The Use of Compounds Derived from Cannabis sativa in the Treatment of Epilepsy, Painful Conditions, and Neuropsychiatric and Neurodegenerative Disorders
Abstract
1. Introduction
2. Neurological Disorders and Cannabis Studies
2.1. Epilepsy
2.2. Neurodegenerative Diseases
2.2.1. Parkinson’s Disease
2.2.2. Alzheimer’s Disease
2.2.3. Multiple Sclerosis
2.2.4. Amyotrophic Lateral Sclerosis
2.2.5. Huntington’s Disease
2.3. Neurodevelopmental Disorders
2.3.1. Attention Deficit-Hyperactivity Disorder
2.3.2. Autism Spectrum Disorder
2.3.3. Tourette Syndrome
2.4. Psychiatric Disorders
2.4.1. Anxiety
2.4.2. Depression
2.4.3. Post-Traumatic Stress Disorder
2.5. Painful Pathological Conditions
2.5.1. Migraine
2.5.2. Neuropathic Pain
2.5.3. Diabetic Neuropathy
2.5.4. Fibromyalgia
2.5.5. Trigeminal Neuralgia
3. Next Steps in the Research Field
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarfo, F.S.; Akassi, J.; Badu, E.; Okorozo, A.; Ovbiagele, B.; Akpalu, A. Profile of Neurological Disorders in an Adult Neurology Clinic in Kumasi, Ghana. eNeurologicalSci 2016, 3, 69–74. [Google Scholar] [CrossRef]
- Carriba, P.; Lorenzón, N.; Dierssen, M. Neurodevelopmental Disorders: 2023 Update. Free Neuropathol. 2023, 4, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Crary, J.F. Neurodegeneration: 2023 Update. Free Neuropathol. 2023, 4, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Williams, N.R.; George, M.S. Beyond Neural Cubism: Promoting a Multidimensional View of Brain Disorders by Enhancing the Integration of Neurology and Psychiatry in Education. Acad. Med. 2015, 90, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Míguez, M.; Navarro-Jiménez, E.; Clemente-Suárez, V.J. Behavioral Patterns of Depression Patients and Control Population. Int. J. Environ. Res. Public Health 2022, 19, 9506. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Simon, N.M. Anxiety Disorders: A Review. JAMA 2022, 328, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Joseph, G.R.; Joy, G.K.; Khanal, S.; Dasireddy, R.R.; Menon, A.; Barrie Mason, I.; Kataria, J.; Patel, T.; Modi, S. Post-Traumatic Stress Disorder: A Narrative Review of Pharmacological and Psychotherapeutic Interventions. Cureus 2023, 15, e44905. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.C.; Prasad, S.; McCrary, A.; Jordan, E.; Sachdeva, V.; Deva, S.; Kumar, H.; Mehta, J.; Neupane, P.; Gupta, A. Adult Attention Deficit Hyperactivity Disorder: A Comprehensive Review. Ann. Med. Surg. 2023, 85, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Doernberg, E.; Hollander, E. Neurodevelopmental Disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr. 2016, 21, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Dan, O. Recognition of Emotional Facial Expressions in Adolescents with Attention Deficit/Hyperactivity Disorder. J. Adolesc. 2020, 82, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Abdoli, N.; Rahmani, A.; Shiri, M.H.; Hashemian, A.H.; Akbari, H.; Mohammadi, M. The Global Prevalence of ADHD in Children and Adolescents: A Systematic Review and Meta-Analysis. Ital. J. Pediatr. 2023, 49, 48. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-Deficit/Hyperactivity Disorder Is Characterized by a Delay in Cortical Maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef]
- Ho, R.C.; Zhang, M.W.; Tsang, T.Y.; Toh, A.H.; Pan, F.; Lu, Y.; Cheng, C.; Yip, P.S.; Lam, L.T.; Lai, C.-M.; et al. The Association between Internet Addiction and Psychiatric Co-Morbidity: A Meta-Analysis. BMC Psychiatry 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Jernelöv, S.; Larsson, Y.; Llenas, M.; Nasri, B.; Kaldo, V. Effects and Clinical Feasibility of a Behavioral Treatment for Sleep Problems in Adult Attention Deficit Hyperactivity Disorder (ADHD): A Pragmatic within-Group Pilot Evaluation. BMC Psychiatry 2019, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, M.; Divan, G.; Koh, Y.-J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global Prevalence of Autism and Other Pervasive Developmental Disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Ripke, S.; Als, T.D.; Mattheisen, M.; Walters, R.K.; Won, H.; Pallesen, J.; Agerbo, E.; Andreassen, O.A.; Anney, R.; et al. Identification of Common Genetic Risk Variants for Autism Spectrum Disorder. Nat. Genet. 2019, 51, 431–444. [Google Scholar] [CrossRef]
- Pringsheim, T.; Edwards, M. Functional Movement Disorders. Neurol. Clin. Pract. 2017, 7, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Serranová, T.; Di Vico, I.; Tinazzi, M. Functional Movement Disorder: Assessment and Treatment. Neurol. Clin. 2023, 41, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Grudzień, J.; Rozenbajgier, M.; Ozga-Stachurska, A.; Pawłowska, P. Tourette Syndrom—A Review of Current Literature. J. Educ. Health Sport 2022, 12, 607–613. [Google Scholar] [CrossRef]
- CDC Data and Statistics on Tourette Syndrome|CDC. Available online: https://www.cdc.gov/ncbddd/tourette/data.html (accessed on 17 April 2024).
- Dong, B.; Xu, R.; Lim, M. The Pathophysiology of Trigeminal Neuralgia: A Molecular Review. J. Neurosurg. 2023, 139, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.; Galvez, R.; Katati, M. Shedding Light on Neuropathic Pain: Current and Emerging Tools for Diagnosis, Screening, and Quantification. SAGE Open Med. 2024, 12, 20503121231218985. [Google Scholar] [CrossRef] [PubMed]
- Rugnath, R.; Orzechowicz, C.; Newell, C.; Carullo, V.; Rugnath, A. A Literature Review: The Mechanisms and Treatment of Neuropathic Pain—A Brief Discussion. Biomedicines 2024, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Doneddu, P.E.; Pensato, U.; Iorfida, A.; Alberti, C.; Nobile-Orazio, E.; Fabbri, A.; Voza, A. Neuropathic Pain in the Emergency Setting: Diagnosis and Management. J. Clin. Med. 2023, 12, 6028. [Google Scholar] [CrossRef] [PubMed]
- Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 26 February 2024).
- Anwar, H.; Khan, Q.U.; Nadeem, N.; Pervaiz, I.; Ali, M.; Cheema, F.F. Epileptic Seizures. Discoveries 2020, 8, e110. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE Classification of the Epilepsies: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, E.T.; Maini, K.; Arya, K.; Sharma, S. Focal Onset Seizure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sarmast, S.T.; Abdullahi, A.M.; Jahan, N. Current Classification of Seizures and Epilepsies: Scope, Limitations and Recommendations for Future Action. Cureus 2020, 12, e10549. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; et al. Instruction Manual for the ILAE 2017 Operational Classification of Seizure Types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Yeni, K. Stigma and Psychosocial Problems in Patients with Epilepsy. Explor. Neurosci. 2023, 2, 251–263. [Google Scholar] [CrossRef]
- Aaberg, K.M.; Bakken, I.J.; Lossius, M.I.; Lund Søraas, C.; Tallur, K.K.; Stoltenberg, C.; Chin, R.; Surén, P. Short-Term Seizure Outcomes in Childhood Epilepsy. Pediatrics 2018, 141, e20174016. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Löscher, W. Drug Resistance in Epilepsy: Putative Neurobiologic and Clinical Mechanisms. Epilepsia 2005, 46, 858–877. [Google Scholar] [CrossRef] [PubMed]
- Teneralli, R.E.; Cepeda, M.S.; Kern, D.M.; Novak, G.P. Individuals Who Develop Drug-Resistant Epilepsy within a Year after Initial Diagnosis Have Higher Burden of Mental and Physical Diseases One-Year Prior to Epilepsy Diagnosis as Compared to Those Whose Seizures Were Controlled during the Same Interval. Epilepsy Behav. 2021, 123, 108243. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and Potential Therapeutic Role in Epilepsy and Other Neuropsychiatric Disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.E.; Jacobson, C. Report of a Parent Survey of Cannabidiol-Enriched Cannabis Use in Pediatric Treatment-Resistant Epilepsy. Epilepsy Behav. 2013, 29, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; DeJean, D.; Clifford, T.; Coyle, D.; Potter, B.K.; Skidmore, B.; Alexander, C.; Repetski, A.E.; Shukla, V.; McCoy, B.; et al. Cannabis-Based Products for Pediatric Epilepsy: A Systematic Review. Epilepsia 2019, 60, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Vyšata, O.; Ťupa, O.; Procházka, A.; Doležal, R.; Cejnar, P.; Bhorkar, A.M.; Dostál, O.; Vališ, M. Classification of Ataxic Gait. Sensors 2021, 21, 5576. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazaleh, A.K.; Zhou, X.; Bhuyan, D.J.; Münch, G.W.; Al-Dalabeeh, E.A.; Jaye, K.; Chang, D. The Neurotherapeutic Arsenal in Cannabis Sativa: Insights into Anti-Neuroinflammatory and Neuroprotective Activity and Potential Entourage Effects. Molecules 2024, 29, 410. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.F.L.; Campos, R.M.P.; Isaac, A.R.; Paes-Colli, Y.; Carvalho, V.M.; Sampaio, L.S.; de Melo Reis, R.A. Long-Term Treatment with Cannabidiol-Enriched Cannabis Extract Induces Synaptic Changes in the Adolescent Rat Hippocampus. Int. J. Mol. Sci. 2023, 24, 11775. [Google Scholar] [CrossRef] [PubMed]
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A Comprehensive Review on Cannabis Sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the Landscape of Cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534. [Google Scholar] [CrossRef] [PubMed]
- Filer, C.N. Acidic Cannabinoid Decarboxylation. Cannabis Cannabinoid Res. 2022, 7, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, O.; Kovalchuk, I. Cannabinoids as Anticancer Therapeutic Agents. Cell Cycle 2020, 19, 961–989. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Rivas-Santisteban, R.; Reyes-Resina, I.; Casanovas, M.; Pérez-Olives, C.; Ferreiro-Vera, C.; Navarro, G.; Sánchez de Medina, V.; Nadal, X. Pharmacological Potential of Varinic-, Minor-, and Acidic Phytocannabinoids. Pharmacol. Res. 2020, 158, 104801. [Google Scholar] [CrossRef]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef] [PubMed]
- Fordjour, E.; Manful, C.F.; Sey, A.A.; Javed, R.; Pham, T.H.; Thomas, R.; Cheema, M. Cannabis: A Multifaceted Plant with Endless Potentials. Front. Pharmacol. 2023, 14, 1200269. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Porter, R.; Facey, P.; Thoms-Rodriguez, C. Chemical Composition and Biological Activities of Jamaican Cannabis Sativa Essential Oils as the Plant Matures. Flavour Fragr. J. 2023, 38, 144–151. [Google Scholar] [CrossRef]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- McDougall, J.J.; McKenna, M.K. Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. Int. J. Mol. Sci. 2022, 23, 7891. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene Synthases from Cannabis Sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef] [PubMed]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis Sativa L. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.C.D.; Baldasso, G.M.; Bicca, M.A.; Paes, R.S.; Capasso, R.; Dutra, R.C. Terpenoids, Cannabimimetic Ligands, beyond the Cannabis Plant. Molecules 2020, 25, 1567. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis Sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Erridge, S.; Mangal, N.; Salazar, O.; Pacchetti, B.; Sodergren, M.H. Cannflavins—From Plant to Patient: A Scoping Review. Fitoterapia 2020, 146, 104712. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.S.; Radwan, M.M.; Metwaly, A.M.; Eissa, I.H.; Hazekamp, A.; Sohly, M.A. Chemistry and Biological Activities of Cannflavins of the Cannabis Plant. Cannabis Cannabinoid Res. 2023, 8, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Tomko, A.M.; Whynot, E.G.; Dupré, D.J. Anti-Cancer Properties of Cannflavin A and Potential Synergistic Effects with Gemcitabine, Cisplatin, and Cannabinoids in Bladder Cancer. J. Cannabis Res. 2022, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- LaVigne, J.E.; Hecksel, R.; Keresztes, A.; Streicher, J.M. Cannabis Sativa Terpenes Are Cannabimimetic and Selectively Enhance Cannabinoid Activity. Sci. Rep. 2021, 11, 8232. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain”, No Gain. Front. Plant Sci. 2018, 9, 1969. [Google Scholar] [CrossRef]
- Chaiton, M.; Kundu, A.; Rueda, S.; Di Ciano, P. Are Vaporizers a Lower-Risk Alternative to Smoking Cannabis? Can. J. Public Health 2021, 113, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sanz, G.; Madiedo, A.; Lynskey, M.; Brown, M.R.D. “Flower Power”: Controlled Inhalation of THC-Predominant Cannabis Flos Improves Health-Related Quality of Life and Symptoms of Chronic Pain and Anxiety in Eligible UK Patients. Biomedicines 2022, 10, 2576. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Acevedo, A.P.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Hladun, O.; Siles, A.; Torrens, M.; Busardo, F.P.; Farré, M. Oral Administration of Cannabis and Δ-9-Tetrahydrocannabinol (THC) Preparations: A Systematic Review. Medicina 2020, 56, 309. [Google Scholar] [CrossRef] [PubMed]
- Lunn, S.; Diaz, P.; O’Hearn, S.; Cahill, S.P.; Blake, A.; Narine, K.; Dyck, J.R.B. Human Pharmacokinetic Parameters of Orally Administered Δ9-Tetrahydrocannabinol Capsules Are Altered by Fed Versus Fasted Conditions and Sex Differences. Cannabis Cannabinoid Res. 2019, 4, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Vandrey, R.; Herrmann, E.S.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; LoDico, C.; Cone, E.J. Pharmacokinetic Profile of Oral Cannabis in Humans: Blood and Oral Fluid Disposition and Relation to Pharmacodynamic Outcomes. J. Anal. Toxicol. 2017, 41, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Schlienz, N.; Spindle, T.; Cone, E.; Herrmann, E.; Bigelow, G.; Mitchell, J.; Flegel, R.; Lodico, C.; Vandrey, R. Pharmacodynamic Dose Effects of Oral Cannabis Ingestion in Healthy Adults Who Infrequently Use Cannabis. Drug Alcohol Depend. 2020, 211, 107969. [Google Scholar] [CrossRef] [PubMed]
