Beta-Hydroxybutyrate Mitigates Sensorimotor and Cognitive Impairments in a Photothrombosis-Induced Ischemic Stroke in Mice
Abstract
:1. Introduction
2. Results
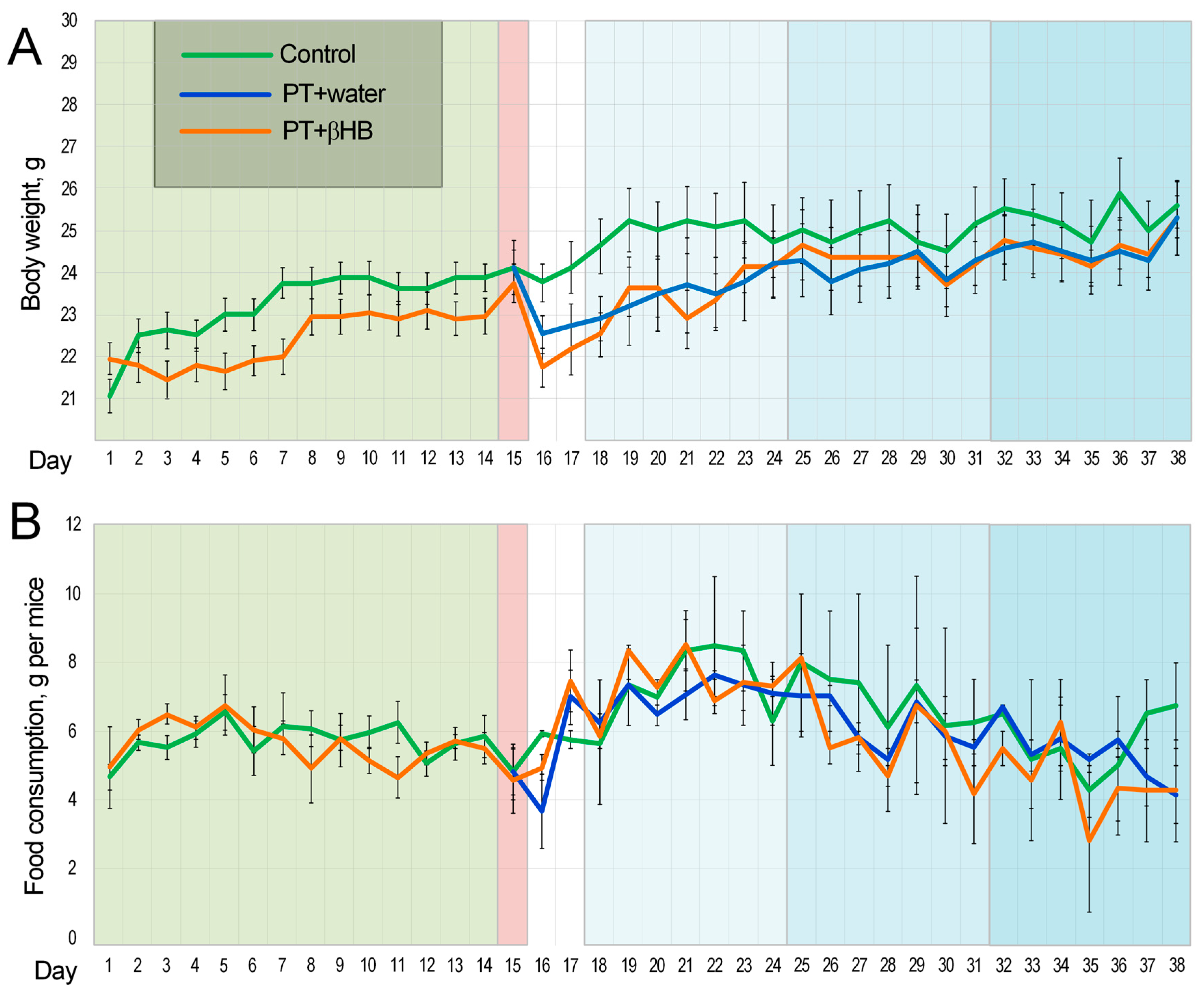
2.1. Changes in Body Weight and Feed Intake Level during the Experiment
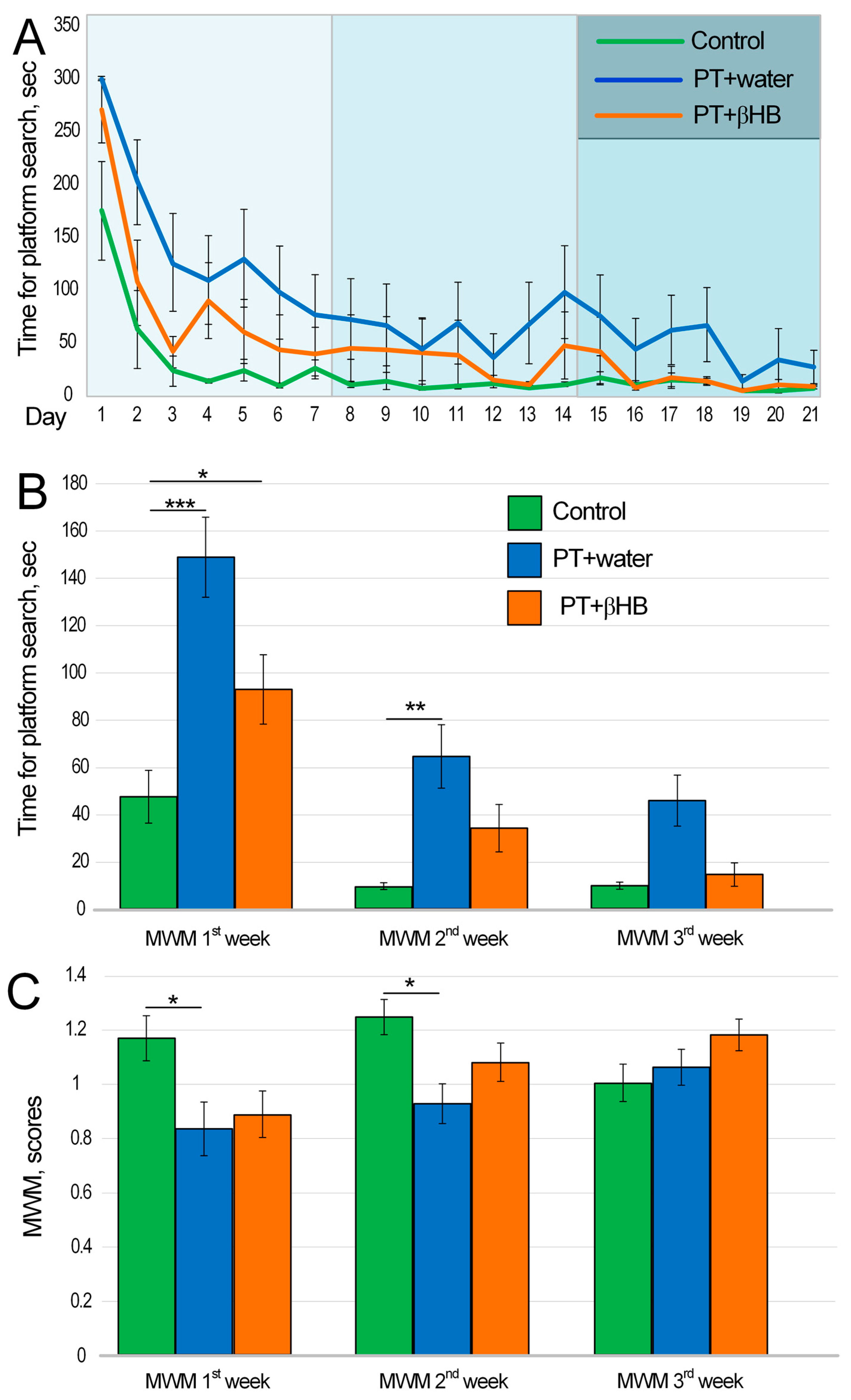
2.2. The Effect of βHB on Cognitive Function of Mice after Photothrombosis
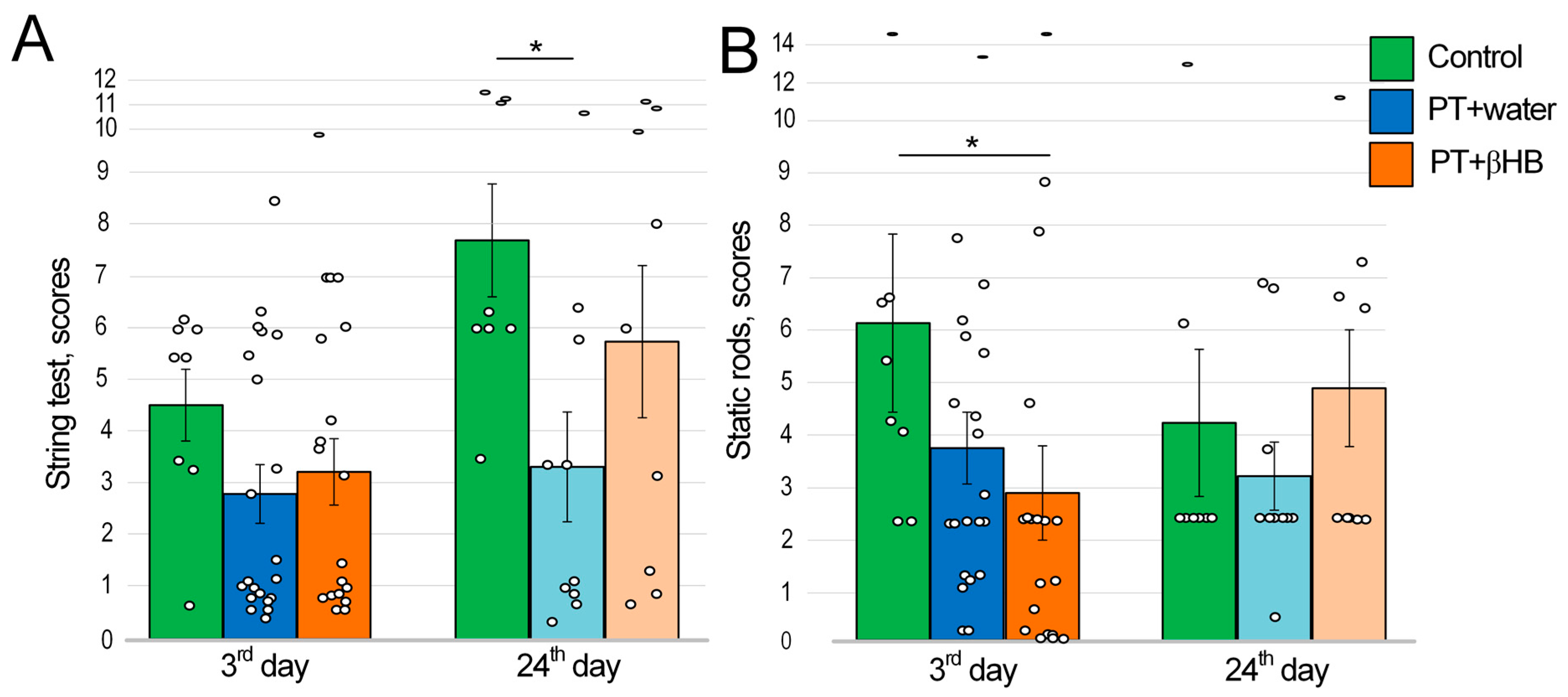
2.3. The Effect of βHB on Strength and Endurance Values of Mice after Photothrombosis
2.4. The Effects of Photothrombosis and βHB on Movement Coordination
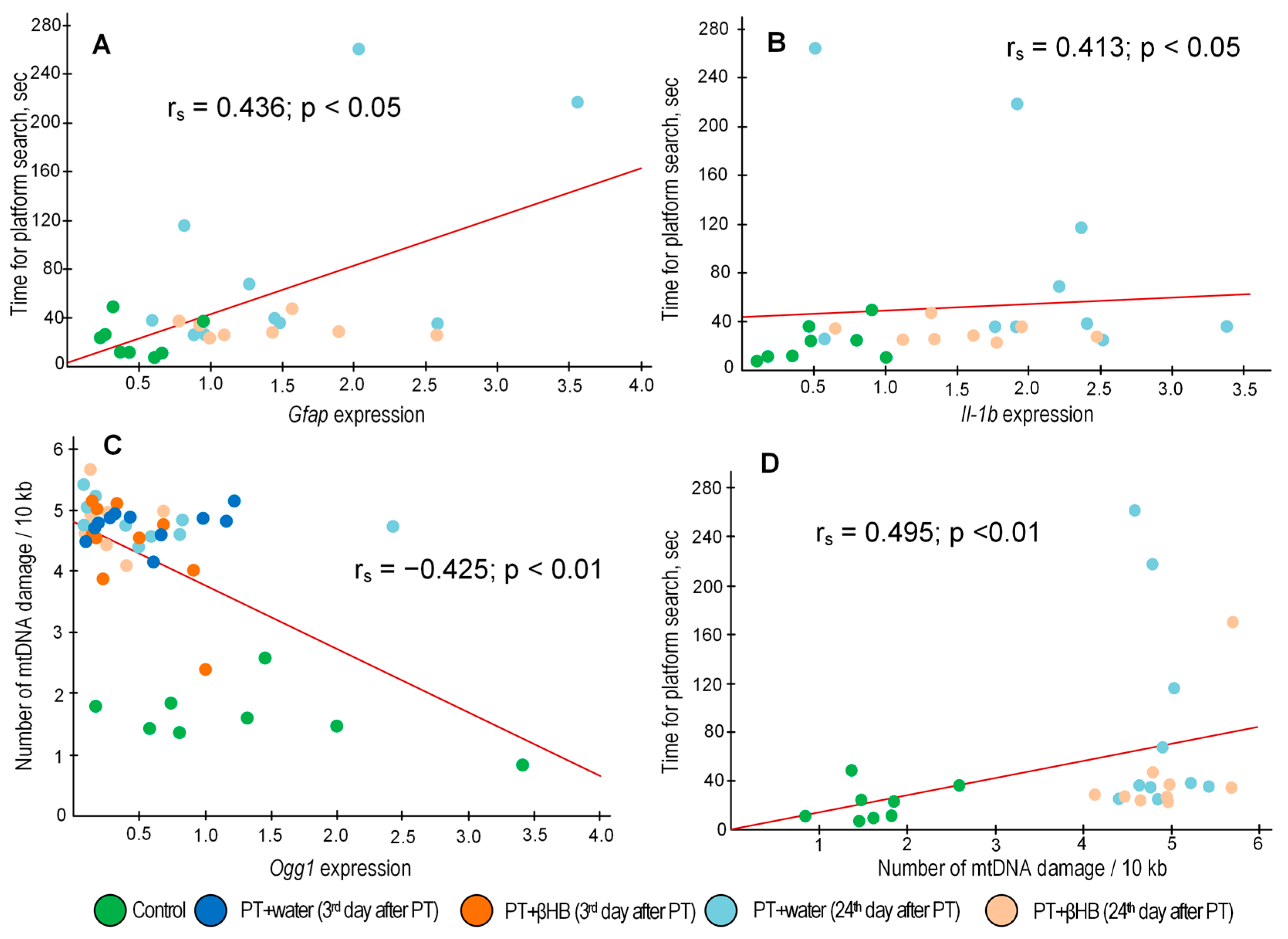
2.5. The Effect of βHB on the Accumulation of mtDNA Damage at Different Stages after Photothrombosis
2.6. The Effect of βHB on Gene Expression at Different Time Points after Photothrombosis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Induction of Photothrombosis
4.4. Morris Water Maze (MWM)
4.5. String Test
4.6. Static Rods
4.7. Nucleic Acids Extraction
4.8. Estimation of the Amount for mtDNA Damage
4.9. Measuring of Gene Expression
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Mehta, T.R. Role of the ketogenic diet in acute neurological diseases. Clin. Neurol. Neurosurg. 2020, 192, 105727. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, M.; Sato, K.; Dohi, S.; Sato, T.; Matsuura, A.; Hiraide, A. Effect of β-hydroxybutyrate, a cerebral function improving agent, on cerebral hypoxia, anoxia and ischemia in mice and rats. Jpn. J. Pharmacol. 2001, 87, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Komaravelli, N.; Tian, B.; Ivanciuc, T.; Mautemps, N.; Brasier, A.R.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic. Biol. Med. 2015, 88, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, B.; Gong, A.Y.; Malhotra, D.K.; Gupta, R.; Dworkin, L.D.; Gong, R. The ketone body β-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults. Kidney Int. 2021, 100, 1037–1053. [Google Scholar] [CrossRef]
- Petri, S.; Körner, S.; Kiaei, M. Nrf2/ARE Signaling Pathway: Key Mediator in Oxidative Stress and Potential Therapeutic Target in ALS. Neurol. Res. Int. 2012, 2012, 878030. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Goodfellow, M.J.; Borcar, A.; Proctor, J.L.; Greco, T.; Rosenthal, R.E.; Fiskum, G. Transcriptional activation of antioxidant gene expression by Nrf2 protects against mitochondrial dysfunction and neuronal death associated with acute and chronic neurodegeneration. Exp. Neurol. 2020, 328, 113247. [Google Scholar] [CrossRef]
