Research on the Medicinal Chemistry and Pharmacology of Taxus × media
Abstract
1. Introduction
2. Origin and Cultivation
2.1. Origin and Geographical Distribution
2.2. Artificial Cultivation and Comparative Analysis
3. Phytochemical Constituents
3.1. Chemical Composition of Different Parts of Taxus × media
3.1.1. Chemical Composition of Taxus × media Leaves and Twigs
3.1.2. Chemical Composition of Taxus × media Barks
3.1.3. Chemical Composition of Taxus × media Seeds
3.2. The Impact of Origin and Growth Duration on the Variation of Active Components in Taxus × media
3.3. Comparison of Chemical Components between Taxus × media and Other Taxus Species
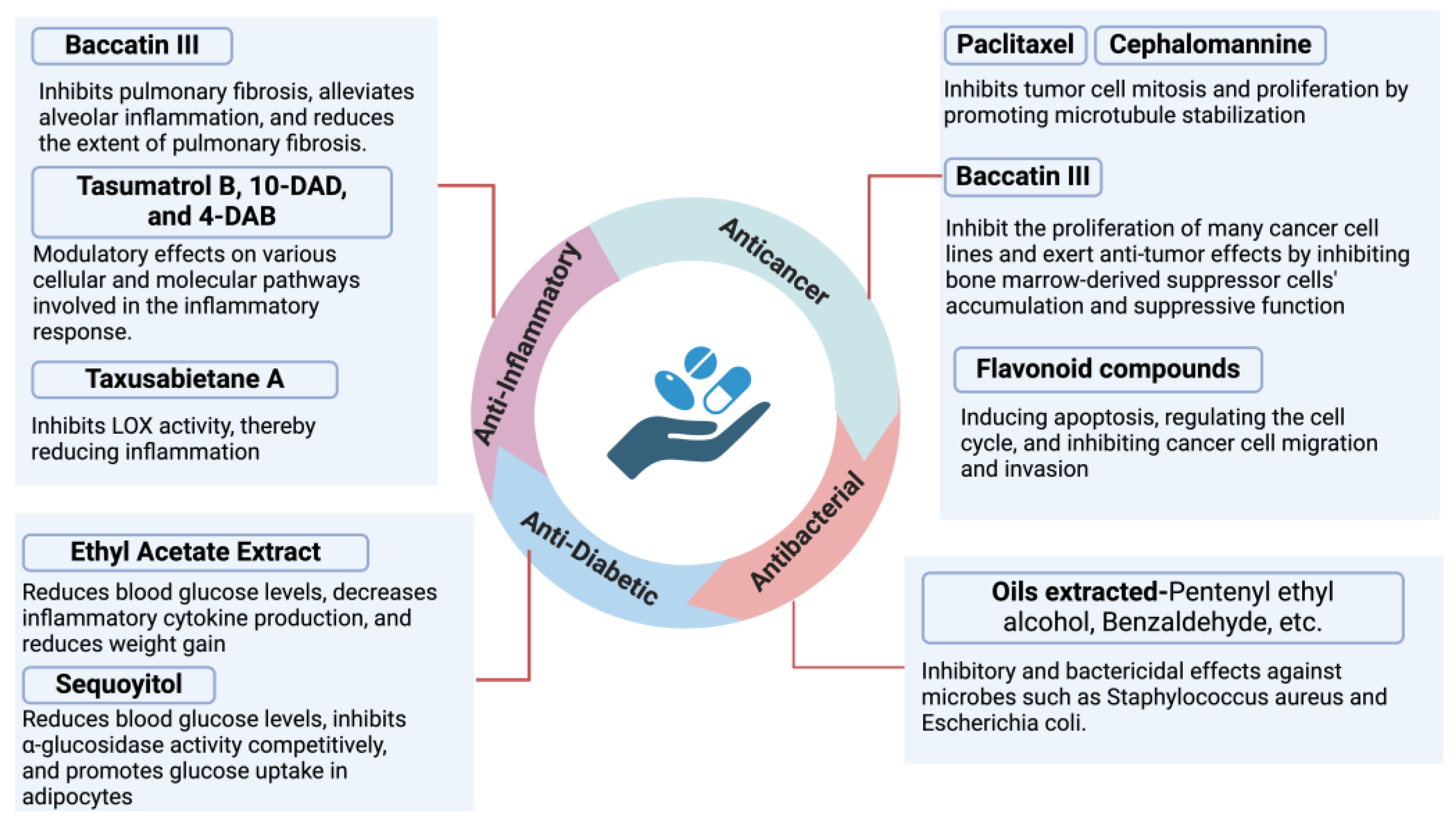
4. Pharmacological Activity
4.1. Anticancer Activity
4.1.1. Anticancer Activity of Monomeric Compounds
Taxane Compounds
Other Non-Taxane Anticancer Components
4.1.2. Anticancer Activity of Extracts
4.2. Antibacterial Activity
4.3. Anti-Diabetic Activity
4.4. Anti-Inflammatory Activity
4.5. Antioxidant Activity
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hao, D.C.; Xiao, P.G.; Peng, Y.; Liu, M.; Huo, L. Research progress and trend analysis of biology and chemistry of Taxus medicinal resources. Yao Xue Xue Bao 2012, 47, 827–835. (In Chinese) [Google Scholar] [PubMed]
- Li, M.; Geng, W.; Wang, Z.; Wang, Q.; Pang, L.; Wang, B.; Wang, P.; Qu, F.; Zhang, X. Analysis of the utilization value of different tissues of Taxus × media based on metabolomics and antioxidant activity. BMC Plant Biol. 2023, 23, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, X.; Zheng, T.; Guo, X.; Chen, Q.; Tang, Z. Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry. Open Life Sci. 2021, 16, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Guo, H.; Shi, X.; Wang, Y.; Wan, Q.; Song, Y.B.; Zhang, L.; Dong, M.; Shen, C. Comparative proteomic analyses of two Taxus species (Taxus × media and Taxus mairei) reveals variations in the metabolisms associated with paclitaxel and other metabolites. Plant Cell Physiol. 2017, 58, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Feng, J.; Chen, C.; Chen, J.; Long, T.; Li, J.; Zang, R.; Li, J. Differential Responses to Climate and Land-Use Changes in Threatened Chinese Taxus Species. Forests 2019, 10, 766. [Google Scholar] [CrossRef]
- Furmanowa, M.; Glowniak, K.; Syklowska-Baranek, K.; Zgórka, G.; Józefczyk, A. Effect of picloram and methyl jasmonate on growth and taxane accumulation in callus culture of Taxus × media var. Hatfieldii. Plant Cell Tissue Organ Cult. 1997, 49, 75–79. [Google Scholar] [CrossRef]
- Parc, G.; Canaguier, A.; Landré, P.; Hocquemiller, R.; Chriqui, D.; Meyer, M. Production of taxoids with biological activity by plants and callus culture from selected Taxus genotypes. Phytochemistry 2002, 59, 725–730. [Google Scholar] [CrossRef]
