneration, and reduces hepatocyte apoptosis in the liver [223]. Pathways involved in the liver include the inhibition of the small heterodimer partner (SHP) and DNL, the activation of PPARα and β-oxidation, and the increased export of VLDL. TR-β also promotes liver regeneration and reduces hepatocyte apoptosis [310].
Selective THR-β agonists improve the conversion of T4 to T3 and likely enhance mitochondrial function, besides the anticipated beneficial metabolic effects [310,311,312]. The THR-β agonist resmetirom (MGL-3196) targets the liver and was used in a 36-week phase 2 trial in patients with MASH and fibrosis. Liver fat content decreased, and lipid metabolism parameters and liver function tests improved. In addition, lipid profile and fibrosis markers improved without affecting body weight [313]. In a phase 3 trial patients with a non-invasive diagnosis of MASLD received 100 mg/daily of resmetirom for 52 weeks. About 50% of treated patients and 8% of placebo-treated patients showed improved liver fat content while liver fibrosis improved in about 20% of treated patients vs. 10% of placebo-treated patients, as confirmed by a non-invasive test. With resmetirom, liver enzymes, lipid metabolism parameters, and inflammatory biomarkers also improve in the absence of major safety concerns. Diarrhea and nausea are the main adverse events [314]. In a phase 3 study in MASH patients with fibrosis (MAESTRO-NASH, NCT03900429), resmetirom use was associated with MASH resolution and improvement in liver fibrosis by at least one stage. In particular, 966 patients with biopsy-confirmed MASH and a fibrosis stage of F1B, F2, or F3 were randomly assigned in a 1:1:1 ratio to receive once-daily resmetirom (322 in the 80-mg resmetirom group, 323 in the 100-mg resmetirom group, and 321 in the placebo group) during 52 weeks. MASH resolution with no worsening of fibrosis was significantly achieved in 25.9% of the patients in the 80-mg resmetirom group and 29.9% of those in the 100-mg resmetirom group, as compared with 9.7% of those in the placebo group. Fibrosis improved by at least one stage with no worsening of the NAFLD activity score occurring in 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group, as compared with 14.2% of those in the placebo group. At week 24 LDL cholesterol levels decreased by −13.6% and −16.3% in the 80-mg and 100-mg resmetirom group, respectively, vs. 0.1% in the placebo group. Diarrhea and nausea were more frequent with resmetirom than with placebo. The incidence of serious adverse events was no more than 13%, and comparable across the three groups [310,315]. Based on these results, resmetirom received accelerated approval by the Food and Drug Administration on March 14, 2024, becoming the first-ever drug approved for the treatment of MASH with stage F2 or F3 fibrosis,
VK2809, a liver-directed THR-β agonist, improved liver fat content in NAFLD patients treated with two different doses in a phase 2 trial [316]. Patients are being recruited in a phase 3 RCT.
4.3.6. Anti-Fibrotic and Anti-Inflammatory Agents
Several additional compounds are tested for their anti-fibrotic and anti-inflammatory effects. Examples are GB1211 targeting galectin 3, DFV890 targeting NLPR-3, or nimacimab targeting CB1 tested in phase 1 or trialstipelukast, a leucotrien, or nitazoxanide, an antiparasitic agent in phase 2 trials. Results about safety and efficacy are therefore awaited.
Other drugs are being tested in more advanced trials, such as cenicriviroc (CVC) a small antagonist molecule administered orally, able to block chemokine 2 and 5 receptors, both involved in liver inflammation and fibrosis. CVC in the CENTAUR phase 2b trial, failed to achieve any histological improvement in NASH but CVC ameliorated liver fibrosis without worsening NASH [232]. In the AURORA phase 3 trial (NCT03028740) the interim analysis did not confirm the efficacy of CVC and the trial was prematurely interrupted.
The TANDEM phase 2b trial (NCT03517540) reported that in patients with biopsy-proven NASH with fibrosis, combined treatment with CVC plus tropifexor was safe, decreased body weight and ALT. Of note, when considering the histological endpoints, the combination of CVC plus tropifexor was not superior to either drug in monotherapy [317].
Galectin-3 is a B-galactoside-binding lectin involved in inflammatory response and fibrosis. The complex carbohydrate belapectin targets Galectin-3 [318] but in the NASH-CX phase 2b clinical trial (NCT02462967) did not have a significant effect on inflammation and fibrosis compared to placebo. Belapectin, however, decreased the hepatic venous pressure gradient in patients with NASH-related cirrhosis without esophageal varices [319]. The NAVIGATE phase 2b/3 trial (NCT04365868) will assess an 18-month course of belapectin compared to a placebo in NASH patients with compensated cirrhosis, to monitor those who will develop new esophageal varices and clinically significant cirrhosis-related events.
Selonsertib is an oral experimental inhibitor of ASK1 (apoptosis signal-regulating kinase-1). The STELLAR-3 and STELLAR-4 phase 3 trials showed that the use of selonsertib was well tolerated but did not improve liver fibrosis without worsening NASH in patients with stage 3 fibrosis or with compensated cirrhosis [236].
4.3.7. Stearoyl-Coenzyme A Desaturase-1 (SCD1) Inhibitors
Aramchol is an oral modulator of liver stearoyl-coenzyme A desaturase-1 (SCD-1), which is involved in fatty acid biosynthesis, liver steatosis, and fibrosis [320,321]. Aramchol improved liver steatosis by reducing hepatic lipid accumulation (−12.5%) after 3 months of treatment in a phase 2 clinical trial [322].
A systematic review and meta-analysis exploring the effects of aramchol vs. placebo in patients with steatotic liver and including 3 clinical trials, documented any effect of this drug on ALT, AP, glycated hemoglobin, total cholesterol, triglycerides, insulin resistance, and insulin levels [323]. However, in the ARREST phase, 2b double-blind trial, aramchol 600 mg/per day for 52 weeks in patients with overweight or obesity and prediabetes was safe, reduced liver fat by −16.7% in hepatic lipid accumulation compared to only a −5% in the placebo group. NASH and fibrosis improved with a 29.1% decrease in serum ALT and a marked improvement in fibrosis less than 1 grade. It must be noted, however, that the decrease in hepatic lipids was not statistically significant [230]. Aramchol is being compared vs. placebo in the ARMOR phase 3 trial (NCT04104321) enrolling patients with advanced fibrosis and NASH checked for NASH resolution, fibrosis improvement, and clinical outcomes during NASH progression.
4.3.8. DGAT Inhibitors and FASN Inhibitors
Trials are in progress with molecules inhibiting the intrahepatic triglyceride synthesis. The enzyme DGAT consists of DGAT1 and DGAT2 isoforms which have substrate specificities [324] and catalyze the conversion of DAG to triglycerides, a step at the end of triglyceride synthesis. DGAT2 is liver-specific and DGAT2-deficient mice develop reduced hepatic lipid accumulation compared to normal mice [325]. The DGAT2i PF-06865571 reduced liver lipid accumulation in a phase I clinical trial, despite the drug increasing the risk of diarrhea [326]. More data are awaited from another phase II clinical trial (NCT04399538) [327].
The lipogenic enzyme fatty acid synthase (FASN) inhibitors like TVB-2640 (NCT03938246, NCT04906421) are currently under evaluation either alone or in combination with other compounds. In a phase 2 study, patients received a placebo or 25 mg or 50 mg of TVB-2640 orally daily for 12 weeks. Compared to baseline, change in lipid was +4.5%, −9.6%, and −28.1% in the control group, TVB 25 mg and TVB 50 mg, respectively. Additional effects were decreased ALT and LDL levels, in a dose- and time-dependent fashion. The small sample size is one limitation of the study [328].
4.3.9. MGAT2 Inhibitors
MGAT2 is overexpressed in the liver and small intestine [329,330]. Selective MGAT2 inhibition can decrease gut TG synthesis, delay fat absorption, and decrease the risk of diarrhea while improving liver steatosis via weight loss. BMS-963,272 is a novel selective MGAT2 inhibitor that in mice with MASH improved liver inflammation and fibrosis without diarrhea. In a phase 1 trial, the use of BMS-963,272 was associated with decreased body weight, increased GLP-1 and PYY levels, and no adverse effects [331].
4.3.10. Dimethyl Peptidase 4 (DPP4) Inhibitors
The DPP4 is expressed on several cell surfaces and functions to cleave different substrates. One target is the GLP-1, and this step has a regulatory effect on diabetes [332]. Notably, individuals with MASLD have increased DPP4 levels along with hepatocyte apoptosis and fibrosis [333]. MASH inflammation and fibrosis improve in mice treated with DPP4 inhibitors [334]. One hypothesis was therefore that the inhibition of DPP4 activity can increase GLP-1 activity, with beneficial effects on MASLD. However, the DPP inhibitor sitagliptin failed to reduce hepatic lipid accumulation and NAS score in a phase 2 trial [335].
4.3.11. Ketohexokinase (KHK) Inhibitors
KHK, the rate-limiting enzyme in fructose metabolism, is responsible for the conversion of fructose to fructose 1-phosphate. Excessive fructose intake is a risk factor for MASLD [336,337,338], and is associated with increased hexokinase levels, deranged fatty acid oxidation, and enhanced DNL. In this context, the hepatic steatosis and insulin signal transduction worsen [339]. Knocking out the liver hexokinase appears to decrease the fructose-induced hepatic damage [340]. PF-06835919 and hexokinase inhibitors in clinical trials decreased hepatic lipid accumulation, despite insulin resistance did not improve [302]. Other trials are awaited.
4.3.12. Miscellanea
Icosabutate, a synthetic omega-3 fatty acid, is an eicosapentaenoic acid that resists oxidation and does not undergo liver accumulation. This molecule might protect against hepatic oxidative stress, inflammation, and fibrosis. In the ICONA phase 2b study (NCT04052516), different doses of icosabutate are used to assess the efficacy of the resolution of MASH (i.e., the disappearance of ballooning with lobular inflammation) without worsening of fibrosis.
Mitochondria are important players during steatosis and can become potential therapeutic targets as part of mitochondrial therapy [41]. The thiazolidinedione MSDC-0602 K modulates the mitochondrial pyruvate carrier (mPC), a protein complex governing the entry of pyruvate into the mitochondria [122]. In patients with biopsy-proven MASH and stage 1–3 fibrosis, the EMMINENCE phase 2b placebo-controlled randomized trial reported a dose-dependent improvement in the glycemic control and liver enzymes but failed to meet the histological outcomes, i.e., ≥2-point NAS improvement without worsening fibrosis, MASH resolution, and fibrosis reduction [341].
