Cartilage-Specific Gene Expression and Extracellular Matrix Deposition in the Course of Mesenchymal Stromal Cell Chondrogenic Differentiation in 3D Spheroid Culture
Abstract
:1. Introduction
2. Results
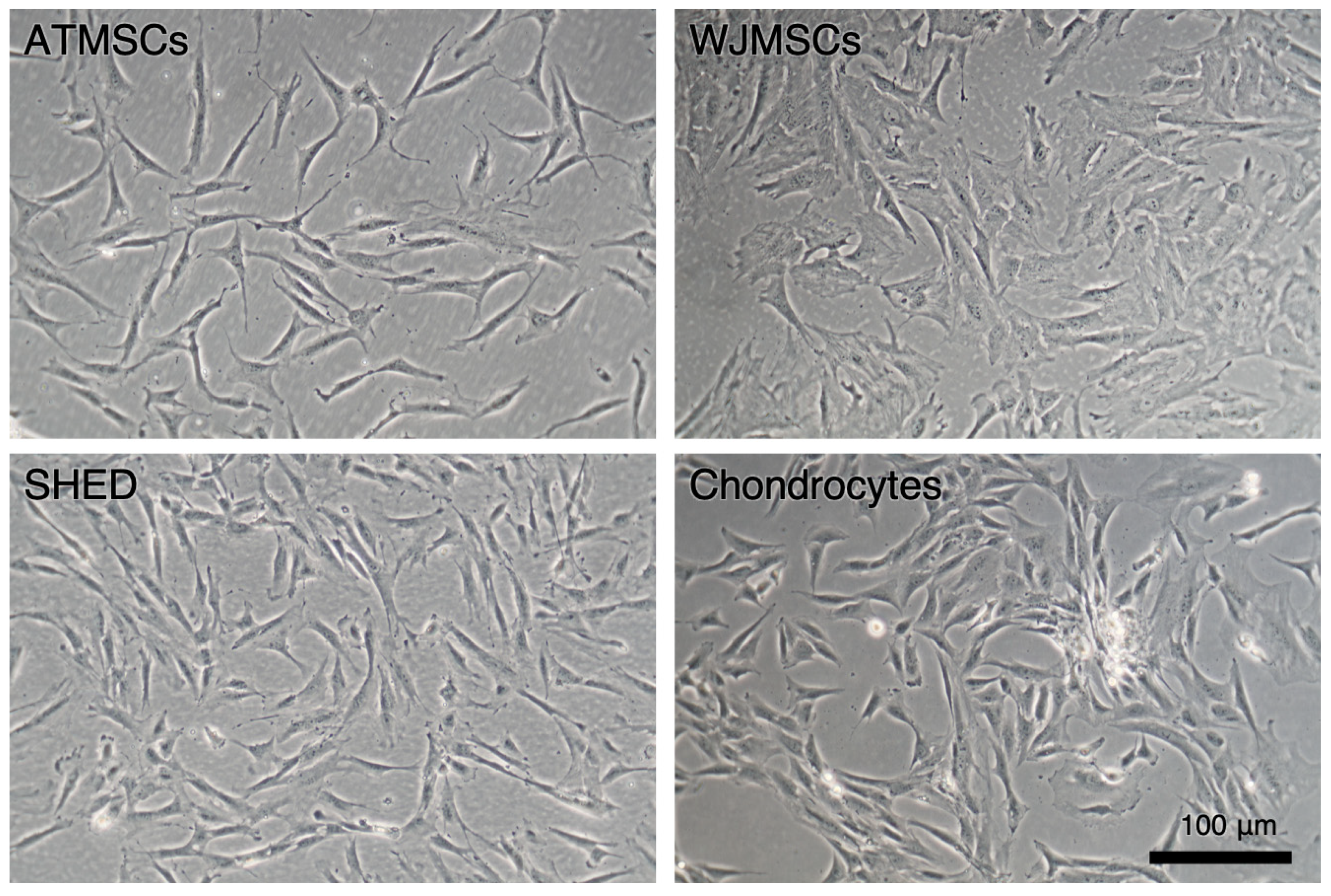
2.1. Primary Cell Cultures
2.2. Spheroid Chondrogenic Differentiation
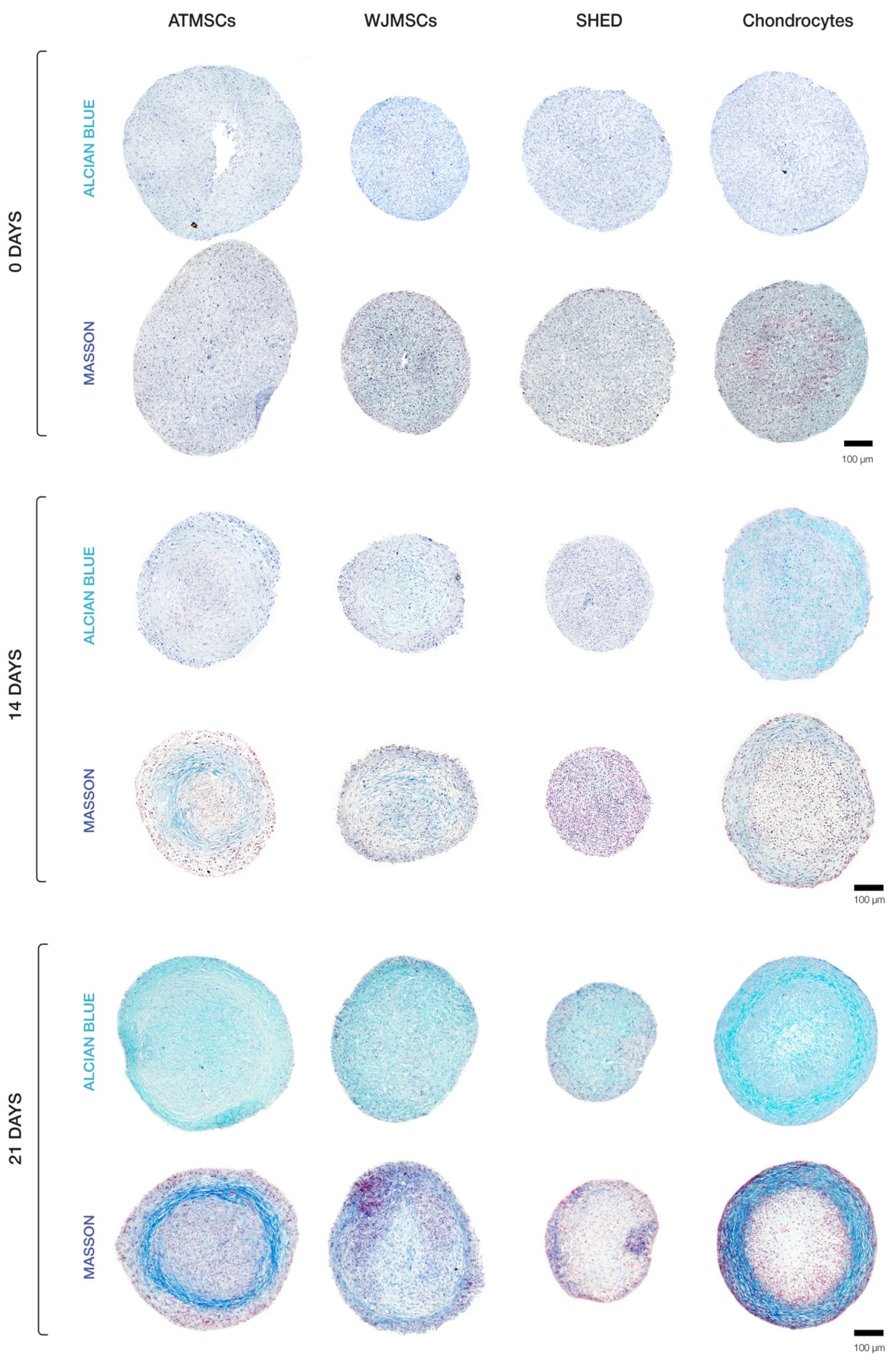
2.3. ECM Deposition in MSC Spheroids
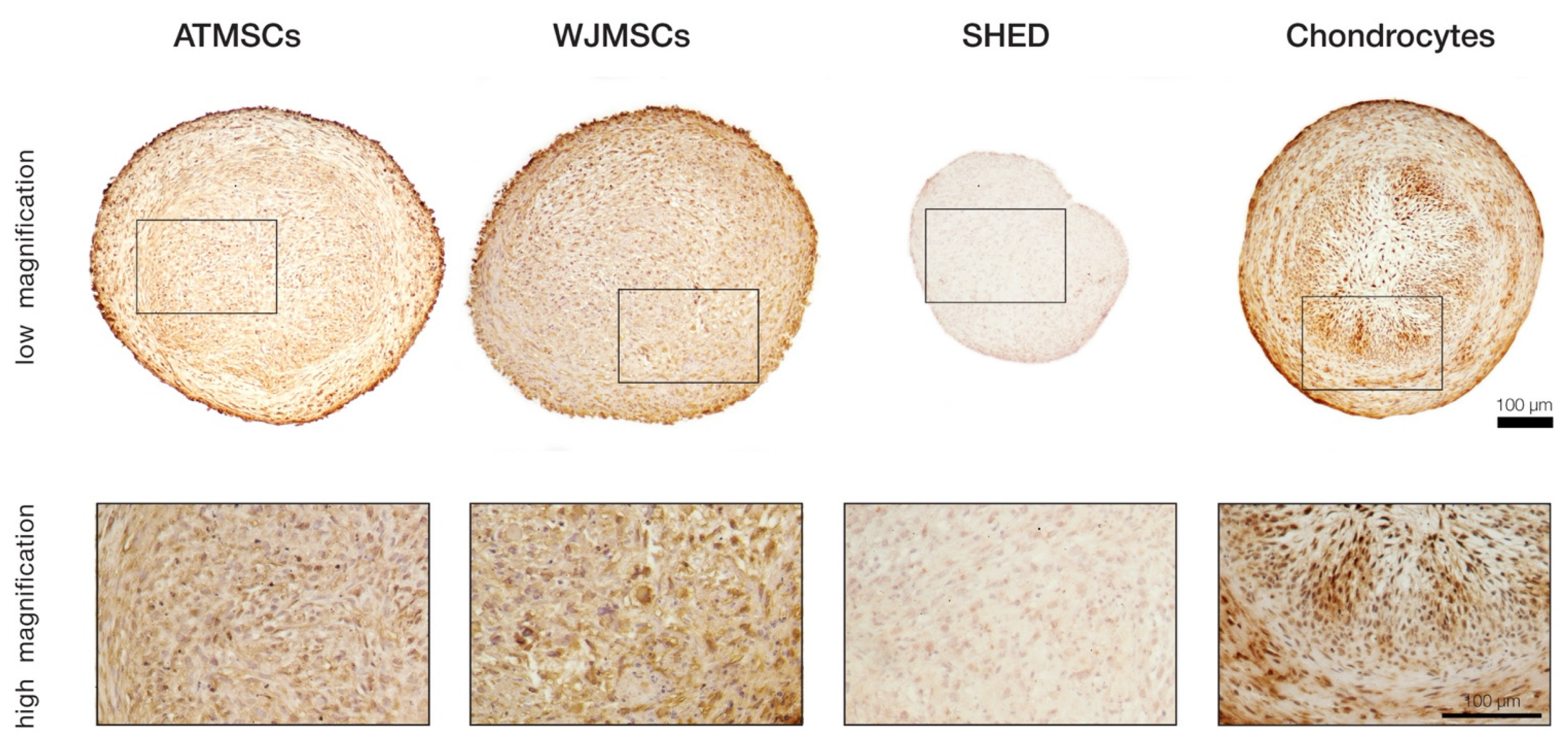
2.4. Collagen II Synthesis
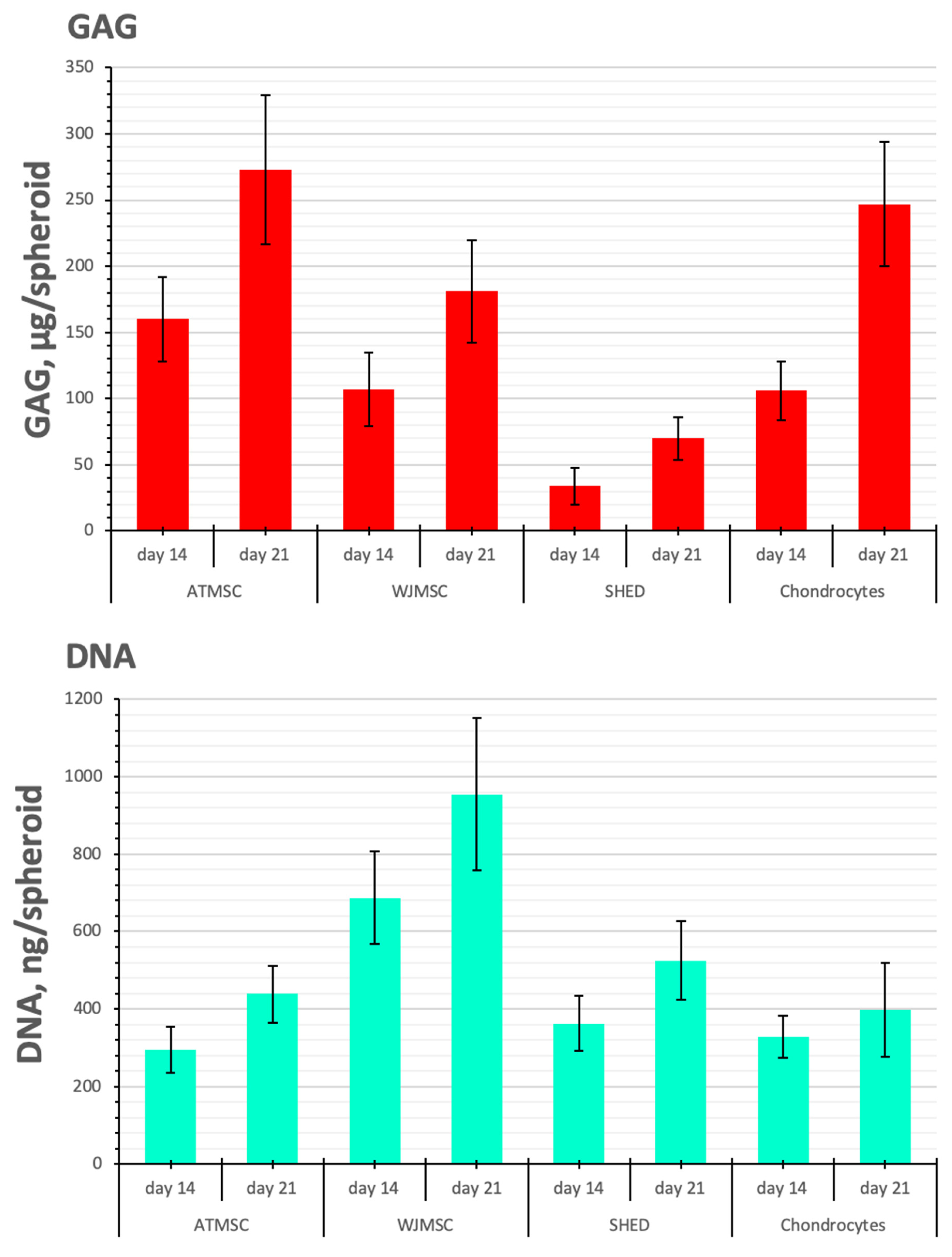
2.5. Biochemical Analysis of GAG/DNA Content
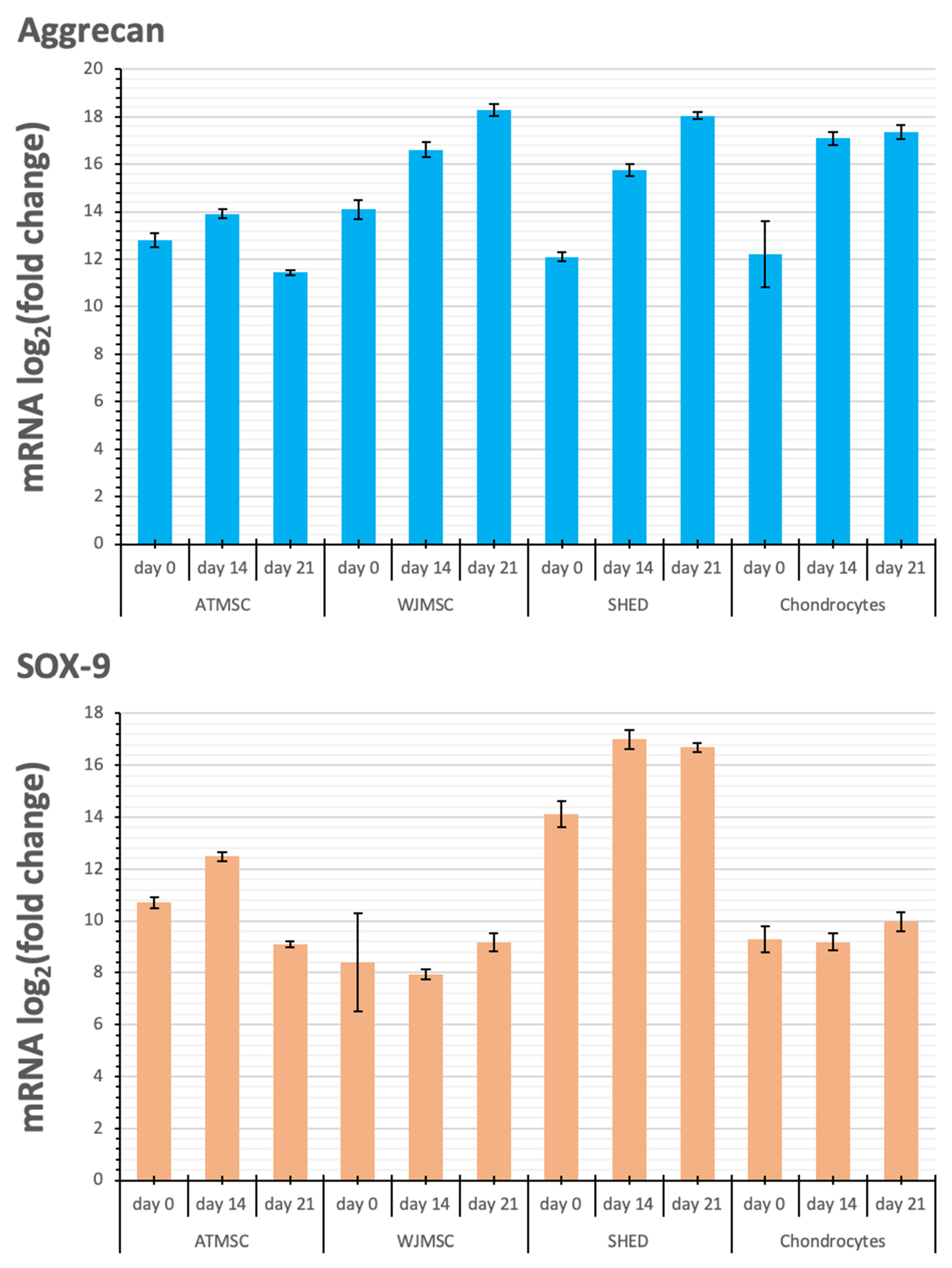
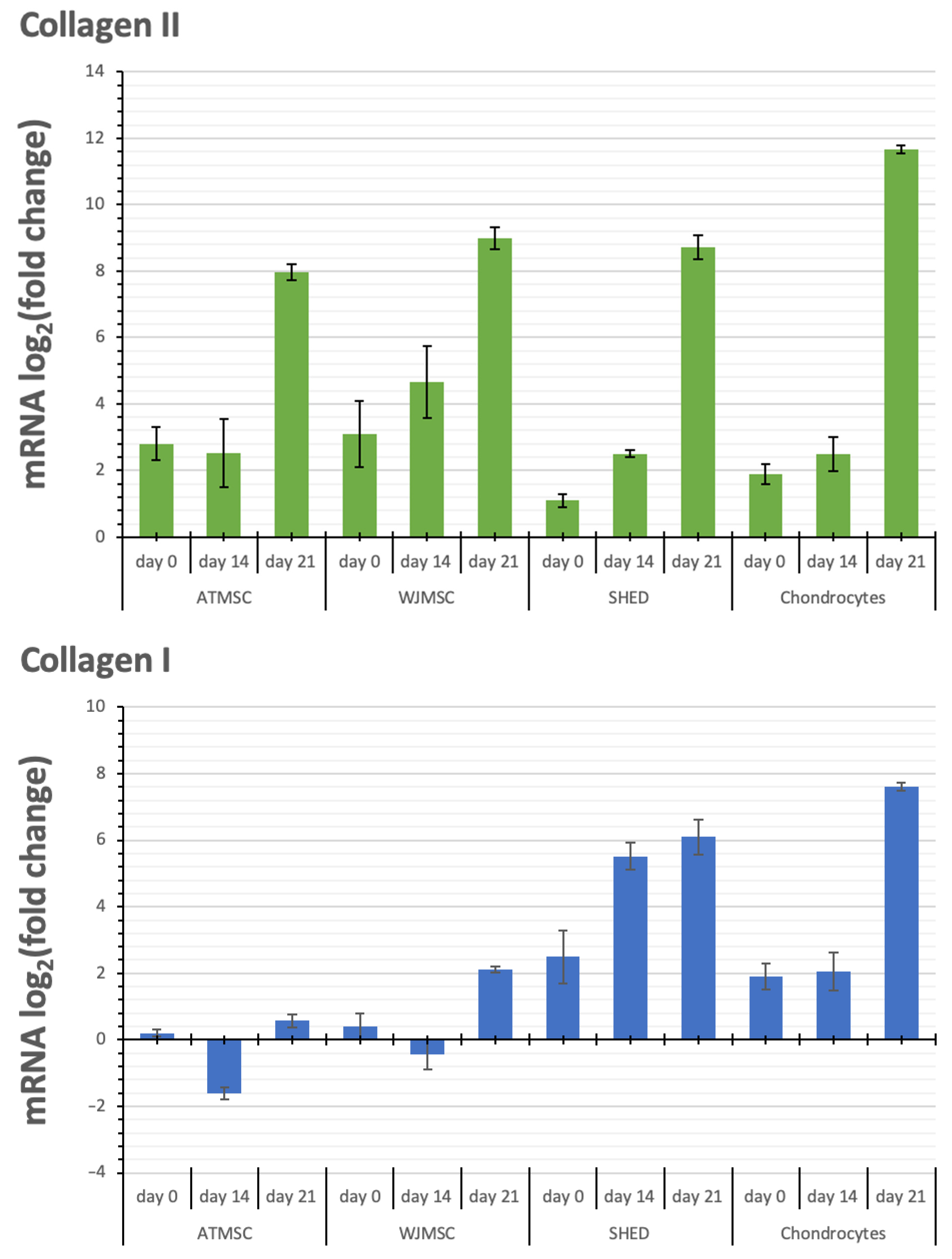
2.6. Changes in Cartilage-Specific Gene Expression
3. Discussion
4. Materials and Methods
4.1. Primary Cell Cultures
4.2. Flow Cytometry
4.3. Osteogenic Differentiation
4.4. Adipogenic Differentiation
4.5. Chondrogenic Differentiation
4.6. Sample Lysis, RNA Extraction, Reverse Transcription, and qPCR Analysis
4.7. DNA Quantification
4.8. Biochemical Quantification of GAG Content
4.9. Histological and Immunohistochemical Staining
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, G.; Lu, K.; Umar, M.; Zhu, Z.; Lu, W.W.; Speakman, J.R.; Chen, Y.; Tong, L.; Chen, D. Risk of Metabolic Abnormalities in Osteoarthritis: A New Perspective to Understand Its Pathological Mechanisms. Bone Res. 2023, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet Lond. Engl. 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Rim, Y.A.; Lee, J.; Ju, J.H. Current Therapeutic Strategies for Stem Cell-Based Cartilage Regeneration. Stem Cells Int. 2018, 2018, 8490489. