CD169+ Skin Macrophages Function as a Specialized Subpopulation in Promoting Psoriasis-like Skin Disease in Mice
Abstract
1. Introduction
2. Results
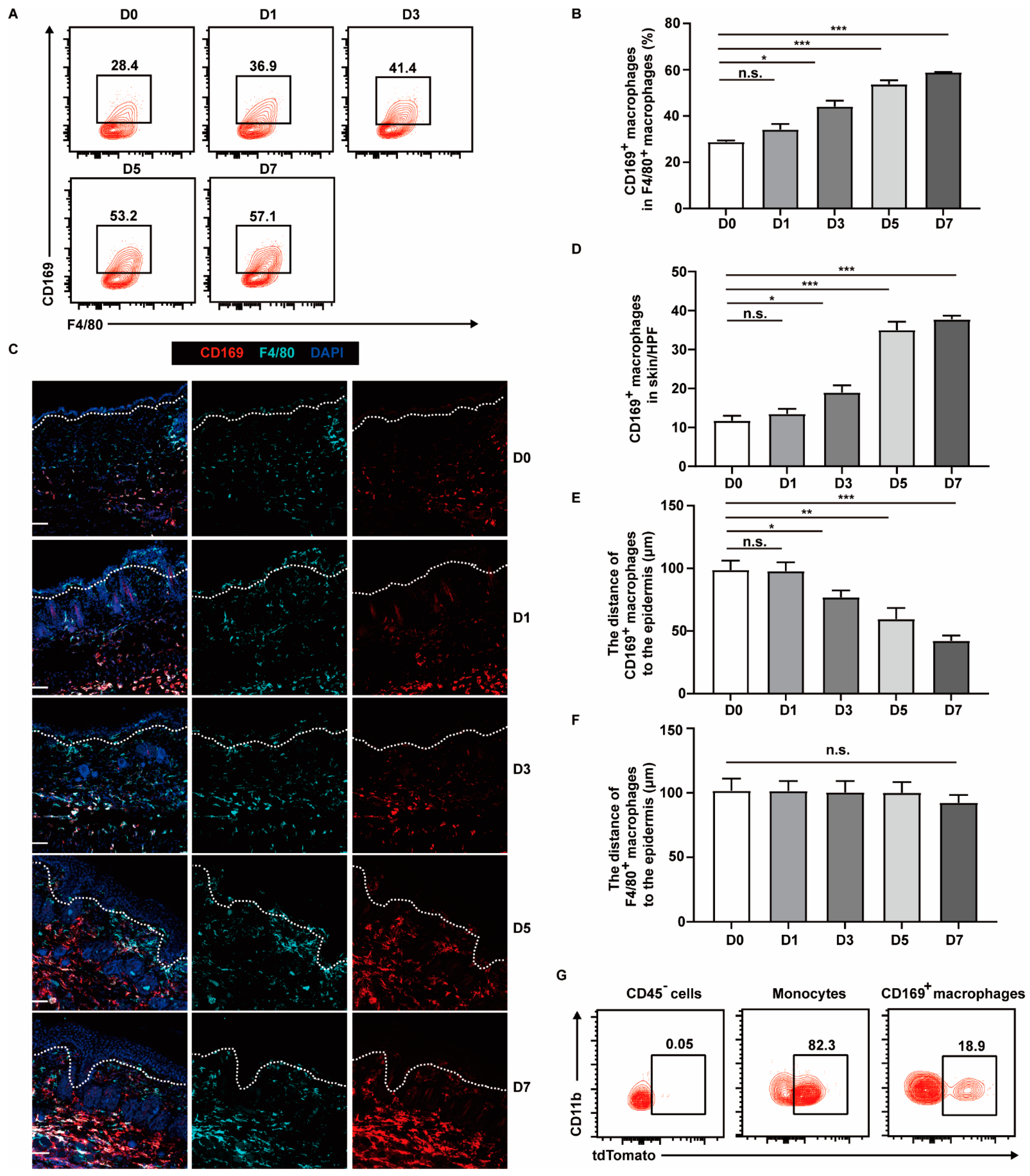
2.1. CD169+ Macrophages Are More Abundant and Appear near Epidermis in Psoriasis-like Lesions
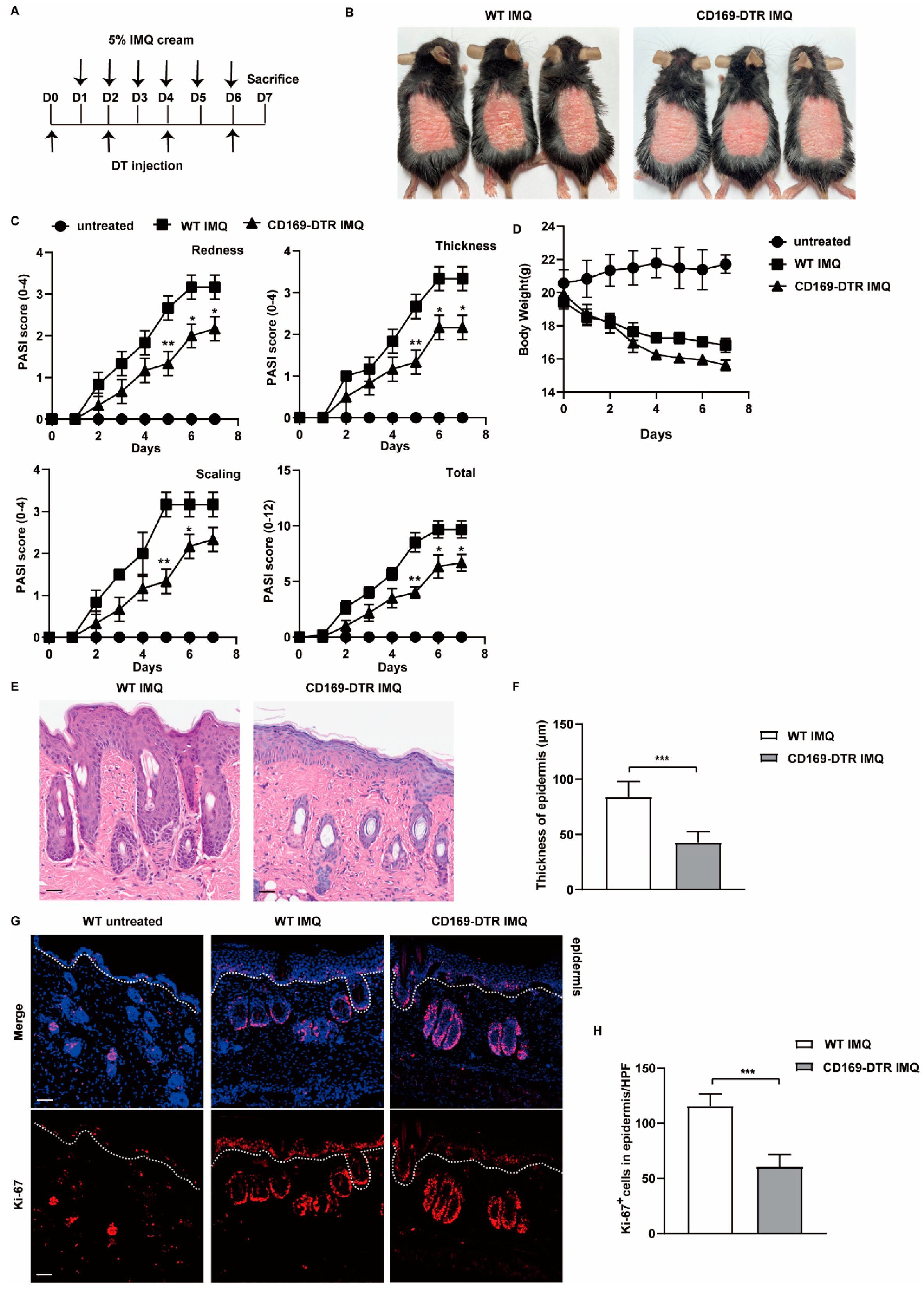
2.2. Depletion of CD169+ Macrophages Inhibits IMQ-Induced Psoriasis
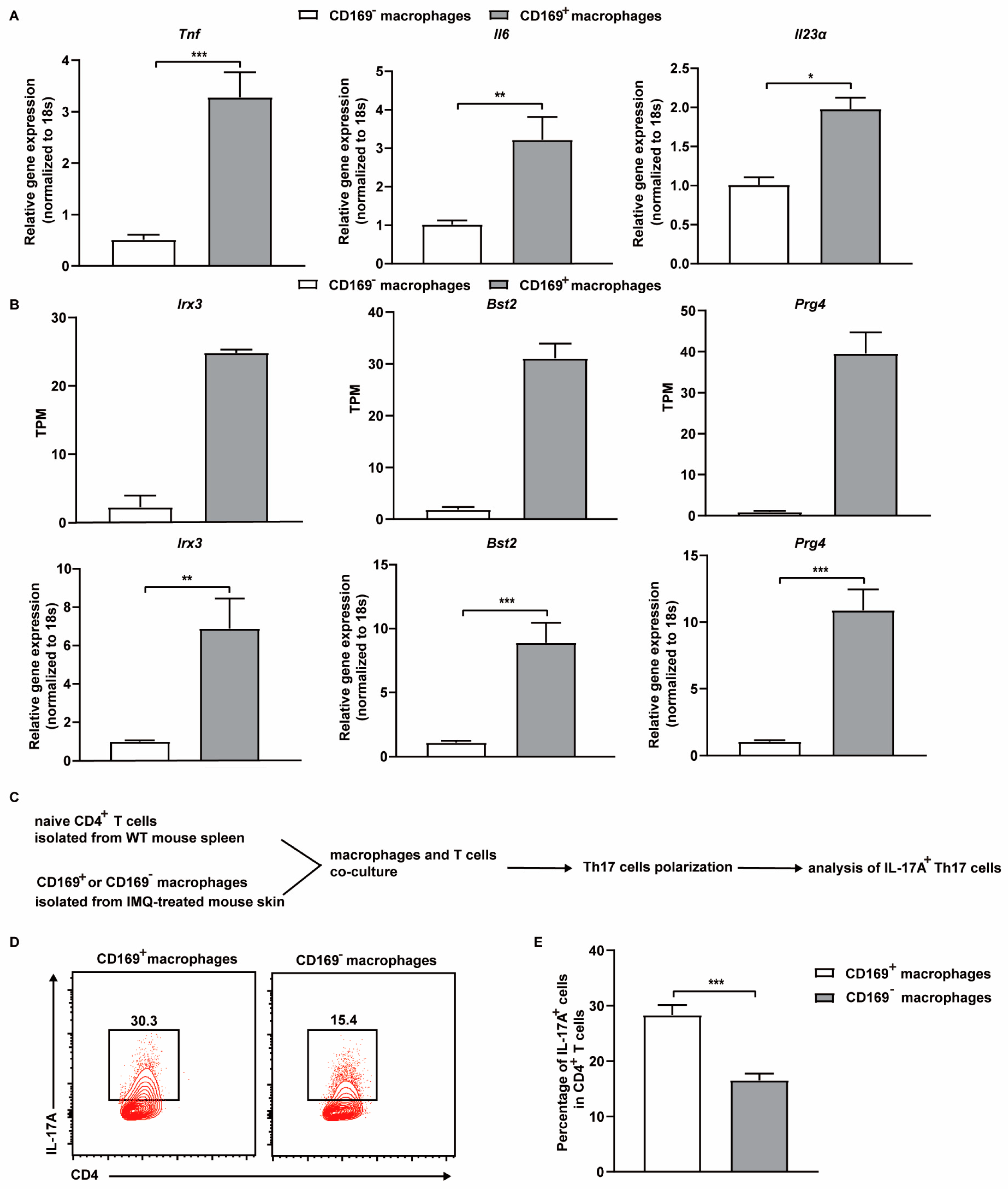
2.3. Depletion of CD169+ Macrophages Suppresses the Production of Psoriasis Related Cytokines and Reduces the Frequency of Th17 Cells in the Skin
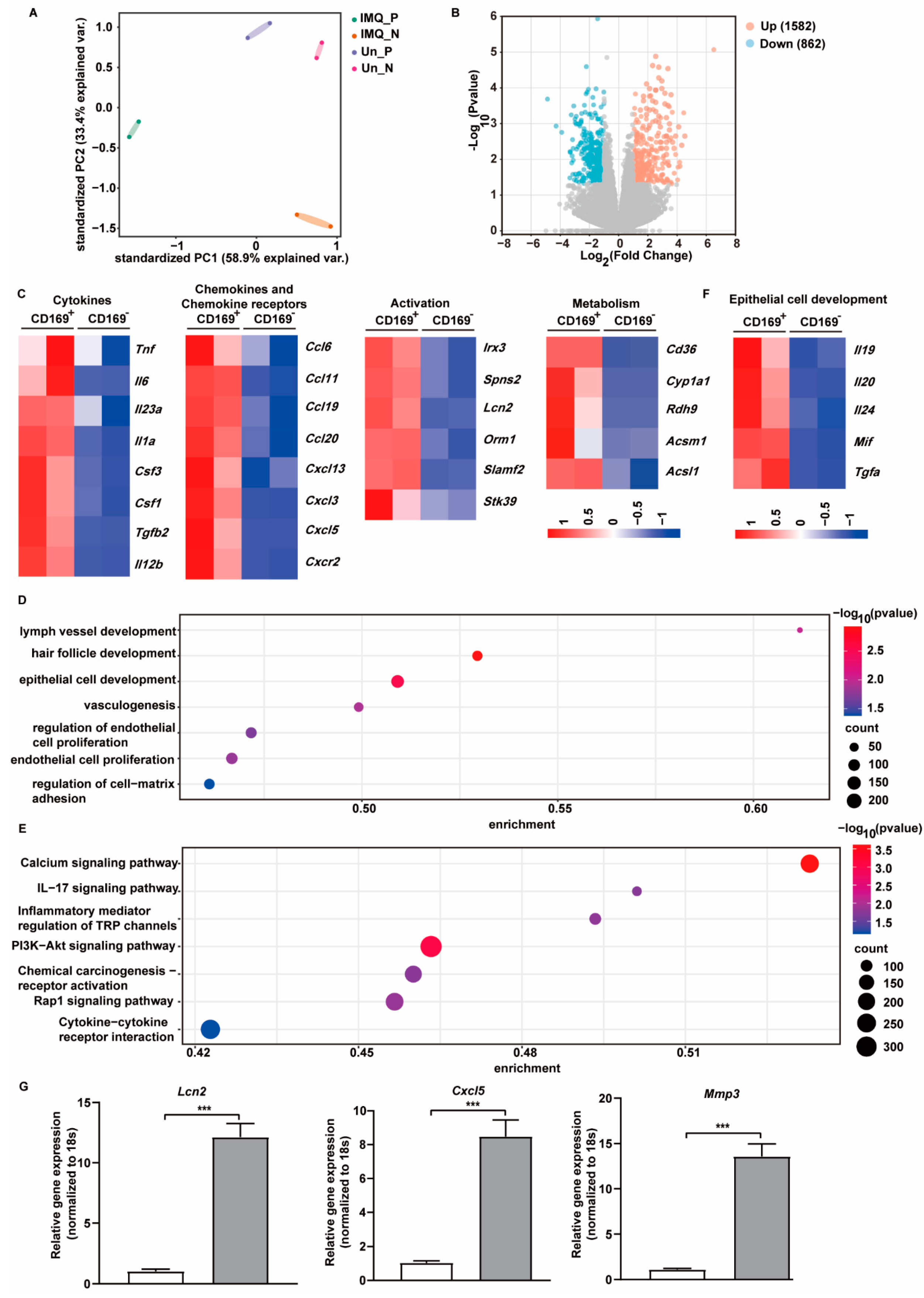
2.4. CD169+ Skin Macrophages Exhibit Unique Gene Expression Profiles upon IMQ Stimulation
2.5. CD169+ Skin Macrophages Promote Th17 Cell Differentiation Ex Vivo
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. IMQ-Induced Mouse Psoriasis Model and DT Injection
4.3. Tamoxifen Administration
4.4. Psoriasis Area Severity Index (PASI) and Bodyweight
4.5. Flow Cytometry
4.6. Skin Macrophages Isolation
4.7. Immunofluorescence Staining
4.8. Hematoxylin and Eosin Staining
4.9. In Vitro Th17 Cell Differentiation
4.10. RNA Extraction and Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
4.11. RNA-Seq
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sewerin, P.; Brinks, R.; Schneider, M.; Haase, I.; Vordenbäumen, S. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2019, 78, 286–287. [Google Scholar] [CrossRef]