PXL065 is a deuterium-stabilized R-pioglitazone lacking PPAR-γ activity. PXL065 has non-genomic target activities via mitochondrial pyruvate carrier and acyl- CoA synthetase 4 inhibition and is being tested in a phase 2 trial in non-cirrhotic patients with NASH (NCT04321343). Preliminary analyses suggest that PXL065 reduces liver fat content in about 40%, and improves at least 1 fibrosis stage in about 30–50% of patients. About 30% of patients showed NASH resolution after 36 weeks of treatment with good safety. Side effects were minimal since PXL065 lacks the PPAR-γ activity of glitazones [342].
Policaptil Gel Retard (PGR) is a natural macromolecular complex covered by a European patent no. 1679009. PGR polysaccharides can reduce carbohydrate and fat absorption rates. PGR was successfully used in adolescents and adults with metabolic syndrome and T2DM and successfully reduced circulating levels of insulin, lipids, and post-prandial triglycerides. The improvement was also evident in insulin resistance and body fat distribution [343,344]. A non-inferiority effect of PGR compared to metformin was also evident in obese adults with metabolic syndrome and T2DM [345]. A recent spontaneous, longitudinal, single-blind, randomized clinical study enrolled 245 individuals with metabolic syndrome and T2DM and randomized to PGR or placebo for 24 weeks when a low-calorie diet and intensified physical activity were allowed. PGR added to lifestyle changes improved lipid and glucose metabolism-related parameters, including insulin resistance, and significantly reduced not only visceral fat but also liver fat content and related liver fibrosis severity. The effect of PGR was likely related to a reduction in the post-meal blood glucose and insulin peaks [346].
The potential role of microbiota manipulation as a therapeutic approach to MASLD must be also considered. Gut microbiota is likely involved in the pathogenesis of MASLD due to reduced bacterial diversity, altered Firmicutes/Bacteroidetes ratio, and a relative abundance of alcohol-producing bacteria [57]. Correction of gut dysbiosis can oppose the disrupted intestinal barrier and hyperpermeability, the flow of bacterial products (i.e., lipopolysaccharides), immune system and inflammatory activation in the intestine, endothelial barrier, in the liver, and at a systemic level [56,57,294]. The close link between overweight/obesity and the steatotic liver opens the venue to potential novel approaches by targeting gut microbiota [55]. For example, a randomized, double-blind, placebo-controlled pilot study with 26 diabesity patients was conducted. Patients received for 6 months a daily dose of a multispecies synbiotic containing 1.5 × 1010 CFU of a blend B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W37, L. casei W56, L. brevis W63, L. salivarius W24, Lc. lactis W58, Lc. lactis W19 plus galacto-, fructo-oligosaccharides, glucomannan, minerals and D3-B2 vitamins. Results showed significant beneficial effects on hip circumference, serum zonulin, and overall quality of life [347]. Further studies are in progress to extend such effects in patients with MASLD/MASH.
There is a rationale to consider the potential benefits of fecal microbiota transplantation (FMT) in MASLD, since the concept of gut–liver axis includes the gut microbiota which appears to be involved in the development of hepatic steatosis [56,57,294]. The gut microbiota may differ between MASLD patients and healthy people [57], and in a phase 2 randomized clinical trial, 21 MASLD patients underwent endoscopic allogeneic or autologous fecal transplantation. Although FMT reduced small intestinal permeability in MASLD, it did not improve insulin resistance or hepatic PDFF lipid accumulation after six months [348]. Interestingly, a single dose injection of A. soehngenii to the duodenum in patients with metabolic syndrome showed robust GLP-1 production and peripheral glycemic homeostasis [349,350].
5. Conclusions and Perspectives
Since 1980, researchers have been focusing on pathophysiological, diagnostic, and therapeutic aspects of NAFLD because of the risk of progression to severe chronic liver diseases. Since then, NAFLD has become the most frequent chronic liver disease and pathogenic mechanisms have been partly unveiled. Starting from this last evidence, between 2020 and 2023 a new nomenclature appeared in the scientific literature, i.e., MAFLD and MASLD, respectively. Despite a still ongoing debate on the final acronym, the robust association of NAFLD/MAFLD/MASLD with metabolic disturbances and cardiovascular risk factors has been globally acknowledged, and risk assessment and effective management of patients with this disorder will require close collaboration between multiple stakeholders of the medical professional community (Figure 8). The high healthcare burden associated with MASLD makes the search for new, effective, and safe drugs a major pressing need while it remains a challenging quest. A unique drug to address all key aspects of MASLD is still missing and is quite unlikely to be found, since MASLD is a disease with complex pathogenesis and highly heterogeneous clinical outcomes, including liver-specific and systemic implications. Clinical trials with single drugs or their limited combinations demonstrate that even the best results are invariably achieved in no more than about half of the treated patients. This experience suggests that lasting success in the management of MASLD will only result from the combination of lifestyle modification and pharmacotherapy targeting multiple molecular targets and taking the genetic predisposition and susceptibilities of individual patients into account. Recent and promising advances indicate that we may soon enter the era of precise and personalized therapy for MASLD/MASH.
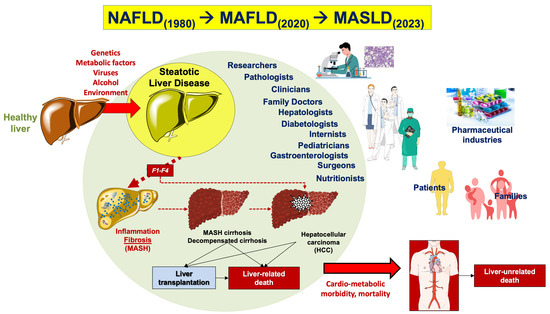
Figure 8.
The process showing the change of nomenclature for liver steatosis (from 1980 to 2023), in relation to the progression of disease and interplay between several professionals and stakeholders. The terminological evolution (i.e., from NAFLD to MAFLD and, finally, to MASLD) has been paralleled by a progressive growth of knowledge about the combined effects of diverse pathogenic factors (i.e., genetic and external factors) in the onset and progression of steatotic liver disease. This evidence, in particular, underscores the association of fat overstorage in the liver not only with the possible progression of hepatic damage but also with systemic metabolic disturbances and cardiovascular risk factors, and the need for a multi-disciplinary and transversal approach to this disease. The cooperation between different stakeholders (including subjects at risk, health professionals, and pharmaceutical industries) could significantly improve either the management of disease and the implementation of primary and secondary prevention measures.
Author Contributions
Conceptualization, P.P. and G.B. and A.D.C.; methodology, M.K., L.M., V.P., V.I. and A.G.; writing—original draft preparation, P.P. and M.K.; writing—review and editing, P.P. and A.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
P.P. is the coordinator of the B4HT project “Box for Health by Tradition & Innovation: promoting sustainable Mediterranean diet by Healthy Foods” funded by the PRIMA project, Section 2—Multi-topic 2022. Project partners: University of Bari Aldo Moro (Italy), University of Genoa (Italy), Lebanese University (Lebanon), and University of Monastir (Tunisia). PP is the recipient of HORIZON-HLTH-2022-STAYHLTH-01-05-two-stage Project 101080329—PAS GRAS with the following partners Universidade De Coimbra, Portugal; Uppsala Universitet, Sweden; Universidade Nova De Lisboa, Portugal; Fundacio Eurecat (Eurecat), Barcelona, Spain; Consiglio Nazionale Delle Ricerche (Cnr), Roma Italy; Instituto Politecnico De Viana De Castelo, Viana Do Castelo 4900-347, Portugal; Technische Universitaet Muenchen (Tum), Muenchen, Germany; Instytut Biologii Doswiadczalnej Im. M. Nenckiego Polskiej Akademii Nauk (Nencki), Warszawa, Poland; Instituto Pedro Nunes Associacao Para A Inovacao E Desenvolvimento Em Ciencia E Tecnologia (Ipn), Coimbra, Portugal; The European Society For Clinical Investigation (Esci), Utrecht, Netherlands; Mediagnost Gesellschaft Fur Forschung Und Herstellung Von Diagnostika Gmbh (Mediagnost), Reutlingen, Germany; Martin-Luther-Universitat Halle-Wittenberg (Mlu), Halle, Germany; Associacao Protectora Dos Diabeticos De Portugal (Apdp), Lisboa 1250-203, Portugal; Agdcentro Associacao De Ginastica Do Centro (Agcentro), Coimbra, Portugal.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are indebted to Paola De Benedictis, Rosa De Venuto, Nicoletta Lamanna, Vito Di Ceglie for excellent technical support.
Conflicts of Interest
Author Valeria Idone was employed by the company Aboca S.p.a. Author Annarita Graziani was employed by the company AllergoSan Pharmazeutische Produkte Forschungs- und Vertriebs GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- NCD Risk Factor Collaboration. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature 2019, 569, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Vecchie, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Fruhbeck, G.; Montecucco, F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.A.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 February 2023).