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T. Intra-Articular Treatment Options for Knee Osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kurenkova, A.D.; Romanova, I.A.; Kibirskiy, P.D.; Timashev, P.; Medvedeva, E.V. Strategies to Convert Cells into Hyaline Cartilage: Magic Spells for Adult Stem Cells. Int. J. Mol. Sci. 2022, 23, 11169. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L.; MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological Characterization of Multipotent Mesenchymal Stromal Cells—The International Society for Cellular Therapy (ISCT) Working Proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Prajwal, G.S.; Jeyaraman, N.; Kanth, V.K.; Jeyaraman, M.; Muthu, S.; Rajendran, S.N.S.; Rajendran, R.L.; Khanna, M.; Oh, E.J.; Choi, K.Y.; et al. Lineage Differentiation Potential of Different Sources of Mesenchymal Stem Cells for Osteoarthritis Knee. Pharmaceuticals 2022, 15, 386. [Google Scholar] [CrossRef]
- Heo, J.S.; Choi, Y.; Kim, H.-S.; Kim, H.O. Comparison of Molecular Profiles of Human Mesenchymal Stem Cells Derived from Bone Marrow, Umbilical Cord Blood, Placenta and Adipose Tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, A.V.; Vakhrushev, I.V.; Basok, Y.B.; Grigor’ev, A.M.; Kirsanova, L.A.; Lupatov, A.Y.; Sevastianov, V.I.; Yarygin, K.N. Chondrogeneic Potential of MSC from Different Sources in Spheroid Culture. Bull. Exp. Biol. Med. 2021, 170, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Sargenti, A.; Pasqua, S.; Leu, M.; Dionisi, L.; Filardo, G.; Grigolo, B.; Gazzola, D.; Santi, S.; Cavallo, C. Adipose Stromal Cell Spheroids for Cartilage Repair: A Promising Tool for Unveiling the Critical Maturation Point. Bioengineering 2023, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering Zonal Cartilage through Bioprinting Collagen Type II Hydrogel Constructs with Biomimetic Chondrocyte Density Gradient. BMC Musculoskelet. Disord. 