- Gureev, A.P.; Sadovnikova, I.S.; Starkov, N.N.; Starkov, A.A.; Popov, V.N. p62-Nrf2-p62 Mitophagy Regulatory Loop as a Target for Preventive Therapy of Neurodegenerative Diseases. Brain Sci. 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Vomhof-Dekrey, E.E.; Picklo, M.J., Sr. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res. 2016, 106, 117–127. [Google Scholar] [CrossRef]
- Liu, L.; Vollmer, M.K.; Ahmad, A.S.; Fernandez, V.M.; Kim, H.; Doré, S. Pretreatment with Korean red ginseng or dimethyl fumarate attenuates reactive gliosis and confers sustained neuroprotection against cerebral hypoxic-ischemic damage by an Nrf2-dependent mechanism. Free Radic. Biol. Med. 2019, 131, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.V.; Dave, K.R.; Saul, I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2-Related Factor 2. Stroke 2015, 46, 1626–1632. [Google Scholar] [CrossRef]
- Mao, L.; Yang, T.; Li, X.; Lei, X.; Sun, Y.; Zhao, Y.; Zhang, W.; Gao, Y.; Sun, B.; Zhang, F. Protective effects of sulforaphane in experimental vascular cognitive impairment: Contribution of the Nrf2 pathway. J. Cereb. Blood Flow Metab. 2019, 39, 352–366. [Google Scholar] [CrossRef]
- Han, J.; Xiao, Q.; Lin, Y.H.; Zheng, Z.Z.; He, Z.D.; Hu, J.; Chen, L.D. Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2-related factor 2 pathway. Neural Regen. Res. 2015, 10, 1989–1996. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, K.; Leng, C.; Zhou, M.; Zhang, J.; Li, Y.; Liu, Y.; Ye, X.; Xu, X.; Sun, B.; et al. Chebulic Acid Prevents Hypoxia Insult via Nrf2/ARE Pathway in Ischemic Stroke. Nutrients 2022, 14, 5390. [Google Scholar] [CrossRef]
- Sun, J.; Hu, H.; Ren, X.; Simpkins, J.W. Tert-butylhydroquinone compromises survival in murine experimental stroke. Neurotoxicol. Teratol. 2016, 54, 15–21. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Rojo, A.I.; Ferreiro, E.; Núñez, Á.; Krause, K.H.; Jaquet, V.; Cuadrado, A. Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol. 2017, 13, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Zweig, J.A.; Caruso, M.; Brandes, M.S.; Gray, N.E. Loss of NRF2 leads to impaired mitochondrial function, decreased synaptic density and exacerbated age-related cognitive deficits. Exp. Gerontol. 2020, 131, 110767. [Google Scholar] [CrossRef] [PubMed]