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Wasniewska, M.; Pepe, G.; Aversa, T.; Bellone, S.; de Sanctis, L.; Di Bonito, P.; Faienza, M.F.; Improda, N.; Licenziati, M.R.; Maffeis, C.; et al. Skeptical Look at the Clinical Implication of Metabolic Syndrome in Childhood Obesity. Children 2023, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Di Sessa, A.; Licenziati, M.R.; Corica, D.; Wasniewska, M.; Umano, G.R.; Morandi, A.; Maffeis, C.; Faienza, M.F.; Mozzillo, E.; et al. Is Metabolic Syndrome Useful for Identifying Youths with Obesity at Risk for NAFLD? Children 2023, 10, 233. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Bonfrate, L.; Stella, A.; Garruti, G.; Lamont, J.T. Metabolic dysfunction-associated gallstone disease: Expecting more from critical care manifestations. Intern. Emerg. Med. 2023, 18, 1897–1918. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5.24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Dietz, W.; Santos-Burgoa, C. Obesity and its Implications for COVID-19 Mortality. Obesity 2020, 28, 1005. [Google Scholar] [CrossRef]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar]
- Schaffner, F.; Thaler, H. Nonalcoholic fatty liver disease. Prog. Liver Dis. 1986, 8, 283–298. [Google Scholar] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.e9. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P. NAFLD, MAFLD, and beyond: One or several acronyms for better comprehension and patient care. Intern. Emerg. Med. 2023, 18, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Harrison, S.A.; Ratziu, V.; Abdelmalek, M.F.; Diehl, A.M.; Caldwell, S.; Shiffman, M.L.; Aguilar Schall, R.; Jia, C.; McColgan, B.; et al. The Natural History of Advanced Fibrosis due to Nonalcoholic Steatohepatitis: Data from the Simtuzumab Trials. Hepatology 2019, 70, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.G.; et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol. Hepatol. 2022, 7, 388–390. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Rafiq, N.; Younossi, Z.M. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: A population-based study. Gut 2010, 59, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Boursier, J.; AFEF Group for the Study of Liver Fibrosis. Confirmatory biomarker diagnostic studies are not needed when transitioning from NAFLD to MASLD. J. Hepatol. 2024, 80, e51–e52. [Google Scholar] [CrossRef]
- Song, S.J.; Lai, J.C.; Wong, G.L.; Wong, V.W.; Yip, T.C. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 2024, 80, e54–e56. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Yoon, E.L.; Kim, M.; Kang, B.K.; Cho, S.; Nah, E.H.; Jun, D.W. Prevalence, distribution, and hepatic fibrosis burden of the different subtypes of steatotic liver disease in primary care settings. Hepatology 2023, 79, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, S.; Han, L.; Liu, J. Pharmacotherapies of NAFLD: Updated opportunities based on metabolic intervention. Nutr. Metab. 2023, 20, 30. [Google Scholar] [CrossRef]
- Ramirez-Mejia, M.M.; Jimenez-Gutierrez, C.; Eslam, M.; George, J.; Mendez-Sanchez, N. Breaking new ground: MASLD vs. MAFLD-which holds the key for risk stratification? Hepatol. Int. 2024, 18, 168–178. [Google Scholar] [CrossRef]
- Spengler, E.K.; Loomba, R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin. Proc. 2015, 90, 1233–1246. [Google Scholar] [CrossRef]
- Palmentieri, B.; de Sio, I.; La Mura, V.; Masarone, M.; Vecchione, R.; Bruno, S.; Torella, R.; Persico, M. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig. Liver Dis. 2006, 38, 485–489. [Google Scholar] [CrossRef]
- Santoro, S.; Khalil, M.; Abdallah, H.; Farella, I.; Noto, A.; Dipalo, G.M.; Villani, P.; Bonfrate, L.; Di Ciaula, A.; Portincasa, P. Early and accurate diagnosis of steatotic liver by artificial intelligence (AI)-supported ultrasonography. Eur. J. Intern. Med. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.M.; Williams, C.D.; Harrison, S.A. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2012, 10, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Noureddin, M.; Alkhouri, N.; Sanyal, A.J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Soni, M.; Cui, J.; Bettencourt, R.; Schork, N.; Chen, C.H.; Ikhwan, M.A.; Bassirian, S.; Cepin, S.; Gonzalez, M.P.; et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J. Clin. Investig. 2017, 127, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Kendall, B.J.; El-Serag, H.B.; Thrift, A.P.; Macdonald, G.A. Hepatocellular and extrahepatic cancer risk in people with non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2024, 9, 159–169. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Passarella, S.; Shanmugam, H.; Noviello, M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Nonalcoholic Fatty Liver Disease (NAFLD). Mitochondria as Players and Targets of Therapies? Int. J. Mol. Sci. 2021, 22, 5375. [Google Scholar] [CrossRef]
- Grattagliano, I.; Di Ciaula, A.; Baj, J.; Molina-Molina, E.; Shanmugam, H.; Garruti, G.; Wang, D.Q.; Portincasa, P. Protocols for Mitochondria as the Target of Pharmacological Therapy in the Context of Nonalcoholic Fatty Liver Disease (NAFLD). Methods Mol. Biol. 2021, 2310, 201–246. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, E.; Shanmugam, H.; Di Palo, D.; Grattagliano, I.; Portincasa, P. Exploring Liver Mitochondrial Function by (13)C-Stable Isotope Breath Tests: Implications in Clinical Biochemistry. Methods Mol. Biol. 2021, 2310, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Calamita, G.; Shanmugam, H.; Khalil, M.; Bonfrate, L.; Wang, D.Q.; Baffy, G.; Portincasa, P. Mitochondria Matter: Systemic Aspects of Nonalcoholic Fatty Liver Disease (NAFLD) and Diagnostic Assessment of Liver Function by Stable Isotope Dynamic Breath Tests. Int. J. Mol. Sci. 2021, 22, 7702. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Seth, D.; Day, C.P. Genetic Factors That Affect Risk of Alcoholic and Nonalcoholic Fatty Liver Disease. Gastroenterology 2016, 150, 1728. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Portincasa, P.; Lammert, F. PNPLA3-associated steatohepatitis: Toward a gene-based classification of fatty liver disease. Semin. Liver Dis. 2013, 33, 369–379. [Google Scholar] [CrossRef]
- Krawczyk, M.; Bonfrate, L.; Portincasa, P. Nonalcoholic fatty liver disease. Best. Pract. Res. Clin. Gastroenterol. 2010, 24, 695–708. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Donati, B.; Fares, R.; Lombardi, R.; Mancina, R.M.; Romeo, S.; Valenti, L. PNPLA3 I148M polymorphism and progressive liver disease. World J. Gastroenterol. 2013, 19, 6969–6978. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Valenti, L.; Rametta, R.; Daly, A.K.; Nobili, V.; Mozzi, E.; Leathart, J.B.; Pietrobattista, A.; Burt, A.D.; Maggioni, M.; et al. Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 267–273. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjaerg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Boren, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef]
- Beer, N.L.; Tribble, N.D.; McCulloch, L.J.; Roos, C.; Johnson, P.R.; Orho-Melander, M.; Gloyn, A.L. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum. Mol. Genet. 2009, 18, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Chantada, M.; Gonzalez-Lahera, A.; Martinez-Arranz, I.; Garcia-Monzon, C.; Regueiro, M.M.; Garcia-Rodriguez, J.L.; Schlangen, K.A.; Mendibil, I.; Rodriguez-Ezpeleta, N.; Lozano, J.J.; et al. Solute carrier family 2 member 1 is involved in the development of nonalcoholic fatty liver disease. Hepatology 2013, 57, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Cheng, X.; Li, A.H.; Xin, Y.; Schurmann, C.; Stevis, P.; Liu, Y.; Kozlitina, J.; Stender, S.; Wood, G.C.; et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N. Engl. J. Med. 2018, 378, 1096–1106. [Google Scholar] [CrossRef]
- Portincasa, P.; Khalil, M.; Graziani, A.; Fruhbeck, G.; Baffy, G.; Garruti, G.; Di Ciaula, A.; Bonfrate, L. Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations? Eur. J. Intern. Med. 2023, 119, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P. The role of microbiota in nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2022, 52, e13768. [Google Scholar] [CrossRef] [PubMed]
- Di Palo, D.M.; Garruti, G.; Di Ciaula, A.; Molina-Molina, E.; Shanmugam, H.; De Angelis, M.; Portincasa, P. Increased Colonic Permeability and Lifestyles as Contributing Factors to Obesity and Liver Steatosis. Nutrients 2020, 12, E564. [Google Scholar] [CrossRef]
- Molina-Molina, E.; Shanmugam, H.; Di Ciaula, A.; Grattagliano, I.; Di Palo, D.M.; Palmieri, V.O.; Portincasa, P. ((13)C)-Methacetin breath test provides evidence of subclinical liver dysfunction linked to fat storage but not lifestyle. JHEP Rep. 2021, 3, 100203. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef]
- Lomonaco, R.; Ortiz-Lopez, C.; Orsak, B.; Webb, A.; Hardies, J.; Darland, C.; Finch, J.; Gastaldelli, A.; Harrison, S.; Tio, F.; et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 1389–1397. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome-Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Ibrabim, S.H.; Gores, G.J.; Malhi, H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: Relevance to NASH pathogenesis. J. Lipid Res. 2016, 57, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Watkins, S.M.; Hotamisligil, G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012, 15, 623–634. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Xia, J.Y.; Holland, W.L.; Kusminski, C.M.; Sun, K.; Sharma, A.X.; Pearson, M.J.; Sifuentes, A.J.; McDonald, J.G.; Gordillo, R.; Scherer, P.E. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015, 22, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Bronneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef]