2016, 17, 301. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, D.; Schwab, A.; Walles, H.; Ehlicke, F. Advanced Therapy Medicinal Products: A Guide for Bone Marrow-Derived MSC Application in Bone and Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2018, 24, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal Stem Cells in Osteoarthritis Therapy: A Review. Am. J. Transl. Res. 2021, 13, 448–461. [Google Scholar] [PubMed]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of Mesenchymal StemCells in Cartilage Regeneration: From Characterization to Application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, J.W. 3D Spheroid Cultures of Stem Cells and Exosome Applications for Cartilage Repair. Life 2022, 12, 939. [Google Scholar] [CrossRef] [PubMed]
- Shestovskaya, M.V.; Bozhkova, S.A.; Sopova, J.V.; Khotin, M.G.; Bozhokin, M.S. Methods of Modification of Mesenchymal Stem Cells and Conditions of Their Culturing for Hyaline Cartilage Tissue Engineering. Biomedicines 2021, 9, 1666. [Google Scholar] [CrossRef]
- Farkhad, N.K.; Mahmoudi, A.; Mahdipour, E. How Similar Are Human Mesenchymal Stem Cells Derived from Different Origins? A Review of Comparative Studies. Curr. Stem Cell Res. Ther. 2021, 16, 980–993. [Google Scholar] [CrossRef]
- Mélou, C.; Pellen-Mussi, P.; Novello, S.; Brézulier, D.; Novella, A.; Tricot, S.; Bellaud, P.; Chauvel-Lebret, D. Spheroid Culture System, a Promising Method for Chondrogenic Differentiation of Dental Mesenchymal Stem Cells. Biomedicines 2023, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jiang, H.; Zuo, H.