- Damiani, G.; Bragazzi, N.L.; Karimkhani Aksut, C.; Wu, D.; Alicandro, G.; McGonagle, D.; Guo, C.; Dellavalle, R.; Grada, A.; Wong, P.; et al. The Global, Regional, and National Burden of Psoriasis: Results and Insights from the Global Burden of Disease 2019 Study. Front. Med. 2021, 8, 743180. [Google Scholar] [CrossRef]
- Guillet, C.; Seeli, C.; Nina, M.; Maul, L.V.; Maul, J.T. The impact of gender and sex in psoriasis: What to be aware of when treating women with psoriasis. Int. J. Women’s Dermatol. 2022, 8, e010. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Liu, S.; He, M.; Jiang, J.; Duan, X.; Chai, B.; Zhang, J.; Tao, Q.; Chen, H. Triggers for the onset and recurrence of psoriasis: A review and update. Cell Commun. Signal. 2024, 22, 108. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Bugaut, H.; Aractingi, S. Major Role of the IL17/23 Axis in Psoriasis Supports the Development of New Targeted Therapies. Front. Immunol. 2021, 12, 621956. [Google Scholar] [CrossRef] [PubMed]
- Calautti, E.; Avalle, L.; Poli, V. Psoriasis: A STAT3-Centric View. Int. J. Mol. Sci. 2018, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, E.; Semerano, L.; Assier, E.; Falgarone, G.; Boissier, M.C. Interleukin-23: A key cytokine in inflammatory diseases. Ann. Med. 2011, 43, 503–511. [Google Scholar] [CrossRef]
- Liu, T.; Li, S.; Ying, S.; Tang, S.; Ding, Y.; Li, Y.; Qiao, J.; Fang, H. The IL-23/IL-17 Pathway in Inflammatory Skin Diseases: From Bench to Bedside. Front. Immunol. 2020, 11, 594735. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Kolter, J.; Feuerstein, R.; Zeis, P.; Hagemeyer, N.; Paterson, N.; d’Errico, P.; Baasch, S.; Amann, L.; Masuda, T.; Lösslein, A.; et al. A Subset of Skin Macrophages Contributes to the Surveillance and Regeneration of Local Nerves. Immunity 2019, 50, 1482–1497.e1487. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.L.; Kumari, S.; Kaur, S.; Khosrotehrani, K. Macrophages in Skin Wounds: Functions and Therapeutic Potential. Biomolecules 2022, 12, 1659. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Duculan, J.; Suárez-Fariñas, M.; Zaba, L.C.; Nograles, K.E.; Pierson, K.C.; Mitsui, H.; Pensabene, C.A.; Kzhyshkowska, J.; Krueger, J.G.; Lowes, M.A. A Subpopulation of CD163-Positive Macrophages Is Classically Activated in Psoriasis. J. Investig. Dermatol. 2010, 130, 2412–2422. [Google Scholar] [CrossRef] [PubMed]
- Marble, D.J.; Gordon, K.B.; Nickoloff, B.J. Targeting TNFalpha rapidly reduces density of dendritic cells and macrophages in psoriatic plaques with restoration of epidermal keratinocyte differentiation. J. Dermatol. Sci. 2007, 48, 87–101. [Google Scholar] [CrossRef]
- Wang, H.; Peters, T.; Kess, D.; Sindrilaru, A.; Oreshkova, T.; Van Rooijen, N.; Stratis, A.; Renkl, A.C.; Sunderkötter, C.; Wlaschek, M.; et al. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J. Clin. Investig. 2006, 116, 2105–2114. [Google Scholar] [CrossRef]
- Tan, R.-z.; Zhong, X.; Han, R.-y.; Xie, K.-h.; Jia, J.; Yang, Y.; Cheng, M.; Yang, C.-y.; Lan, H.-y.; Wang, L. Macrophages mediate psoriasis via Mincle-dependent mechanism in mice. Cell Death Discov. 2023, 9, 140. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [PubMed]