- Li, Z.L.; Lu, X.H.; Wang, X.J.; Zhou, X.J.; Li, J.; Qian, S.H. Chemical constituents from twig and leaves of Taxus media. Chin. Tradit. Herb. Drugs 2018, 49, 3226–3231. (In Chinese) [Google Scholar]
- Hanano, A.; Perez-Matas, E.; Shaban, M.; Cusido, R.M.; Murphy, D.J. Characterization of lipid droplets from a Taxus media cell suspension and their potential involvement in trafficking and secretion of paclitaxel. Plant Cell Rep. 2022, 41, 853–871. [Google Scholar] [CrossRef]
- Li, N.; Pan, Z.; Zhang, D.; Wang, H.X.; Yu, B.; Zhao, S.P.; Guo, J.J.; Wang, J.W.; Yao, L.; Cao, W.G. Chemical Components, Biological Activities, and Toxicological Evaluation of the Fruit (Aril) of Two Precious Plant Species from Genus Taxus. Chem. Biodivers. 2017, 14, e1700305. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, N.; Xie, W. Advancements in the Cultivation, Active Components, and Pharmacological Activities of Taxus mairei. Molecules 2024, 29, 1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wang, Q.; Ruan, X.; Zhang, Y.Y. Research Status and Utilization Strategies of Rare Medicinal Plants in Taxus. Sci. Silvae Sin. 2012, 48, 116–125. (In Chinese) [Google Scholar]
- Tan, F.; Pang, Y.Z.; Xiong, N.X.; Deng, C.Y. Introduction, propagation and taxol’s accun nulation in leaves of Taxus media. J. Southwest China Norm. Univ. (Nat. Sci. Ed.) 2000, 12, 86–87. (In Chinese) [Google Scholar]
- Wang, Q.; Zhao, X.; Jiang, Y.; Jin, B.; Wang, L. Functions of Representative Terpenoids and Their Biosynthesis Mechanisms in Medicinal Plants. Biomolecules 2023, 13, 1725. [Google Scholar] [CrossRef] [PubMed]
- Du, C.L. Study on Pharmacognosy of Taxus media and Identification of DNA Molecules between in Taxus chinensis var. mairei. Master’s Thesis, Nanchang University, Nanchang, China, 2018. [Google Scholar]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yan, Y.; Song, J.; Li, J.; Mao, J.; Wang, N.; Wang, W.; Du, F.K. Recent Fragmentation May Not Alter Genetic Patterns in Endangered Long-Lived Species: Evidence From Taxus cuspidata. Front. Plant Sci. 2018, 9, 1571. [Google Scholar] [CrossRef]
- Poudel, R.C.; Gao, L.M.; Möller, M.; Baral, S.R.; Uprety, Y.; Liu, J.; Li, D.Z. Yews (Taxus) along the Hindu Kush-Himalayan region: Exploring the ethnopharmacological relevance among communities of Mongol and Caucasian origins. J. Ethnopharmacol. 2013, 147, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Poudel, R.C.; Moeller, M.; Li, D.Z.; Shah, A.; Gao, L.M. Genetic diversity, demographical history and conservation aspects of the endangered yew tree Taxus contorta (syn. Taxus fuana) in Pakistan. Tree Genet. Genomes 2014, 10, 653–665. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Onrubia, M.; Moyano, E.; Bonfill, M.; Palazón, J.; Pedreño, M.A.; Cusidó, R.M. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus × media cell cultures. Plant Biotechnol. J. 2014, 12, 1075–1084. [Google Scholar] [CrossRef]
