Exploring the Role of MMP-9 and MMP-9/TIMP-1 Ratio in Subacute Stroke Recovery: A Prospective Observational Study
Abstract
:1. Introduction
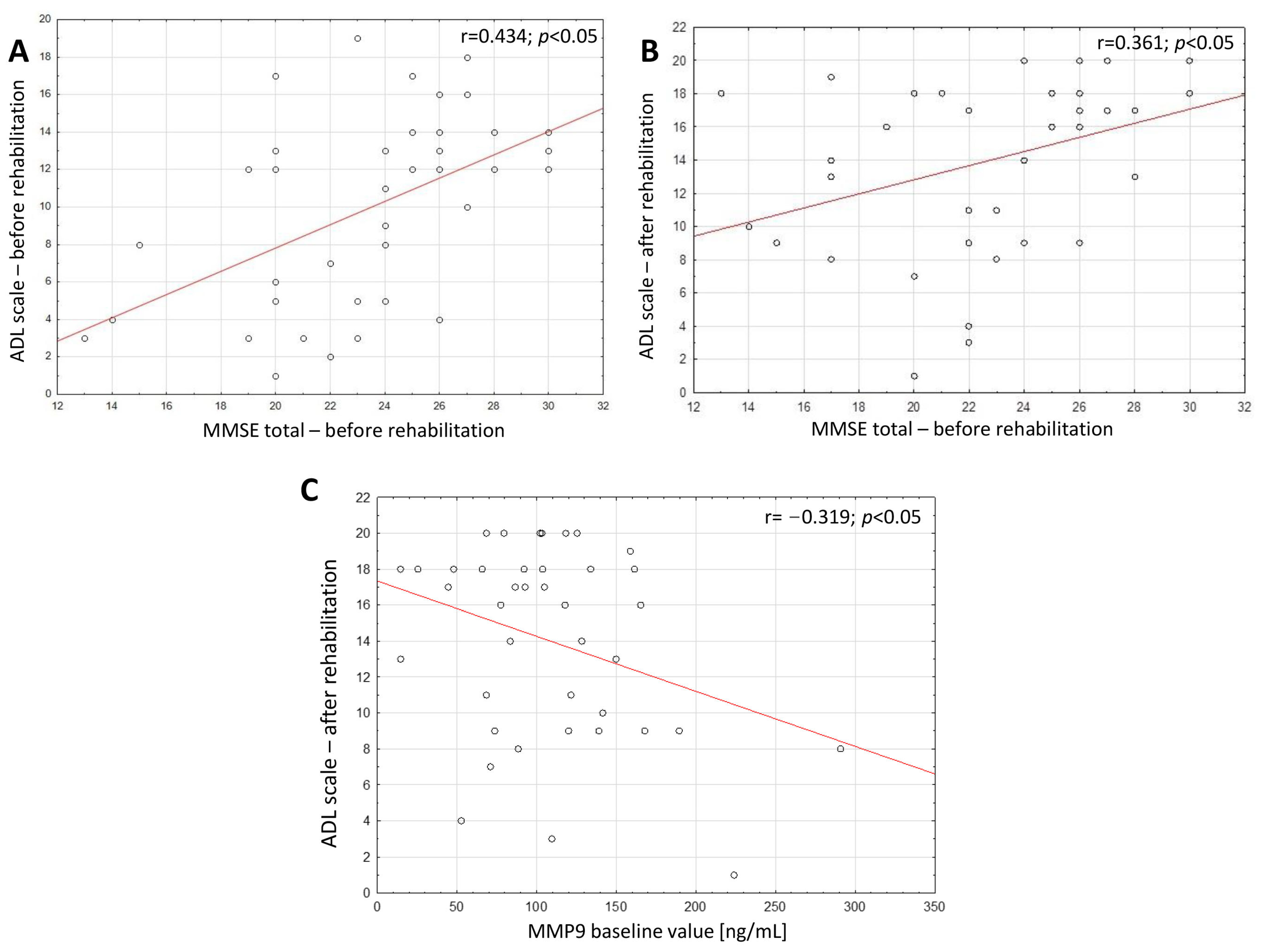
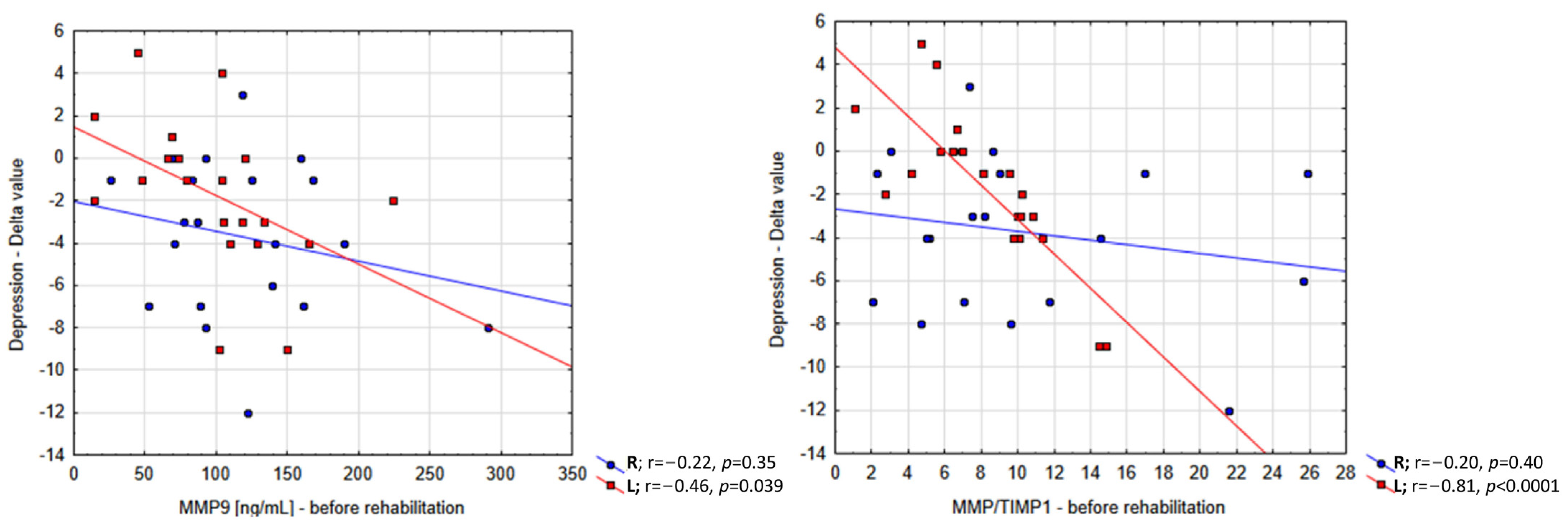
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Subjects
4.2. Outcome Evaluation
4.3. Biochemical Assessment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [PubMed]
- Guzik, A.; Bushnell, C. Stroke epidemiology and risk factor management. Contin. Lifelong Learn. Neurol. 2017, 23, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Cassidy, J.M.; Cramer, S.C. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl. Stroke Res. 2017, 8, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, L.; Cichoń, N.; Karbownik, M.S.; Saso, L.; Saluk, J.; Miller, E. Circulating Serum VEGF, IGF-1 and MMP-9 and Expression of Their Genes as Potential Prognostic Markers of Recovery in Post-Stroke Rehabilitation—A Prospective Observational Study. Brain Sci. 2023, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139 (Suppl. S2), 91–114. [Google Scholar] [CrossRef]
- Ferrer-Ferrer, M.; Dityatev, A. Shaping Synapses by the Neural Extracellular Matrix. Front. Neuroanat. 2018, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, P.; Wawrzyniak, M.; Alot, P.; Szczot, M.; Wyrembek, P.; Mercik, K.; Medvedev, N.; Wilczek, E.; De Roo, M.; Zuschratter, W.; et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 2011, 124, 3369–3380. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagi, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, J.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006, 26, 1923–1934. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Luo, S.; Huang, H.; Huang, X.; Li, M.; Huang, Y.; Chen, Y.; Wu, Z. Predictive value of longitudinal changes of serum matrix metalloproteinase-9 and brain-derived neurotrophic factor in acute ischemic stroke. Front. Aging Neurosci. 2022, 14, 952038. [Google Scholar] [CrossRef]
- Mechtouff, L.; Bochaton, T.; Paccalet, A.; Crola Da Silva, C.; Buisson, M.; Amaz, C.; Bouin, M.; Derex, L.; Ong, E.; Berthezene, Y. Matrix metalloproteinase-9 relationship with infarct growth and hemorrhagic transformation in the era of thrombectomy. Front. Neurol. 2020, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; You, Y.; Wang, X. Predictive value of serum matrix metalloproteinase 9 combined with tissue inhibitor of metalloproteinase 1 for post-stroke cognitive impairment. J. Clin. Neurosci. 2022, 105, 103–108. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Meng, L.; Wang, F.; Li, Q.; Yang, F.; Wang, M.; Yu, M.; Zhang, J.; Li, S.; et al. Relationship between MMP-9 serum levels and tHcy levels and total imaging load and cognitive dysfunction. J. Stroke Cerebrovasc. Dis. 2022, 31, 106759. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Qu, J. Plasma MMP-9/TIMP-1 ratio serves as a novel potential biomarker in Alzheimer’s disease. Neuroreport 2023, 34, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Nagase, H.; Meng, Q.; Malinovskii, V.; Huang, W.; Chung, L.; Bode, W.; Maskos, K.; Brew, K. Engineering of selective TIMPs. Ann. N. Y. Acad. Sci. 1999, 878, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.A.; Kirik, S.Y.; Sumer, S.; Ural, O.; Kiratlı, H.E.; Vatansev, H.; Hayatsal, E.P.; Arslan, U.; Cebeci, H.; Demir, L.S. An evaluation of matrix metalloproteinase-9 (Mmp-9) and tissue inhibitor metalloproteinase-1 (Timp-1) Serum levels and the Mmp-9/Timp-1 Ratio in Covid-19 patients. Afr. Health Sci. 2023, 23, 37–43. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D. Agreed definitions and a shared vision for new standards in stroke recovery research: The stroke recovery and rehabilitation roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Einstad, M.S.; Saltvedt, I.; Lydersen, S.; Ursin, M.H.; Munthe-Kaas, R.; Ihle-Hansen, H.; Knapskog, A.-B.; Askim, T.; Beyer, M.K.; Næss, H. Associations between post-stroke motor and cognitive function: A cross-sectional study. BMC Geriatr. 2021, 21, 103. [Google Scholar] [CrossRef]
- Ben Assayag, E.; Shenhar-Tsarfaty, S.; Korczyn, A.D.; Kliper, E.; Hallevi, H.; Shopin, L.; Auriel, E.; Giladi, N.; Mike, A.; Halevy, A. Gait measures as predictors of poststroke cognitive function: Evidence from the TABASCO study. Stroke 2015, 46, 1077–1083. [Google Scholar] [CrossRef]
- VanGilder, J.L.; Hooyman, A.; Peterson, D.S.; Schaefer, S.Y. Post-stroke cognitive impairments and responsiveness to motor rehabilitation: A review. Curr. Phys. Med. Rehabil. Rep. 2020, 8, 461–468. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, J.; Xu, T.; Peng, Y.; Wang, A.; Wang, J.; Peng, H.; Li, Q.; Ju, Z.; Geng, D.; et al. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology 2017, 89, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Ma, Y.; Jiang, L.; Mu, Z.; Jiang, Z.; Chen, X.; Wang, Y.; Yang, G.Y.; Zhang, Z. Hypoxia Response Element-Regulated MMP-9 Promotes Neurological Recovery via Glial Scar Degradation and Angiogenesis in Delayed Stroke. Mol. Ther. 2017, 25, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, P.; Khoshneviszadeh, M.; Jandke, S.; Schreiber, S.; Dityatev, A. Interplay between perivascular and perineuronal extracellular matrix remodelling in neurological and psychiatric diseases. Eur. J. Neurosci. 2021, 53, 3811–3830. [Google Scholar] [CrossRef] [PubMed]