- Liu, Y.; Xia, Y.; Qiu, C.H. Functions of CD169 positive macrophages in human diseases (Review). Biomed. Rep. 2021, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Park, J.H.; Kim, H.C.; Kim, C.W.; Kang, I.; Lee, H.K. Blood monocyte-derived CD169+ macrophages contribute to antitumor immunity against glioblastoma. Nat. Commun. 2022, 13, 6211. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Huggins, M.; Ahmed, J.; Hashimoto, D.; Lucas, D.; Kunisaki, Y.; Pinho, S.; Leboeuf, M.; Noizat, C.; van Rooijen, N.; et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat. Med. 2013, 19, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kang, B.H.; Kim, H.J.; Oh, J.E.; Lee, H.K. A Microbiota-Dependent Subset of Skin Macrophages Protects against Cutaneous Bacterial Infection. Front. Immunol. 2022, 13, 799598. [Google Scholar] [CrossRef] [PubMed]
- Thornley, T.B.; Fang, Z.; Balasubramanian, S.; Larocca, R.A.; Gong, W.; Gupta, S.; Csizmadia, E.; Degauque, N.; Kim, B.S.; Koulmanda, M.; et al. Fragile TIM-4-expressing tissue resident macrophages are migratory and immunoregulatory. J. Clin. Investig. 2014, 124, 3443–3454. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, Y.; Chakarov, S.; Bleriot, C.; Kwok, I.; Chen, X.; Shin, A.; Huang, W.; Dress, R.J.; Dutertre, C.A.; et al. Fate Mapping via Ms4a3-Expression History Traces Monocyte-Derived Cells. Cell 2019, 178, 1509–1525.e19. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Singh, R.; Koppu, S.; Perche, P.O.; Feldman, S.R. The Cytokine Mediated Molecular Pathophysiology of Psoriasis and Its Clinical Implications. Int. J. Mol. Sci. 2021, 22, 12793. [Google Scholar] [CrossRef]