- Barrus, D.G.; Capogrossi, K.L.; Cates, S.C.; Gourdet, C.K.; Peiper, N.C.; Novak, S.P.; Lefever, T.W.; Wiley, J.L. Tasty THC: Promises and Challenges of Cannabis Edibles; Methods Report; RTI Press: Research Triangle Park, NC, USA, 2016; Volume 2016. [Google Scholar] [CrossRef]
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franzè, S.; Rocco, P.; Giuliani, C.; Fico, G.; Minghetti, P.; et al. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Ritter, S.; Yassin, M. Comparing Sublingual and Inhaled Cannabis Therapies for Low Back Pain: An Observational Open-Label Study. Rambam Maimonides Med. J. 2022, 13, e0026. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudinoodezh, H.; Telukutla, S.R.; Bhangu, S.K.; Bachari, A.; Cavalieri, F.; Mantri, N. The Transdermal Delivery of Therapeutic Cannabinoids. Pharmaceutics 2022, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Makhakhe, L. Topical Cannabidiol (CBD) in Skin Pathology—A Comprehensive Review and Prospects for New Therapeutic Opportunities. S. Afr. Fam. Pract. 2022, 64, 5493. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Gul, W.; Walker, L.A. Pharmacokinetics and Tolerability of Δ9-THC-Hemisuccinate in a Suppository Formulation as an Alternative to Capsules for the Systemic Delivery of Δ9-THC. Med. Cannabis Cannabinoids 2018, 1, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, R.; Egli, A.; Elsohly, M.; Henn, V.; Spiess, Y. The Effect of Orally and Rectally Administered Δ 9-Tetrahydrocannabinol on Spasticity: A Pilot Study with 2 Patients. Int. J. Clin. Pharmacol. Ther. 1996, 34, 446–452. [Google Scholar] [PubMed]
- Dell, D.D.; Stein, D.P. Exploring the Use of Medical Marijuana for Supportive Care of Oncology Patients. J. Adv. Pract. Oncol. 2021, 12, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Elsohly, M.; Hill, K.P. Cannabidiol Interactions with Medications, Illicit Substances, and Alcohol: A Comprehensive Review. J. Gen. Intern. Med. 2021, 36, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Buchtova, T.; Lukac, D.; Skrott, Z.; Chroma, K.; Bartek, J.; Mistrik, M. Drug–Drug Interactions of Cannabidiol with Standard-of-Care Chemotherapeutics. Int. J. Mol. Sci. 2023, 24, 2885. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kim, J.-K. The Role of Cannabidiol in Liver Disease: A Systemic Review. Int. J. Mol. Sci. 2024, 25, 2370. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Vecchinni Rodríguez, C.M.; Escalona, Y.; Flores-Otero, J. Cannabinoid Receptors and Ligands: Lessons from CNS Disorders and the Quest for Novel Treatment Venues. Adv. Exp. Med. Biol. 2021, 1297, 43–64. [Google Scholar] [CrossRef]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R.B. Adverse Health Effects of Marijuana Use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef]
- Wong, G.; Greenhalgh, T.; Westhorp, G.; Pawson, R. Development of Methodological Guidance, Publication Standards and Training Materials for Realist and Meta-Narrative Reviews: The RAMESES (Realist and Meta-Narrative Evidence Syntheses—Evolving Standards) Project; Health Services and Delivery Research; NIHR Journals Library: Southampton, UK, 2014. [Google Scholar]
- Lee, Y.H. Strengths and Limitations of Meta-Analysis. Korean J. Med. 2019, 94, 391–395. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Sirven, J.I. Epilepsy: A Spectrum Disorder. Cold Spring Harb. Perspect. Med. 2015, 5, a022848. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Heron, S.E.; Scheffer, I.E.; Berkovic, S.F.; Dibbens, L.M.; Mulley, J.C. Channelopathies in Idiopathic Epilepsy. Neurotherapeutics 2007, 4, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Cavirani, B.; Spagnoli, C.; Caraffi, S.G.; Cavalli, A.; Cesaroni, C.A.; Cutillo, G.; De Giorgis, V.; Frattini, D.; Marchetti, G.B.; Masnada, S.; et al. Genetic Epilepsies and Developmental Epileptic Encephalopathies with Early Onset: A Multicenter Study. Int. J. Mol. Sci. 2024, 25, 1248. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-S.; Huang, T.-H.; Lai, M.-C.; Huang, C.-W. The Role of Glutamate Receptors in Epilepsy. Biomedicines 2023, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Costa, R.O.; Duarte, C.B. Alterations in GABAA-Receptor Trafficking and Synaptic Dysfunction in Brain Disorders. Front. Cell. Neurosci. 2019, 13, 77. [Google Scholar] [CrossRef]
- Hanada, T. Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors. Biomolecules 2020, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, E.; Polat, A.K.; Eroglu, E.; Kullu, I.; Angelopoulou, E.; Paudel, Y.N. Revisiting the Role of Neurotransmitters in Epilepsy: An Updated Review. Life Sci. 2021, 265, 118826. [Google Scholar] [CrossRef] [PubMed]
- Sumadewi, K.T.; Harkitasari, S.; Tjandra, D.C. Biomolecular Mechanisms of Epileptic Seizures and Epilepsy: A Review. Acta Epileptol. 2023, 5, 28. [Google Scholar] [CrossRef]
- Foiadelli, T.; Santangelo, A.; Costagliola, G.; Costa, E.; Scacciati, M.; Riva, A.; Volpedo, G.; Smaldone, M.; Bonuccelli, A.; Clemente, A.M.; et al. Neuroinflammation and Status Epilepticus: A Narrative Review Unraveling a Complex Interplay. Front. Pediatr. 2023, 11, 1251914. [Google Scholar] [CrossRef]
- Borowicz-Reutt, K.K.; Czuczwar, S.J. Role of Oxidative Stress in Epileptogenesis and Potential Implications for Therapy. Pharmacol. Rep. 2020, 72, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Henshall, D.C.; Kobow, K. Epigenetics and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022731. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, M.; Uliel-Siboni, S.; Linder, I.; Kramer, U.; Epstein, O.; Menascu, S.; Nissenkorn, A.; Yosef, O.B.; Hyman, E.; Granot, D.; et al. CBD-Enriched Medical Cannabis for Intractable Pediatric Epilepsy: The Current Israeli Experience. Seizure 2016, 35, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Porcari, G.S.; Fu, C.; Doll, E.D.; Carter, E.G.; Carson, R.P. Efficacy of Artisanal Preparations of Cannabidiol for the Treatment of Epilepsy: Practical Experiences in a Tertiary Medical Center. Epilepsy Behav. 2018, 80, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, F.A.; da Silva, L.R.; Coan, A.C. Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-Analysis. Front. Neurol. 2018, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Dravet, C.; Oguni, H. Dravet Syndrome (Severe Myoclonic Epilepsy in Infancy). Handb. Clin. Neurol. 2013, 111, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.; Gozal, D.; Carney, P. Channelopathy of Dravet Syndrome and Potential Neuroprotective Effects of Cannabidiol. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211048045. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S.; Cannabidiol in Dravet Syndrome Study Group. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.A.; Whalley, B.J. The Proposed Mechanisms of Action of CBD in Epilepsy. Epileptic Disord. 2020, 22, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Sharir, H.; Abood, M.E. Pharmacological Characterization of GPR55, a Putative Cannabinoid Receptor. Pharmacol. Ther. 2010, 126, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.C.; Chamberland, S.; Bazelot, M.; Nebet, E.R.; Wang, X.; McKenzie, S.; Jain, S.; Greenhill, S.; Wilson, M.; Marley, N.; et al. Cannabidiol Modulates Excitatory-Inhibitory Ratio to Counter Hippocampal Hyperactivity. Neuron 2023, 111, 1282–1300.e8. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Li, J. TRPV1 Receptor Mediates Glutamatergic Synaptic Input to Dorsolateral Periaqueductal Gray (Dl-PAG) Neurons. J. Neurophysiol. 2007, 97, 503–511. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Ribolsi, M.; Kusayanagi, H.; Monteleone, F.; Mantovani, V.; Buttari, F.; Marasco, E.; Bernardi, G.; Maccarrone, M.; Centonze, D. TRPV1 Channels Regulate Cortical Excitability in Humans. J. Neurosci. 2012, 32, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.-J.; Guo, W.; Zheng, D.-H.; Zhang, C.-Q.; Li, S.; Liu, S.-Y.; Yin, Q.; Yang, H.; Shu, H.-F. Increased Expression of TRPV1 in the Cortex and Hippocampus from Patients with Mesial Temporal Lobe Epilepsy. J. Mol. Neurosci. 2013, 49, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- The Effect of a Pharmaceutical Formulation of Pure Cannabidiol on Human CNS Expressed Voltage Gated Sodium Channels. Available online: https://aesnet.org/abstractslisting/the-effect-of-a-pharmaceutical-formulation-of-pure-cannabidiol-on-human-cns-expressed-voltage-gated-sodium-channels (accessed on 9 April 2024).
- Dunwiddie, T.V. Endogenously Released Adenosine Regulates Excitability in the in Vitro Hippocampus. Epilepsia 1980, 21, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Chen, J.-F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.-M. Adenosine and Brain Function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, A.M.; Ribeiro, J.A. Adenosine Receptors and the Central Nervous System. In Adenosine Receptors in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2009; pp. 471–534. [Google Scholar] [CrossRef]
- Lotankar, S.; Prabhavalkar, K.S.; Bhatt, L.K. Biomarkers for Parkinson’s Disease: Recent Advancement. Neurosci. Bull. 2017, 33, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, D.M.; Goyal, V. Parkinson’s Disease: A Review. Neurol. India 2018, 66, S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s Disease across North America. NPJ Park. Dis. 2018, 4, 21. [Google Scholar] [CrossRef]
- Surmeier, D.J. Determinants of Dopaminergic Neuron Loss in Parkinson’s Disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-Synuclein in Parkinson’s Disease and Other Synucleinopathies: From Overt Neurodegeneration Back to Early Synaptic Dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease—Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Kong, J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Isik, S.; Yeman Kiyak, B.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Fakhari, D.; Cantuti-Castelvetri, I.; Fan, Z.; Rockenstein, E.; Masliah, E.; Hyman, B.T.; McLean, P.J.; Unni, V.K. Distinct Roles In Vivo for the Ubiquitin–Proteasome System and the Autophagy–Lysosomal Pathway in the Degradation of α-Synuclein. J. Neurosci. 2011, 31, 14508–14520. [Google Scholar] [CrossRef]
- Ihara, Y.; Morishima-Kawashima, M.; Nixon, R. The Ubiquitin–Proteasome System and the Autophagic–Lysosomal System in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006361. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Cardoso, I.L.; Teixeira, D.; Cardoso, I.L. Genes Involved in the Development of Parkinson. Open J. Park. Dis. Treat. 2017, 1, 39–51. [Google Scholar] [CrossRef]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; DiFrancisco-Donoghue, J. Cannabidiol and Tetrahydrocannabinol Use in Parkinson’s Disease: An Observational Pilot Study. Cureus 2023, 15, e42391. [Google Scholar] [CrossRef] [PubMed]
- Venderová, K.; Růzicka, E.; Vorísek, V.; Visnovský, P. Survey on Cannabis Use in Parkinson’s Disease: Subjective Improvement of Motor Symptoms. Mov. Disord. 2004, 19, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Choi, D.-K. Promising Cannabinoid-Based Therapies for Parkinson’s Disease: Motor Symptoms to Neuroprotection. Mol. Neurodegener. 2015, 10, 17. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Sancesario, A.; Morace, R.; Centonze, D.; Iezzi, E. Cannabinoids in Parkinson’s Disease. Cannabis Cannabinoid Res. 2017, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lotan, I.; Treves, T.A.; Roditi, Y.; Djaldetti, R. Cannabis (Medical Marijuana) Treatment for Motor and Non-Motor Symptoms of Parkinson Disease: An Open-Label Observational Study. Clin. Neuropharmacol. 2014, 37, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Segovia, G.; Mora, F.; Crossman, A.R.; Brotchie, J.M. Effects of CB1 Cannabinoid Receptor Modulating Compounds on the Hyperkinesia Induced by High-Dose Levodopa in the Reserpine-Treated Rat Model of Parkinson’s Disease. Mov. Disord. 2003, 18, 138–149. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.A.M.; Vanwersch, R.A.P.; Jongsma, M.J.; Olivier, B.; Philippens, I.H.C.H.M. Therapeutic Effects of Delta9-THC and Modafinil in a Marmoset Parkinson Model. Eur. Neuropsychopharmacol. 2008, 18, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, A.C.; Malenka, R.C. Endocannabinoid-Mediated Rescue of Striatal LTD and Motor Deficits in Parkinson’s Disease Models. Nature 2007, 445, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Brotchie, J.M. Adjuncts to Dopamine Replacement: A Pragmatic Approach to Reducing the Problem of Dyskinesia in Parkinson’s Disease. Mov. Disord. 1998, 13, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Espejo, E.; Caraballo, I.; Rodriguez de Fonseca, F.; Ferrer, B.; Banoua, F.E.; Flores, J.A.; Galan-Rodriguez, B. Experimental Parkinsonism Alters Anandamide Precursor Synthesis, and Functional Deficits Are Improved by AM404: A Modulator of Endocannabinoid Function. Neuropsychopharmacol 2004, 29, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- García-Arencibia, M.; Ferraro, L.; Tanganelli, S.; Fernández-Ruiz, J. Enhanced Striatal Glutamate Release after the Administration of Rimonabant to 6-Hydroxydopamine-Lesioned Rats. Neurosci. Lett. 2008, 438, 10–13. [Google Scholar] [CrossRef] [PubMed]
