Prolonged Response to Afatinib and Crizotinib in a Rare Case of EGFR-, HER2-, MET- and ROS1-Alterated Lung Adenocarcinoma
Abstract
1. Introduction
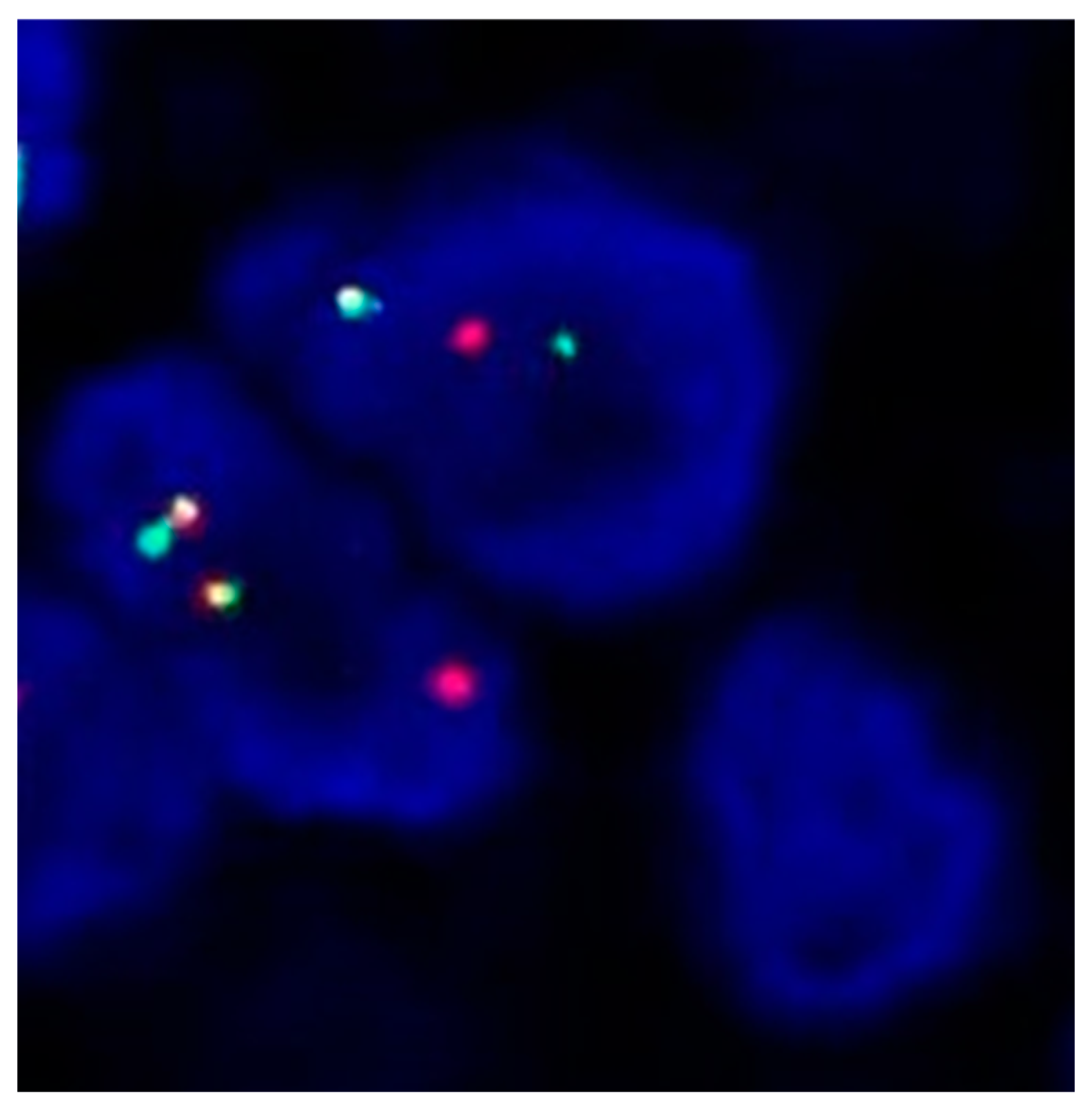
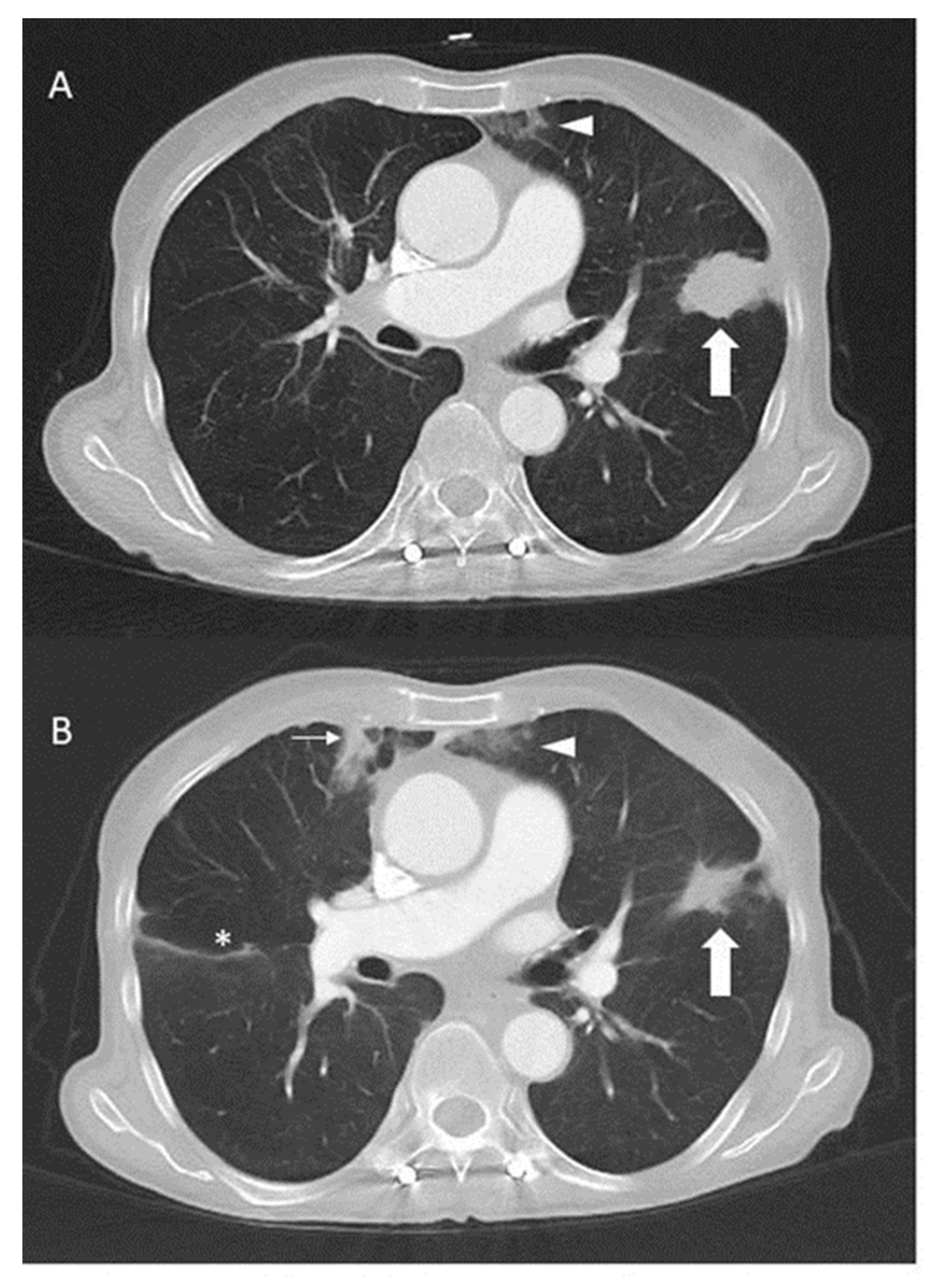
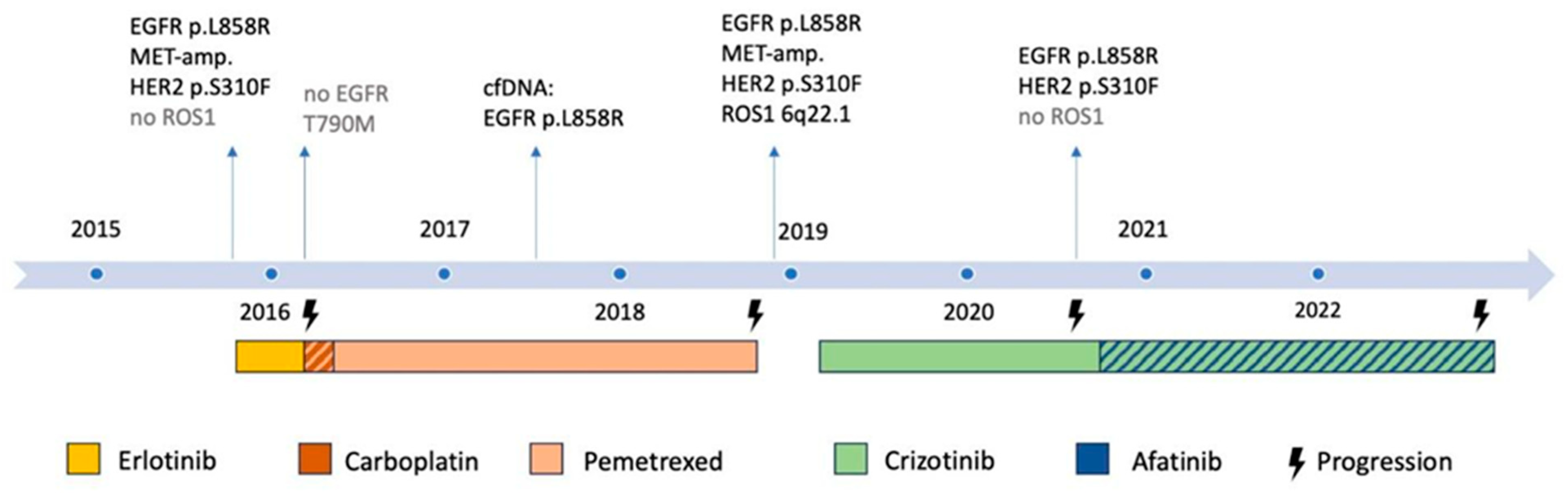
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Hanna, N.H.; Robinson, A.G.; Temin, S.; Baker, S.; Brahmer, J.R.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non–Small-Cell Lung Cancer with Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2021, 39, 1040–1091. [Google Scholar] [CrossRef]
- Burnett, H.; Emich, H.; Carroll, C.; Stapleton, N.; Mahadevia, P.; Li, T. Epidemiological and Clinical Burden of EGFR Exon 20 Insertion in Advanced Non-Small Cell Lung Cancer: A Systematic Literature Review. PLoS ONE 2021, 16, e0247620. [Google Scholar] [CrossRef]
- Dearden, S.; Stevens, J.; Wu, Y.-L.; Blowers, D. Mutation Incidence and Coincidence in Non Small-Cell Lung Cancer: Meta-Analyses by Ethnicity and Histology (MutMap). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2371–2376. [Google Scholar] [CrossRef]
- Jordan, E.J.; Kim, H.R.; Arcila, M.E.; Barron, D.; Chakravarty, D.; Gao, J.; Chang, M.T.; Ni, A.; Kundra, R.; Jonsson, P.; et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov. 2017, 7, 596–609. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus Chemotherapy as First-Line Treatment for Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802): A Multicentre, Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Final Overall Survival Results from a Randomised, Phase III Study of Erlotinib versus Chemotherapy as First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1877–1883. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.-M.; Zhao, X.; Christensen, J.; et al. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Kang, J.; Chen, H.-J.; Tu, H.-Y.; Zhou, Q.; Ye, J.-Y.; Wu, Y.-L.; Yang, J.-J. Co-Occurring Alterations in Driver Genes Impact on EGFR-Targeted Therapy among Patients with EGFR-Mutant Advanced Non–Small Cell Lung Cancer. Ann. Oncol. 2018, 29, ix158–ix159. [Google Scholar] [CrossRef]