- Alalaiwe, A.; Chen, C.Y.; Chang, Z.Y.; Sung, J.T.; Chuang, S.Y.; Fang, J.Y. Psoriasiform Inflammation Is Associated with Mitochondrial Fission/GDAP1L1 Signaling in Macrophages. Int. J. Mol. Sci. 2021, 22, 10410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Merana, G.R.; Harris-Tryon, T.; Scharschmidt, T.C. Skin immunity: Dissecting the complex biology of our body’s outer barrier. Mucosal Immunol. 2022, 15, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Malissen, B.; Tamoutounour, S.; Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 2014, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Yanez, D.A.; Lacher, R.K.; Vidyarthi, A.; Colegio, O.R. The role of macrophages in skin homeostasis. Pflug. Arch. Eur. J. Physiol. 2017, 469, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-specific macrophages: How they develop and choreograph tissue biology. Nat. Rev. Immunol. 2023, 23, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef]
- Nakamizo, S.; Dutertre, C.A.; Khalilnezhad, A.; Zhang, X.M.; Lim, S.; Lum, J.; Koh, G.; Foong, C.; Yong, P.J.A.; Tan, K.J.; et al. Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J. Exp. Med. 2021, 218, e20202345. [Google Scholar] [CrossRef]
- Marrocco, A.; Ortiz, L.A. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front. Immunol. 2022, 13, 936167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, M.; Huang, W.; Chen, W.; Zhao, Y.; Schulte, M.L.; Volberding, P.; Gerbec, Z.; Zimmermann, M.T.; Zeighami, A.; et al. Mitochondrial Metabolic Reprogramming by CD36 Signaling Drives Macrophage Inflammatory Responses. Circ. Res. 2019, 125, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wu, D.; Zhang, C.; Yan, T.; Zhao, Y.; Shen, H.; Xue, K.; Huang, X.; Wang, Z.; Qiu, Y. Macrophage IRX3 promotes diet-induced obesity and metabolic inflammation. Nat. Immunol. 