- Afthinos, A.; Themistocleous, C.; Herrmann, O.; Fan, H.; Lu, H.; Tsapkini, K. The contribution of working memory areas to verbal learning and recall in primary progressive aphasia. Front. Neurol. 2022, 13, 698200. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Murphy, E.S.; Elijah, I.E.; Holtfreter, K.L.; Davis, C.J.; Olson, M.L.; Muhunthan, K.; Harding, J.W. Influence of hippocampectomy on habituation, exploratory behavior, and spatial memory in rats. Brain Res. 2004, 1023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Chang, T.T.; Majid, A.; Caspers, S.; Eickhoff, S.B.; Menon, V. Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cereb. Cortex 2009, 19, 2930–2945. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D. The brain basis of language processing: From structure to function. Physiol. Rev. 2011, 91, 1357–1392. [Google Scholar] [CrossRef]
- Shu, H.; Zheng, G.Q.; Wang, X.; Sun, Y.; Liu, Y.; Weaver, J.M.; Shen, X.; Liu, W.; Jin, X. Activation of matrix metalloproteinase in dorsal hippocampus drives improvement in spatial working memory after intra-VTA nicotine infusion in rats. J. Neurochem. 2015, 135, 357–367. [Google Scholar] [CrossRef]
- Matusiak, M.; Oziębło, D.; Obrycka, A.; Ołdak, M.; Kaczmarek, L.; Skarżyński, P.; Skarżyński, H. Functional Polymorphism of MMP9 and BDNF as Potential Biomarker of Auditory Neuroplasticity in Prelingual Deafness Treatment with Cochlear Implantation-A Retrospective Cohort Analysis. Trends Hear. 2021, 25, 23312165211002140. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, M.; Oziębło, D.; Ołdak, M.; Rejmak, E.; Kaczmarek, L.; Dobek, D.; Skarżyński, H. MMP-9 plasma level as biomarker of cochlear implantation outcome in cohort study of deaf children. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 4361–4369. [Google Scholar] [CrossRef]
- Che, B.; Zhong, C.; Ge, J.; Li, R.; Zhu, Z.; Bu, X.; Xu, T.; Ju, Z.; Liu, J.; Zhang, J.; et al. Serum Matrix Metalloproteinase-9 Is Associated with Depression After Acute Ischemic Stroke. Circ. J. 2019, 83, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.A.; Nassan, M.; Jenkins, G.D.; Kung, S.; Veldic, M.; Palmer, B.A.; Feeder, S.E.; Tye, S.J.; Choi, D.S.; Biernacka, J.M. Feasibility of investigating differential proteomic expression in depression: Implications for biomarker development in mood disorders. Transl. Psychiatry 2015, 5, e689. [Google Scholar] [CrossRef] [PubMed]
- Bobińska, K.; Szemraj, J.; Czarny, P.; Gałecki, P. Expression and Activity of Metalloproteinases in Depression. Med. Sci. Monit. 2016, 22, 1334–1341. [Google Scholar] [CrossRef]
- Rybakowski, J.K.; Remlinger-Molenda, A.; Czech-Kucharska, A.; Wojcicka, M.; Michalak, M.; Losy, J. Increased serum matrix metalloproteinase-9 (MMP-9) levels in young patients during bipolar depression. J. Affect. Disord. 2013, 146, 286–289. [Google Scholar] [CrossRef]