- Sadovnikova, I.S.; Gureev, A.P.; Ignatyeva, D.A.; Gryaznova, M.V.; Chernyshova, E.V.; Krutskikh, E.P.; Novikova, A.G.; Popov, V.N. Nrf2/ARE Activators Improve Memory in Aged Mice via Maintaining of Mitochondrial Quality Control of Brain and the Modulation of Gut Microbiome. Pharmaceuticals 2021, 14, 607. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.R.; Ziaee, P.; Motevalizadeh, S.A.; Rohani, M.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur. J. Pharmacol. 2018, 826, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, S.; Wang, Y.; Yang, X.; Jiang, J.; Wu, D.; Qu, X.; Fan, H.; Yao, R. Quercetin ameliorates learning and memory via the Nrf2-ARE signaling pathway in d-galactose-induced neurotoxicity in mice. Biochem. Biophys. Res. Commun. 2017, 491, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Akhtar, M.; Madaan, T.; Vohora, D.; Abdin, M.Z.; Islamuddin, M.; Najmi, A.K. Tannins Enriched Fraction of Emblica officinalis Fruits Alleviates High-Salt and Cholesterol Diet-Induced Cognitive Impairment in Rats via Nrf2-ARE Pathway. Front. Pharmacol. 2018, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Krutskikh, E.P.; Potanina, D.V.; Samoylova, N.A.; Gryaznova, M.V.; Sadovnikova, I.S.; Gureev, A.P.; Popov, V.N. Brain Protection by Methylene Blue and Its Derivative, Azur B, via Activation of the Nrf2/ARE Pathway in Cisplatin-Induced Cognitive Impairment. Pharmaceuticals 2022, 15, 815. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hur, B.E.; Bokara, K.K.; Yang, W.; Cho, H.J.; Park, K.A.; Lee, W.T.; Lee, K.M.; Lee, J.E. Agmatine improves cognitive dysfunction and prevents cell death in a streptozotocin-induced Alzheimer rat model. Yonsei Med. J. 2014, 55, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Stewart, T.; Bai, L.; Li, X.; Xu, T.; Iliff, J.; Shi, M.; Zheng, D.; Yuan, L.; Wei, T.; et al. Coniferaldehyde attenuates Alzheimer’s pathology via activation of Nrf2 and its targets. Theranostics 2020, 10, 179–200. [Google Scholar] [CrossRef]
- Monir, D.M.; Mahmoud, M.E.; Ahmed, O.G.; Rehan, I.F.; Abdelrahman, A. Forced exercise activates the NrF2 pathway in the striatum and ameliorates motor and behavioral manifestations of Parkinson’s disease in rotenone-treated rats. Behav. Brain Funct. 2020, 16, 9. [Google Scholar] [CrossRef]
- Jin, H.; Qi, F.; Chu, F.; Liu, C.; Qian, T.; Zeng, W.; Wang, Q.; Wang, X.; Xiao, J. Morin improves functional recovery after spinal cord injury in rats by enhancing axon regeneration via the Nrf2/HO-1 pathway. Phytother. Res. 2021, 35, 5754–5766. [Google Scholar] [CrossRef] [PubMed]
