Stress Affects Mast Cell Proteases in Murine Skin in a Model of Atopic Dermatitis-like Allergic Inflammation
Abstract
:1. Introduction
2. Results
2.1. Immunohistomorphometry Reveals Stress-Induced Increase of mMCP4+ MCs in AlD
2.2. Immunohistomorphometry Suggests mMCP4 Production and Release
2.3. Blocking NGF Reduces mMCP4+ MC in NiS+AlD Skin
2.4. Immunohistomorphometry Does Not Reveal Changes in mMCP6 Expression
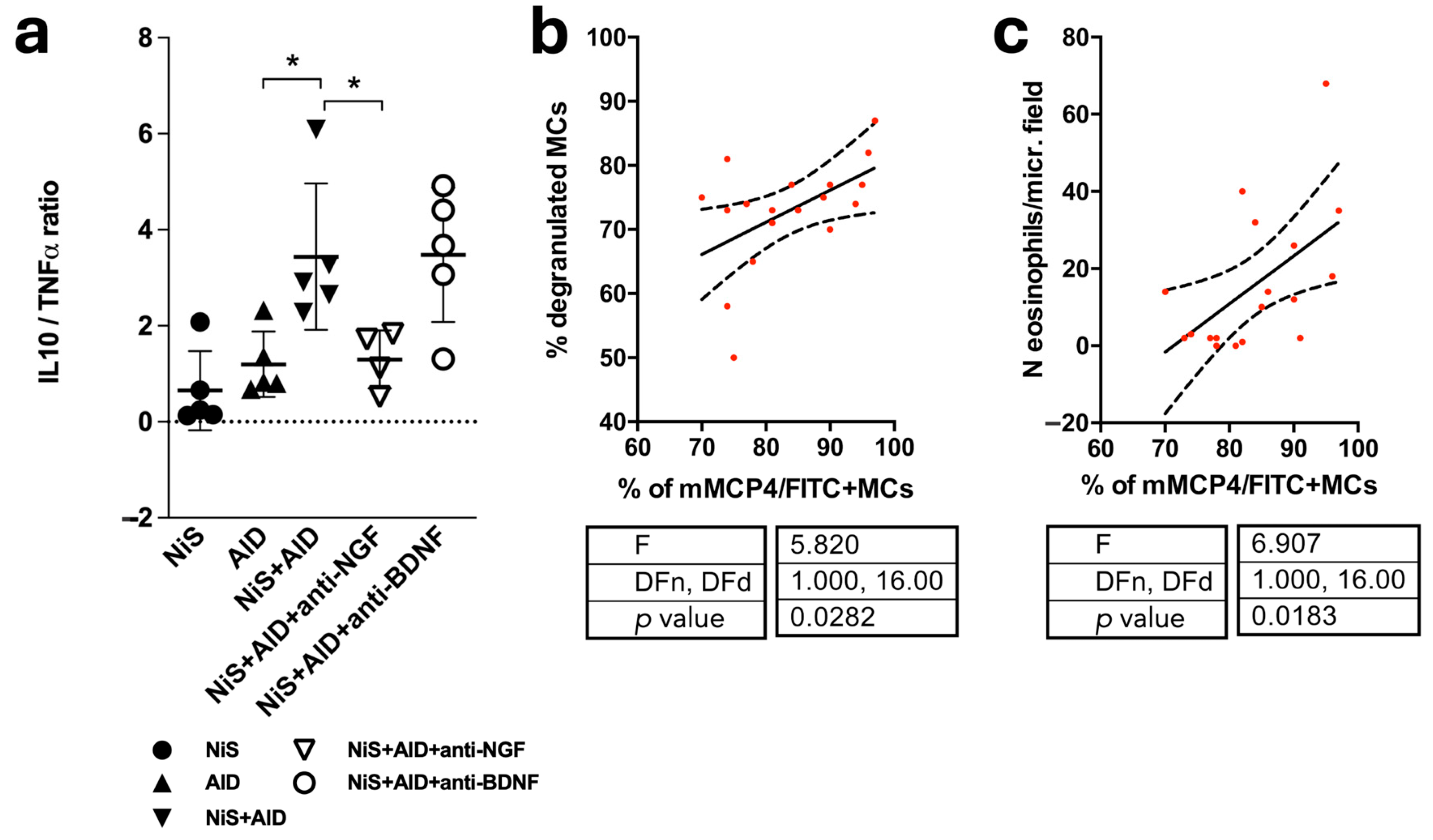
2.5. Skipping the Immune Balance and Positive Correlation of mMCP4+ MCs and Indicators of Disease Severity
2.6. In Cultured MC, SP Induces MCP4 mRNA in the Presence of Chrna7
2.7. No Significant Effect of SP on MCP6 in Cultured MC
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Genotyping
4.3. The NiS-AlD Model
4.4. Tissue Collection
4.5. Immunohistochemistry
4.6. Determination of Disease Severity
4.7. Peritoneal Mast Cell Culture (PMCC)
4.8. RNA Isolation and qRTPCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liezmann, C.; Klapp, B.; Peters, E.M. Stress, atopy and allergy: A re-evaluation from a psychoneuroimmunologic persepective. Dermatoendocrinology 2011, 3, 37–40. [Google Scholar] [CrossRef]
- Woźniak, E.; Owczarczyk-Saczonek, A.; Placek, W. Psychological Stress, Mast Cells, and Psoriasis—Is There Any Relationship? Int. J. Mol. Sci. 2021, 22, 13252. [Google Scholar] [CrossRef]
- Traina, G. The role of mast cells in the gut and brain. J. Integr. Neurosci. 2021, 20, 185–196. [Google Scholar] [CrossRef]
- Theoharides, T.C. Effect of Stress on Neuroimmune Processes. Clin. Ther. 2020, 42, 1007–1014. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J. The mast cell-nerve functional unit: A key component of physiologic and pathophysiologic responses. Chem. Immunol. Allergy 2012, 98, 196–221. [Google Scholar] [CrossRef]
- Jones, H.P. Immune cells listen to what stress is saying: Neuroendocrine receptors orchestrate immune function. Methods Mol. Biol. 2012, 934, 77–87. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Tsai, M.; Grimbaldeston, M.; Galli, S.J. Mast cells and immunoregulation/immunomodulation. Adv. Exp. Med. Biol. 2011, 716, 186–211. [Google Scholar] [CrossRef]
- Kleij, H.P.; Bienenstock, J. Significance of Conversation between Mast Cells and Nerves. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2005, 1, 65–80. [Google Scholar] [CrossRef]
- Granstein, R.D.; Wagner, J.A.; Stohl, L.L.; Ding, W. Calcitonin gene-related peptide: Key regulator of cutaneous immunity. Acta Physiol. 2015, 213, 586–594. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Magerl, M.; Peters, E.M.; Hendrix, S.; Metz, M.; Maurer, M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J. Allergy Clin. Immunol. 2008, 121, 955–961. [Google Scholar] [CrossRef]
- Arck, P.C.; Slominski, A.; Theoharides, T.C.; Peters, E.M.; Paus, R. Neuroimmunology of stress: Skin takes center stage. J. Investig. Dermatol. 2006, 126, 1697–1704. [Google Scholar] [CrossRef]