- Bobińska, K.; Szemraj, J.; Gałecki, P.; Talarowska, M. The role of MMP genes in recurrent depressive disorders and cognitive functions. Acta Neuropsychiatr. 2016, 28, 221–231. [Google Scholar] [CrossRef]
- Hüfner, K.; Koudouovoh-Tripp, P.; Kandler, C.; Hochstrasser, T.; Malik, P.; Giesinger, J.; Semenitz, B.; Humpel, C.; Sperner-Unterweger, B. Differential changes in platelet reactivity induced by acute physical compared to persistent mental stress. Physiol. Behav. 2015, 151, 284–291. [Google Scholar] [CrossRef]
- Baccaro, A.; Wang, Y.P.; Brunoni, A.R.; Candido, M.; Conforto, A.B.; da Costa Leite, C.; Lotufo, P.A.; Benseñor, I.M.; Goulart, A.C. Does stroke laterality predict major depression and cognitive impairment after stroke? Two-year prospective evaluation in the EMMA study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109639. [Google Scholar] [CrossRef]
- Abdullaev, Y.; Kennedy, B.L.; Tasman, A. Changes in neural circuitry of language before and after treatment of major depression. Hum. Brain Mapp. 2002, 17, 156–167. [Google Scholar] [CrossRef]
- Abdelnaseer, M.M.; Elfauomy, N.M.; Esmail, E.H.; Kamal, M.M.; Elsawy, E.H. Matrix Metalloproteinase-9 and Recovery of Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Chiti, G.; Pantoni, L. Use of Montreal Cognitive Assessment in patients with stroke. Stroke 2014, 45, 3135–3140. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Sheikh, J.I. 9/Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Marc, L.G.; Raue, P.J.; Bruce, M.L. Screening performance of the 15-item geriatric depression scale in a diverse elderly home care population. Am. J. Geriatr. Psychiatry 2008, 16, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Steer, R.A.; Rissmiller, D.J.; Beck, A.T. Use of the Beck Depression Inventory-II with depressed geriatric inpatients. Behav. Res. Ther. 2000, 38, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Farooque, U.; Lohano, A.K.; Kumar, A.; Karimi, S.; Yasmin, F.; Bollampally, V.C.; Ranpariya, M.R. Validity of National Institutes of Health Stroke Scale for Severity of Stroke to Predict Mortality Among Patients Presenting with Symptoms of Stroke. Cureus 2020, 12, e10255. [Google Scholar] [CrossRef] [PubMed]
- Kwah, L.K.; Diong, J. National institutes of health stroke scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.; Collin, C. The Barthel ADL Index: A standard measure of physical disability? Int. Disabil. Stud. 1988, 10, 64–67. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, F.; Peng, X.; Li, Q.; Wang, F.; Xu, Z.; Cai, R.; Ji, D.; Zhang, J.; Wang, M.; et al. Is Matrix Metalloproteinase-9 Associated with Post-Stroke Cognitive Impairment or Dementia? J. Integr. Neurosci. 2022, 21, 160. [Google Scholar] [CrossRef]