- Martinez, L.; Torres, S.; Baulies, A.; Alarcon-Vila, C.; Elena, M.; Fabrias, G.; Casas, J.; Caballeria, J.; Fernandez-Checa, J.C.; Garcia-Ruiz, C. Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget 2015, 6, 41479–41496. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Colell, A.; Mari, M.; Morales, A.; Fernandez-Checa, J.C. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 1997, 272, 11369–11377. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carreras, M.; Del Hoyo, P.; Martin, M.A.; Rubio, J.C.; Martin, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- DiPilato, L.M.; Ahmad, F.; Harms, M.; Seale, P.; Manganiello, V.; Birnbaum, M.J. The Role of PDE3B Phosphorylation in the Inhibition of Lipolysis by Insulin. Mol. Cell Biol. 2015, 35, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, R.P.; Collins, S. A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clin. Sci. 2020, 134, 473–512. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K.; Pettiti, M.; Hardies, J.; Miyazaki, Y.; Berria, R.; Buzzigoli, E.; Sironi, A.M.; Cersosimo, E.; Ferrannini, E. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007, 133, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Juurinen, L.; Tiikkainen, M.; Vehkavaara, S.; Yki-Jarvinen, H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 2008, 135, 122–130. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Shi, Y.; Cheng, D. Beyond triglyceride synthesis: The dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E10–E18. [Google Scholar] [CrossRef]
- Wang, D.Q.H.; Neuschwander-Tetri, B.A.; Portincasa, P. The Biliary System, 2nd ed.; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2017; Volume 8, pp. 460–490. [Google Scholar]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos–Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.A.; Lee, D.P. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004, 43, 134–176. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Oosterveer, M.H.; Schoonjans, K. Hepatic glucose sensing and integrative pathways in the liver. Cell Mol. Life Sci. 2014, 71, 1453–1467. [Google Scholar] [CrossRef]
- Bianchi, A.; Evans, J.L.; Iverson, A.J.; Nordlund, A.C.; Watts, T.D.; Witters, L.A. Identification of an isozymic form of acetyl-CoA carboxylase. J. Biol. Chem. 1990, 265, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Smith, S. The animal fatty acid synthase: One gene, one polypeptide, seven enzymes. FASEB J. 1994, 8, 1248–1259. [Google Scholar] [CrossRef]
- Brindley, D.; Matsumura, S.; Bloch, K. Mycobacterium phlei fatty acid synthetase—A bacterial multienzyme complex. Nature 1969, 224, 666–669. [Google Scholar] [CrossRef]
- Majerus, P.W.; Alberts, A.W.; Vagelos, P.R. The Acyl Carrier Protein of Fatty Acid Synthesis: Purification, Physical Properties, and Substrate Binding Site. Proc. Natl. Acad. Sci. USA 1964, 51, 1231–1238. [Google Scholar] [CrossRef]
- Maier, T.; Leibundgut, M.; Ban, N. The crystal structure of a mammalian fatty acid synthase. Science 2008, 321, 1315–1322. [Google Scholar] [CrossRef]
- Smith, S.; Tsai, S.C. The type I fatty acid and polyketide synthases: A tale of two megasynthases. Nat. Prod. Rep. 2007, 24, 1041–1072. [Google Scholar] [CrossRef]
- Wakil, S.J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 1989, 28, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.M.; Dils, R.; Hansen, H.J. Short communications. Chain-length specificity for termination of atty acid biosynthesis by fatty acid synthetase complexes from lactating rabbit mamary gland and rat liver. Biochem. J. 1970, 117, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.W.; Bloom, B. The synthesis of fatty acids by rat liver slices in tritiated water. J. Biol. Chem. 1963, 238, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Campbell, R.D. Characterization of a human lysophosphatidic acid acyltransferase that is encoded by a gene located in the class III region of the human major histocompatibility complex. J. Biol. Chem. 1998, 273, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Mannaerts, G.P.; Foster, D.W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Investig. 1977, 60, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Arner, P.; Yki-Jarvinen, H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006, 42, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.E.; Nelson, D.W.; Yen, M.I. Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism. J. Lipid Res. 2015, 56, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Laurencikiene, J.; Skurk, T.; Kulyte, A.; Heden, P.; Astrom, G.; Sjolin, E.; Ryden, M.; Hauner, H.; Arner, P. Regulation of lipolysis in small and large fat cells of the same subject. J. Clin. Endocrinol. Metab. 2011, 96, E2045–E2049. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, s4–s14. [Google Scholar] [CrossRef]
- Chavez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef]
- Beaven, S.W.; Matveyenko, A.; Wroblewski, K.; Chao, L.; Wilpitz, D.; Hsu, T.W.; Lentz, J.; Drew, B.; Hevener, A.L.; Tontonoz, P. Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab. 2013, 18, 106–117. [Google Scholar] [CrossRef]
- Cave, M.C.; Clair, H.B.; Hardesty, J.E.; Falkner, K.C.; Feng, W.; Clark, B.J.; Sidey, J.; Shi, H.; Aqel, B.A.; McClain, C.J.; et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim. Biophys. Acta 2016, 1859, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shu, X.-B.; Yao, Z.; Ji, G.; Zhang, L. Is vitamin D receptor a druggable target for non-alcoholic steatohepatitis? World J. Gastroenterol. 2020, 26, 5812. [Google Scholar] [CrossRef]
- Watanabe, A.; Sohail, M.A.; Gomes, D.A.; Hashmi, A.; Nagata, J.; Sutterwala, F.S.; Mahmood, S.; Jhandier, M.N.; Shi, Y.; Flavell, R.A.; et al. Inflammasome-mediated regulation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1248–G1257. [Google Scholar] [CrossRef] [PubMed]
- Flannery, C.; Dufour, S.; Rabol, R.; Shulman, G.I.; Petersen, K.F. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes 2012, 61, 2711–2717. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef]
- Uyeda, K.; Repa, J.J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006, 4, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Bindesboll, C.; Fan, Q.; Norgaard, R.C.; MacPherson, L.; Ruan, H.B.; Wu, J.; Pedersen, T.A.; Steffensen, K.R.; Yang, X.; Matthews, J.; et al. Liver X receptor regulates hepatic nuclear O-GlcNAc signaling and carbohydrate responsive element-binding protein activity. J. Lipid Res. 2015, 56, 771–785. [Google Scholar] [CrossRef]
- Nagai, Y.; Yonemitsu, S.; Erion, D.M.; Iwasaki, T.; Stark, R.; Weismann, D.; Dong, J.; Zhang, D.; Jurczak, M.J.; Loffler, M.G.; et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009, 9, 252–264. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Richart, C.; Auguet, T.; Terra, X. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 193–194; author reply 195. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Bazick, J.; Donithan, M.; Neuschwander-Tetri, B.A.; Kleiner, D.; Brunt, E.M.; Wilson, L.; Doo, E.; Lavine, J.; Tonascia, J.; Loomba, R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients with Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care 2015, 38, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Zhao, Y.; Sun, Q.; Zhang, J.; Shen, D.; Wu, J.; Shen, N.; Fu, X.; Sun, X.; et al. Geranylgeranyl diphosphate synthase (GGPPS) regulates non-alcoholic fatty liver disease (NAFLD)-fibrosis progression by determining hepatic glucose/fatty acid preference under high-fat diet conditions. J. Pathol. 2018, 246, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Xiao, C.; Wang, R.H.; Lahusen, T.; Xu, X.; Vassilopoulos, A.; Vazquez-Ortiz, G.; Jeong, W.I.; Park, O.; Ki, S.H.; et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010, 12, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, K.; Yao, W.; Zheng, R.; He, Q.; Xia, J.; Li, J.; Shao, Y.; Zhang, L.; Huang, L.; et al. Acetylation of lactate dehydrogenase B drives NAFLD progression by impairing lactate clearance. J. Hepatol. 2021, 74, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Satapati, S.; Sunny, N.E.; Kucejova, B.; Fu, X.; He, T.T.; Mendez-Lucas, A.; Shelton, J.M.; Perales, J.C.; Browning, J.D.; Burgess, S.C. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 2012, 53, 1080–1092. [Google Scholar] [CrossRef]
- Passarella, S.; Schurr, A.; Portincasa, P. Mitochondrial Transport in Glycolysis and Gluconeogenesis: Achievements and Perspectives. Int. J. Mol. Sci. 2021, 22, 12620. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Kamagate, A.; Qu, S.; Perdomo, G.; Su, D.; Kim, D.H.; Slusher, S.; Meseck, M.; Dong, H.H. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Investig. 2008, 118, 2347–2364. [Google Scholar] [CrossRef] [PubMed]
- Avramoglu, R.K.; Basciano, H.; Adeli, K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin. Chim. Acta 2006, 368, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Tian, X.; Wu, H.; Huang, J.; Li, M.; Mei, Z.; Zhou, L.; Xie, H.; Zheng, S. Metabolic Changes of Hepatocytes in NAFLD. Front. Physiol. 2021, 12, 710420. [Google Scholar] [CrossRef]
- Passarella, S.; Atlante, A.; Valenti, D.; de Bari, L. The role of mitochondrial transport in energy metabolism. Mitochondrion 2003, 2, 319–343. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef]
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef]
- Kurtz, T.W.; Kabra, P.M.; Booth, B.E.; Al-Bander, H.A.; Portale, A.A.; Serena, B.G.; Tsai, H.C.; Morris, R.C., Jr. Liquid-chromatographic measurements of inosine, hypoxanthine, and xanthine in studies of fructose-induced degradation of adenine nucleotides in humans and rats. Clin. Chem. 1986, 32, 782–786. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Bronfman, M.; Vanneste, R.; Hers, H.G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem. J. 1977, 162, 601–609. [Google Scholar] [CrossRef]
- Smith, C.M.; Rovamo, L.M.; Raivio, K.O. Fructose-induced adenine nucleotide catabolism in isolated rat hepatocytes. Can. J. Biochem. 1977, 55, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Maenpaa, P.H.; Raivio, K.O.; Kekomaki, M.P. Liver adenine nucleotides: Fructose-induced depletion and its effect on protein synthesis. Science 1968, 161, 1253–1254. [Google Scholar] [CrossRef] [PubMed]
- Bawden, S.J.; Stephenson, M.C.; Ciampi, E.; Hunter, K.; Marciani, L.; Macdonald, I.A.; Aithal, G.P.; Morris, P.G.; Gowland, P.A. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: A (31)P MRS study. Clin. Nutr. 2016, 35, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef]
- Ritze, Y.; Bardos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; Beutheu, S.; Ventura, G.; Sarfati, G.; Nubret, E.; Kapel, N.; Waligora-Dupriet, A.J.; Bergheim, I.; Cynober, L.; De-Bandt, J.P. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clin. Nutr. 2016, 35, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; Beutheu, S.; Freese, K.; Waligora-Dupriet, A.J.; Nubret, E.; Butel, M.J.; Bergheim, I.; De Bandt, J.P. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Brit. J. Nutr. 2016, 116, 191–203. [Google Scholar] [CrossRef]
- Massafra, V.; Pellicciari, R.; Gioiello, A.; van Mil, S.W.C. Progress and challenges of selective Farnesoid X receptor modulation. Pharmacol. Ther. 2018, 191, 162–177. [Google Scholar] [CrossRef]