- Clement, M.S.; Ebert, E.B.F.; Meldgaard, P.; Sorensen, B.S. Co-Occurring MET Amplification Predicts Inferior Clinical Response to First-Line Erlotinib in Advanced Stage EGFR-Mutated NSCLC Patients. Clin. Lung Cancer 2021, 22, e870–e877. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Singh, V.; Baca, Y.; Sukari, A.; Kim, C.; Mamdani, H.; Spira, A.I.; Uprety, D.; Bepler, G.; Kim, E.S.; et al. The Effects of HER2 Alterations in EGFR Mutant Non-Small Cell Lung Cancer. Clin. Lung Cancer 2022, 23, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Hsieh, M.-S.; Wu, S.-G.; Chang, Y.-L.; Yu, C.-J.; Yang, J.C.-H.; Yang, P.-C.; Shih, J.-Y. Efficacy of Pemetrexed-Based Chemotherapy in Patients with ROS1 Fusion-Positive Lung Adenocarcinoma Compared with in Patients Harboring Other Driver Mutations in East Asian Populations. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 1140–1152. [Google Scholar] [CrossRef]
- Uguen, A.; Schick, U.; Quéré, G. A Rare Case of ROS1 and ALK Double Rearranged Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, e71–e72. [Google Scholar] [CrossRef][Green Version]
- Lambros, L.; Guibourg, B.; Uguen, A. ROS1-Rearranged Non-Small Cell Lung Cancers With Concomitant Oncogenic Driver Alterations: About Some Rare Therapeutic Dilemmas. Clin. Lung Cancer 2018, 19, e73–e74. [Google Scholar] [CrossRef]