- Steinhoff, M.; Stander, S.; Seeliger, S.; Ansel, J.C.; Schmelz, M.; Luger, T. Modern aspects of cutaneous neurogenic inflammation. Arch. Dermatol. 2003, 139, 1479–1488. [Google Scholar] [CrossRef]
- Black, P.H. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav. Immun. 2002, 16, 622–653. [Google Scholar] [CrossRef]
- Pavlovic, S.; Daniltchenko, M.; Tobin, D.J.; Hagen, E.; Hunt, S.P.; Klapp, B.F.; Arck, P.C.; Peters, E.M. Further exploring the brain-skin connection: Stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J. Investig. Dermatol. 2008, 128, 434–446. [Google Scholar] [CrossRef]
- Peters, E.M.; Liezmann, C.; Spatz, K.; Daniltchenko, M.; Joachim, R.; Gimenez-Rivera, A.; Hendrix, S.; Botchkarev, V.A.; Brandner, J.M.; Klapp, B.F. Nerve growth factor partially recovers inflamed skin from stress-induced worsening in allergic inflammation. J. Investig. Dermatol. 2011, 131, 735–743. [Google Scholar] [CrossRef]
- Peters, E.M.; Handjiski, B.; Kuhlmei, A.; Hagen, E.; Bielas, H.; Braun, A.; Klapp, B.F.; Paus, R.; Arck, P.C. Neurogenic inflammation in stress-induced termination of murine hair growth is promoted by nerve growth factor. Am. J. Pathol. 2004, 165, 259–271. [Google Scholar] [CrossRef]
- Raychaudhuri, S.P.; Raychaudhuri, S.K. Role of NGF and neurogenic inflammation in the pathogenesis of psoriasis. Prog. Brain Res. 2004, 146, 433–437. [Google Scholar] [CrossRef]
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front Cell Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell proteases as protective and inflammatory mediators. Adv. Exp. Med. Biol. 2011, 716, 212–234. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef]
- Pejler, G.; Ronnberg, E.; Waern, I.; Wernersson, S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef]
- Caughey, G.H. Roles of mast cell tryptase and chymase in airway function. Am. J. Physiol. 1989, 257, L39–L46. [Google Scholar] [CrossRef]
- Ali, F.; Vyas, J.; Finlay, A.Y. Counting the Burden: Atopic Dermatitis and Health-related Quality of Life. Acta Derm. Venereol. 2020, 100, adv00161. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann. Allergy Asthma Immunol. 2018, 121, 340–347. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, S.; Wang, X.; Guo, B.; Liu, J.; Xu, D.; Liu, F. The Specific microRNA Profile and Functional Networks for Children with Allergic Asthma. J. Asthma Allergy 2022, 15, 1179–1194. [Google Scholar] [CrossRef]
- Soeberdt, M.; Kilic, A.; Abels, C. Current and emerging treatments targeting the neuroendocrine system for disorders of the skin and its appendages. Exp. Dermatol. 2020, 29, 801–813. [Google Scholar] [CrossRef]
- Bosmans, G.; Shimizu Bassi, G.; Florens, M.; Gonzalez-Dominguez, E.; Matteoli, G.; Boeckxstaens, G.E. Cholinergic Modulation of Type 2 Immune Responses. Front. Immunol. 2017, 8, 1873. [Google Scholar] [CrossRef]