- Jung, Y.; Koo, B.K.; Jang, S.Y.; Kim, D.; Lee, H.; Lee, D.H.; Joo, S.K.; Jung, Y.J.; Park, J.H.; Yoo, T.; et al. Association between circulating bile acid alterations and nonalcoholic steatohepatitis independent of obesity and diabetes mellitus. Liver Int. 2021, 41, 2892–2902. [Google Scholar] [CrossRef]
- Rao, A.; Kosters, A.; Mells, J.E.; Zhang, W.; Setchell, K.D.; Amanso, A.M.; Wynn, G.M.; Xu, T.; Keller, B.T.; Yin, H.; et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 2016, 8, 357ra122. [Google Scholar] [CrossRef] [PubMed]
- Pineda Torra, I.; Claudel, T.; Duval, C.; Kosykh, V.; Fruchart, J.C.; Staels, B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol. Endocrinol. 2003, 17, 259–272. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Savkur, R.S.; Bramlett, K.S.; Michael, L.F.; Burris, T.P. Regulation of pyruvate dehydrogenase kinase expression by the farnesoid X receptor. Biochem. Biophys. Res. Commun. 2005, 329, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wong, K.; Walsh, K.; Gao, B.; Zang, M. Retinoic acid receptor beta stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J. Biol. Chem. 2013, 288, 10490–10504. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Kir, S.; Beddow, S.A.; Samuel, V.T.; Miller, P.; Previs, S.F.; Suino-Powell, K.; Xu, H.E.; Shulman, G.I.; Kliewer, S.A.; Mangelsdorf, D.J. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 2011, 331, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; John, L.M.; Adams, S.H.; Yu, X.X.; Tomlinson, E.; Renz, M.; Williams, P.M.; Soriano, R.; Corpuz, R.; Moffat, B.; et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004, 145, 2594–2603. [Google Scholar] [CrossRef]
- Alvarez-Sola, G.; Uriarte, I.; Latasa, M.U.; Fernandez-Barrena, M.G.; Urtasun, R.; Elizalde, M.; Barcena-Varela, M.; Jimenez, M.; Chang, H.C.; Barbero, R.; et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: Development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut 2017, 66, 1818–1828. [Google Scholar] [CrossRef]
- Tomlinson, E.; Fu, L.; John, L.; Hultgren, B.; Huang, X.; Renz, M.; Stephan, J.P.; Tsai, S.P.; Powell-Braxton, L.; French, D.; et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002, 143, 1741–1747. [Google Scholar] [CrossRef]
- Watanabe, M.; Horai, Y.; Houten, S.M.; Morimoto, K.; Sugizaki, T.; Arita, E.; Mataki, C.; Sato, H.; Tanigawara, Y.; Schoonjans, K.; et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J. Biol. Chem. 2011, 286, 26913–26920. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, X.; Heemstra, L.A.; Chen, W.D.; Xu, J.; Smith, J.L.; Ma, H.; Kasim, N.; Edwards, P.A.; Novak, C.M. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol. Endocrinol. 2012, 26, 272–280. [Google Scholar] [CrossRef]
- Prawitt, J.; Abdelkarim, M.; Stroeve, J.H.; Popescu, I.; Duez, H.; Velagapudi, V.R.; Dumont, J.; Bouchaert, E.; van Dijk, T.H.; Lucas, A.; et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 2011, 60, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, S.C.; Kim, J.; Anakk, S.; Lee, J.M.; Tseng, H.T.; Yechoor, V.; Park, J.; Choi, J.S.; Jang, H.C.; et al. Dissociation of diabetes and obesity in mice lacking orphan nuclear receptor small heterodimer partner. J. Lipid Res. 2011, 52, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Jiang, C.; Shi, J.; Gao, X.; Sun, D.; Sun, L.; Wang, T.; Takahashi, S.; Anitha, M.; Krausz, K.W.; et al. An Intestinal Farnesoid X Receptor-Ceramide Signaling Axis Modulates Hepatic Gluconeogenesis in Mice. Diabetes 2017, 66, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xie, C.; Li, F.; Zhang, L.; Nichols, R.G.; Krausz, K.W.; Cai, J.; Qi, Y.; Fang, Z.Z.; Takahashi, S.; et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 386–402. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Trabelsi, M.S.; Daoudi, M.; Prawitt, J.; Ducastel, S.; Touche, V.; Sayin, S.I.; Perino, A.; Brighton, C.A.; Sebti, Y.; Kluza, J.; et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 2015, 6, 7629. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Van Nierop, F.S.; Scheltema, M.J.; Eggink, H.M.; Pols, T.W.; Sonne, D.P.; Knop, F.K.; Soeters, M.R. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 2017, 5, 224–233. [Google Scholar] [CrossRef]
- Yuan, L.; Bambha, K. Bile acid receptors and nonalcoholic fatty liver disease. World J. Hepatol. 2015, 7, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig. Liver Dis. 2018, 50, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D.; Vennstrom, B. Functions of thyroid hormone receptors in mice. Thyroid. 2000, 10, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Lammel Lindemann, J.A.; Angajala, A.; Engler, D.A.; Webb, P.; Ayers, S.D. Thyroid hormone induction of human cholesterol 7 alpha-hydroxylase (Cyp7a1) in vitro. Mol. Cell Endocrinol. 2014, 388, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.Y.; Kim, H.H.; Kim, Y.A.; Kim, M.; Ohn, J.H.; Chung, S.S.; Lee, Y.K.; Park, D.J.; Park, K.S.; Moore, D.D.; et al. Thyroid Hormone Regulates the mRNA Expression of Small Heterodimer Partner through Liver Receptor Homolog-1. Endocrinol. Metab. 2015, 30, 584–592. [Google Scholar] [CrossRef]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid 2019, 29, 1173–1191. [Google Scholar] [CrossRef]
- Shneider, B.L.; Dawson, P.A.; Christie, D.M.; Hardikar, W.; Wong, M.H.; Suchy, F.J. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J. Clin. Investig. 1995, 95, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chiang, J.Y. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): Roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J. Biol. Chem. 2001, 276, 41690–41699. [Google Scholar] [CrossRef]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Soroka, C.J.; Ballatori, N.; Boyer, J.L. Organic solute transporter, OSTα-OSTβ: Its role in bile acid transport and cholestasis. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2010; pp. 178–185. [Google Scholar]
- Song, K.H.; Li, T.; Owsley, E.; Strom, S.; Chiang, J.Y. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 2009, 49, 297–305. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Bonfrate, L.; Khalil, M.; Garruti, G.; Portincasa, P. Contribution of the microbiome for better phenotyping of people living with obesity. Rev. Endocr. Metab. Disord. 2023, 24, 839–870. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Schnabl, B.; Knight, R.; Loomba, R. Current Concepts, Opportunities, and Challenges of Gut Microbiome-Based Personalized Medicine in Nonalcoholic Fatty Liver Disease. Cell Metab. 2021, 33, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Fei, N.; Bruneau, A.; Zhang, X.; Wang, R.; Wang, J.; Rabot, S.; Gerard, P.; Zhao, L. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio 2020, 11, 10-1128. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Kacprowski, T.; Ruhlemann, M.; Pietzner, M.; Bang, C.; Franke, A.; Nauck, M.; Volker, U.; Volzke, H.; Dorr, M.; et al. Long-term instability of the intestinal microbiome is associated with metabolic liver disease, low microbiota diversity, diabetes mellitus and impaired exocrine pancreatic function. Gut 2021, 70, 522–530. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Amiar, M.R.; Ocana-Wilhelmi, L.; Soler-Humanes, R.; Arranz-Salas, I.; Garrido-Sanchez, L.; Gutierrez-Repiso, C.; Tinahones, F.J. Non-alcoholic fatty liver disease in patients with morbid obesity: The gut microbiota axis as a potential pathophysiology mechanism. J. Gastroenterol. 2024, 59, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect against High-Fat Diet-Induced Obesity via a PPARgamma-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Baumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Mon, K.K.Z.; Nguyen, H.; Chanthavixay, G.; Liou, M.; Velazquez, E.M.; Kutter, L.; Alcantara, M.A.; Byndloss, M.X.; Tiffany, C.R.; et al. Commensal Enterobacteriaceae Protect against Salmonella Colonization through Oxygen Competition. Cell Host Microbe 2019, 25, 128–139.e5. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Keenan, M.J.; Zhou, J.; McCutcheon, K.L.; Raggio, A.M.; Bateman, H.G.; Todd, E.; Jones, C.K.; Tulley, R.T.; Melton, S.; Martin, R.J.; et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity 2006, 14, 1523–1534. [Google Scholar] [CrossRef]
- Aziz, A.A.; Kenney, L.S.; Goulet, B.; Abdel-Aal, E.S. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J. Nutr. 2009, 139, 1881–1889. [Google Scholar] [CrossRef]
- Cho, I.; Yamanishi, S.; Cox, L.; Methe, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Ilie, I.; Oprea, L.; Picu, A.; Petcu, L.M.; Burlibasa, L.; Chifiriuc, M.C.; Musat, M. Microbiome, Mycobiome and Related Metabolites Alterations in Patients with Metabolic Syndrome-A Pilot Study. Metabolites 2022, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, T.; Moens de Hase, E.; Van Hul, M.; Paquot, A.; Pelicaen, R.; Regnier, M.; Depommier, C.; Druart, C.; Everard, A.; Maiter, D.; et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut 2022, 71, 534–543. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Chitturi, S.; Wong, V.W.; Chan, W.K.; Wong, G.L.; Wong, S.K.; Sollano, J.; Ni, Y.H.; Liu, C.J.; Lin, Y.C.; Lesmana, L.A.; et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: Management and special groups. J. Gastroenterol. Hepatol. 2018, 33, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Linge, J.; Nasr, P.; Sanyal, A.J.; Dahlqvist Leinhard, O.; Ekstedt, M. Adverse muscle composition is a significant risk factor for all-cause mortality in NAFLD. JHEP Rep. 2023, 5, 100663. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Bae, S.J.; Lee, M.J.; Kim, E.H.; Park, H.; Kim, H.S.; Cho, Y.K.; Jung, C.H.; Lee, W.J.; Choe, J. Association of Visceral Fat Obesity, Sarcopenia, and Myosteatosis with Non-Alcoholic Fatty Liver Disease without Obesity. Clin. Mol. Hepatol. 2023, 29, 987–1001. [Google Scholar] [CrossRef]
- Molina-Molina, E.; Lunardi Baccetto, R.; Wang, D.Q.; de Bari, O.; Krawczyk, M.; Portincasa, P. Exercising the hepatobiliary-gut axis. The impact of physical activity performance. Eur. J. Clin. Investig. 2018, 48, e12958. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, J.; Francque, S.; De Block, C. Therapeutic approaches for non-alcoholic steatohepatitis. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211034300. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals with Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8. [Google Scholar] [CrossRef]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.A.K.; Santos, H.O.; Gaman, M.A.; Cerqueira, H.S.; Zaher, E.A.; Alromaih, W.R.; Arafat, N.S.; Adi, A.R.; Adly, H.M.; Alyoubi, R.; et al. Effects of intermittent fasting regimens on glycemic, hepatic, anthropometric, and clinical markers in patients with non-alcoholic fatty liver disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2024, 59, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; So, R.; Shida, T.; Matsuo, T.; Kim, B.; Akiyama, K.; Isobe, T.; Okamoto, Y.; Tanaka, K.; Shoda, J. High-Intensity Aerobic Exercise Improves both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci. Rep. 2017, 7, 43029. [Google Scholar] [CrossRef]