-D. Factors Affecting Osteogenesis and Chondrogenic Differentiation of Mesenchymal Stem Cells in Osteoarthritis. World J. Stem Cells 2023, 15, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Desroches, A.; Queinnec, S.; FlouzatLachaniette, C.H.; Poignard, A.; Allain, J.; Chevallier, N.; Rouard, H. Morbidity of Graft Harvesting versus Bone Marrow Aspiration in Cell Regenerative Therapy. Int. Orthop. 2014, 38, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Kasir, R.; Vernekar, V.N.; Laurencin, C.T. Regenerative Engineering of Cartilage Using Adipose-Derived Stem Cells. Regen. Eng. Transl. Med. 2015, 1, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, A.; Kałucka, M.; Kokoszka-Mikołaj, E.; Wilczok, A. Osteogenic Differentiation of Human Mesenchymal Stem Cells from Adipose Tissue and Wharton’s Jelly of the Umbilical Cord. Acta Biochim. Pol. 2017, 64, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Jiang, B.; Cui, J.; Li, G.; Yu, M.; Wang, F.; Zhang, G.; Nan, X.; Yue, W.; Xu, X.; et al. Superior Osteogenic Capacity of Different Mesenchymal Stem Cells for Bone Tissue Engineering. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e324–e332. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of Rat Mesenchymal Stem Cells Derived from Bone Marrow, Synovium, Periosteum, Adipose Tissue, and Muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, O.R.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 2015, 43, 2738–2746. [Google Scholar] [CrossRef]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of Proliferative and Multilineage Differentiation Potential of Human Mesenchymal Stem Cells Derived from Umbilical Cord and Bone Marrow. Stem Cells Dayt. Ohio 2007, 25, 1384–1392. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells Dayt. Ohio 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Kamolz, L.