- Lipsanen, A.; Jolkkonen, J. Experimental approaches to study functional recovery following cerebral ischemia. Cell. Mol. Life Sci. 2011, 68, 18. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Cardozo-Pelaez, F.; Song, S.; Parthasarathy, A.; Hazzi, C.; Naidu, K.; Sanchez-Ramos, J. Oxidative DNA damage in the aging mouse brain. Mov. Disord. 1999, 14, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Measuring motor coordination in mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Starkov, A.A.; Popov, V.N. Simplified qPCR method for detecting excessive mtDNA damage induced by exogenous factors. Toxicology 2017, 382, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Larivière, S.; Ward, N.S.; Boudrias, M.H. Disrupted functional network integrity and flexibility after stroke: Relation to motor impairments. Neuroimage Clin. 2018, 19, 883–891. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, Y.Y.; Lee, S.H.; Jung, C.; Kim, M.H.; Kim, D.Y. Neuroprotective Effect of Macrophage Migration Inhibitory Factor (MIF) in a Mouse Model of Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 6975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Li, Y.; Zhang, Z.G.; Chopp, M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J. Neurol. Sci. 2000, 174, 141–146. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, M.S.; Kim, Y.S.; Moon, K.S.; Joo, S.P.; Kim, T.S.; Kim, J.H.; Kim, S.H. Photochemically induced cerebral ischemia in a mouse model. Surg. Neurol. 2007, 67, 620–625. [Google Scholar] [CrossRef]
- Monsalves-Alvarez, M.; Morales, P.E.; Castro-Sepulveda, M.; Sepulveda, C.; Rodriguez, J.M.; Chiong, M.; Eisner, V.; Lavandero, S.; Troncoso, R. β-Hydroxybutyrate Increases Exercise Capacity Associated with Changes in Mitochondrial Function in Skeletal Muscle. Nutrients 2020, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, Y.; Chai, X.; Wang, S.; Zhao, Y.; Hou, Y.; Ma, Y.; Chen, S.; Zhao, S.; Zhu, X. β-Hydroxybutyric acid improves cognitive function in a model of heat stress by promoting adult hippocampal neurogenesis. Stress Biol. 2022, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Kovács, Z.; Juhasz, G.; Murdun, C.; Goldhagen, C.R.; Koutnik, A.P.; Poff, A.M.; Kesl, S.L.; D’Agostino, D.P. Exogenous Ketone Supplements Reduce Anxiety-Related Behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk Rats. Front. Mol. Neurosci. 2016, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Mayr, K.A.; Kwok, C.H.T.; Eaton, S.E.A.; Baker, G.B.; Whelan, P.J. The Effects of a Ketogenic Diet on Sensorimotor Function in a Thoracolumbar Mouse Spinal Cord Injury Model. eNeuro 2020, 7, ENEURO.0178-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Matsuo, A.; Toide, K. Pharmacological studies of a novel prolyl endopeptidase inhibitor, JTP-4819, in rats with middle cerebral artery occlusion. Eur. J. Pharmacol. 