| MMP9 Baseline Value (ng/mL) | Delta MMP9 (log%) | TIMP1 Baseline Value (ng/mL) | MMP9/TIMP1 Baseline Value | Delta MMP9/TIMP1 (log%) | ||
|---|---|---|---|---|---|---|
| MMSE total | Before rehabilitation | r = −0.00, p = 0.999 | r = −0.11, p = 0.490 | r = −0.13, p = 0.439 | r = 0.06, p = 0.726 | r = −0.19, p = 0.253 |
| After rehabilitation | r = −0.10, p = 0.564 | r = 0.01, p = 0.955 | r = −0.16, p = 0.319 | r = −0.03, p = 0.852 | r = −0.08, p = 0.633 | |
| Delta value | r = −0.17, p = 0.312 | r = 0.26, p = 0.108 | r = −0.01, p = 0.937 | r = −0.18, p = 0.277 | r = 0.26, p = 0.104 | |
| Registration | Before rehabilitation | r = −0.41, p = 0.009 | r = 0.22, p = 0.173 | r = −0.39, p = 0.015 | r = 0.03, p = 0.851 | r = 0.00, p = 0.998 |
| Delta value | r = 0.06, p = 0.720 | r = −0.36, p = 0.026 | r = −0.15, p = 0.348 | r = 0.11, p = 0.497 | r = −0.33, p = 0.041 | |
| Attention and Calculation | Delta value | r = 0.01, p = 0.943 | r = 0.43, p = 0.006 | r = −0.00, p = 0.987 | r = 0.06, p = 0.737 | r = 0.37, p = 0.019 |
| Language | Delta value | r = −0.46, p = 0.003 | r = 0.13, p = 0.426 | r = −0.15, p = 0.346 | r = −0.38, p = 0.017 | r = 0.06, p = 0.729 |
| MMP9 Baseline Value (ng/mL) | Delta MMP9 (log%) | TIMP1 Baseline Value (ng/mL) | Delta TIMP1 (log%) | MMP9/TIMP1 Baseline Value | Delta MMP9/TIMP1 (log%) | ||
|---|---|---|---|---|---|---|---|
| Attention and Calculation | Before rehabilitation | r = −0.40, p = 0.077 | r = 0.08, p = 0.734 | r = −0.24, p = 0.300 | r = 0.26, p = 0.271 | r = −0.10, p = 0.690 | r = −0.08, p = 0.745 |
| After rehabilitation | r = −0.47, p = 0.035 | r = 0.45, p = 0.047 | r = −0.31, p = 0.180 | r = 0.39, p = 0.091 | r = −0.04, p = 0.858 | r = 0.15, p = 0.514 | |
| Delta value | r = 0.06, p = 0.802 | r = 0.41, p = 0.069 | r = 0, p = 0.984 | r = 0.06, p = 0.792 | r = 0.10, p = 0.685 | r = 0.31, p = 0.188 | |
| Language | Before rehabilitation | r = 0.26, p = 0.277 | r = −0.23, p = 0.323 | r = −0.08, p = 0.730 | r = 0.23, p = 0.324 | r = 0.50, p = 0.025 | r = −0.32, p = 0.165 |
| After rehabilitation | r = −0.23, p = 0.337 | r = −0.18, p = 0.443 | r = −0.42, p = 0.064 | r = 0.51, p = 0.021 | r = 0.16, p = 0.500 | r = −0.44, p = 0.054 | |
| Delta value | r = −0.45, p = 0.048 | r = 0.19, p = 0.412 | r = −0.13, p = 0.589 | r = −0.01, p = 0.961 | r = −0.54, p = 0.013 | r = 0.17, p = 0.482 | |
| MMP9 Baseline Value (ng/mL) | Delta MMP9 (log%) | TIMP1 Baseline Value (ng/mL) | Delta TIMP1 (log%) | MMP9/TIMP1 Baseline Value | Delta MMP9/TIMP1 (log%) | ||
|---|---|---|---|---|---|---|---|