- Wiesweg, M.; Eberhardt, W.E.E.; Reis, H.; Ting, S.; Savvidou, N.; Skiba, C.; Herold, T.; Christoph, D.C.; Meiler, J.; Worm, K.; et al. High Prevalence of Concomitant Oncogene Mutations in Prospectively Identified Patients with ROS1-Positive Metastatic Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Van Der Steen, N.; Mentens, Y.; Ramael, M.; Leon, L.G.; Germonpré, P.; Ferri, J.; Gandara, D.R.; Giovannetti, E.; Peters, G.J.; Pauwels, P.; et al. Double Trouble: A Case Series on Concomitant Genetic Aberrations in NSCLC. Clin. Lung Cancer 2018, 19, 35–41. [Google Scholar] [CrossRef]
- Christensen, J.G.; Zou, H.Y.; Arango, M.E.; Li, Q.; Lee, J.H.; McDonnell, S.R.; Yamazaki, S.; Alton, G.R.; Mroczkowski, B.; Los, G. Cytoreductive Antitumor Activity of PF-2341066, a Novel Inhibitor of Anaplastic Lymphoma Kinase and c-Met, in Experimental Models of Anaplastic Large-Cell Lymphoma. Mol. Cancer Ther. 2007, 6, 3314–3322. [Google Scholar] [CrossRef]
- Shaw, A.T.; Riely, G.J.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer (NSCLC): Updated Results, Including Overall Survival, from PROFILE 1001. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-H.I. Crizotinib: A Novel and First-in-Class Multitargeted Tyrosine Kinase Inhibitor for the Treatment of Anaplastic Lymphoma Kinase Rearranged Non-Small Cell Lung Cancer and Beyond. Drug Des. Devel. Ther. 2011, 5, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Le, A.T.; Theodoro, M.F.; Skokan, M.C.; Aisner, D.L.; Berge, E.M.; Terracciano, L.M.; Cappuzzo, F.; Incarbone, M.; Roncalli, M.; et al. Identifying and Targeting ROS1 Gene Fusions in Non-Small Cell Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 4570–4579. [Google Scholar] [CrossRef]
- Li, D.; Ambrogio, L.; Shimamura, T.; Kubo, S.; Takahashi, M.; Chirieac, L.R.; Padera, R.F.; Shapiro, G.I.; Baum, A.; Himmelsbach, F.; et al. BIBW2992, an Irreversible EGFR/HER2 Inhibitor Highly Effective in Preclinical Lung Cancer Models. Oncogene 2008, 27, 4702–4711. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Yang, J.C.-H.; Park, K.; Kim, J.-H.; Bennouna, J.; Chen, Y.