1996, 305, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, K.; Koshino, H.; Toyoshita, Y.; Tanaka, M.; Hirai, T. Effect of mastication on functional recoveries after permanent middle cerebral artery occlusion in rats. J. Stroke Cerebrovasc. Dis. 2010, 19, 398–403. [Google Scholar] [CrossRef]
- Pavlichenko, N.; Sokolova, I.; Vijde, S.; Shvedova, E.; Alexandrov, G.; Krouglyakov, P.; Fedotova, O.; Gilerovich, E.G.; Polyntsev, D.G.; Otellin, V.A. Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008, 1233, 203–213. [Google Scholar] [CrossRef]
- Rogers, D.C.; Hunter, A.J. Photothrombotic lesions of the rat cortex impair acquisition of the water maze. Pharmacol. Biochem. Behav. 1997, 56, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, S.; Wang, Y.; Shi, J. Photochemically induced thalamus infarction impairs cognition in a mouse model. Stroke Vasc. Neurol. 2023, 8, e002235. [Google Scholar] [CrossRef]
- Kharlamov, A.; Zivkovic, I.; Polo, A.; Armstrong, D.M.; Costa, E.; Guidotti, A. LIGA20, a lyso derivative of ganglioside GM1, given orally after cortical thrombosis reduces infarct size and associated cognition deficit. Proc. Natl. Acad. Sci. USA 1994, 91, 6303–6307. [Google Scholar] [CrossRef]
- Kliper, E.; Bashat, D.B.; Bornstein, N.M.; Shenhar-Tsarfaty, S.; Hallevi, H.; Auriel, E.; Shopin, L.; Bloch, S.; Berliner, S.; Giladi, N.; et al. Cognitive decline after stroke: Relation to inflammatory biomarkers and hippocampal volume. Stroke 2013, 44, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, A.; Loh, V.; Stranahan, B.N.; Palmer, C.M. Case report: Ketogenic diet acutely improves cognitive function in patient with Down syndrome and Alzheimer’s disease. Front. Psychiatry 2023, 13, 1085512. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Ford, J.M. The Role of Ketogenic Metabolic Therapy on the Brain in Serious Mental Illness: A Review. J. Psychiatr. Brain Sci. 2022, 7, e220009. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Wang, L.; Liu, H.; Zhang, Y.; Shen, W. Post-stroke cognitive impairment and synaptic plasticity: A review about the mechanisms and Chinese herbal drugs strategies. Front. Neurosci. 2023, 17, 1123817. [Google Scholar] [CrossRef] [PubMed]