- van der Stelt, M.; Fox, S.H.; Hill, M.; Crossman, A.R.; Petrosino, S.; Di Marzo, V.; Brotchie, J.M. A Role for Endocannabinoids in the Generation of Parkinsonism and Levodopa-Induced Dyskinesia in MPTP-Lesioned Non-Human Primate Models of Parkinson’s Disease. FASEB J. 2005, 19, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Espejo, E.; Caraballo, I.; de Fonseca, F.R.; El Banoua, F.; Ferrer, B.; Flores, J.A.; Galan-Rodriguez, B. Cannabinoid CB1 Antagonists Possess Antiparkinsonian Efficacy Only in Rats with Very Severe Nigral Lesion in Experimental Parkinsonism. Neurobiol. Dis. 2005, 18, 591–601. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Scorticati, C.; García-Arencibia, M.; de Miguel, R.; Ramos, J.A.; Fernández-Ruiz, J. Effects of Rimonabant, a Selective Cannabinoid CB1 Receptor Antagonist, in a Rat Model of Parkinson’s Disease. Brain Res. 2006, 1073–1074, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Hill, M.P.; Bisogno, T.; Crossman, A.R.; Brotchie, J.M. Enhanced Levels of Endogenous Cannabinoids in the Globus Pallidus Are Associated with a Reduction in Movement in an Animal Model of Parkinson’s Disease. FASEB J. 2000, 14, 1432–1438. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Cebeira, M.; de Ceballos, M.L.; Zeng, B.Y.; Jenner, P.; Ramos, J.A.; Fernández-Ruiz, J.J. Increased Cannabinoid CB1 Receptor Binding and Activation of GTP-Binding Proteins in the Basal Ganglia of Patients with Parkinson’s Syndrome and of MPTP-Treated Marmosets. Eur. J. Neurosci. 2001, 14, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Luquin, N.; Rico, A.J.; Gómez-Bautista, V.; Roda, E.; Dopeso-Reyes, I.G.; Vázquez, A.; Martínez-Pinilla, E.; Labandeira-García, J.L.; Franco, R.; et al. Detection of Cannabinoid Receptors CB1 and CB2 within Basal Ganglia Output Neurons in Macaques: Changes Following Experimental Parkinsonism. Brain Struct. Funct. 2015, 220, 2721–2738. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.Y.; Dass, B.; Owen, A.; Rose, S.; Cannizzaro, C.; Tel, B.C.; Jenner, P. Chronic L-DOPA Treatment Increases Striatal Cannabinoid CB1 Receptor mRNA Expression in 6-Hydroxydopamine-Lesioned Rats. Neurosci. Lett. 1999, 276, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H.; Henry, B.; Hill, M.; Crossman, A.; Brotchie, J. Stimulation of Cannabinoid Receptors Reduces Levodopa-Induced Dyskinesia in the MPTP-Lesioned Nonhuman Primate Model of Parkinson’s Disease. Mov. Disord. 2002, 17, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, B.; Asbrock, N.; Kathuria, S.; Piomelli, D.; Giuffrida, A. Effects of Levodopa on Endocannabinoid Levels in Rat Basal Ganglia: Implications for the Treatment of Levodopa-Induced Dyskinesias. Eur. J. Neurosci. 2003, 18, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Morgese, M.G.; Cassano, T.; Cuomo, V.; Giuffrida, A. Anti-Dyskinetic Effects of Cannabinoids in a Rat Model of Parkinson’s Disease: Role of CB1 and TRPV1 Receptors. Exp. Neurol. 2007, 208, 110–119. [Google Scholar] [CrossRef]
- Alex, M.; Teresa, M.; Grazia, M.M.; Trabace, L.; Andrea, G. The Cannabinoid Agonist WIN55212-2 Decreases L-DOPA-Induced PKA Activation and Dyskinetic Behavior in 6-OHDA-Treated Rats. Neurosci. Res. 2012, 72, 236–242. [Google Scholar] [CrossRef]
- Walsh, S.; Gorman, A.M.; Finn, D.P.; Dowd, E. The Effects of Cannabinoid Drugs on Abnormal Involuntary Movements in Dyskinetic and Non-Dyskinetic 6-Hydroxydopamine Lesioned Rats. Brain Res. 2010, 1363, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.A.; Morgese, M.G.; Pisanu, A.; Macheda, T.; Paquette, M.A.; Seillier, A.; Cassano, T.; Carta, A.R.; Giuffrida, A. Activation of PPAR Gamma Receptors Reduces Levodopa-Induced Dyskinesias in 6-OHDA-Lesioned Rats. Neurobiol. Dis. 2015, 74, 295–304. [Google Scholar] [CrossRef] [PubMed]
- González-Aparicio, R.; Moratalla, R. Oleoylethanolamide Reduces L-DOPA-Induced Dyskinesia via TRPV1 Receptor in a Mouse Model of Parkinson’s Disease. Neurobiol. Dis. 2014, 62, 416–425. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-C.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Herrmann, N.; Ruthirakuhan, M.; Gallagher, D.; Verhoeff, N.P.L.G.; Kiss, A.; Black, S.E.; Lanctôt, K.L. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am. J. Geriatr. Psychiatry 2019, 27, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, G.A.; Tobben, L.; Ahmed, A.I.; Verkes, R.J.; Kramers, C.; Marijnissen, R.M.; Olde Rikkert, M.G.; van der Marck, M.A. Effects of Tetrahydrocannabinol on Balance and Gait in Patients with Dementia: A Randomised Controlled Crossover Trial. J. Psychopharmacol. 2017, 31, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; De Filippis, D.; Maiuri, M.C.; De Stefano, D.; Carnuccio, R.; Iuvone, T. Cannabidiol Inhibits Inducible Nitric Oxide Synthase Protein Expression and Nitric Oxide Production in Beta-Amyloid Stimulated PC12 Neurons through P38 MAP Kinase and NF-kappaB Involvement. Neurosci. Lett. 2006, 399, 91–95. [Google Scholar] [CrossRef]
- Libro, R.; Diomede, F.; Scionti, D.; Piattelli, A.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Expression of Alzheimer’s Disease-Related Genes in Mesenchymal Stem Cells. Int. J. Mol. Sci. 2016, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol Promotes Amyloid Precursor Protein Ubiquitination and Reduction of Beta Amyloid Expression in SHSY5YAPP+ Cells through PPARγ Involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.K.; de Freitas, B.S.; da Silva Dornelles, A.; Nery, L.R.; Falavigna, L.; Ferreira, R.D.P.; Bogo, M.R.; Hallak, J.E.C.; Zuardi, A.W.; Crippa, J.A.S.; et al. Cannabidiol Normalizes Caspase 3, Synaptophysin, and Mitochondrial Fission Protein DNM1L Expression Levels in Rats with Brain Iron Overload: Implications for Neuroprotection. Mol. Neurobiol. 2014, 49, 222–233. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.K.; de Freitas, B.S.; Garcia, R.C.L.; Monteiro, R.T.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.S.; Schröder, N. Antiapoptotic Effects of Cannabidiol in an Experimental Model of Cognitive Decline Induced by Brain Iron Overload. Transl. Psychiatry 2018, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Kepchia, D.; Liang, Z.; Dargusch, R.; Goldberg, J.; Maher, P. Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 7719–7730. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Soriano-Castell, D.; Kepchia, D.; Duggan, B.M.; Currais, A.; Schubert, D.; Maher, P. Cannabinol Inhibits Oxytosis/Ferroptosis by Directly Targeting Mitochondria Independently of Cannabinoid Receptors. Free Radic. Biol. Med. 2022, 180, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Tafti, D.; Ehsan, M.; Xixis, K.L. Multiple Sclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and Genetic Risk Factors for MS: An Integrated Review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef] [PubMed]
- Patsopoulos, N.A.; Barcellos, L.F.; Hintzen, R.Q.; Schaefer, C.; van Duijn, C.M.; Noble, J.A.; Raj, T.; IMSGC; ANZgene; Gourraud, P.-A.; et al. Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects. PLoS Genet. 2013, 9, e1003926. [Google Scholar] [CrossRef]
- Gomez-Gaitan, E.A.; Garcia-Ortega, Y.E.; Saldaña-Cruz, A.M.; Contreras-Haro, B.; Gamez-Nava, J.I.; Perez-Guerrero, E.E.; Nava-Valdivia, C.A.; Gallardo-Moya, S.; Martinez-Hernandez, A.; Gonzalez Lopez, L.; et al. Genetic Variant HLA-DRB1*0403 and Therapeutic Response to Disease-Modifying Therapies in Multiple Sclerosis: A Case-Control Study. Int. J. Mol. Sci. 2023, 24, 14594. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.M.; Hutton, J.; Baecher-Allan, C.; Hafler, D.A. Multiple Sclerosis and Regulatory T Cells. J. Clin. Immunol. 2008, 28, 697–706. [Google Scholar] [CrossRef]
- Trapp, B.D.; Nave, K.-A. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Dokmak, G.; Karaman, R. The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms. Life 2022, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Kraft, G.H.; Freal, J.E.; Coryell, J.K. Disability, Disease Duration, and Rehabilitation Service Needs in Multiple Sclerosis: Patient Perspectives. Arch. Phys. Med. Rehabil. 1986, 67, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Filippini, G.; Minozzi, S.; Borrelli, F.; Cinquini, M.; Dwan, K. Cannabis and Cannabinoids for Symptomatic Treatment for People with Multiple Sclerosis. Cochrane Database Syst. Rev. 2022, 5, CD013444. [Google Scholar] [CrossRef]
- Rainka, M.M.; Aladeen, T.S.; Mattle, A.G.; Lewandowski, E.; Vanini, D.; McCormack, K.; Mechtler, L. Multiple Sclerosis and Use of Medical Cannabis: A Retrospective Review of a Neurology Outpatient Population. Int. J. MS Care 2023, 25, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.P.; Hobart, J.C.; Slade, A.; Barnes, D.; Mattison, P.G.; MUSEC Research Group. Multiple Sclerosis and Extract of Cannabis: Results of the MUSEC Trial. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Afshar, B.; Khalifehzadeh-Esfahani, Z.; Seyfizadeh, N.; Rezaei Danbaran, G.; Hemmatzadeh, M.; Mohammadi, H. The Role of Immune Regulatory Molecules in Multiple Sclerosis. J. Neuroimmunol. 2019, 337, 577061. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Jian, C.; Liao, Y.; Huang, Q.; Wu, Y.; Liu, X.; Zou, D.; Wu, Y. The Role of Microglia in Multiple Sclerosis. Neuropsychiatr. Dis. Treat. 2017, 13, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 Receptor and Its Role as a Regulator of Inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W.; Newton, C.; Zhu, W.; Daaka, Y.; Friedman, H. Delta 9-Tetrahydrocannabinol, Cytokines, and Immunity to Legionella Pneumophila. Proc. Soc. Exp. Biol. Med. 1995, 209, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Derocq, J.M.; Ségui, M.; Marchand, J.; Le Fur, G.; Casellas, P. Cannabinoids Enhance Human B-Cell Growth at Low Nanomolar Concentrations. FEBS Lett. 1995, 369, 177–182. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.H.; Haskó, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of Cannabinoid Receptor 2 Attenuates Leukocyte-Endothelial Cell Interactions and Blood-Brain Barrier Dysfunction under Inflammatory Conditions. J. Neurosci. 2012, 32, 4004–4016. [Google Scholar] [CrossRef]
- Pacher, P.; Mechoulam, R. Is Lipid Signaling through Cannabinoid 2 Receptors Part of a Protective System? Prog. Lipid Res. 2011, 50, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Dulamea, A.O. Role of Oligodendrocyte Dysfunction in Demyelination, Remyelination and Neurodegeneration in Multiple Sclerosis. Adv. Exp. Med. Biol. 2017, 958, 91–127. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.; Scaroni, F.; Gabrielli, M.; Raffaele, S.; Bonfanti, E.; Filipello, F.; Giussani, P.; Picciolini, S.; de Rosbo, N.K.; Uccelli, A.; et al. Extracellular Vesicles Released by Microglia and Macrophages Carry Endocannabinoids Which Foster Oligodendrocyte Differentiation. Front. Immunol. 2024, 15, 1331210. [Google Scholar] [CrossRef] [PubMed]
- van der Stelt, M.; Veldhuis, W.B.; Bär, P.R.; Veldink, G.A.; Vliegenthart, J.F.G.; Nicolay, K. Neuroprotection by Δ9-Tetrahydrocannabinol, the Main Active Compound in Marijuana, against Ouabain-Induced In Vivo Excitotoxicity. J. Neurosci. 2001, 21, 6475–6479. [Google Scholar] [CrossRef] [PubMed]
- Kubiliene, A.; Mickute, K.; Baranauskaite, J.; Marksa, M.; Liekis, A.; Sadauskiene, I. The Effects of Cannabis Sativa L. Extract on Oxidative Stress Markers In Vivo. Life 2021, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Guilhaumou, R.; Micallef, J.; Bruneteau, G.; Desnuelle, C.; Blin, O. Cannabis for the Treatment of Amyotrophic Lateral Sclerosis: What Is the Patients’ View? Rev. Neurol. 2023, 179, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Štětkářová, I.; Ehler, E. Diagnostics of Amyotrophic Lateral Sclerosis: Up to Date. Diagnostics 2021, 11, 231. [Google Scholar] [CrossRef]
- Doi, Y.; Atsuta, N.; Sobue, G.; Morita, M.; Nakano, I. Prevalence and Incidence of Amyotrophic Lateral Sclerosis in Japan. J. Epidemiol. 2014, 24, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Riva, N.; Mora, G.; Sorarù, G.; Lunetta, C.; Ferraro, O.E.; Falzone, Y.; Leocani, L.; Fazio, R.; Comola, M.; Comi, G.; et al. Safety and Efficacy of Nabiximols on Spasticity Symptoms in Patients with Motor Neuron Disease (CANALS): A Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2019, 18, 155–164. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, C.S.; Li, D.; Wilton, S.D.; Aung-Htut, M.T. Polyglutamine Ataxias: Our Current Molecular Understanding and What the Future Holds for Antisense Therapies. Biomedicines 2021, 9, 1499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Handley, R.R.; Lehnert, K.; Snell, R.G. From Pathogenesis to Therapeutics: A Review of 150 Years of Huntington’s Disease Research. Int. J. Mol. Sci. 2023, 24, 13021. [Google Scholar] [CrossRef] [PubMed]
- Martí-Martínez, S.; Valor, L.M. A Glimpse of Molecular Biomarkers in Huntington’s Disease. Int. J. Mol. Sci. 2022, 23, 5411. [Google Scholar] [CrossRef]
- Fiszer, A.; Nowak, B. Strategie wyciszania ekspresji zmutowanego genu w terapii choroby Huntingtona: Inhibicja genu huntingtyny. Postępy Biochem. 2020, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef]
- Polyglutamine (PolyQ) Diseases: Genetics to Treatments. Available online: https://journals.sagepub.com/doi/epub/10.3727/096368914x678454 (accessed on 25 January 2024).