- Tapia, N.; Zamilpa, A.; Bonfill, M.; Ventura, E.; Cruz-Vega, D.; Villar, A.; Cruz-Sosa, F.; Osuna, L. Effect of the culture medium and biotic stimulation on taxane production in Taxus globosa Schltdl in vitro cultures. Acta Physiol. Plant. 2013, 35, 3447–3455. [Google Scholar] [CrossRef]
- Zhou, T.; Luo, X.; Zhang, C.; Xu, X.; Yu, C.; Jiang, Z.; Zhang, L.; Yuan, H.; Zheng, B.; Pi, E. Comparative metabolomic analysis reveals the variations in taxoids and flavonoids among three Taxus species. BMC Plant Biol. 2019, 19, 529. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Wang, T.; Zhou, S.; Wu, Y.; Huang, X.; Lin, H.; Su, X. First report of Taxus media branch blight caused by Neopestalotiopsis clavispora in China. Plant Dis. 2022, 106, 12. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.H.; Wang, Y.F.; Zhang, X.P.; Li, C.F.; Kiyota, H. A New Metabolite with a New Substitution Pattern from the Seeds of the Chinese Yew, Taxus mairei. Chem. Biodivers. 2010, 4, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xu, Z. Effects of harvesting season and age on main active components of Taxus media. Chin. J. Tradit. Chin. Med. 2010, 35, 2538–2540. (In Chinese) [Google Scholar]
- Xu, R.; Wu, T.; Fan, J. GC-MS Analysis of the Chemical Constituents of the Essential Oil from Taxus × Media by Different Extraction Method. J. Nanchang Univ. (Eng. Technol.) 2013, 35, 22–28. (In Chinese) [Google Scholar]
- Ma, S.; Liu, J.; Ma, L.; Wang, Y.; Cao, X.; Luo, J. Comparative analysis on contents of 10-deacetyl-bacratin III, cephalomannine, taxol of Taxus media from different habitats and growth years. Chin. Tradit. Herb. Drugs 2017, 48, 4979–4985. (In Chinese) [Google Scholar]
- Jiang, Y.; Shen, C.; Zhou, D.; Yu, Q. Comparative study of taxanes content in the branches and leaves of different species of Taxus. J. Zhejiang Agric. Sci. 2018, 59, 4. (In Chinese) [Google Scholar]
- Cui, H.; Ge, F. Studies on constituents from Taxus chinensis var. mairei Bark. J. Chin. Med. Mater. 2004, 27, 566–568. (In Chinese) [Google Scholar]
- Miyazaki, M.; Shimizu, K.; Mishima, H.; Kurabayashi, M. The Constituent of the Heartwood of Taxus cuspidata Sieb. et Zucc. Chem. Pharm. Bull. 1968, 16, 546–548. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, D.; Liu, H.; Guo, J.; Liang, X. Synthesis and drug resistant reversal activities of taxane-like multi-drug resistant reversal agents. Acta Pharm. Sin. 2003, 38, 424–429. (In Chinese) [Google Scholar]
- Wianowska, D.; Hajnos, M.; Dawidowicz, A.L.; Oniszczuk, A.; Waksmundzka-Hajnos, M.; Gowniak, K. Extraction Methods of 10-Deacetylbaccatin III, Paclitaxel, and Cephalomannine from Taxus baccata L. Twigs: A Comparison. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 589–601. [Google Scholar] [CrossRef]