- Ertle, C.M.; Rommel, F.R.; Tumala, S.; Moriwaki, Y.; Klein, J.; Kruse, J.; Gieler, U.; Peters, E.M.J. New Pathways for the Skin’s Stress Response: The Cholinergic Neuropeptide SLURP-1 Can Activate Mast Cells and Alter Cytokine Production in Mice. Front Immunol. 2021, 12, 631881. [Google Scholar] [CrossRef] [PubMed]
- Parrish, W.R.; Rosas-Ballina, M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yang, L.H.; Hudson, L.; Lin, X.; Patel, N.; Johnson, S.M.; et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 2008, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, P.S.; Rosas-Ballina, M.; Levine, Y.A.; Tracey, K.J. Rethinking inflammation: Neural circuits in the regulation of immunity. Immunol. Rev. 2012, 248, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kodama, T.; Lee, J.; Utsunomiya, N.; Hayashi, S.; Sakamoto, H.; Kuramoto, H.; Kadowaki, M. Anti-allergic role of cholinergic neuronal pathway via alpha7 nicotinic ACh receptors on mucosal mast cells in a murine food allergy model. PLoS ONE 2014, 9, e85888. [Google Scholar] [CrossRef]
- Rommel, F.R.; Raghavan, B.; Paddenberg, R.; Kummer, W.; Tumala, S.; Lochnit, G.; Gieler, U.; Peters, E.M. Suitability of Nicotinic Acetylcholine Receptor alpha7 and Muscarinic Acetylcholine Receptor 3 Antibodies for Immune Detection: Evaluation in Murine Skin. J. Histochem.Cytochem. Off. J. Histochem. Soc. 2015, 63, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanova, E.N.; Shulepko, M.A.; Kudryavtsev, D.; Bychkov, M.L.; Kulbatskii, D.S.; Kasheverov, I.E.; Astapova, M.V.; Feofanov, A.V.; Thomsen, M.S.; Mikkelsen, J.D.; et al. Human Secreted Ly-6/uPAR Related Protein-1 (SLURP-1) Is a Selective Allosteric Antagonist of alpha7 Nicotinic Acetylcholine Receptor. PLoS ONE 2016, 11, e0149733. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, S.; Liezmann, C.; Blois, S.M.; Joachim, R.; Kruse, J.; Romani, N.; Klapp, B.F.; Peters, E.M. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J. Immunol. 2011, 186, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.; Kuhlmei, A.; Tobin, D.J.; Muller-Rover, S.; Klapp, B.F.; Arck, P.C. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav. Immun. 2005, 19, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, Y.; Yoshikawa, K.; Fukuda, H.; Fujii, Y.X.; Misawa, H.; Kawashima, K. Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sci. 2007, 80, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Yang, W.K.; Jo, E.H.; Shin, S.H.; Lee, Y.C.; Park, M.C.; Kim, S.H. NCM 1921, a Mixture of Several Ingredients, Including Fatty Acids and Choline, Attenuates Atopic Dermatitis in 1-Chloro-2,4-Dinitrobenzene-Treated NC/Nga Mice. Nutrients 2020, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Marko, M.; Pawliczak, R. Real-life efficiency and safety comparison study of emollient ointment based on glycerophosphoinositol (GPI) salt of choline and other emollient products in patients with atopic dermatitis. J. Dermatolog. Treat 2022, 33, 999–1010. [Google Scholar] [CrossRef]