- Aguareles, J.; Paraíso-Luna, J.; Palomares, B.; Bajo-Grañeras, R.; Navarrete, C.; Ruiz-Calvo, A.; García-Rincón, D.; García-Taboada, E.; Guzmán, M.; Muñoz, E.; et al. Oral Administration of the Cannabigerol Derivative VCE-003.2 Promotes Subventricular Zone Neurogenesis and Protects against Mutant Huntingtin-Induced Neurodegeneration. Transl. Neurodegener. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Valdeolivas, S.; Navarrete, C.; Cantarero, I.; Bellido, M.L.; Muñoz, E.; Sagredo, O. Neuroprotective Properties of Cannabigerol in Huntington’s Disease: Studies in R6/2 Mice and 3-Nitropropionate-Lesioned Mice. Neurotherapeutics 2015, 12, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; Murphy, A.J.; England, T.J.; O’Sullivan, S.E. A Systematic Review of Minor Phytocannabinoids with Promising Neuroprotective Potential. Br. J. Pharmacol. 2020, 177, 4330–4352. [Google Scholar] [CrossRef] [PubMed]
- Parrella, N.-F.; Hill, A.T.; Enticott, P.G.; Barhoun, P.; Bower, I.S.; Ford, T.C. A Systematic Review of Cannabidiol Trials in Neurodevelopmental Disorders. Pharmacol. Biochem. Behav. 2023, 230, 173607. [Google Scholar] [CrossRef]
- Márquez-Caraveo, M.E.; Rodríguez-Valentín, R.; Pérez-Barrón, V.; Vázquez-Salas, R.A.; Sánchez-Ferrer, J.C.; De Castro, F.; Allen-Leigh, B.; Lazcano-Ponce, E. Children and Adolescents with Neurodevelopmental Disorders Show Cognitive Heterogeneity and Require a Person-Centered Approach. Sci. Rep. 2021, 11, 18463. [Google Scholar] [CrossRef] [PubMed]
- Barchel, D.; Stolar, O.; De-Haan, T.; Ziv-Baran, T.; Saban, N.; Fuchs, D.O.; Koren, G.; Berkovitch, M. Oral Cannabidiol Use in Children with Autism Spectrum Disorder to Treat Related Symptoms and Co-Morbidities. Front. Pharmacol. 2018, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Cayam-Rand, D. Medical Cannabis in Children. Rambam. Maimonides Med. J. 2020, 11, e0003. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, F.D.; Pimenta, S.; Soares, S.; Gonzaga, D.; Vaz-Matos, I.; Prior, C. El Papel de Los Cannabinoides En Los Trastornos Del Neurodesarrollo de Niños y Adolescentes. Rev. Neurol. 2022, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Magnus, W.; Nazir, S.; Anilkumar, A.C.; Shaban, K. Attention Deficit Hyperactivity Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Turiaco, F.; Cullotta, C.; Mannino, F.; Bruno, A.; Squadrito, F.; Pallio, G.; Irrera, N. Attention Deficit Hyperactivity Disorder (ADHD) and Polyphenols: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1536. [Google Scholar] [CrossRef]
- Cooper, R.E.; Williams, E.; Seegobin, S.; Tye, C.; Kuntsi, J.; Asherson, P. Cannabinoids in Attention-Deficit/Hyperactivity Disorder: A Randomised-Controlled Trial. Eur. Neuropsychopharmacol. 2017, 27, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.; Casey, B.J.; van Erp, T.G.M.; Tamm, L.; Epstein, J.N.; Buss, C.; Bjork, J.M.; Molina, B.S.G.; Velanova, K.; Mathalon, D.H.; et al. ADHD and Cannabis Use in Young Adults Examined Using fMRI of a Go/NoGo Task. Brain Imaging Behav. 2016, 10, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Bossong, M.G.; Mehta, M.A.; van Berckel, B.N.M.; Howes, O.D.; Kahn, R.S.; Stokes, P.R.A. Further Human Evidence for Striatal Dopamine Release Induced by Administration of ∆9-Tetrahydrocannabinol (THC): Selectivity to Limbic Striatum. Psychopharmacology 2015, 232, 2723–2729. [Google Scholar] [CrossRef] [PubMed]
- Bossong, M.G.; van Berckel, B.N.M.; Boellaard, R.; Zuurman, L.; Schuit, R.C.; Windhorst, A.D.; van Gerven, J.M.A.; Ramsey, N.F.; Lammertsma, A.A.; Kahn, R.S. Delta 9-Tetrahydrocannabinol Induces Dopamine Release in the Human Striatum. Neuropsychopharmacology 2009, 34, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Voruganti, L.N.; Slomka, P.; Zabel, P.; Mattar, A.; Awad, A.G. Cannabis Induced Dopamine Release: An in-Vivo SPECT Study. Psychiatry Res. 2001, 107, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Kollins, S.H.; Wigal, T.L.; Newcorn, J.H.; Telang, F.W.; Fowler, J.S.; Logan, J.; Wong, C.T.; et al. Methylphenidate-Elicited Dopamine Increases in Ventral Striatum Are Associated with Long-Term Symptom Improvement in Adults with Attention Deficit Hyperactivity Disorder. J. Neurosci. 2012, 32, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Stokes, P.R.A.; Egerton, A.; Watson, B.; Reid, A.; Breen, G.; Lingford-Hughes, A.; Nutt, D.J.; Mehta, M.A. Significant Decreases in Frontal and Temporal [11C]-Raclopride Binding after THC Challenge. Neuroimage 2010, 52, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Barkus, E.; Morrison, P.D.; Vuletic, D.; Dickson, J.C.; Ell, P.J.; Pilowsky, L.S.; Brenneisen, R.; Holt, D.W.; Powell, J.; Kapur, S.; et al. Does Intravenous Δ9-Tetrahydrocannabinol Increase Dopamine Release? A SPET Study. J. Psychopharmacol. 2011, 25, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Strohbeck-Kühner, P.; Skopp, G.; Mattern, R. Cannabis Improves Symptoms of ADHD. Cannabinoids 2008, 3, 1–3. [Google Scholar]
- Hupli, A.M.M. Medical Cannabis for Adult Attention Deficit Hyperactivity Disorder: Sociological Patient Case Report of Cannabinoid Therapeutics in Finland. Med. Cannabis Cannabinoids 2019, 1, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Motlani, V.; Motlani, G.; Thool, A. Asperger Syndrome (AS): A Review Article. Cureus 2022, 14, e31395. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, B.; Gérardin, P. Asperger’s Syndrome: What to Consider? Encephale 2019, 45, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Cassuto, H.; Lubotzky, A.; Wattad, N.; Hazan, E. Brief Report: Cannabidiol-Rich Cannabis in Children with Autism Spectrum Disorder and Severe Behavioral Problems-A Retrospective Feasibility Study. J. Autism. Dev. Disord. 2019, 49, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Fleury-Teixeira, P.; Caixeta, F.V.; Ramires da Silva, L.C.; Brasil-Neto, J.P.; Malcher-Lopes, R. Effects of CBD-Enriched Cannabis Sativa Extract on Autism Spectrum Disorder Symptoms: An Observational Study of 18 Participants Undergoing Compassionate Use. Front. Neurol. 2019, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Bar-Lev Schleider, L.; Mechoulam, R.; Saban, N.; Meiri, G.; Novack, V. Real Life Experience of Medical Cannabis Treatment in Autism: Analysis of Safety and Efficacy. Sci. Rep. 2019, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Kwan Cheung, K.A.; Mitchell, M.D.; Heussler, H.S. Cannabidiol and Neurodevelopmental Disorders in Children. Front. Psychiatry 2021, 12, 643442. [Google Scholar] [CrossRef] [PubMed]
- Panayotis, N.; Ehinger, Y.; Felix, M.S.; Roux, J.-C. State-of-the-Art Therapies for Rett Syndrome. Dev. Med. Child. Neurol. 2023, 65, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Chahil, G.; Bollu, P.C. Rett Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Desnous, B.; Beretti, T.; Muller, N.; Neveu, J.; Villeneuve, N.; Lépine, A.; Daquin, G.; Milh, M. Efficacy and Tolerance of Cannabidiol in the Treatment of Epilepsy in Patients with Rett Syndrome. Epilepsia Open 2024, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.M.; Downey, L.; Conboy, M.; Finn, D.P.; Roche, M. Alterations in the Endocannabinoid System in the Rat Valproic Acid Model of Autism. Behav. Brain Res. 2013, 249, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Karhson, D.S.; Krasinska, K.M.; Dallaire, J.A.; Libove, R.A.; Phillips, J.M.; Chien, A.S.; Garner, J.P.; Hardan, A.Y.; Parker, K.J. Plasma Anandamide Concentrations Are Lower in Children with Autism Spectrum Disorder. Mol. Autism 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Servadio, M.; Melancia, F.; Manduca, A.; di Masi, A.; Schiavi, S.; Cartocci, V.; Pallottini, V.; Campolongo, P.; Ascenzi, P.; Trezza, V. Targeting Anandamide Metabolism Rescues Core and Associated Autistic-like Symptoms in Rats Prenatally Exposed to Valproic Acid. Transl. Psychiatry 2016, 6, e902. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.M.; Macrì, S.; Laviola, G. Critical Age Windows for Neurodevelopmental Psychiatric Disorders: Evidence from Animal Models. Neurotox. Res. 2011, 19, 286–307. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Casanova, M.F.; Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Brown, G.L.; Edelson, S.M.; Slattery, J.C.; Adams, J.B. Neuropathological Mechanisms of Seizures in Autism Spectrum Disorder. Front. Neurosci. 2016, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Cifelli, P.; Ruffolo, G.; De Felice, E.; Alfano, V.; van Vliet, E.A.; Aronica, E.; Palma, E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? Int. J. Mol. Sci. 2020, 21, 723. [Google Scholar] [CrossRef]
- Silvestro, S.; Mammana, S.; Cavalli, E.; Bramanti, P.; Mazzon, E. Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules 2019, 24, 1459. [Google Scholar] [CrossRef]
- Lai, M.-C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of Co-Occurring Mental Health Diagnoses in the Autism Population: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol Induces Rapid-Acting Antidepressant-like Effects and Enhances Cortical 5-HT/Glutamate Neurotransmission: Role of 5-HT1A Receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Oluwabusi, O.O.; Parke, S.; Ambrosini, P.J. Tourette Syndrome Associated with Attention Deficit Hyperactivity Disorder: The Impact of Tics and Psychopharmacological Treatment Options. World J. Clin. Pediatr. 2016, 5, 128–135. [Google Scholar] [CrossRef]
- Set, K.K.; Warner, J.N. Tourette Syndrome in Children: An Update. Curr. Probl. Pediatr. Adolesc. Health Care 2021, 51, 101032. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Schneider, U.; Koblenz, A.; Jöbges, M.; Kolbe, H.; Daldrup, T.; Emrich, H.M. Treatment of Tourette’s Syndrome with Delta 9-Tetrahydrocannabinol (THC): A Randomized Crossover Trial. Pharmacopsychiatry 2002, 35, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.; Zalomek, C.; Korczyn, A.D.; Rosenberg, A.; Giladi, N.; Gurevich, T. Medical Cannabis for Gilles de La Tourette Syndrome: An Open-Label Prospective Study. Behav. Neurol. 2022, 2022, 5141773. [Google Scholar] [CrossRef] [PubMed]
- Abi-Jaoude, E.; Bhikram, T.; Parveen, F.; Levenbach, J.; Lafreniere-Roula, M.; Sandor, P. A Double-Blind, Randomized, Controlled Crossover Trial of Cannabis in Adults with Tourette Syndrome. Cannabis Cannabinoid Res. 2023, 8, 835–845. [Google Scholar] [CrossRef] [PubMed]
- López-Ramírez, G.; Sánchez-Zavaleta, R.; Ávalos-Fuentes, A.; José Sierra, J.; Paz-Bermúdez, F.; Leyva-Gómez, G.; Segovia Vila, J.; Cortés, H.; Florán, B. D2 Autoreceptor Switches CB2 Receptor Effects on [3H]-Dopamine Release in the Striatum. Synapse 2020, 74, e22139. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-P.; Onaivi, E.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.; Brusco, A.; Uhl, G. Cannabinoid CB2 Receptors: Immunohistochemical Localization in Rat Brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Lanciego, J.; Barroso-Chinea, P.; Rico, A.J.; Conte, L.; Callén, L.; Roda, E.; Gómez-Bautista, V.; Lopez, I.; Lluis, C.; Labandeira-Garcia, J.; et al. Expression of the mRNA Coding the Cannabinoid Receptor 2 in the Pallidal Complex of Macaca Fascicularis. J. Psychopharmacol. 2011, 25, 97–104. [Google Scholar] [CrossRef]
- Bova, A.; Gaidica, M.; Hurst, A.; Iwai, Y.; Hunter, J.; Leventhal, D.K. Precisely Timed Dopamine Signals Establish Distinct Kinematic Representations of Skilled Movements. eLife 2020, 9, e61591. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.D.; Cavazos, C.; Morales, C.A.; Lopez, S.M.; Amodeo, D.A. Impact of Specific Serotonin Receptor Modulation on Restricted Repetitive Behaviors. Front. Behav. Neurosci. 2022, 16, 1078983. [Google Scholar] [CrossRef] [PubMed]
- Gorberg, V.; Borisov, V.; Greig, I.R.; Pertwee, R.G.; McCaffery, P.; Anavi-Goffer, S. Motor-like Tics Are Mediated by CB2 Cannabinoid Receptor-Dependent and Independent Mechanisms Associated with Age and Sex. Mol. Neurobiol. 2022, 59, 5070–5083. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Bindila, L.; Lutz, B.; Musshoff, F.; Skripuletz, T.; Baumgaertel, C.; Sühs, K.-W. Cerebrospinal Fluid Endocannabinoid Levels in Gilles de La Tourette Syndrome. Neuropsychopharmacology 2020, 45, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Depression and Other Common Mental Disorders: Global Health Estimates. 2017. Available online: https://www.afro.who.int/publications/depression-and-other-common-mental-disorders-global-health-estimates-2017 (accessed on 27 February 2024).