- Hoeffer, C.A.; Klann, E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.C.; Gesualdo, C.; Herman, H.; Gharbia, S.; Balta, C.; Lepre, C.C.; Russo, M.; Itro, A.; D’Amico, G.; Peluso, L.; et al. Systemic Beta-Hydroxybutyrate Affects BDNF and Autophagy into the Retina of Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 10184. [Google Scholar] [CrossRef]
- McDaniel, S.S.; Rensing, N.R.; Thio, L.L.; Yamada, K.A.; Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011, 52, e7–e11. [Google Scholar] [CrossRef]
- Seira, O.; Kolehmainen, K.; Liu, J.; Streijger, F.; Haegert, A.; Lebihan, S.; Boushel, R.; Tetzlaff, W. Ketogenesis controls mitochondrial gene expression and rescues mitochondrial bioenergetics after cervical spinal cord injury in rats. Sci. Rep. 2021, 11, 16359. [Google Scholar] [CrossRef]
- Gureev, A.P.; Silachev, D.N.; Sadovnikova, I.S.; Krutskikh, E.P.; Chernyshova, E.V.; Volodina, D.E.; Samoylova, N.A.; Potanina, D.V.; Burakova, I.Y.; Smirnova, Y.D.; et al. The Ketogenic Diet but not Hydroxycitric Acid Keeps Brain Mitochondria Quality Control and mtDNA Integrity Under Focal Stroke. Mol. Neurobiol. 2023, 60, 4288–4303. [Google Scholar] [CrossRef]
- Bendavit, G.; Aboulkassim, T.; Hilmi, K.; Shah, S.; Batist, G. Nrf2 Transcription Factor Can Directly Regulate mTOR: Linking Cytoprotective Gene Expression to a Major Metabolic Regulator That Generates Redox Activity. J. Biol. Chem. 2016, 291, 25476–25488. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hodes, G.E.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M.; et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, Z.; Zhou, K.; Jiang, S. The Predictive Role of Systemic Inflammation Response Index (SIRI) in the Prognosis of Stroke Patients. Clin. Interv. Aging 2021, 16, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, M.; Peng, C.; Zhao, G.; Gu, R. GFAP expression in injured astrocytes in rats. Exp. Ther. Med. 2017, 14, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Amalia, L. Glial Fibrillary Acidic Protein (GFAP): Neuroinflammation Biomarker in Acute Ischemic Stroke. J. Inflamm. Res. 2021, 14, 7501–7506. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, M.H.; Ha, S.; Bang, E.J.; Lee, Y.; Lee, A.K.; Lee, J.; Yu, B.P.; Chung, H.Y. Anti-inflammatory action of β-hydroxybutyrate via modulation of PGC-1α and FoxO1, mimicking calorie restriction. Aging 2019, 11, 1283–1304. [Google Scholar] [CrossRef] [PubMed]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflammation 2020, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.J.; Pekkurnaz, G. Powerhouse of the mind: Mitochondrial plasticity at the synapse. Curr. Opin. Neurobiol. 2019, 57, 149–155. [Google Scholar] [CrossRef]