- Peters, E.M.; Michenko, A.; Kupfer, J.; Kummer, W.; Wiegand, S.; Niemeier, V.; Potekaev, N.; Lvov, A.; Gieler, U. Mental stress in atopic dermatitis--neuronal plasticity and the cholinergic system are affected in atopic dermatitis and in response to acute experimental mental stress in a randomized controlled pilot study. PLoS ONE 2014, 9, e113552. [Google Scholar] [CrossRef]
- Waern, I.; Jonasson, S.; Hjoberg, J.; Bucht, A.; Abrink, M.; Pejler, G.; Wernersson, S. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J. Immunol. 2009, 183, 6369–6376. [Google Scholar] [CrossRef] [PubMed]
- Orr-Urtreger, A.; Goldner, F.M.; Saeki, M.; Lorenzo, I.; Goldberg, L.; De Biasi, M.; Dani, J.A.; Patrick, J.W.; Beaudet, A.L. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J. Neurosci. 1997, 17, 9165–9171. [Google Scholar] [CrossRef] [PubMed]
- Moser, N.; Mechawar, N.; Jones, I.; Gochberg-Sarver, A.; Orr-Urtreger, A.; Plomann, M.; Salas, R.; Molles, B.; Marubio, L.; Roth, U.; et al. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J. Neurochem. 2007, 102, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Gonda, A.; Gál, I.; Szántó, S.; Sarraj, B.; Glant, T.T.; Hunyadi, J.; Mikecz, K. CD44, but not l-selectin, is critically involved in leucocyte migration into the skin in a murine model of allergic dermatitis. Exp. Dermatol. 2005, 14, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Strickland, I.; Tomkinson, A.; Fehringer, A.P.; Gelfand, E.W.; Leung, D.Y. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J. Investig. Dermatol. 2001, 116, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Enjo, S.; Hazama, Y.; Kimura, S.; Morimoto, Y.; Ueda, H. Effect of ultrasound treatment of the skin on activation of Langerhans cells and antibody production in rodents. J. Adv. Pharm. Technol. Res. 2023, 14, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Matsuhara, H.; Tanaka, H.; Inagaki, N.; Tsutsui, M. Oral allergy induction through skin exposure to previously tolerated food antigens in murine models. J. Pharmacol. Sci. 2023, 152, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Lee, Y.J.; Kang, D.G.; Lee, H.S.; Kim, D.K.; Park, M.C. Effects of Sohamhyoong-Tang on Ovalbumin-Induced Allergic Reaction in BALB/c Mice. Evid. Based Complement. Alternat. Med. 2016, 2016, 6286020. [Google Scholar] [CrossRef]
- Ki, H.H.; Hwang, S.W.; Lee, J.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. A dichloromethane fraction of Triticum aestivum sprouts reduces allergic immune response through inhibiting Th2 differentiation in ovalbumin-immunized mice. Mol. Med. Rep. 2017, 16, 3535–3541. [Google Scholar] [CrossRef]
- Sawada, K.; Nagai, H.; Basaki, Y.; Yamaya, H.; Ikizawa, K.; Watanabe, M.; Kojima, M.; Matsuura, N.; Kiniwa, M. The expression of murine cutaneous late phase reaction requires both IgE antibodies and CD4 T cells. Clin. Exp. Allergy 1997, 27, 225–231. [Google Scholar] [CrossRef]