- Haller, J. Anxiety Modulation by Cannabinoids—The Role of Stress Responses and Coping. Int. J. Mol. Sci. 2023, 24, 15777. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; McEwen, B.S. Involvement of the Endocannabinoid System in the Neurobehavioural Effects of Stress and Glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The Endocannabinoid System in Guarding against Fear, Anxiety and Stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Aliczki, M.; Haller, J. Interactions between Cannabinoid Signaling and Anxiety: A Comparative Analysis of Intervention Tools and Behavioral Effects; Springer: Berlin/Heidelberg, Germany, 2015; pp. 73–96. [Google Scholar] [CrossRef]
- Barsky, A.J. Defining Psychiatry in Primary Care: Origins, Opportunities, and Obstacles. Compr. Psychiatry 1980, 21, 221–232. [Google Scholar] [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm. J. 2019, 23, 18–041. [Google Scholar] [CrossRef] [PubMed]
- Hundal, H.; Lister, R.; Evans, N.; Antley, A.; Englund, A.; Murray, R.M.; Freeman, D.; Morrison, P.D. The Effects of Cannabidiol on Persecutory Ideation and Anxiety in a High Trait Paranoid Group. J. Psychopharmacol. 2018, 32, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, E.P.; Stevenson, C.W. Cannabinoid Regulation of Fear and Anxiety: An Update. Curr. Psychiatry Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.C.; Bertoglio, L.J.; Guimarães, F.S.; Stevenson, C.W. Cannabidiol Regulation of Emotion and Emotional Memory Processing: Relevance for Treating Anxiety-related and Substance Abuse Disorders. Br. J. Pharmacol. 2017, 174, 3242–3256. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Guimarães, F.S. Involvement of 5HT1A Receptors in the Anxiolytic-like Effects of Cannabidiol Injected into the Dorsolateral Periaqueductal Gray of Rats. Psychopharmacology 2008, 199, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Luoto, S.; Krams, I.; Karlsson, H. Depression Subtyping Based on Evolutionary Psychiatry: Proximate Mechanisms and Ultimate Functions. Brain Behav. Immun. 2018, 69, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, A.; Spanagel, R. Medical Cannabinoids: A Pharmacology-Based Systematic Review and Meta-Analysis for All Relevant Medical Indications. BMC Med. 2022, 20, 259. [Google Scholar] [CrossRef] [PubMed]
- Allsop, D.J.; Copeland, J.; Lintzeris, N.; Dunlop, A.J.; Montebello, M.; Sadler, C.; Rivas, G.R.; Holland, R.M.; Muhleisen, P.; Norberg, M.M.; et al. Nabiximols as an Agonist Replacement Therapy during Cannabis Withdrawal: A Randomized Clinical Trial. JAMA Psychiatry 2014, 71, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.M.; Soliman, A.; Quilty, L.C.; Fischer, B.; Rehm, J.; Selby, P.; Barnes, A.J.; Huestis, M.A.; George, T.P.; Streiner, D.L.; et al. Nabiximols Combined with Motivational Enhancement/Cognitive Behavioral Therapy for the Treatment of Cannabis Dependence: A Pilot Randomized Clinical Trial. PLoS ONE 2018, 13, e0190768. [Google Scholar] [CrossRef]
- Lev-Ran, S.; Roerecke, M.; Le Foll, B.; George, T.P.; McKenzie, K.; Rehm, J. The Association between Cannabis Use and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Psychol. Med. 2014, 44, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Sinclair, J.; Karamacoska, D.; Davidson, M.; Firth, J. Medicinal Cannabis for Psychiatric Disorders: A Clinically-Focused Systematic Review. BMC Psychiatry 2020, 20, 24. [Google Scholar] [CrossRef]
- Bryant, R.A. Post-traumatic Stress Disorder: A State-of-the-art Review of Evidence and Challenges. World Psychiatry 2019, 18, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-Traumatic Stress Disorder. Nat. Rev. Dis. Primers 2015, 1, 15057. [Google Scholar] [CrossRef] [PubMed]
- Jetly, R.; Heber, A.; Fraser, G.; Boisvert, D. The Efficacy of Nabilone, a Synthetic Cannabinoid, in the Treatment of PTSD-Associated Nightmares: A Preliminary Randomized, Double-Blind, Placebo-Controlled Cross-over Design Study. Psychoneuroendocrinology 2015, 51, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Bonn-Miller, M.O.; Sisley, S.; Riggs, P.; Yazar-Klosinski, B.; Wang, J.B.; Loflin, M.J.E.; Shechet, B.; Hennigan, C.; Matthews, R.; Emerson, A.; et al. The Short-Term Impact of 3 Smoked Cannabis Preparations versus Placebo on PTSD Symptoms: A Randomized Cross-over Clinical Trial. PLoS ONE 2021, 16, e0246990. [Google Scholar] [CrossRef] [PubMed]
- Kesner, A.J.; Mateo, Y.; Abrahao, K.P.; Ramos-Maciel, S.; Pava, M.J.; Gracias, A.L.; Paulsen, R.T.; Carlson, H.B.; Lovinger, D.M. Changes in Striatal Dopamine Release, Sleep, and Behavior during Spontaneous Δ-9-Tetrahydrocannabinol Abstinence in Male and Female Mice. Neuropsychopharmacology 2022, 47, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Goodwin, R.S.; Schwilke, E.; Schroeder, J.R.; Schwope, D.M.; Kelly, D.L.; Ortemann-Renon, C.; Bonnet, D.; Huestis, M.A. Around-the-Clock Oral THC Effects on Sleep in Male Chronic Daily Cannabis Smokers. Am. J. Addict. 2013, 22, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Pandi-Perumal, S.R. Clinical Management of Sleep and Sleep Disorders with Cannabis and Cannabinoids: Implications to Practicing Psychiatrists. Clin. Neuropharmacol. 2022, 45, 27. [Google Scholar] [CrossRef] [PubMed]
- Kolla, B.P.; Hayes, L.; Cox, C.; Eatwell, L.; Deyo-Svendsen, M.; Mansukhani, M.P. The Effects of Cannabinoids on Sleep. J. Prim. Care Community Health 2022, 13, 21501319221081277. [Google Scholar] [CrossRef] [PubMed]
- Kuhathasan, N.; Minuzzi, L.; MacKillop, J.; Frey, B.N. The Use of Cannabinoids for Insomnia in Daily Life: Naturalistic Study. J. Med. Internet Res. 2021, 23, e25730. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gaurkar, S.S. Migraine: An Underestimated Neurological Condition Affecting Billions. Cureus 2022, 14, e28347. [Google Scholar] [CrossRef]
- Silvestro, M.; Iannone, L.F.; Orologio, I.; Tessitore, A.; Tedeschi, G.; Geppetti, P.; Russo, A. Migraine Treatment: Towards New Pharmacological Targets. Int. J. Mol. Sci. 2023, 24, 12268. [Google Scholar] [CrossRef]
- Pescador Ruschel, M.A.; De Jesus, O. Migraine Headache. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Stith, S.S.; Diviant, J.P.; Brockelman, F.; Keeling, K.; Hall, B.; Lucern, S.; Vigil, J.M. Alleviative Effects of Cannabis Flower on Migraine and Headache. J. Integr. Med. 2020, 18, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, C.; Spradlin, A.; Cleveland, M.J.; Craft, R.M. Short- and Long-Term Effects of Cannabis on Headache and Migraine. J. Pain 2020, 21, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Francavilla, M.; Demartini, C.; Zanaboni, A.M.; Sodergren, M.H.; Facchetti, S.; Pacchetti, B.; Palmisani, M.; Franco, V.; Tassorelli, C. Characterization of the Biochemical and Behavioral Effects of Cannabidiol: Implications for Migraine. J. Headache Pain 2023, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a Non-Psychotropic Plant-Derived Cannabinoid, Decreases Inflammation in a Murine Model of Acute Lung Injury: Role for the Adenosine A2A Receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Colleoni, M.; Conti, S.; Parolaro, D.; Franke, C.; Trovato, A.E.; Giagnoni, G. Oral Anti-Inflammatory Activity of Cannabidiol, a Non-Psychoactive Constituent of Cannabis, in Acute Carrageenan-Induced Inflammation in the Rat Paw. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 294–299. [Google Scholar] [CrossRef]
- Sepulveda, D.E.; Morris, D.P.; Raup-Konsavage, W.M.; Sun, D.; Vrana, K.E.; Graziane, N.M. Evaluating the Antinociceptive Efficacy of Cannabidiol Alone or in Combination with Morphine Using the Formalin Test in Male and Female Mice. Cannabis Cannabinoid Res. 2022, 7, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Franjo Grotenhermen. Med. Cannabis Cannabinoids 2018, 1, 5. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Dykukha, I.; Malessa, R.; Essner, U.; Überall, M.A. Nabiximols in Chronic Neuropathic Pain: A Meta-Analysis of Randomized Placebo-Controlled Trials. Pain Med. 2021, 22, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Schimrigk, S.; Marziniak, M.; Neubauer, C.; Kugler, E.M.; Werner, G.; Abramov-Sommariva, D. Dronabinol Is a Safe Long-Term Treatment Option for Neuropathic Pain Patients. Eur. Neurol. 2017, 78, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, B.; Marcotte, T.; Deutsch, R.; Gouaux, B.; Sakai, S.; Donaghe, H. Low-Dose Vaporized Cannabis Significantly Improves Neuropathic Pain. J. Pain 2013, 14, 136–148. [Google Scholar] [CrossRef]
- Pertwee, R.G. The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: Δ9-Tetrahydrocannabinol, Cannabidiol and Δ9-Tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Borgonetti, V.; Mugnaini, C.; Corelli, F.; Galeotti, N. The Selective CB2 Agonist COR167 Reduced Symptoms in a Mice Model of Trauma-Induced Peripheral Neuropathy through HDAC-1 Inhibition. Biomedicines 2023, 11, 1546. [Google Scholar] [CrossRef] [PubMed]
- Adamson Barnes, N.S.; Mitchell, V.A.; Kazantzis, N.P.; Vaughan, C.W. Actions of the Dual FAAH/MAGL Inhibitor JZL195 in a Murine Neuropathic Pain Model. Br. J. Pharmacol. 2016, 173, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Johnson, D.S.; Mileni, M.; Beidler, D.; Long, J.Z.; McKinney, M.K.; Weerapana, E.; Sadagopan, N.; Liimatta, M.; Smith, S.E.; et al. Discovery and Characterization of a Highly Selective FAAH Inhibitor That Reduces Inflammatory Pain. Chem. Biol. 2009, 16, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, S.G.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Fatty Acid Amide Hydrolase and Monoacylglycerol Lipase Inhibitors Produce Anti-Allodynic Effects in Mice through Distinct Cannabinoid Receptor Mechanisms. J. Pain 2010, 11, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Ignatowska-Jankowska, B.M.; Ghosh, S.; Crowe, M.S.; Kinsey, S.G.; Niphakis, M.J.; Abdullah, R.A.; Tao, Q.; O’Neal, S.T.; Walentiny, D.M.; Wiley, J.L.; et al. In Vivo Characterization of the Highly Selective Monoacylglycerol Lipase Inhibitor KML29: Antinociceptive Activity without Cannabimimetic Side Effects. Br. J. Pharmacol. 2014, 171, 1392–1407. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, S.G.; Wise, L.E.; Ramesh, D.; Abdullah, R.; Selley, D.E.; Cravatt, B.F.; Lichtman, A.H. Repeated Low-Dose Administration of the Monoacylglycerol Lipase Inhibitor JZL184 Retains Cannabinoid Receptor Type 1-Mediated Antinociceptive and Gastroprotective Effects. J. Pharmacol. Exp. Ther. 2013, 345, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Brotchie, J.M.; Fitzjohn, S.M. Cannabinoids Decrease Corticostriatal Synaptic Transmission via an Effect on Glutamate Uptake. J. Neurosci. 2003, 23, 11073–11077. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacological Actions of Cannabinoids. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–51. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Jin, X.; Jo, S.; Zhang, H.B.; Fujita, A.; Bean, B.P.; Yan, N. Cannabidiol Inhibits Nav Channels through Two Distinct Binding Sites. Nat. Commun. 2023, 14, 3613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-X.B.; Bean, B.P. Cannabidiol Inhibition of Murine Primary Nociceptors: Tight Binding to Slow Inactivated States of Nav1.8 Channels. J. Neurosci. 2021, 41, 6371–6387. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory Effects of Cannabidiol on Voltage-Dependent Sodium Currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef]