-P.; Keck, M.; Kasper, C. Wharton’s Jelly Mesenchymal Stem Cells Promote Wound Healing and Tissue Regeneration. Stem Cell Res. Ther. 2014, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Stanko, P.; Altanerova, U.; Jakubechova, J.; Repiska, V.; Altaner, C. Dental Mesenchymal Stem/Stromal Cells and Their Exosomes. Stem Cells Int. 2018, 2018, 8973613. [Google Scholar] [CrossRef]
- Westin, C.B.; Trinca, R.B.; Zuliani, C.; Coimbra, I.B.; Moraes, Â.M. Differentiation of Dental Pulp Stem Cells into Chondrocytes upon Culture on Porous Chitosan-Xanthan Scaffolds in the Presence of Kartogenin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.L.; Shimomura, K.; Asperti, A.; Pinheiro, C.C.G.; Caetano, H.V.A.; Oliveira, C.R.G.C.M.; Nakamura, N.; Hernandez, A.J.; Bueno, D.F. Development of a Novel Large Animal Model to Evaluate Human Dental Pulp Stem Cells for Articular Cartilage Treatment. Stem Cell Rev. Rep. 2018, 14, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.C.; MacArthur, B.D.; Tare, R.S.; Oreffo, R.O.C.; Please, C.P. Extracellular Matrix Deposition in Engineered Micromass Cartilage Pellet Cultures: Measurements and Modelling. PLoS ONE 2016, 11, e0147302. [Google Scholar] [CrossRef]
- Ahmed, S.; Chauhan, V.M.; Ghaemmaghami, A.M.; Aylott, J.W. New Generation of Bioreactors That Advance Extracellular Matrix Modelling and Tissue Engineering. Biotechnol. Lett. 2019, 41, 1–25. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Basok, Y.B.; Grigor’ev, A.M.; Kirsanova, L.A.; Vasilets, V.N. Formation of Tissue-Engineered Construct of Human Cartilage Tissue in a Flow-Through Bioreactor. Bull. Exp. Biol. Med. 2017, 164, 269–273. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Basok, Y.B.; Kirsanova, L.A.; Grigoriev, A.M.; Kirillova, A.D.; Nemets, E.A.; Subbot, A.M.; Gautier, S.V. A Comparison of the Capacity of Mesenchymal Stromal Cells for Cartilage Regeneration Depending on Collagen-Based Injectable Biomimetic Scaffold Type. Life 2021, 11, 756. [Google Scholar] [CrossRef]
- Körsmeier, K.; Claßen, T.; Kamminga, M.; Rekowski, J.; Jäger, M.; Landgraeber, S. Arthroscopic Three-Dimensional Autologous Chondrocyte Transplantation Using Spheroids for the Treatment of Full-Thickness Cartilage Defects of the Hip Joint. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2016, 24, 2032–2037. [Google Scholar] [CrossRef]
- Shimizu, M.; Matsumoto, T.; Kikuta, S.; Ohtaki, M.; Kano, K.; Taniguchi, H.; Saito, S.; Nagaoka, M.; Tokuhashi, Y. Transplantation of Dedifferentiated Fat Cell-Derived Micromass Pellets Contributed to Cartilage Repair in the Rat Osteochondral Defect Model. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2018, 23, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Merlo, B.; Teti, G.; Mazzotti, E.; Ingrà, L.; Salvatore, V.; Buzzi, M.; Cerqueni, G.; Dicarlo, M.; Lanci, A.; Castagnetti, C.; et al. Wharton’s Jelly Derived Mesenchymal Stem Cells: Comparing Human and Horse. Stem Cell Rev. Rep. 2018, 14, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Choi, G.T.; Park, J.; Lee, J.; Do, J.T. Comparative Analysis of Porcine Adipose- and Wharton’s Jelly-Derived Mesenchymal Stem Cells. Animals 2023, 13, 2947. [Google Scholar] [CrossRef]