- Orci, L.A.; Gariani, K.; Oldani, G.; Delaune, V.; Morel, P.; Toso, C. Exercise-based Interventions for Nonalcoholic Fatty Liver Disease: A Meta-analysis and Meta-regression. Clin. Gastroenterol. Hepatol. 2016, 14, 1398–1411. [Google Scholar] [CrossRef]
- Sung, K.C.; Ryu, S.; Lee, J.Y.; Kim, J.Y.; Wild, S.H.; Byrne, C.D. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J. Hepatol. 2016, 65, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shida, T.; Yamagishi, K.; Tanaka, K.; So, R.; Tsujimoto, T.; Shoda, J. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: A retrospective study. Hepatology 2015, 61, 1205–1215. [Google Scholar] [CrossRef]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kistler, K.D.; Brunt, E.M.; Clark, J.M.; Diehl, A.M.; Sallis, J.F.; Schwimmer, J.B.; Group, N.C.R. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2011, 106, 460–468; quiz 469. [Google Scholar] [CrossRef]
- Shanmugam, H.; Di Ciaula, A.; Di Palo, D.M.; Molina-Molina, E.; Garruti, G.; Faienza, M.F.; vanErpecum, K.; Portincasa, P. Multiplying effects of COVID-19 lockdown on metabolic risk and fatty liver. Eur. J. Clin. Investig. 2021, 51, e13597. [Google Scholar] [CrossRef]
- Chen, G.; Banini, B.A.; Do, A.; Gunderson, C.; Zaman, S.; Lim, J.K. Exercise Does Not Independently Improve Histological Outcomes in Biopsy-Proven Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Genes 2023, 14, 1811. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, A.M.; de Vries, M.; El-Morabit, F.; Bac, S.T.; Mundt, M.W.; van der Schuit, L.E.; Hirdes, M.M.C.; Kara, M.; de Bruijne, J.; van Meer, S.; et al. Intra-gastric balloon with lifestyle modification: A promising therapeutic option for overweight and obese patients with metabolic dysfunction-associated steatotic liver disease. Intern. Emerg. Med. 2023, 18, 2271–2280. [Google Scholar] [CrossRef]
- Chavez-Tapia, N.C.; Tellez-Avila, F.I.; Barrientos-Gutierrez, T.; Mendez-Sanchez, N.; Lizardi-Cervera, J.; Uribe, M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst. Rev. 2010, 2010, CD007340. [Google Scholar] [CrossRef]
- Lee, Y.; Doumouras, A.G.; Yu, J.; Brar, K.; Banfield, L.; Gmora, S.; Anvari, M.; Hong, D. Complete Resolution of Nonalcoholic Fatty Liver Disease after Bariatric Surgery: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1040–1060.e11. [Google Scholar] [CrossRef]
- Klein, S.; Mittendorfer, B.; Eagon, J.C.; Patterson, B.; Grant, L.; Feirt, N.; Seki, E.; Brenner, D.; Korenblat, K.; McCrea, J. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology 2006, 130, 1564–1572. [Google Scholar] [CrossRef]
- Aguilar-Olivos, N.E.; Almeda-Valdes, P.; Aguilar-Salinas, C.A.; Uribe, M.; Mendez-Sanchez, N. The role of bariatric surgery in the management of nonalcoholic fatty liver disease and metabolic syndrome. Metabolism 2016, 65, 1196–1207. [Google Scholar] [CrossRef]
- Verrastro, O.; Panunzi, S.; Castagneto-Gissey, L.; De Gaetano, A.; Lembo, E.; Capristo, E.; Guidone, C.; Angelini, G.; Pennestri, F.; Sessa, L.; et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): A multicentre, open-label, randomised trial. Lancet 2023, 401, 1786–1797. [Google Scholar] [CrossRef] [PubMed]
- Aoko, O.; Maharaj, T.; Boland, F.; Cheriyan, D.; Ryan, J. Meta-analysis: Impact of intragastric balloon therapy on NAFLD-related parameters in patients with obesity. Aliment. Pharmacol. Ther. 2024, 59, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Portincasa, P. Contrasting obesity: Is something missing here? Intern. Emerg. Med. 2024, 19, 265–269. [Google Scholar] [CrossRef]
- Sangro, P.; de la Torre Alaez, M.; Sangro, B.; D’Avola, D. Metabolic dysfunction-associated fatty liver disease (MAFLD): An update of the recent advances in pharmacological treatment. J. Physiol. Biochem. 2023, 79, 869–879. [Google Scholar] [CrossRef]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic Steatohepatitis Is the Second Leading Etiology of Liver Disease Among Adults Awaiting Liver Transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- Dufour, J.F.; Caussy, C.; Loomba, R. Combination therapy for non-alcoholic steatohepatitis: Rationale, opportunities and challenges. Gut 2020, 69, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; Investigators, N.N. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Eguchi, Y.; Yoneda, M.; Imajo, K.; Tamaki, N.; Suganami, H.; Nojima, T.; Tanigawa, R.; Iizuka, M.; Iida, Y.; et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha), versus placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2021, 54, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; de Guevara, L.; Safadi, R.; Poordad, F.; Fuster, F.; Flores-Figueroa, J.; Arrese, M.; Fracanzani, A.L.; Ben Bashat, D.; Lackner, K.; et al. Aramchol in patients with nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 2021, 27, 1825–1835. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wong, V.W.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients With Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Sanyal, A.J.; Kowdley, K.V.; Robuck, P.R.; Hoofnagle, J.; Kleiner, D.E.; Unalp, A.; Tonascia, J.; Group, N.C.R. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp. Clin. Trials 2009, 30, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-alpha/gamma Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Wong, V.W.; Okanoue, T.; Bzowej, N.; Vuppalanchi, R.; Younes, Z.; Kohli, A.; Sarin, S.; Caldwell, S.H.; Alkhouri, N.; et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J. Hepatol. 2020, 73, 26–39. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Ning, H.; Allen, N.B.; Ajmera, V.; Lewis, C.E.; Carr, J.J.; Lloyd-Jones, D.M.; Terrault, N.A.; Siddique, J. Alcohol Use and Cardiovascular Disease Risk in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 153, 1260–1272.e3. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Neuschwander-Tetri, B.A.; Wong, V.W.; Abdelmalek, M.F.; Younossi, Z.M.; Yuan, J.; Pecoraro, M.L.; Seyedkazemi, S.; Fischer, L.; Bedossa, P.; et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design. Contemp. Clin. Trials 2020, 89, 105922. [Google Scholar] [CrossRef] [PubMed]
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Orsak, B.; Bril, F.; Lomonaco, R.; Hecht, J.; Ortiz-Lopez, C.; Tio, F.; Hardies, J.; Darland, C.; Musi, N.; et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann. Intern. Med. 2016, 165, 305–315. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Butrico, G.M.; Kalpage, H.A.; Goedeke, L.; Hubbard, B.T.; Vatner, D.F.; Gaspar, R.C.; Zhang, X.M.; Cline, G.W.; Nakahara, K.; et al. Metformin, phenformin, and galegine inhibit complex IV activity and reduce glycerol-derived gluconeogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122287119. [Google Scholar] [CrossRef]
- European Association for the Study of The Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Barzilai, N.; Simonson, D.C. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J. Clin. Endocrinol. Metab. 1991, 73, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.A.; Hawa, M.I.; Jaspan, J.B.; Sim, B.M.; Disilvio, L.; Featherbe, D.; Kurtz, A.B. Mechanism of metformin action in non-insulin-dependent diabetes. Diabetes 1987, 36, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E. The target of metformin in type 2 diabetes. N. Engl. J. Med. 2014, 371, 1547–1548. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Ma, A.; Bird, D.M.; Paterson, C.A.; Ravenscroft, P.J.; Cameron, D.P. Metformin increases insulin sensitivity and basal glucose clearance in type 2 (non-insulin dependent) diabetes mellitus. Aust. N. Z. J. Med. 1991, 21, 714–719. [Google Scholar] [CrossRef]
- Stumvoll, M.; Nurjhan, N.; Perriello, G.; Dailey, G.; Gerich, J.E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 550–554. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Hawley, S.A.; Gadalla, A.E.; Olsen, G.S.; Hardie, D.G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 2002, 51, 2420–2425. [Google Scholar] [CrossRef]
- Shaw, R.J. Metformin trims fats to restore insulin sensitivity. Nat. Med. 2013, 19, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Fontaine, R.N.; Wang, P.; Subbiah, M.T.; Weber, K.; Illig, E.; Streicher, P.; Sieve-Smith, L.; Tracy, T.M.; Lang, J.E.; et al. Metformin reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metab. Clin. Exp. 2001, 50, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Buckley, N.S.; Kahn, J.A.; Salpeter, E.E. Meta-analysis: Metformin treatment in persons at risk for diabetes mellitus. Am. J. Med. 2008, 121, 149–157.e2. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C. The U.K. Prospective Diabetes Study. A review. Diabetes Care 1998, 21 (Suppl. S3), C35–C38. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhao, Y.; Xu, X.; Ling, S. Advance of Metformin in Liver Disease. Curr. Med. Chem. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wang, B.; Wang, J.; Chen, D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed. Rep. 2013, 1, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, O.E.; Alalalmeh, S.O.; Shahwan, M.; Jairoun, A.A.; Alourfi, M.M.; Bokhari, G.A.; Alkhattabi, A.; Alsharif, S.; Aljehani, M.A.; Alsabban, A.M.; et al. Exploring Promising Therapies for Non-Alcoholic Fatty Liver Disease: A ClinicalTrials.gov Analysis. Diabetes Metab. Syndr. Obes. 2024, 17, 545–561. [Google Scholar] [CrossRef]
- Jang, H.; Kim, Y.; Lee, D.H.; Joo, S.K.; Koo, B.K.; Lim, S.; Lee, W.; Kim, W. Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease. JAMA Intern. Med. 2024, 184, 375–383. [Google Scholar] [CrossRef]
- Galli, A.; Crabb, D.W.; Ceni, E.; Salzano, R.; Mello, T.; Svegliati-Baroni, G.; Ridolfi, F.; Trozzi, L.; Surrenti, C.; Casini, A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology 2002, 122, 1924–1940. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Xiong, J.; Solis-Herrera, C.; Merovci, A.; Eldor, R.; Tripathy, D.; DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Dapagliflozin Enhances Fat Oxidation and Ketone Production in Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 2036–2041. [Google Scholar] [CrossRef] [PubMed]