- Watkins, A.R. Cannabinoid Interactions with Ion Channels and Receptors. Channels 2019, 13, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Chidiac, C.; Herault, Y.; Gaveriaux-Ruff, C. Pain Behavior in SCN9A (Nav1.7) and SCN10A (Nav1.8) Mutant Rodent Models. Neurosci. Lett. 2021, 753, 135844. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.K.; Souza, I.A.; Gandini, M.A.; Gadotti, V.M.; Ali, M.Y.; Huang, S.; Antunes, F.T.T.; Trang, T.; Zamponi, G.W. Differential Regulation of Cav 3.2 and Cav 2.2 Calcium Channels by CB1 Receptors and Cannabidiol. Br. J. Pharmacol. 2023, 180, 1616–1633. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Nabissi, M.; Santoni, G.; Ligresti, A. Actions and Regulation of Ionotropic Cannabinoid Receptors. Adv. Pharmacol. 2017, 80, 249–289. [Google Scholar] [CrossRef] [PubMed]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The Direct Actions of Cannabidiol and 2-Arachidonoyl Glycerol at GABAA Receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Kossakowski, R.; Schlicker, E.; Toczek, M.; Weresa, J.; Malinowska, B. Cannabidiol Affects the Bezold-Jarisch Reflex via TRPV1 and 5-HT3 Receptors and Has Peripheral Sympathomimetic Effects in Spontaneously Hypertensive and Normotensive Rats. Front. Pharmacol. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Li, T.; Kumari, S.; Wang, S.; Asgar, J.; Chung, M.-K. Capsaicin-Induced Depolymerization of Axonal Microtubules Mediates Analgesia for Trigeminal Neuropathic Pain. Pain 2022, 163, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Gavva, N.R. Therapeutic Potential of Vanilloid Receptor TRPV1 Agonists and Antagonists as Analgesics: Recent Advances and Setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Cui, T.; Cheng, K.; Yang, F.; Chen, S.-R.; Willenbring, D.; Guan, Y.; Pan, H.-L.; Ren, K.; Xu, Y.; et al. Cannabinoids Suppress Inflammatory and Neuropathic Pain by Targeting A3 Glycine Receptors. J. Exp. Med. 2012, 209, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Okine, B.N.; Gaspar, J.C.; Finn, D.P. PPARs and Pain. Br. J. Pharmacol. 2019, 176, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Finn, D.P. Cannabinoids and Pain: Sites and Mechanisms of Action. Adv. Pharmacol. 2017, 80, 437–475. [Google Scholar] [CrossRef] [PubMed]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol Protects an in Vitro Model of the Blood-Brain Barrier from Oxygen-Glucose Deprivation via PPARγ and 5-HT1A Receptors. Br. J. Pharmacol. 2016, 173, 815–825. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. Cannabinoids Go Nuclear: Evidence for Activation of Peroxisome Proliferator-Activated Receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.R.; Gomes, F.I.F.; Lopes, A.H.P.; Cortez, I.L.; Dos Santos, J.C.; Silva, C.E.A.; Mechoulam, R.; Gomes, F.V.; Cunha, T.M.; Guimarães, F.S. The Cannabidiol Analog PECS-101 Prevents Chemotherapy-Induced Neuropathic Pain via PPARγ Receptors. Neurotherapeutics 2022, 19, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Rock, E.M.; Guenther, K.; Limebeer, C.L.; Stevenson, L.A.; Haj, C.; Smoum, R.; Parker, L.A.; Mechoulam, R. Cannabidiolic Acid Methyl Ester, a Stable Synthetic Analogue of Cannabidiolic Acid, Can Produce 5-HT1A Receptor-Mediated Suppression of Nausea and Anxiety in Rats. Br. J. Pharmacol. 2018, 175, 100–112. [Google Scholar] [CrossRef]
- Jesus, C.H.A.; Redivo, D.D.B.; Gasparin, A.T.; Sotomaior, B.B.; de Carvalho, M.C.; Genaro, K.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Zanoveli, J.M.; et al. Cannabidiol Attenuates Mechanical Allodynia in Streptozotocin-Induced Diabetic Rats via Serotonergic System Activation through 5-HT1A Receptors. Brain Res. 2019, 1715, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol Inhibits Paclitaxel-Induced Neuropathic Pain through 5-HT1A Receptors without Diminishing Nervous System Function or Chemotherapy Efficacy. Br. J. Pharmacol. 2014, 171, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C. Δ9-THC and COX-2 Signaling. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 729–738. ISBN 978-0-12-800756-3. [Google Scholar]
- Szczyrba, S.; Kozera, G.; Bieniaszewski, L.; Nyka, W.M. Neuropatia Cukrzycowa—Patogeneza, Rozpoznawanie, Zapobieganie, Leczenie. Forum Med. Rodz. 2010, 4, 339–355. [Google Scholar]
- Yang, K.; Wang, Y.; Li, Y.; Chen, Y.; Xing, N.; Lin, H.; Zhou, P.; Yu, X. Progress in the Treatment of Diabetic Peripheral Neuropathy. Biomed. Pharmacother. 2022, 148, 112717. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.-D. Challenges of Neuropathic Pain: Focus on Diabetic Neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.S.; Marcotte, T.D.; Umlauf, A.; Gouaux, B.; Atkinson, J.H. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J. Pain 2015, 16, 616–627. [Google Scholar] [CrossRef]
- Wallace, M.S.; Marcotte, T.D.; Atkinson, J.H.; Padovano, H.T.; Bonn-Miller, M. A Secondary Analysis from a Randomized Trial on the Effect of Plasma Tetrahydrocannabinol Levels on Pain Reduction in Painful Diabetic Peripheral Neuropathy. J. Pain 2020, 21, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Bagher, A.M. The Endocannabinoid System as a Therapeutic Target in Diabetic Peripheral Neuropathic Pain: A Review. J. Microsc. Ultrastruct. 2021, 10, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Fibromyalgia: Recent Advances in Diagnosis, Classification, Pharmacotherapy and Alternative Remedies. Int. J. Mol. Sci. 2020, 21, 7877. [Google Scholar] [CrossRef]
- Scaturro, D.; Vitagliani, F.; Signa, G.; Tomasello, S.; Tumminelli, L.G.; Picelli, A.; Smania, N.; Letizia Mauro, G. Neck Pain in Fibromyalgia: Treatment with Exercise and Mesotherapy. Biomedicines 2023, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; van Velzen, M. An Experimental Randomized Study on the Analgesic Effects of Pharmaceutical-Grade Cannabis in Chronic Pain Patients with Fibromyalgia. Pain 2019, 160, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Chaves, C.; Bittencourt, P.C.T.; Pelegrini, A. Ingestion of a THC-Rich Cannabis Oil in People with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pain Med. 2020, 21, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Fitzcharles, M.-A.; Joseph, L.; Shir, Y. The Effects of Nabilone on Sleep in Fibromyalgia: Results of a Randomized Controlled Trial. Anesth Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef]
- Anthony, A.T.; Rahmat, S.; Sangle, P.; Sandhu, O.; Khan, S.; Anthony, A.T.; Rahmat, S.; Sangle, P.; Sandhu, O.; Khan, S. Cannabinoid Receptors and Their Relationship with Chronic Pain: A Narrative Review. Cureus 2020, 12, e10436. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Klose, P.; Fitzcharles, M.-A.; Phillips, T.; Häuser, W. Cannabinoids for Fibromyalgia. Cochrane Database Syst. Rev. 2016, 1–26. [Google Scholar] [CrossRef]
- Safakish, R.; Ko, G.; Salimpour, V.; Hendin, B.; Sohanpal, I.; Loheswaran, G.; Yoon, S.Y.R. Medical Cannabis for the Management of Pain and Quality of Life in Chronic Pain Patients: A Prospective Observational Study. Pain Med. 2020, 21, 3073–3086. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, H.; Slitt, A.L.; Seeram, N.P. Inhibitory Effect of Cannabidiol on the Activation of NLRP3 Inflammasome Is Associated with Its Modulation of the P2X7 Receptor in Human Monocytes. J. Nat. Prod. 2020, 83, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells. Front. Physiol. 2016, 7, 559. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Cronstein, B.N. Adenosine: An Endogenous Regulator of Innate Immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gambeta, E.; Chichorro, J.G.; Zamponi, G.W. Trigeminal Neuralgia: An Overview from Pathophysiology to Pharmacological Treatments. Mol. Pain 2020, 16, 1744806920901890. [Google Scholar] [CrossRef]
- Peciu-Florianu, I.; Régis, J.; Levivier, M.; Dedeciusova, M.; Reyns, N.; Tuleasca, C. Trigeminal Neuralgia Secondary to Meningiomas and Vestibular Schwannoma Is Improved after Stereotactic Radiosurgery: A Systematic Review and Meta-Analysis. Stereotact. Funct. Neurosurg. 2021, 99, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Jang, H.-Y.; Won, H.-S.; Kim, J.-S.; Kim, Y.-D. Epidemiology of Trigeminal Neuralgia: An Electronic Population Health Data Study in Korea. Korean J. Pain 2021, 34, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Mechtler, L.; Hart, P.; Bargnes, V.; Saikali, N. Medical Cannabis Treatment in Patients with Trigeminal Neuralgia (P5.10-020). Neurology 2019, 92, 5–10. [Google Scholar] [CrossRef]
- Hill, A.J.; Weston, S.E.; Jones, N.A.; Smith, I.; Bevan, S.A.; Williamson, E.M.; Stephens, G.J.; Williams, C.M.; Whalley, B.J. Δ9-Tetrahydrocannabivarin Suppresses in Vitro Epileptiform and in Vivo Seizure Activity in Adult Rats. Epilepsia 2010, 51, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Palomo-Garo, C.; García-Arencibia, M.; Ramos, J.; Pertwee, R.; Fernández-Ruiz, J. Symptom-Relieving and Neuroprotective Effects of the Phytocannabinoid Δ9-THCV in Animal Models of Parkinson’s Disease. Br. J. Pharmacol. 2011, 163, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Espadas, I.; Keifman, E.; Palomo-Garo, C.; Burgaz, S.; García, C.; Fernández-Ruiz, J.; Moratalla, R. Beneficial Effects of the Phytocannabinoid Δ9-THCV in L-DOPA-Induced Dyskinesia in Parkinson’s Disease. Neurobiol. Dis. 2020, 141, 104892. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M.G.; Zamberletti, E.; Marini, P.; Parolaro, D.; Pertwee, R.G. The Phytocannabinoid, Δ9-Tetrahydrocannabivarin, Can Act through 5-HT1A Receptors to Produce Antipsychotic Effects. Br. J. Pharmacol. 2015, 172, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Jones, N.A.; Smith, I.; Hill, C.L.; Williams, C.M.; Stephens, G.J.; Whalley, B.J. Voltage-Gated Sodium (NaV) Channel Blockade by Plant Cannabinoids Does Not Confer Anticonvulsant Effects per Se. Neurosci. Lett. 2014, 566, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef]
- Fleisher-Berkovich, S.; Ventura, Y.; Amoyal, M.; Dahan, A.; Feinshtein, V.; Alfahel, L.; Israelson, A.; Bernstein, N.; Gorelick, J.; Ben-Shabat, S. Therapeutic Potential of Phytocannabinoid Cannabigerol for Multiple Sclerosis: Modulation of Microglial Activation In Vitro and In Vivo. Biomolecules 2023, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A Cannabigerol Quinone Alleviates Neuroinflammation in a Chronic Model of Multiple Sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cueto, C.; Santos-García, I.; García-Toscano, L.; Espejo-Porras, F.; Bellido, M.; Fernández-Ruiz, J.; Muñoz, E.; de Lago, E. Neuroprotective Effects of the Cannabigerol Quinone Derivative VCE-003.2 in SOD1G93A Transgenic Mice, an Experimental Model of Amyotrophic Lateral Sclerosis. Biochem. Pharmacol. 2018, 157, 217–226. [Google Scholar] [CrossRef]