| Language | Before rehabilitation | r = 0.15, p = 0.535 | r = 0.07, p = 0.781 | r = 0.17, p = 0.487 | r = −0.36, p = 0.129 | r = 0.10, p = 0.671 | r = 0.30, p = 0.207 |
| After rehabilitation | r = −0.12, p = 0.612 | r = 0.09, p = 0.724 | r = 0.10, p = 0.690 | r = −0.28, p = 0.244 | r = −0.07, p = 0.780 | r = 0.27, p = 0.265 | |
| Delta value | r = −0.47, p = 0.044 | r = 0.02, p = 0.947 | r = −0.15, p = 0.548 | r = 0.20, p = 0.419 | r = −0.29, p = 0.221 | r = −0.11, p = 0.647 | |
| Parameter | Mean (SD) or Number (Frequency) |
|---|---|
| Sociodemographic | |
| Sex—female | 14 (35%) |
| Sex—male | 26 (65%) |
| Age (years) | 68 (10.8) |
| Comorbidity and treatment | |
| Hypertension | 24 (60%) |
| Diabetes | 11 (28%) |
| Atherosclerosis | 7 (18%) |
| Thrombolytic treatment | 5 (13%) |
| Blood parameters | |
| Sodium | 138.9 (2.87) |
| Potassium | 4.3 (0.36) |
| WBC | 7.55 (1.96) |
| RBC | 4.41 (0.57) |
| Hb | 13.31 (1.54) |
| HCT | 40.8 (4.26) |
| Urea | 32.8 (15.1) |
| Creatinine | 0.82 (0.24) |
| Methods | Description |
|---|---|
| Neurophysiological session (morning) | A 30 min session focused on implementing techniques derived from daily activities, along with an additional 30 min dedicated to repetitive task exercises or balance exercises |
| Psychotherapy | A 15 min session designated for psychotherapeutic interventions |
| Aerobic training | Aerobic exercise sessions were conducted 2–3 times daily, each one lasting 10 min, with intervals spaced at 60 min intervals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, L.; Cichon, N.; Karbownik, M.S.; Saluk, J.; Miller, E. Exploring the Role of MMP-9 and MMP-9/TIMP-1 Ratio in Subacute Stroke Recovery: A Prospective Observational Study. Int. J. Mol. Sci. 2024, 25, 5745. https://doi.org/10.3390/ijms25115745
Włodarczyk L, Cichon N, Karbownik MS, Saluk J, Miller E. Exploring the Role of MMP-9 and MMP-9/TIMP-1 Ratio in Subacute Stroke Recovery: A Prospective Observational Study. International Journal of Molecular Sciences. 2024; 25(11):5745. https://doi.org/10.3390/ijms25115745
Chicago/Turabian StyleWłodarczyk, Lidia, Natalia Cichon, Michał Seweryn Karbownik, Joanna Saluk, and Elzbieta Miller. 2024. "Exploring the Role of MMP-9 and MMP-9/TIMP-1 Ratio in Subacute Stroke Recovery: A Prospective Observational Study" International Journal of Molecular Sciences 25, no. 11: 5745. https://doi.org/10.3390/ijms25115745
APA StyleWłodarczyk, L., Cichon, N., Karbownik, M. S., Saluk, J., & Miller, E. (2024). Exploring the Role of MMP-9 and MMP-9/TIMP-1 Ratio in Subacute Stroke Recovery: A Prospective Observational Study. International Journal of Molecular Sciences, 25(11), 5745. https://doi.org/10.3390/ijms25115745