2021, 22, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, T.; Pettersson, U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica 1978, 157, 238–244. [Google Scholar] [CrossRef]
- Lou, F.; Sun, Y.; Wang, H. Protocol for Flow Cytometric Detection of Immune Cell Infiltration in the Epidermis and Dermis of a Psoriasis Mouse Model. STAR Protoc. 2020, 1, 100115. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yu, W.; Liu, Z.; Liu, S. CD169+ Skin Macrophages Function as a Specialized Subpopulation in Promoting Psoriasis-like Skin Disease in Mice. Int. J. Mol. Sci. 2024, 25, 5705. https://doi.org/10.3390/ijms25115705
Li M, Yu W, Liu Z, Liu S. CD169+ Skin Macrophages Function as a Specialized Subpopulation in Promoting Psoriasis-like Skin Disease in Mice. International Journal of Molecular Sciences. 2024; 25(11):5705. https://doi.org/10.3390/ijms25115705
Chicago/Turabian StyleLi, Mengyao, Wenjing Yu, Zhiduo Liu, and Siming Liu. 2024. "CD169+ Skin Macrophages Function as a Specialized Subpopulation in Promoting Psoriasis-like Skin Disease in Mice" International Journal of Molecular Sciences 25, no. 11: 5705. https://doi.org/10.3390/ijms25115705
APA StyleLi, M., Yu, W., Liu, Z., & Liu, S. (2024). CD169+ Skin Macrophages Function as a Specialized Subpopulation in Promoting Psoriasis-like Skin Disease in Mice. International Journal of Molecular Sciences, 25(11), 5705. https://doi.org/10.3390/ijms25115705