- Díaz-Alonso, J.; Paraíso-Luna, J.; Navarrete, C.; Del Río, C.; Cantarero, I.; Palomares, B.; Aguareles, J.; Fernández-Ruiz, J.; Bellido, M.L.; Pollastro, F.; et al. VCE-003.2, a Novel Cannabigerol Derivative, Enhances Neuronal Progenitor Cell Survival and Alleviates Symptomatology in Murine Models of Huntington’s Disease. Sci. Rep. 2016, 6, 29789. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carreiro, S.; Navarro, E.; Muñoz, E.; Fernández-Ruiz, J. The Cannabigerol Derivative VCE-003.2 Exerts Therapeutic Effects in 6-Hydroxydopamine-Lesioned Mice: Comparison with The Classic Dopaminergic Replacement Therapy. Brain Sci. 2023, 13, 1272. [Google Scholar] [CrossRef] [PubMed]
- Burgaz, S.; García, C.; Gómez-Cañas, M.; Muñoz, E.; Fernández-Ruiz, J. Development of An Oral Treatment with the PPAR-γ-Acting Cannabinoid VCE-003.2 Against the Inflammation-Driven Neuronal Deterioration in Experimental Parkinson’s Disease. Molecules 2019, 24, 2702. [Google Scholar] [CrossRef]
- García, C.; Gómez-Cañas, M.; Burgaz, S.; Palomares, B.; Gómez-Gálvez, Y.; Palomo-Garo, C.; Campo, S.; Ferrer-Hernández, J.; Pavicic, C.; Navarrete, C.; et al. Benefits of VCE-003.2, a Cannabigerol Quinone Derivative, against Inflammation-Driven Neuronal Deterioration in Experimental Parkinson’s Disease: Possible Involvement of Different Binding Sites at the PPARγ Receptor. J. Neuroinflamm. 2018, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Shinjyo, N.; Di Marzo, V. The Effect of Cannabichromene on Adult Neural Stem/Progenitor Cells. Neurochem. Int. 2013, 63, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Valeri, A.; Chiricosta, L.; D’Angiolini, S.; Pollastro, F.; Salamone, S.; Mazzon, E. Cannabichromene Induces Neuronal Differentiation in NSC-34 Cells: Insights from Transcriptomic Analysis. Life 2023, 13, 742. [Google Scholar] [CrossRef] [PubMed]
- Raup-Konsavage, W.M.; Sepulveda, D.E.; Wang, J.; Dokholyan, N.V.; Vrana, K.E.; Graziane, N.M. Antinociceptive Effects of Cannabichromene (CBC) in Mice: Insights from von Frey, Tail-Flick, Formalin, and Acetone Tests. Biomedicines 2023, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Kim, J.-H.; Han, J.-H.; Ryu, B.-R.; Lim, Y.-S.; Lim, J.-D.; Park, S.-H.; Kim, C.-H.; Lee, S.-U.; Kwon, T.-H. In Vitro and In Vivo Anti-Inflammatory Potential of Cannabichromene Isolated from Hemp. Plants 2023, 12, 3966. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.; Dickson, K.E.; Carty, D.R.; Ashpole, N.M.; Willett, K.L. Cannabis Constituents Reduce Seizure Behavior in Chemically-Induced and Scn1a-Mutant Zebrafish. Epilepsy Behav. 2020, 110, 107152. [Google Scholar] [CrossRef] [PubMed]
- Bourque, J.; Potvin, S. Cannabis and Cognitive Functioning: From Acute to Residual Effects, From Randomized Controlled Trials to Prospective Designs. Front. Psychiatry 2021, 12, 596601. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.L.; Ellingson, J.M.; Bidwell, L.C.; Bryan, A.D.; Hutchison, K.E. Are the Acute Effects of THC Different in Aging Adults? Brain Sci. 2021, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Hasbi, A.; Madras, B.K.; George, S.R. Endocannabinoid System and Exogenous Cannabinoids in Depression and Anxiety: A Review. Brain Sci. 2023, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Bialas, P.; Fitzcharles, M.-A.; Klose, P.; Häuser, W. Long-Term Observational Studies with Cannabis-Based Medicines for Chronic Non-Cancer Pain: A Systematic Review and Meta-Analysis of Effectiveness and Safety. Eur. J. Pain 2022, 26, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Alessandria, G.; Meli, R.; Infante, M.T.; Vestito, L.; Capello, E.; Bandini, F. Long-Term Assessment of the Cognitive Effects of Nabiximols in Patients with Multiple Sclerosis: A Pilot Study. Clin. Neurol. Neurosurg. 2020, 196, 105990. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L. Cannabis and the Brain. Brain 2003, 126, 1252–1270. [Google Scholar] [CrossRef] [PubMed]
- Ronan, P.J.; Wongngamnit, N.; Beresford, T.P. Molecular Mechanisms of Cannabis Signaling in the Brain. In Progress in Molecular Biology and Translational Science; Rahman, S., Ed.; The Molecular Basis of Drug Addiction; Academic Press: Cambridge, MA, USA, 2016; Volume 137, pp. 123–147. [Google Scholar]
- Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals 2021, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Kilaru, A.; Chapman, K.D. The Endocannabinoid System. Essays Biochem. 2020, 64, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Batalla, A.; Bos, J.; Postma, A.; Bossong, M.G. The Impact of Cannabidiol on Human Brain Function: A Systematic Review. Front. Pharmacol. 2021, 11, 618184. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weizman, A.; Weinstein, A. Modulatory Effects of Cannabinoids on Brain Neurotransmission. Eur. J. Neurosci. 2019, 50, 2322–2345. [Google Scholar] [CrossRef]
- Adel, Y.; Alexander, S.P.H. Neuromolecular Mechanisms of Cannabis Action. Adv. Exp. Med. Biol. 2021, 1264, 15–28. [Google Scholar] [CrossRef]
- Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. [Google Scholar] [CrossRef] [PubMed]
- Babayeva, M.; Loewy, Z.G. Cannabis Pharmacogenomics: A Path to Personalized Medicine. Curr. Issues Mol. Biol. 2023, 45, 3479–3514. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.D.; Craft, R.M. Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 2018, 43, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Arkell, T.R.; Kevin, R.C.; Vinckenbosch, F.; Lintzeris, N.; Theunissen, E.; Ramaekers, J.G.; McGregor, I.S. Sex Differences in Acute Cannabis Effects Revisited: Results from Two Randomized, Controlled Trials. Addict. Biol. 2022, 27, e13125. [Google Scholar] [CrossRef]
- Zamberletti, E.; Rubino, T. Dos(e)Age: Role of Dose and Age in the Long-Term Effect of Cannabinoids on Cognition. Molecules 2022, 27, 1411. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Blair, P. Routes of Administration, Pharmacokinetics and Safety of Medicinal Cannabis. In Medicinal Cannabis and CBD in Mental Healthcare; O’Brien, K., Blair, P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 513–557. ISBN 978-3-030-78559-8. [Google Scholar]
- McGilveray, I.J. Pharmacokinetics of Cannabinoids. Pain Res. Manag. 2005, 10 (Suppl. SA), 15A–22A. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.D.; MacCallum, C.; Margolese, S.; Walsh, Z.; Wright, P.; Daeninck, P.J.; Mandarino, E.; Lacasse, G.; Kaur Deol, J.; de Freitas, L.; et al. Clinical Practice Guidelines for Cannabis and Cannabinoid-Based Medicines in the Management of Chronic Pain and Co-Occurring Conditions. Cannabis Cannabinoid Res. 2024, 9, 669–687. [Google Scholar] [CrossRef]
- Hordowicz, M.; Jarosz, J.; Czaplińska, M.; Leonhard, A.; Klimkiewicz, A. Polish Physicians’ Perspectives on Medical Cannabis Policy and Educational Needs: Results of An Online Survey. J. Clin. Med. 2021, 10, 4545. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.J.; Mokbel, M.A.; Clauw, D.J.; Boehnke, K.F. Assessing Health Care Providers’ Knowledge of Medical Cannabis. Cannabis Cannabinoid Res. 2022, 7, 501–507. [Google Scholar] [CrossRef]
- St Pierre, M.; Matthews, L.; Walsh, Z. Cannabis Education Needs Assessment among Canadian Physicians-in-Training. Complement. Ther. Med. 2020, 49, 102328. [Google Scholar] [CrossRef] [PubMed]
- Philpot, L.M.; Ebbert, J.O.; Hurt, R.T. A Survey of the Attitudes, Beliefs and Knowledge about Medical Cannabis among Primary Care Providers. BMC Fam. Pract. 2019, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Jankie, S.; Sewdass, K.; Smith, W.; Naraynsingh, C.; Johnson, J.; Farnon, N.; Mahadeo, K.; Motilal, S. A Cross-Sectional Survey of Prospective Healthcare Professionals’ Knowledge, Attitudes and Perceptions of Medical Cannabis. Explor. Res. Clin. Soc. Pharm. 2023, 10, 100275. [Google Scholar] [CrossRef] [PubMed]

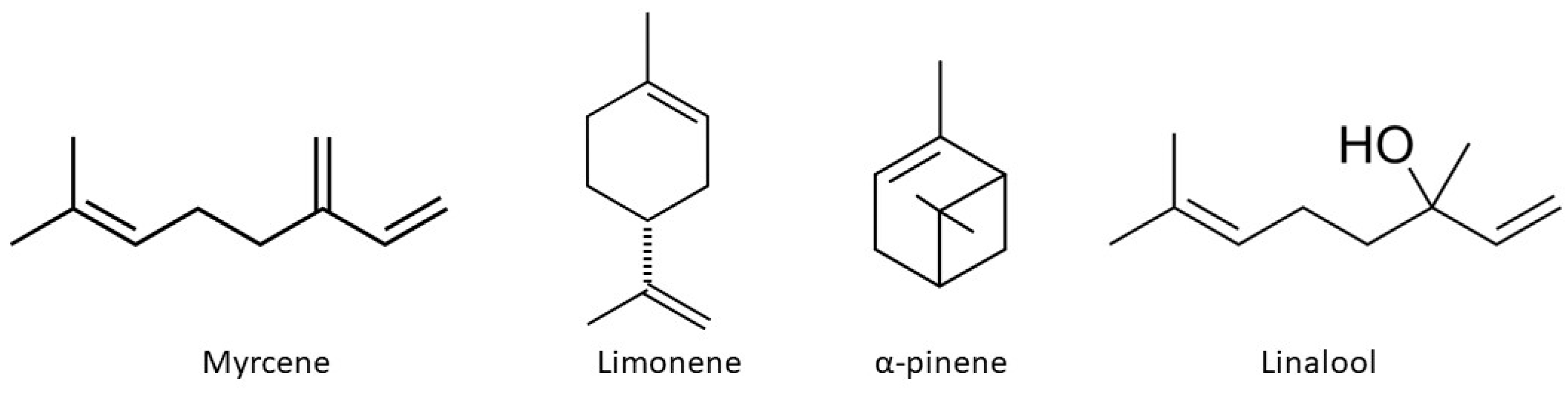
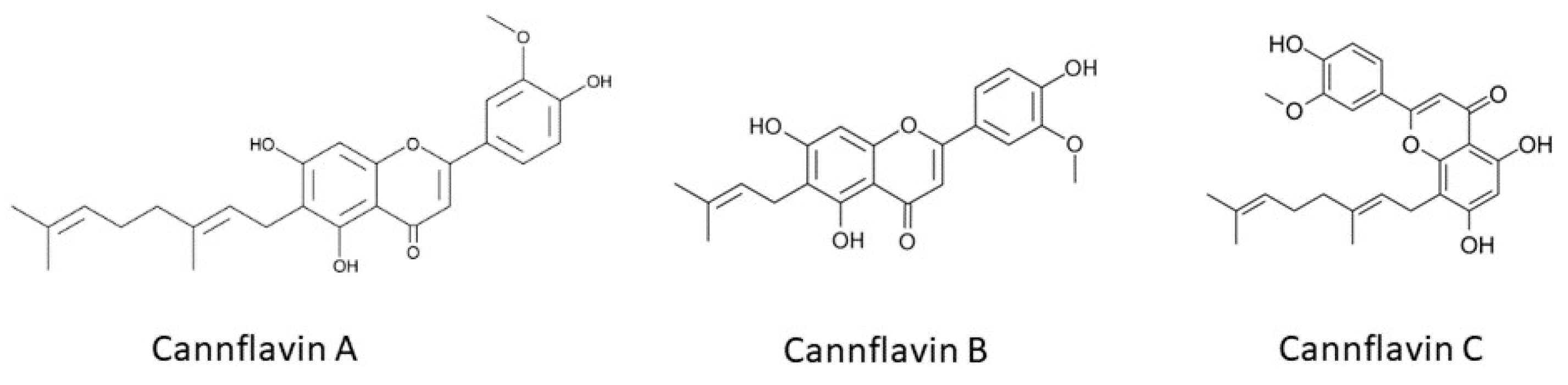

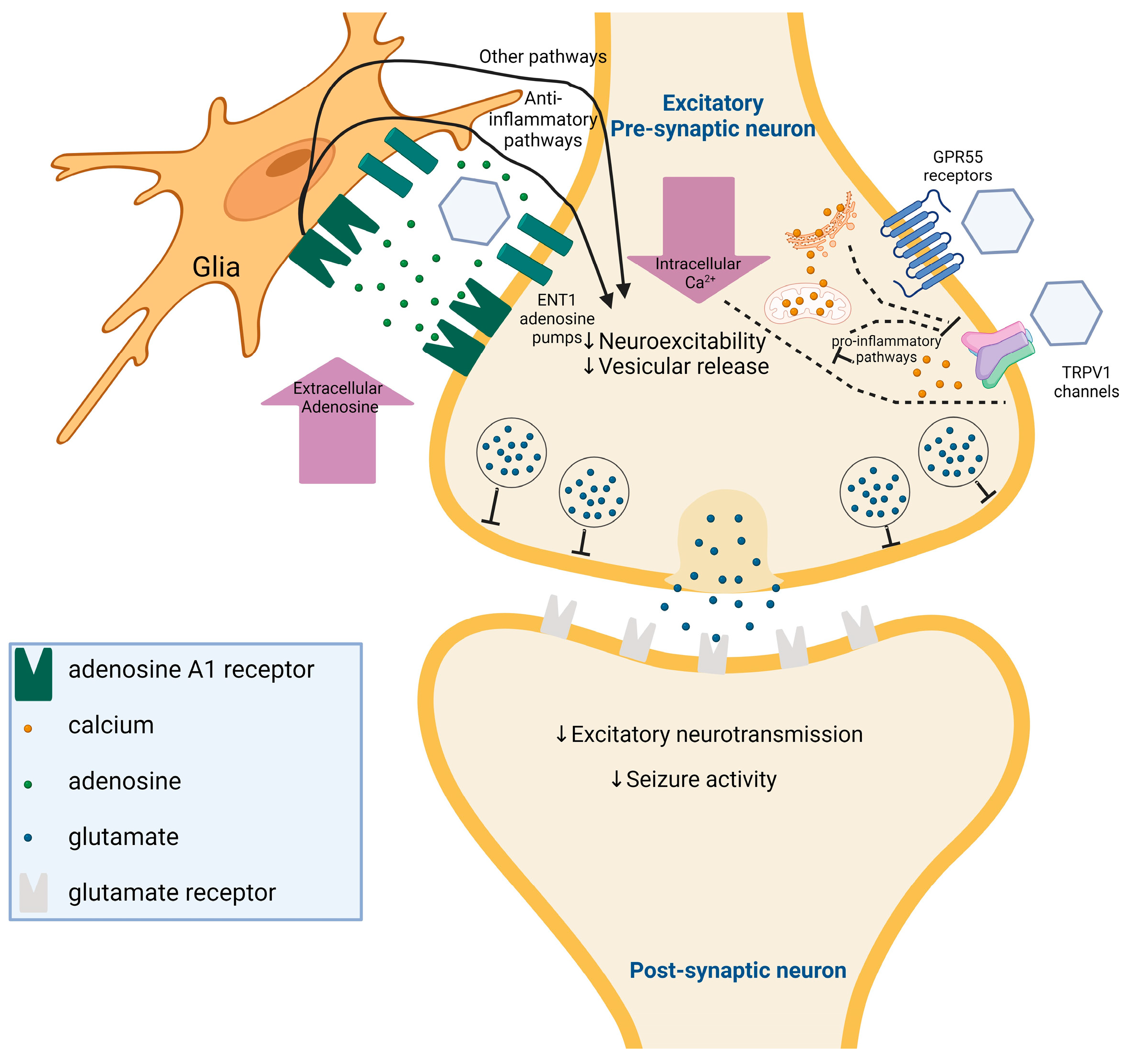
| Disease | Preparation | Study | Sample Size | Outcomes | Observed Adverse Events |
|---|---|---|---|---|---|
| Epilepsy | CBD-enriched medical cannabis | Tzadok et al. [99] | 74 patients | - 89% reduction in seizure frequency - Improvement in behavior in 44% of patients | Seizure aggravation, somnolence/fatigue |
| Epilepsy | Artisanal CBD preparations | Porcari et al. [100] | 209 patients | - Reduction in seizure frequency of at least 50% observed in 33% of the CBD group, 44% of the CBD + clobazam group, and 38% of the clobazam group - Minimal differences between groups suggested that CBD may provide comparable benefits regardless of clobazam use | Sedation (more frequent in the clobazam group) |
| Epilepsy | CBD-based products | Pamplona et al. [101] | 670 patients | - CBD significantly reduced seizure frequency, with 64% of patients showing improvement - Studies with CBD-rich cannabis extracts (71%) reported more improvement than those with purified CBD (46%) - Secondary health improvements were reported in 52% of patients, including improvements in awareness, sleep quality, mood, behavior, language, and motor skills | Appetite alteration, sleepiness, gastrointestinal disturbances, weight changes, fatigue, nausea |
| Dravet syndrome | CBD oral solution | Devinsky et al. [104] | 120 patients | - Resulted in a more significant reduction in convulsive seizure frequency than placebo - Patients who became seizure-free was 5% with CBD | Diarrhea, vomiting, fatigue, pyrexia, somnolence, abnormal results on liver-function tests |
| Alzheimer’s disease | Nabilone | Herrmann et al. [158] | 39 patients | - Nabilone showed efficacy in reducing agitation with a medium effect size | Sedation |
| Dementia | Oral THC | van den Elsen et al. [159] | 22 patients | - Significant increases in dynamic balance, stride length, and gait velocity after THC administration compared to placebo | Dizziness, somnolence, balance disorders, and falls |
| Parkinson’s disease | CBD/THC product | Sousa et al. [130] | 15 patients | - Pain reduction, anxiety relief, and improved sleep quality | Exhibited poorer cognitive ability |
| Multiple sclerosis | Synthetic or herbal and plant-derived cannabinoids | Haddad et al. [176] | 3763 patients | - Reduction in the severity of multiple sclerosis-induced spasticity | Not found |
| Multiple sclerosis | Medical cannabis | Rainka et al. [177] | 141 patients | - 72% experienced pain relief, 48% reported reduced muscle spasticity, and 40% improved sleep. Lesser improvements were noted in gait (11%), anxiety (11%), and quality of life (7%) | Not found |
| Multiple sclerosis | Extract of cannabis | Zajicek et al. [178] | 279 patients | - Degree of muscle stiffness decreased | Not found |
| Amyotrophic lateral sclerosis | Nabiximols | Riva et al. [194] | 59 patients | - 0.11 improvement on the Modified Ashworth scale | Not found |