-M.; Chouaid, C.; De Marinis, F.; Feng, J.-F.; Grossi, F.; et al. Afatinib beyond Progression in Patients with Non-Small-Cell Lung Cancer Following Chemotherapy, Erlotinib/Gefitinib and Afatinib: Phase III Randomized LUX-Lung 5 Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 417–423. [Google Scholar] [CrossRef]
- Jia, Y.; Ali, S.M.; Saad, S.; Chan, C.A.; Miller, V.A.; Halmos, B. Successful Treatment of a Patient with Li-Fraumeni Syndrome and Metastatic Lung Adenocarcinoma Harboring Synchronous EGFR L858R and ERBB2 Extracellular Domain S310F Mutations with the Pan-HER Inhibitor Afatinib. Cancer Biol. Ther. 2014, 15, 970–974. [Google Scholar] [CrossRef]
- Mazieres, J.; Kim, T.M.; Lim, B.K.; Wislez, M.; Dooms, C.; Finocchiaro, G.; Hayashi, H.; Liam, C.K.; Raskin, J.; Tho, L.M.; et al. LBA52 Tepotinib + Osimertinib for EGFRm NSCLC with MET Amplification (METamp) after Progression on First-Line (1L) Osimertinib: Initial Results from the INSIGHT 2 Study. Ann. Oncol. 2022, 33, S1419–S1420. [Google Scholar] [CrossRef]
- Tan, D.S.-W.; Kim, T.M.; Guarneri, V.; VOON, P.J.; Lim, B.K.; Wislez, M.; Huang, C.; Liam, C.K.; Mazieres, J.; Tho, L.M.; et al. Tepotinib + Osimertinib for EGFR Mutant (EGFRm) NSCLC with MET Amplification (METamp) after First-Line (1L) Osimertinib. J. Clin. Oncol. 2023, 41, 9021. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Zhong, W.-Z.; Zhang, X.-C.; Su, J.; Yang, X.-N.; Chen, Z.-H.; Yang, J.-J.; Zhou, Q.; Yan, H.-H.; An, S.-J.; et al. EGFR Mutation Heterogeneity and the Mixed Response to EGFR Tyrosine Kinase Inhibitors of Lung Adenocarcinomas. The Oncol. 2012, 17, 978–985. [Google Scholar] [CrossRef]