- Wu, S.-H.; Yu, J.-H.; Liao, Y.-T.; Liu, K.-H.; Chiang, E.-R.; Chang, M.-C.; Wang, J.-P. Comparison of the Infant and Adult Adipose-Derived Mesenchymal Stem Cells in Proliferation, Senescence, Anti-Oxidative Ability and Differentiation Potential. Tissue Eng. Regen. Med. 2022, 19, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of Mesenchymal Stem Cell: Machinery, Markers, and Strategies of Fighting. Cell. Mol. Biol. Lett. 2022, 27, 69. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.A.; Yousefi, M.; Talebi, M.; Shamsasenjan, K. Regenerative Potential of Wharton’s Jelly-Derived Mesenchymal Stem Cells: A New Horizon of Stem Cell Therapy. J. Cell. Physiol. 2020, 235, 9230–9240. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Yun, B.G.; Lim, M.J.; Kim, S.J.; Lim, M.H.; Lim, J.Y.; Park, S.H.; Kim, S.W. Rapid Cartilage Regeneration of Spheroids Composed of Human Nasal Septum-Derived Chondrocyte in Rat Osteochondral Defect Model. Tissue Eng. Regen. Med. 2020, 17, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Park, K.-H. Regulation and Function of SOX9 during Cartilage Development and Regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef]
- Kim, J.; Tomida, K.; Matsumoto, T.; Adachi, T. Spheroid Culture for Chondrocytes Triggers the Initial Stage of Endochondral Ossification. Biotechnol. Bioeng. 2022, 119, 3311–3318. [Google Scholar] [CrossRef]




| Gene Symbol and Name | Qiagen Catalogue Number |
|---|---|
| GAPDH, glyceraldehyde-3-phosphate dehydrogenase | 330001 PPH00150F |
| Col1A1, Collagen, Type I, alpha 1 | 330001 PPH01299F |
| Col2A1, Collagen, Type II, alpha 1 | 330001 PPH02134F |
| ACAN, Aggrecan | 330001 PPH06097E |