| Autism spectrum disorder | Oral drops of CBD oil | Barchel et al. [206] | 53 patients | - Reduction in aggressive behavior and self-injury, hyperarousal, and sleep symptoms improved | Drowsiness and loss of appetite |
| Autism Spectrum Disorder | CBD-rich cannabis | Aran et al. [223] | 60 patients | - Improvements in aggressive behavior, anxiety | Sleep disturbances, loss of appetite, and irritability |
| Autism Spectrum Disorder | CBD-Enriched Cannabis sativa extract | Fleury-Teixeira et al. [224] | 18 patients | - Sleep and behavioral disorders, as well as in motor development, communication, social interaction, and cognitive performance | Drowsiness, irritability, diarrhea, increased appetite, conjunctival congestion, and hyperthermia |
| Autism Spectrum Disorder | Cannabis oil containing 30% CBD and 1.5% THC | Bar-lev Schleider et al. [225] | 188 patients | - Significant improvement in Autism Spectrum Disorder | Drowsiness, psychoactive symptoms, increased appetite, digestive disturbances, dry mouth, lack of appetite |
| Rett syndrome | Epidyolex®, CBD, 100 mg/mL oral solution | Desnous et al. [229] | 46 patients | - Reduced the incidence of seizures, a reduction in agitation or anxiety attacks, improvement in spasticity | No aggravation of symptoms or side effects was observed |
| Tourette syndrome | Medical cannabis | Anis et al. [243] | 18 patients | - Subjective reduction in tics | Dry mouth, followed by fatigue, sedation, and dizziness; three patients suffered from psychiatric side effects, including worsening obsessive-compulsive disorder (treatment discontinuation), panic attacks, anxiety, visuospatial disorientation, confusion, slow processing speed, and attention |
| Tourette Syndrome | vaporized 0.25 g dose of THC 10%, THC/CBD 9%/9%, CBD 13% | Abi-Jaoude et al. [244] | 12 patients | - In terms of Tourette syndrome tics, there was no statistically significant difference for any of the cannabis products | Sedation, psychomotor effects, dizziness, cough, burning throat, dry mouth, feeling cold and feeling high, burning throat and dry mouth, while sedation, psychomotor effects, and dizziness |
| Attention Deficit-Hyperactivity Disorder | Sativex, oromucosal spray | Cooper et al. [211] | 30 patients | - Reduction of symptoms and no cognitive impairments following cannabinoid use | Light-headedness and diarrhea, one participant taking the active medication experienced sudden onset of muscular seizures/spasms |
| Attention Deficit-Hyperactivity Disorder | Cannabis | Rasmussen et al. [212] | 88 patients | - Subcortical regions like the right caudate exhibited reduced activation, an interaction - The effect between ADHD and cannabis was observed in the right hippocampus and - Cerebellar vermis, cannabis use did not impact behavioral response inhibition | Not found |
| Anxiety | CBD | Shannon et al. [258] | 72 patients | - Decrease anxiety or sleep problems, reduce distressing symptoms, | Not found |
| Anxiety | CBD | Hundal et al. [259] | 32 patients | - Effective in treating anxiety | Not found |
| Post-traumatic stress disorder | Nabilone | Jetly et al. [273] | Ten patients | - The results revealed a significant reduction in CAPS (Clinician-Administered PTSD Scale) Recurring and Distressing Dream Scores among the participants who received nabilone compared to those who received a placebo | Dry mouth, headache |
| Post-traumatic Stress Disorder | Cannabis | Bonn-Miller et al. [274] | 76 patients | - Both THC + CBD and High THC led to decreased depression and social anxiety | Cough, throat irritation, anxiety |
| Neuropathic pain | Dronabinol | Schimrigk et al. [293] | 240 patients | - Pain intensity during 16 weeks of dronabinol and placebo treatment was reduced by 1.92 and 1.81 - No signs of drug abuse and only one possible case of dependency occurred | Restlessness, irritability, sleep interference, decreased appetite, excessive sweating |
| Neuropathic pain | Cannabis | Wilsey et al. [294] | 39 patients | - Pain relief appears to be maximal after the second dosing at 180 min postbaseline, but the peak effect drops off 1 to 2 h later - Cannabis has analgesic efficacy, with the low dose being as effective a pain reliever as the medium dose | There were no study-related serious adverse events |
| Fibromyalgia | Cannabis | Van de Donk et al. [334] | 20 patients | - Cannabis varieties containing THC caused a significant increase in pressure pain threshold relative to placebo | Deterioration in mood and alertness, sore throat and sour taste, coughed during inhalation, nausea without vomiting |
| Fibromyalgia | THC-rich cannabis oil | Chaves et al. [335] | 17 patients | - Group presented significant improvement on the “feel good”, “pain”, “do work”, and “fatigue” scores on the FIQ - THC-rich cannabis oil improved well-being, reduced pain and fatigue, and enhanced work quality | Somnolence, dizziness, mouth dryness, improved mood, improved libido |
| Fibromyalgia | Nabilone | Ware et al. [336] | 29 patients | - Nabilone improved sleep - Nabilone was less effective during awakening, while it performed better during rest | Nausea, dizziness, dry mouth |
| Trigeminal neuralgia | Medical cannabis | Mechtler et al. [346] | 42 patients | >50% improvement in reducing trigeminal neuralgia symptoms, 50% of patients were able to reduce opioids | Fatigue, reducing appetite, nausea, vomiting, diarrhea |
| Migraine | Cannabis | Stith et al. [283] | 669 patients | - 94% of users felt relief. Symptoms dropped 3.3 points on the 1–10 vas scale | Not found |
| Migraine | Cannabis | Cuttler et al. [284] | 1306 patients | - 56% reduction of headache and migraine symptoms | Not found |
| Diabetic neuropathy | Cannabis | Wallace et al. [329] | 16 patients | - Reduction in spontaneous and evoked pain scores - Cognitive function worsening at the 7% THC dose | Euphoria, sedation |
| Diabetic Peripheral Neuropathy | 1%, 4%, or 7% THC dose of cannabis | Wallace et al. [330] | 16 patients | - Minimal cognitive effects observed at low plasma THC levels during pain relief | Not found |
| Concerns | Explanation |
|---|---|
| Clinical trials with specific cannabinoids | There is a lot of research on Δ9-THC and CBD. However, other cannabinoids have not been extensively studied. Future research could focus on clinical trials to investigate the therapeutic potential of these lesser-known cannabinoids and their derivatives, which have proven in vitro or in vivo biological activity, such as the following: THCV—antiepileptic activity [347], potential against Parkinson’s disease [348,349], antipsychotic effects [350], CBG—anticonvulsant activity [351], counteract neuroinflammation [352], neuroprotective in Huntington’s disease [202], in multiple sclerosis [353], CBG derivatives—anti-inflammatory in multiple sclerosis [354], in amyotrophic lateral sclerosis [355], neuroprotective potential in Huntington’s disease [356], in Parkinson’s disease [357,358,359], CBC—promotes neural stem/progenitor cells viability [360], induces neuronal differentiation in NSC-34 Cells [361], antinociceptive activity [362], anti-inflammatory [363], CBN—neuroprotective activity [167], antiseizure potential [364]. |
| Long-term side effects | Cannabis use can have both short-term and long-term adverse effects, influenced by factors like frequency, THC strength, age, and individual susceptibility. Prolonged use may lead to cognitive decline, especially in younger patients, while also increasing the risk of psychiatric disorders and addiction [365,366,367]. However, understanding the long-term effects of medicinal cannabis remains limited. A meta-analysis found long-term medicinal cannabis use safe for chronic non-cancer pain (12 months) [368]. Still, research results on patients with multiple sclerosis vary, with some indicating potential benefits for cognition while others warn of increased psychiatric risks [176,369]. More research is needed to clarify the long-term safety and efficacy of medical cannabis. |
| Mechanisms of action | Although we have some understanding of how cannabinoids interact with the endocannabinoid system and other neurotransmitter systems in the brain [370,371,372,373,374,375,376], further research is needed to elucidate the precise mechanisms of action underlying the therapeutic effects of cannabis in neurological disorders. |
| Individual responses to cannabis | The delivery route, individual traits (age, sex), and genetic predispositions might influence the responses of patients to cannabis [377,378,379,380,381,382,383,384]. Future research should explore genetic, epigenetic, and pharmacogenomic factors to understand individual variability in cannabis response better and personalize treatments accordingly. |
| Clinical guidelines and education | Standardized dosing for cannabis-based treatments remains elusive. Establishing a consensus and clear clinical guidelines for the use of cannabis in neurological disorders is essential [385]. More research is imperative to develop evidence-based guidelines for healthcare providers; there also is a need for enhanced education among healthcare professionals and patients alike regarding the potential benefits and risks of cannabis-based treatments [386,387,388,389,390]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiłowicz-Krzemień, A.; Nogalska, W.; Maszewska, Z.; Maleszka, M.; Dobroń, M.; Szary, A.; Kępa, A.; Żarowski, M.; Hojan, K.; Lukowicz, M.; et al. The Use of Compounds Derived from Cannabis sativa in the Treatment of Epilepsy, Painful Conditions, and Neuropsychiatric and Neurodegenerative Disorders. Int. J. Mol. Sci. 2024, 25, 5749. https://doi.org/10.3390/ijms25115749
Stasiłowicz-Krzemień A, Nogalska W, Maszewska Z, Maleszka M, Dobroń M, Szary A, Kępa A, Żarowski M, Hojan K, Lukowicz M, et al. The Use of Compounds Derived from Cannabis sativa in the Treatment of Epilepsy, Painful Conditions, and Neuropsychiatric and Neurodegenerative Disorders. International Journal of Molecular Sciences. 2024; 25(11):5749. https://doi.org/10.3390/ijms25115749
Chicago/Turabian StyleStasiłowicz-Krzemień, Anna, Wiktoria Nogalska, Zofia Maszewska, Mateusz Maleszka, Maria Dobroń, Agnieszka Szary, Aleksandra Kępa, Marcin Żarowski, Katarzyna Hojan, Malgorzata Lukowicz, and et al. 2024. "The Use of Compounds Derived from Cannabis sativa in the Treatment of Epilepsy, Painful Conditions, and Neuropsychiatric and Neurodegenerative Disorders" International Journal of Molecular Sciences 25, no. 11: 5749. https://doi.org/10.3390/ijms25115749
APA StyleStasiłowicz-Krzemień, A., Nogalska, W., Maszewska, Z., Maleszka, M., Dobroń, M., Szary, A., Kępa, A., Żarowski, M., Hojan, K., Lukowicz, M., & Cielecka-Piontek, J. (2024). The Use of Compounds Derived from Cannabis sativa in the Treatment of Epilepsy, Painful Conditions, and Neuropsychiatric and Neurodegenerative Disorders. International Journal of Molecular Sciences, 25(11), 5749. https://doi.org/10.3390/ijms25115749