- Zachou, M.; Flevari, P.; Nasiri-Ansari, N.; Varytimiadis, C.; Kalaitzakis, E.; Kassi, E.; Androutsakos, T. The role of anti-diabetic drugs in NAFLD. Have we found the Holy Grail? A narrative review. Eur. J. Clin. Pharmacol. 2024, 80, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An Antidiabetic Thiazolidinedione Is a High Affinity Ligand for Peroxisome Proliferator-activated Receptor γ (PPARγ)∗. J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Deng, C.; Gu, Y.; He, Y.; Yang, L.; Shi, J. Effects of dapagliflozin in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101876. [Google Scholar] [CrossRef]
- Juurlink, D.N.; Gomes, T.; Lipscombe, L.L.; Austin, P.C.; Hux, J.E.; Mamdani, M.M. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: Population based cohort study. BMJ 2009, 339, b2942. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Rubenstrunk, A.; Noel, B.; Rigou, G.; Delataille, P.; Millatt, L.J.; Baron, M.; Lucas, A.; Tailleux, A.; Hum, D.W.; et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013, 58, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Hanf, R.; Lambert-Porcheron, S.; Zair, Y.; Sauvinet, V.; Noel, B.; Flet, L.; Vidal, H.; Staels, B.; Laville, M. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care 2013, 36, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Ueda, P.; Svanstrom, H.; Melbye, M.; Eliasson, B.; Svensson, A.M.; Franzen, S.; Gudbjornsdottir, S.; Hveem, K.; Jonasson, C.; Pasternak, B. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: Nationwide register based cohort study. BMJ 2018, 363, k4365. [Google Scholar] [CrossRef]
- Kahl, S.; Gancheva, S.; Strassburger, K.; Herder, C.; Machann, J.; Katsuyama, H.; Kabisch, S.; Henkel, E.; Kopf, S.; Lagerpusch, M.; et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care 2020, 43, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Latva-Rasku, A.; Honka, M.-J.; Kullberg, J.; Mononen, N.; Lehtimäki, T.; Saltevo, J.; Kirjavainen, A.K.; Saunavaara, V.; Iozzo, P.; Johansson, L. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: A randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care 2019, 42, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Rha, S.W.; Kang, H.J.; Bae, J.W.; Choi, B.J.; Choi, S.Y.; Gwon, H.C.; Bae, J.H.; Hong, B.K.; Choi, D.H.; et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). J. Clin. Lipidol. 2012, 6, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Hyogo, H.; Kimura, Y.; Ishitobi, T.; Arihiro, K.; Aikata, H.; Takahashi, S.; Chayama, K. Efficacy of rosuvastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: An open-label, pilot study. Hepatol. Res. 2012, 42, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Lee, H.W.; Lee, K.S.; Lee, H.S.; Park, J.Y. Effects of Statin Use on the Development and Progression of Nonalcoholic Fatty Liver Disease: A Nationwide Nested Case-Control Study. Am. J. Gastroenterol. 2021, 116, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Sfikas, G.; Psallas, M.; Koumaras, C.; Imprialos, K.; Perdikakis, E.; Doumas, M.; Giouleme, O.; Karagiannis, A.; Athyros, V.G. Prevalence, diagnosis, and treatment with 3 different statins of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis in military personnel. Do genetics play a role? Curr. Vasc. Pharmacol. 2021, 19, 572–581. [Google Scholar] [CrossRef]
- Pineda, C.; Rios, R.; Raya, A.I.; Rodriguez, M.; Aguilera-Tejero, E.; Lopez, I. Hypocaloric Diet Prevents the Decrease in FGF21 Elicited by High Phosphorus Intake. Nutrients 2018, 10, 1496. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Mangelsdorf, D.J. A Dozen Years of Discovery: Insights into the Physiology and Pharmacology of FGF21. Cell Metab. 2019, 29, 246–253. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef]
- Gong, Q.; Hu, Z.; Zhang, F.; Cui, A.; Chen, X.; Jiang, H.; Gao, J.; Chen, X.; Han, Y.; Liang, Q.; et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 2016, 64, 425–438. [Google Scholar] [CrossRef]
- Sanyal, A.; Charles, E.D.; Neuschwander-Tetri, B.A.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019, 392, 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, J.; Ji, S.; Ma, F.; Wang, G.; Xue, Y.; Liu, Z.; Gao, J.; Han, J.; Tai, P.; et al. The Effects of B1344, a Novel Fibroblast Growth Factor 21 Analog, on Nonalcoholic Steatohepatitis in Nonhuman Primates. Diabetes 2020, 69, 1611–1623. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Charles, E.D.; Sanyal, A.J.; Harrison, S.A.; Neuschwander-Tetri, B.A.; Goodman, Z.; Ehman, R.A.; Karsdal, M.; Nakajima, A.; Du, S.; et al. The FALCON program: Two phase 2b randomized, double-blind, placebo-controlled studies to assess the efficacy and safety of pegbelfermin in the treatment of patients with nonalcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Contemp. Clin. Trials 2021, 104, 106335. [Google Scholar] [CrossRef]
- Jani, R.H.; Pai, V.; Jha, P.; Jariwala, G.; Mukhopadhyay, S.; Bhansali, A.; Joshi, S. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI). Diabetes Technol. Ther. 2014, 16, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Idowu, M.O.; Parmar, D.; Borg, B.B.; Denham, D.; Loo, N.M.; Lazas, D.; Younes, Z.; Sanyal, A.J. A Phase 2 Double Blinded, Randomized Controlled Trial of Saroglitazar in Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2021, 19, 2670–2672. [Google Scholar] [CrossRef]
- Boeckmans, J.; Natale, A.; Rombaut, M.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, M.R. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells 2019, 9, 37. [Google Scholar] [CrossRef]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Ratziu, V.; Bedossa, P.; Dufour, J.-F.; Kruger, F.; Schattenberg, J.; Francque, S.; Arrese, M.; George, J.; Bugianesi, E. RESOLVE-IT Phase 3 Trial of Elafibranor in NASH: Final Results of the Week 72 Interim Surrogate Efficacy Analysis. In Proceedings of the Liver Meeting Digital Experience 2020 of American Association for the Study of Liver Diseases, Online, 11–16 November 2020; Available online: https://www.natap.org/2020/AASLD/AASLD_162.htm (accessed on 1 April 2024).
- Mayerson, A.B.; Hundal, R.S.; Dufour, S.; Lebon, V.; Befroy, D.; Cline, G.W.; Enocksson, S.; Inzucchi, S.E.; Shulman, G.I.; Petersen, K.F. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 2002, 51, 797–802. [Google Scholar] [CrossRef]
- Stayrook, K.R.; Bramlett, K.S.; Savkur, R.S.; Ficorilli, J.; Cook, T.; Christe, M.E.; Michael, L.F.; Burris, T.P. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology 2005, 146, 984–991. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Baj, J.; Khalil, M.; Garruti, G.; Stellaard, F.; Wang, H.H.; Wang, D.Q.; Portincasa, P. Recent Advances in the Digestive, Metabolic and Therapeutic Effects of Farnesoid X Receptor and Fibroblast Growth Factor 19: From Cholesterol to Bile Acid Signaling. Nutrients 2022, 14, 4950. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Khalil, M.; Portincasa, P. The interaction of bile acids and gut inflammation influences the pathogenesis of inflammatory bowel disease. Intern. Emerg. Med. 2023, 18, 2181–2197. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.; Molina-Molina, E.; Lunardi Baccetto, R.; Calamita, G.; Palmieri, V.O.; Portincasa, P. Bile Acids and Cancer: Direct and Environmental-Dependent Effects. Ann. Hepatol. 2017, 16, s87–s105. [Google Scholar] [CrossRef] [PubMed]
- Garruti, G.; Di Ciaula, A.; Wang, H.H.; Wang, D.Q.; Portincasa, P. Cross-Talk Between Bile Acids and Gastro-Intestinal and Thermogenic Hormones: Clues from Bariatric Surgery. Ann. Hepatol. 2017, 16, s68–s82. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lu, Y.; Li, X.Y. Farnesoid X receptor: A master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin. 2015, 36, 44–50. [Google Scholar] [CrossRef]
- Panzitt, K.; Wagner, M. FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166133. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Lucas, K.J.; Francque, S.M.; Abdelmalek, M.F.; Sanyal, A.J.; Ratziu, V.; Gadano, A.C.; Rinella, M.E.; Charlton, M.R.; Loomba, R. Safety and efficacy of Tropifexor plus Cenicriviroc combination therapy in adult patients with fibrotic NASH: 48 week results from the phase 2b tandem study. Hepatology 2021, 74, 96A–97A. [Google Scholar]
- Alkhouri, N.; Herring, R.; Kabler, H.; Kayali, Z.; Hassanein, T.; Kohli, A.; Huss, R.S.; Zhu, Y.; Billin, A.N.; Damgaard, L.H.; et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial. J. Hepatol. 2022, 77, 607–618. [Google Scholar] [CrossRef]
- Lawitz, E.J.; Poordad, F.; Coste, A.; Loo, N.; Djedjos, C.S.; McColgan, B.; Jia, C.; Xu, R.; Myers, R.P.; Subramanian, G.M.; et al. Acetyl-CoA carboxylase (ACC) inhibitor GS-0976 leads to suppression of hepatic de novo lipogenesis and significant improvements in MRI-PDFF, MRE, and markers of fibrosis after 12 weeks of therapy in patients with NASH. J. Hepatol. 2017, 66, S34. [Google Scholar] [CrossRef]
- Calle, R.A.; Amin, N.B.; Carvajal-Gonzalez, S.; Ross, T.T.; Bergman, A.; Aggarwal, S.; Crowley, C.; Rinaldi, A.; Mancuso, J.; Aggarwal, N.; et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: Two parallel, placebo-controlled, randomized phase 2a trials. Nat. Med. 2021, 27, 1836–1848. [Google Scholar] [CrossRef]
- Ratziu, V.; Rinella, M.E.; Neuschwander-Tetri, B.A.; Lawitz, E.; Denham, D.; Kayali, Z.; Sheikh, A.; Kowdley, K.V.; Desta, T.; Elkhashab, M.; et al. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J. Hepatol. 2022, 76, 506–517. [Google Scholar] [CrossRef]
- Loomba, R.; Kowdley, K.V.; Vuppalanchi, R.; Hassanein, T.; Rojter, S.E.; Sheikh, M.Y.; Moussa, S.; Chung, D.; Eng, C.; Marmon, T.; et al. Liver-distributed FXR agonist TERN-101 demonstrates favorable safety and efficacy profile in NASH phase 2a lift study. Hepatology 2021, 74, 97a–98a. [Google Scholar]
- Talukdar, S.; Kharitonenkov, A. FGF19 and FGF21: In NASH we trust. Mol. Metab. 2021, 46, 101152. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Neff, G.; Guy, C.D.; Bashir, M.R.; Paredes, A.H.; Frias, J.P.; Younes, Z.; Trotter, J.F.; Gunn, N.T.; Moussa, S.E.; et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology 2021, 160, 219–231.e1. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Abdelmalek, M.F.; Neff, G.; Gunn, N.; Guy, C.D.; Alkhouri, N.; Bashir, M.R.; Freilich, B.; Kohli, A.; Khazanchi, A.; et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 2022, 7, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Selective Agonists of Thyroid Hormone Receptor Beta for the Treatment of NASH. N. Engl. J. Med. 2024, 390, 559–561. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Mantovani, A.; Nascimbeni, F.; Lugari, S.; Targher, G. Pathogenesis of hypothyroidism-induced NAFLD: Evidence for a distinct disease entity? Dig. Liver Dis. 2019, 51, 462–470. [Google Scholar] [CrossRef]