- Conover, J.C.; Yancopoulos, G.D. Neurotrophin regulation of the developing nervous system: Analyses of knockout mice. Rev. Neurosci. 1997, 8, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, S.; Picker, B.; Liezmann, C.; Peters, E.M. Skin and hair follicle innervation in experimental models: A guide for the exact and reproducible evaluation of neuronal plasticity. Exp. Dermatol. 2008, 17, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Tharp, M.D.; Seelig, L.L., Jr.; Tigelaar, R.E.; Bergstresser, P.R. Conjugated avidin binds to mast cell granules. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1985, 33, 27–32. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]



| Substance/Company | Concentration | Action |
|---|---|---|
| Substance P (Sigma-Aldrich, St. Louis, MA, USA, Cat No. S6883) | 1 µM | Stressor |
| SLURP1 (Abnova, Taipei, Taiwan, Cat No. H00057152-P01–10 µg) | 5 ng/mL | Chrna7 agonist |
| Gene | Sequence |
|---|---|
| Caspase 3 | Forward: GCATTGAGACAGACAGTGGGAC Reverse: CTCCAGGAATAGTAACCAGGTGC Taqman: 6FAM-TAAGCATACAGGAAGTCAGCCTCCACCG--BBQ |
| c-Kit | Forward: CCTTTCTGGTGTCCAACTCTGAT Reverse: AGATACATTCTGGACCTGTACGTCC Taqman: 6FAM-CCAGTGCTTCCGTGACATTCAACGT--BBQ |
| IL10 | Forward: GACTTTCTTTCAAACAAAGGACC Reverse: GCTTGGCAACCCAAGTAA Taqman: 6FAM-ACTGCTAACCGACTCCTTAATGCAGG- -BBQ |
| mMCP4 | Forward: GGCTGGAGCTGAGGAGATTATT Reverse: AGTGTGCAGCAGTCAACACAAAT Taqman: FAM-CCACTGAGAGAGGGTTCACAGCTACCTGT-BBQ |
| mMCP6 | Forward: GTGCAGCTTCGTGAGCAGT Reverse: GGTGGGAGAGGCTCGTCATTAT Taqman: FAM-AGCTCCTCTCTTTGAACAGGATCGTGGT-BBQ |
| PCNA | Forward: CAACTTGGAATCCCAGAACAGG Reverse: GAACAGGCTCATTCATCTCTATGGTTA Taqman: 6FAM-TTGCACGTATATGCCGAGACCTTAGCCA--BBQ |
| TBP | Forward: GTGAATCTTGGCTGTAAACTTGACCT Reverse: GCAGTTGTCCGTGGCTC Taqman: 6FAM-AAATGCTGAATATAATCCCAAGCGATTTGC--BBQ |
| TNFα | Forward: GCCTATGTCTCAGCCTCTTCTCATT Reverse: CCACTTGGTGGTTTGCTACGA Taqman: 6FAM-CCATAGAACTGATGAGAGGGAGGCCATTT- -BBQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rommel, F.R.; Tumala, S.; Urban, A.-L.; Siebenhaar, F.; Kruse, J.; Gieler, U.; Peters, E.M.J. Stress Affects Mast Cell Proteases in Murine Skin in a Model of Atopic Dermatitis-like Allergic Inflammation. Int. J. Mol. Sci. 2024, 25, 5738. https://doi.org/10.3390/ijms25115738
Rommel FR, Tumala S, Urban A-L, Siebenhaar F, Kruse J, Gieler U, Peters EMJ. Stress Affects Mast Cell Proteases in Murine Skin in a Model of Atopic Dermatitis-like Allergic Inflammation. International Journal of Molecular Sciences. 2024; 25(11):5738. https://doi.org/10.3390/ijms25115738
Chicago/Turabian StyleRommel, Frank R., Susanne Tumala, Anna-Lena Urban, Frank Siebenhaar, Johannes Kruse, Uwe Gieler, and Eva M. J. Peters. 2024. "Stress Affects Mast Cell Proteases in Murine Skin in a Model of Atopic Dermatitis-like Allergic Inflammation" International Journal of Molecular Sciences 25, no. 11: 5738. https://doi.org/10.3390/ijms25115738