- Smart, D.J.; Chipman, J.K.; Hodges, N.J. Activity of OGG1 variants in the repair of pro-oxidant-induced 8-oxo-2’-deoxyguanosine. DNA Repair 2006, 5, 1337–1345. [Google Scholar] [CrossRef]
- Zinovkina, L.A. Mechanisms of Mitochondrial DNA Repair in Mammals. Biochemistry 2018, 83, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Hardingham, G.E. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Aspects Med. 2009, 30, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, M.Y.; Ivanov, A.V.; Virus, E.D.; Nikiforova, K.A.; Ochtova, F.R.; Suanova, E.T.; Kruglova, M.P.; Piradov, M.A.; Kubatiev, A.A. Impact of glutathione on acute ischemic stroke severity and outcome: Possible role of aminothiols redox status. Redox Rep. 2021, 26, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, J. Thioredoxin Reductase 2 (Txnrd2) Regulates Mitochondrial Integrity in the Progression of Age-Related Heart Failure. J. Am. Heart Assoc. 2015, 4, e002278. [Google Scholar] [CrossRef] [PubMed]
- Hattori, F.; Murayama, N.; Noshita, T.; Oikawa, S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J. Neurochem. 2003, 86, 860–868. [Google Scholar] [CrossRef]
- Romanello, K.S.; Teixeira, K.K.L.; Silva, J.P.M.O.; Nagamatsu, S.T.; Bezerra, M.A.C.; Domingos, I.F.; Martins, D.A.P.; Araujo, A.S.; Lanaro, C.; Breyer, C.A.; et al. Global analysis of erythroid cells redox status reveals the involvement of Prdx1 and Prdx2 in the severity of beta thalassemia. PLoS ONE 2018, 13, e0208316. [Google Scholar] [CrossRef]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef]





| Day | Start | Goal | Day | Start | Goal | Day | Start | Goal |
|---|---|---|---|---|---|---|---|---|
| 1st Week | 2nd Week | 3rd Week | ||||||
| 1 | N | SE | 8 | E | SE | 15 | N | SE |
| 2 | E | NE | 9 | W | NW | 16 | S | SW |
| 3 | S | SW | 10 | S | SE | 17 | N | NE |
| 4 | W | SE | 11 | E | SW | 18 | S | NW |
| 5 | S | NE | 12 | N | SW | 19 | E | NW |
| 6 | N | NW | 13 | E | NW | 20 | W | SW |
| 7 | W | NE | 14 | W | NE | 21 | N | SE |
| Fragment | Forward Primer 5′–3′ | Reverse Primer 5′–3′ |
|---|---|---|
| 12s–16s rRNA | TAAATTTCGTGCCAGCCACC | ATGCTACCTTTGCACGGTCA |
| 16s rRNA-Nd1 | CGAGGGTCCAACTGTCTCTTA | CCGGCTGCGTATTCTACGTT |
| Nd1-Nd2 | CTAGCAGAAACAAACCGGGC | TTAGGGCTTTGAAGGCTCGC |
| Nd5 | TCATTCTTCTACTATCCCCAATCC | TGGTTTGGGAGATTGGTTGATG |
| Nd6–CytB | TCATTCTTCTACTATCCCCAATCC | GGTGGGGAGTAGCTCCTTCTT |