- Taniguchi, K.; Okami, J.; Kodama, K.; Higashiyama, M.; Kato, K. Intratumor Heterogeneity of Epidermal Growth Factor Receptor Mutations in Lung Cancer and Its Correlation to the Response to Gefitinib. Cancer Sci. 2008, 99, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Petronczki, M.; Solca, F.; Maemondo, M. Tumor Clonality and Resistance Mechanisms in EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Implications for Therapeutic Sequencing. Future Oncol. Lond. Engl. 2019, 15, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef]
- Xu, C.; Li, D.; Duan, W.; Tao, M. TPD52L1-ROS1 Rearrangement as a New Acquired Resistance Mechanism to Osimertinib That Responds to Crizotinib in Combination With Osimertinib in Lung Adenocarcinoma. JTO Clin. Res. Rep. 2020, 1, 100034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plomer, E.; Früh, M.; Lauber, A.; Demmer, I.; Jochum, W.; Koster, K.-L. Prolonged Response to Afatinib and Crizotinib in a Rare Case of EGFR-, HER2-, MET- and ROS1-Alterated Lung Adenocarcinoma. Int. J. Mol. Sci. 2024, 25, 5698. https://doi.org/10.3390/ijms25115698
Plomer E, Früh M, Lauber A, Demmer I, Jochum W, Koster K-L. Prolonged Response to Afatinib and Crizotinib in a Rare Case of EGFR-, HER2-, MET- and ROS1-Alterated Lung Adenocarcinoma. International Journal of Molecular Sciences. 2024; 25(11):5698. https://doi.org/10.3390/ijms25115698
Chicago/Turabian StylePlomer, Eva, Martin Früh, Arno Lauber, Izadora Demmer, Wolfram Jochum, and Kira-Lee Koster. 2024. "Prolonged Response to Afatinib and Crizotinib in a Rare Case of EGFR-, HER2-, MET- and ROS1-Alterated Lung Adenocarcinoma" International Journal of Molecular Sciences 25, no. 11: 5698. https://doi.org/10.3390/ijms25115698
APA StylePlomer, E., Früh, M., Lauber, A., Demmer, I., Jochum, W., & Koster, K.-L. (2024). Prolonged Response to Afatinib and Crizotinib in a Rare Case of EGFR-, HER2-, MET- and ROS1-Alterated Lung Adenocarcinoma. International Journal of Molecular Sciences, 25(11), 5698. https://doi.org/10.3390/ijms25115698