- Wirth, E.K.; Puengel, T.; Spranger, J.; Tacke, F. Thyroid hormones as a disease modifier and therapeutic target in nonalcoholic steatohepatitis. Expert Rev. Endocrinol. Metab. 2022, 17, 425–434. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Harrison, S.; Taub, R.; Neff, G.; Moussa, S.; Alkhouri, N.; Bashir, M. Primary data analyses of MAESTRO-NAFLD-1 a 52 week double-blind placebo-controlled phase 3 clinical trial of resmetirom in patients with NAFLD. J. Hepatol. 2022, 77, S14. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Loomba, R.; Neutel, J.; Margaritescu, C.; Homer, K.; Luk, A.; Mancini, M.; Ji, S.; Barker, G.; Severance, R.; et al. VK2809, a novel liver-directed thyroid receptor agonist, produces durable reductions in liver fat in patients with non-alcoholic fatty liver disease: Results of 4-week follow-up assessment from a 12-week phase 2 randomized, placebo-controlled trial. J. Hepatol. 2020, 73, S53. [Google Scholar] [CrossRef]
- Pedrosa, M.; Seyedkazemi, S.; Francque, S.; Sanyal, A.; Rinella, M.; Charlton, M.; Loomba, R.; Ratziu, V.; Kochuparampil, J.; Fischer, L.; et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp. Clin. Trials 2020, 88, 105889. [Google Scholar] [CrossRef]
- Di Lella, S.; Sundblad, V.; Cerliani, J.P.; Guardia, C.M.; Estrin, D.A.; Vasta, G.R.; Rabinovich, G.A. When galectins recognize glycans: From biochemistry to physiology and back again. Biochemistry 2011, 50, 7842–7857. [Google Scholar] [CrossRef]
- Chalasani, N.; Abdelmalek, M.F.; Garcia-Tsao, G.; Vuppalanchi, R.; Alkhouri, N.; Rinella, M.; Noureddin, M.; Pyko, M.; Shiffman, M.; Sanyal, A.; et al. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology 2020, 158, 1334–1345.e5. [Google Scholar] [CrossRef]
- Iruarrizaga-Lejarreta, M.; Varela-Rey, M.; Fernandez-Ramos, D.; Martinez-Arranz, I.; Delgado, T.C.; Simon, J.; Juan, V.G.; delaCruz-Villar, L.; Azkargorta, M.; Lavin, J.L.; et al. Role of Aramchol in steatohepatitis and fibrosis in mice. Hepatol. Commun. 2017, 1, 911–927. [Google Scholar] [CrossRef]
- Miyazaki, M.; Flowers, M.T.; Sampath, H.; Chu, K.; Otzelberger, C.; Liu, X.; Ntambi, J.M. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007, 6, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Safadi, R.; Konikoff, F.M.; Mahamid, M.; Zelber-Sagi, S.; Halpern, M.; Gilat, T.; Oren, R.; Group, F. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2085–2091.e1. [Google Scholar] [CrossRef]
- Malik, A.; Nadeem, M.; Amjad, W.; Malik, M.I.; Javaid, S.; Farooq, U.; Naseem, K.; Khan, A. Effects of Aramchol in patients with nonalcoholic fatty liver disease (NAFLD). A systematic review and meta-analysis. Prz. Gastroenterol. 2023, 18, 67–75. [Google Scholar] [CrossRef]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Gabriel, K.R.; Chitraju, C.; Bronson, R.T.; Mejhert, N.; Boland, S.; Wang, K.; Lai, Z.W.; Farese, R.V., Jr.; Walther, T.C. Hepatocyte Deletion of Triglyceride-Synthesis Enzyme Acyl CoA: Diacylglycerol Acyltransferase 2 Reduces Steatosis Without Increasing Inflammation or Fibrosis in Mice. Hepatology 2019, 70, 1972–1985. [Google Scholar] [CrossRef]
- Saxena, A.; Chidsey, K.; Somayaji, V.; Ogden, A.; Duvvuri, S. Diacylglycerol acyltransferase 2 (DGAT2) inhibitor PF-06865571 reduces liver fat by MRI-PDFF after 2 weeks in adults with NAFLD. Hepatology 2019, 70, 1260A. [Google Scholar]
- Amin, N.B.; Darekar, A.; Anstee, Q.M.; Wong, V.W.; Tacke, F.; Vourvahis, M.; Lee, D.S.; Charlton, M.; Alkhouri, N.; Nakajima, A.; et al. Efficacy and safety of an orally administered DGAT2 inhibitor alone or coadministered with a liver-targeted ACC inhibitor in adults with non-alcoholic steatohepatitis (NASH): Rationale and design of the phase II, dose-ranging, dose-finding, randomised, placebo-controlled MIRNA (Metabolic Interventions to Resolve NASH with fibrosis) study. BMJ Open 2022, 12, e056159. [Google Scholar] [CrossRef]
- Loomba, R.; Mohseni, R.; Lucas, K.J.; Gutierrez, J.A.; Perry, R.G.; Trotter, J.F.; Rahimi, R.S.; Harrison, S.A.; Ajmera, V.; Wayne, J.D.; et al. TVB-2640 (FASN Inhibitor) for the Treatment of Nonalcoholic Steatohepatitis: FASCINATE-1, a Randomized, Placebo-Controlled Phase 2a Trial. Gastroenterology 2021, 161, 1475–1486. [Google Scholar] [CrossRef]
- Cao, J.; Lockwood, J.; Burn, P.; Shi, Y. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 2003, 278, 13860–13866. [Google Scholar] [CrossRef]
- Yen, C.L.; Farese, R.V., Jr. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 2003, 278, 18532–18537. [Google Scholar] [CrossRef]
- Cheng, D.; Zinker, B.A.; Luo, Y.; Shipkova, P.; De Oliveira, C.H.; Krishna, G.; Brown, E.A.; Boehm, S.L.; Tirucherai, G.S.; Gu, H.; et al. MGAT2 inhibitor decreases liver fibrosis and inflammation in murine NASH models and reduces body weight in human adults with obesity. Cell Metab. 2022, 34, 1732–1748.e5. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Gaggini, M.; Daniele, G.; Ciociaro, D.; Cersosimo, E.; Tripathy, D.; Triplitt, C.; Fox, P.; Musi, N.; DeFronzo, R.; et al. Exenatide Improves both Hepatic and Adipose Tissue Insulin Resistance: A Dynamic Positron Emission Tomography Study. Hepatology 2016, 64, 2028–2037. [Google Scholar] [CrossRef]
- Gimeno, R.E.; Briere, D.A.; Seeley, R.J. Leveraging the Gut to Treat Metabolic Disease. Cell Metab. 2020, 31, 679–698. [Google Scholar] [CrossRef]
- Mells, J.E.; Fu, P.P.; Sharma, S.; Olson, D.; Cheng, L.; Handy, J.A.; Saxena, N.K.; Sorescu, D.; Anania, F.A. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G225–G235. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Tetri, L.H.; Basaranoglu, M.; Brunt, E.M.; Yerian, L.M.; Neuschwander-Tetri, B.A. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G987–G995. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, M.F.; Suzuki, A.; Guy, C.; Unalp-Arida, A.; Colvin, R.; Johnson, R.J.; Diehl, A.M.; Clini, N.S. Increased Fructose Consumption Is Associated with Fibrosis Severity in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2010, 51, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, M.; Basaranoglu, G.; Sabuncu, T.; Senturk, H. Fructose as a key player in the development of fatty liver disease. World J. Gastroenterol. 2013, 19, 1166–1172. [Google Scholar] [CrossRef]
- Ding, X.; Saxena, N.K.; Lin, S.; Gupta, N.A.; Anania, F.A. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006, 43, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; De Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011, 31, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Alkhouri, N.; Davison, B.A.; Sanyal, A.; Edwards, C.; Colca, J.R.; Lee, B.H.; Loomba, R.; Cusi, K.; Kolterman, O.; et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J. Hepatol. 2020, 72, 613–626. [Google Scholar] [CrossRef]
- Harrison, S. PXL065 (deuterium-stabilized R-enantiomer of pioglitazone) reduces liver fat content and improves liver histology without PPARG-mediated side effects in patients with NASH: Analysis of a 36 week placebo-controlled Phase 2 trial (DESTINY1). In Proceedings of the 2022 AASLD the Liver Meeting, Washington, DC, USA, 4–8 November 2022; pp. 4–8. [Google Scholar]
- Stagi, S.; Ricci, F.; Bianconi, M.; Sammarco, M.A.; Municchi, G.; Toni, S.; Lenzi, L.; Verrotti, A.; de Martino, M. Retrospective Evaluation of Metformin and/or Metformin Plus a New Polysaccharide Complex in Treating Severe Hyperinsulinism and Insulin Resistance in Obese Children and Adolescents with Metabolic Syndrome. Nutrients 2017, 9, 524. [Google Scholar] [CrossRef]
- Stagi, S.; Lapi, E.; Seminara, S.; Pelosi, P.; Del Greco, P.; Capirchio, L.; Strano, M.; Giglio, S.; Chiarelli, F.; de Martino, M. Policaptil Gel Retard significantly reduces body mass index and hyperinsulinism and may decrease the risk of type 2 diabetes mellitus (T2DM) in obese children and adolescents with family history of obesity and T2DM. Ital. J. Pediatr. 2015, 41, 10. [Google Scholar] [CrossRef]
- Guarino, G.; Della Corte, T.; Strollo, F.; Gentile, S.; Nefrocenter Research Study, G. Policaptil Gel Retard in adult subjects with the metabolic syndrome: Efficacy, safety, and tolerability compared to metformin. Diabetes Metab. Syndr. 2021, 15, 901–907. [Google Scholar] [CrossRef]
- Guarino, G.; Strollo, F.; Della Corte, T.; Satta, E.; Gentile, S. Effect of Policaptil Gel Retard on Liver Fat Content and Fibrosis in Adults with Metabolic Syndrome and Type 2 Diabetes: A Non-invasive Approach to MAFLD. Diabetes Ther. 2023, 14, 2089–2108. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Leber, B.; Feldbacher, N.; Tripolt, N.; Rainer, F.; Blesl, A.; Trieb, M.; Marsche, G.; Sourij, H.; Stadlbauer, V. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: A randomized, double-blind, placebo-controlled pilot study. Eur. J. Nutr. 2020, 59, 2969–2983. [Google Scholar] [CrossRef] [PubMed]
- Craven, L.; Rahman, A.; Nair Parvathy, S.; Beaton, M.; Silverman, J.; Qumosani, K.; Hramiak, I.; Hegele, R.; Joy, T.; Meddings, J.; et al. Allogenic Fecal Microbiota Transplantation in Patients with Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020, 115, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Koopen, A.; Witjes, J.; Wortelboer, K.; Majait, S.; Prodan, A.; Levin, E.; Herrema, H.; Winkelmeijer, M.; Aalvink, S.; Bergman, J.; et al. Duodenal Anaerobutyricum soehngenii infusion stimulates GLP-1 production, ameliorates glycaemic control and beneficially shapes the duodenal transcriptome in metabolic syndrome subjects: A randomised double-blind placebo-controlled cross-over study. Gut 2022, 71, 1577–1587. [Google Scholar] [CrossRef]
- Witjes, J.; Koopen, A.; Wortelboer, K.; Majait, S.; Prodan, A.; Levin, E.; Herrema, H.; Winkelmeijer, M.; Aalvink, S.; Bergman, J. Duodenal Infusion of Anaerobutyricum Soehngenii Ameliorates Glycemic Control and Postprandial GLP-1 Responses and Alters the Transcriptional Profile of Small Intestine in Subjects with Metabolic Syndrome. In Proceedings of the 1st International Electronic Conference on Nutrients—Nutritional and Microbiota Effects on Chronic Disease, Online, 2–15 November 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).