| D-loop | AAGAAGGAGCTACTCCCCACC | GTTGACACGTTTTACGCCGA |
| Short fragment | CGAGGGTCCAACTGTCTCTTA | AGCTCCATAGGGTCTTCTCGT |
| Gene | Forward Primer 5′–3′ | Reverse Primer 5′–3′ |
|---|---|---|
| Gapdh | GGCTCCCTAGGCCCCTCCTG | TCCCAACTCGGCCCCCAACA |
| Akt1 | TGATCAAGATGACAGCATGGAGTG | GATGATCCATGCGGGGCTT |
| Bdnf | AAGGACGCGGACTTGTACAC | CGCTAATACTGTCACACACGC |
| Gclc | GGGGTGACGAGGTGGAGTA | GTTGGGGTTTGTCCTCTCCC |
| Gfap | CAACGTTAAGCTAGCCCTGGACAT | CTCACCATCCCGCATCTCCACAGT |
| Gpx1 | AGTCCACCGTGTATGCCTTCT | GAGACGCGACATTCTCAATGA |
| Il-1b | TTGCGGACCCCAAAAGATG | AGAAGGTGCTCATGTCCTCA |
| Il-6 | CGGAGAGGAGACTTCACAGAG | CATTTCCACGATTTCCCAGA |
| Mtor | AGATAAGCTCACTGGTCGGG | GTGGTTTTCCAGGCCTCAGT |
| Nfe2l2 | CTCTCTGAACTCCTGGACGG | GGGTCTCCGTAAATGGAAG |
| Ogg1 | GAGACGACAGCCAGGTGTGAG | CCGTTCCACCATGCCAGTA |
| Sqstm1 | GCCAGAGGAACAGATGGAGT | TCCGATTCTGGCATCTGTAG |
| Pink1 | GAGCAGACTCCCAGTTCTCG | GTCCCACTCCACAAGGATGT |
| Prdx3 | GTGGTTTGGGCCACATGAAC | TGGCTTGATCGTAGGGGACT |
| Ptsg2 | AGTCCGGGTACAGTCACACTT | TTCCAATCCATGTCAAAACCGT |
| Sod2 | CAGACCTGCCTTACGACTATGG | CTCGGTGGCGTTGAGATTGTT |
| Tnf | TATGGCTCAGGGTCCAACTC | GGAAAGCCCATTTGAGTCCT |
| Txnrd2 | GATCCGGTGGCCTAGCTTG | TCGGGGAGAAGGTTCCACAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gureev, A.P.; Sadovnikova, I.S.; Chernyshova, E.V.; Tsvetkova, A.D.; Babenkova, P.I.; Nesterova, V.V.; Krutskikh, E.P.; Volodina, D.E.; Samoylova, N.A.; Andrianova, N.V.; et al. Beta-Hydroxybutyrate Mitigates Sensorimotor and Cognitive Impairments in a Photothrombosis-Induced Ischemic Stroke in Mice. Int. J. Mol. Sci. 2024, 25, 5710. https://doi.org/10.3390/ijms25115710
Gureev AP, Sadovnikova IS, Chernyshova EV, Tsvetkova AD, Babenkova PI, Nesterova VV, Krutskikh EP, Volodina DE, Samoylova NA, Andrianova NV, et al. Beta-Hydroxybutyrate Mitigates Sensorimotor and Cognitive Impairments in a Photothrombosis-Induced Ischemic Stroke in Mice. International Journal of Molecular Sciences. 2024; 25(11):5710. https://doi.org/10.3390/ijms25115710
Chicago/Turabian StyleGureev, Artem P., Irina S. Sadovnikova, Ekaterina V. Chernyshova, Arina D. Tsvetkova, Polina I. Babenkova, Veronika V. Nesterova, Ekaterina P. Krutskikh, Daria E. Volodina, Natalia A. Samoylova, Nadezda V. Andrianova, and et al. 2024. "Beta-Hydroxybutyrate Mitigates Sensorimotor and Cognitive Impairments in a Photothrombosis-Induced Ischemic Stroke in Mice" International Journal of Molecular Sciences 25, no. 11: 5710. https://doi.org/10.3390/ijms25115710