| SOX-9, SRY (sex-determining region Y)-box 9 | 330001 PPH02125A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakhrushev, I.V.; Basok, Y.B.; Baskaev, K.K.; Novikova, V.D.; Leonov, G.E.; Grigoriev, A.M.; Belova, A.D.; Kirsanova, L.A.; Lupatov, A.Y.; Burunova, V.V.; et al. Cartilage-Specific Gene Expression and Extracellular Matrix Deposition in the Course of Mesenchymal Stromal Cell Chondrogenic Differentiation in 3D Spheroid Culture. Int. J. Mol. Sci. 2024, 25, 5695. https://doi.org/10.3390/ijms25115695
Vakhrushev IV, Basok YB, Baskaev KK, Novikova VD, Leonov GE, Grigoriev AM, Belova AD, Kirsanova LA, Lupatov AY, Burunova VV, et al. Cartilage-Specific Gene Expression and Extracellular Matrix Deposition in the Course of Mesenchymal Stromal Cell Chondrogenic Differentiation in 3D Spheroid Culture. International Journal of Molecular Sciences. 2024; 25(11):5695. https://doi.org/10.3390/ijms25115695
Chicago/Turabian StyleVakhrushev, Igor V., Yulia B. Basok, Konstantin K. Baskaev, Victoria D. Novikova, Georgy E. Leonov, Alexey M. Grigoriev, Aleksandra D. Belova, Ludmila A. Kirsanova, Alexey Y. Lupatov, Veronika V. Burunova, and et al. 2024. "Cartilage-Specific Gene Expression and Extracellular Matrix Deposition in the Course of Mesenchymal Stromal Cell Chondrogenic Differentiation in 3D Spheroid Culture" International Journal of Molecular Sciences 25, no. 11: 5695. https://doi.org/10.3390/ijms25115695
APA StyleVakhrushev, I. V., Basok, Y. B., Baskaev, K. K., Novikova, V. D., Leonov, G. E., Grigoriev, A. M., Belova, A. D., Kirsanova, L. A., Lupatov, A. Y., Burunova, V. V., Kovalev, A. V., Makarevich, P. I., Sevastianov, V. I., & Yarygin, K. N. (2024). Cartilage-Specific Gene Expression and Extracellular Matrix Deposition in the Course of Mesenchymal Stromal Cell Chondrogenic Differentiation in 3D Spheroid Culture. International Journal of Molecular Sciences, 25(11), 5695. https://doi.org/10.3390/ijms25115695