- Cui, H.; Zheng, W.; Zhang, Z.; Fu, Y.; Li, X.; Fu, Y.; Gu, C. Determination and Analysis of Seven Taxane s in Different Taxus Species by HPLC Method. For. Eng. 2022, 38, 118–124. (In Chinese) [Google Scholar]
- Li, S.; Fu, Y.; Zu, Y.; Sun, R.; Wang, Y.; Zhang, L.; Luo, H.; Gu, C.; Efferth, T. Determination of paclitaxel and other six taxoids in Taxus species by high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lu, J.; Wang, J.; Han, L.; Yang, X. Isolation and identification of chemical constituents from needles of cultivated Taxus media. J. Shenyang Pharm. Univ. 2009, 26, 789–799. (In Chinese) [Google Scholar]
- Dong, Q.F.; Liu, J.J.; Yu, R.M. Taxol content comparison in different parts of Taxus media and Taxus chinensis var. mairei by HPLC. Zhong Yao Cai 2010, 33, 1048–1051. [Google Scholar] [PubMed]
- Li, Z.; Lu, X.; Wang, X.; Zhou, X.; Li, J.; Qian, S. Study on chemical constituents in branches and leaves of Taxus media. Chin. Tradit. Herb. Drugs 2018, 49, 3226–3231. (In Chinese) [Google Scholar]
- Wu, C.; Jiang, L.; Yang, Y.; Tang, Y.; Chen, Q.; Duan, F.; Qiu, D. Comparative, regression and cluster analysis on contents of six taxanes in Taxus spp. Chin. J. Chin. Mater. Medica 2021, 52, 538–543. (In Chinese) [Google Scholar]
- Su, C. Study on Dynamic Changes of Biomass and 10-DAB Content of Taxus media. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2017. [Google Scholar]
- Yang, G.; Ye, F.; Li, L.; Zhang, C.; Wei, J.; Hu, P. Content Comparison of Taxol in Different Parts of Taxus Madia and Taxus Chinensis var. Mairei. China Pharm. 2015, 18, 1844–1847. (In Chinese) [Google Scholar]
- Wei, Q.; Li, Q.Z.; Wang, R.L. Flavonoid Components, Distribution, and Biological Activities in Taxus: A review. Molecules 2023, 28, 1713. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Su, Y.; Xu, X.; Wang, J. Response surface method was used to optimize the extraction of total flavonoids from Taxus media and its antioxidant activity. North. Hortic. 2021, 5, 94–102. (In Chinese) [Google Scholar]
- Mao, M. Study on the Annual Change of Main Medicinal Components in Different Branches of Taxus media. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2023. [Google Scholar]
- Zhen, D.; Xu, L.; Zhen, Y.; Lin, Q. Extraction of Maire Yew Polysaccharide and Its Structure. Chem. Ind. For. Prod. 2012, 32, 102–106. (In Chinese) [Google Scholar]
- Xu, D.; Su, C.; Zhang, Y. Study on essential oil components of Taxus media and their antibacterial activity. J. Mianyang Teach. Coll. 2021, 50, 75–79. (In Chinese) [Google Scholar]
- Zhang, J.; Yuan, K.; Jin, Y.C. Comparison of Chemical Composition and Antimicrobial Activities of the Essential Oil of Taxus Media and Taxus Chinensis Var. Mairei Leaves. Adv. Mater. Res. 2012, 343–344, 1092–1097. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Y.; Han, L.; Li, Y.; Zhang, J.; Lu, J. Taxane diterpenoids from the needles of Taxus media ‘Hicksii’. Chin. J. Med. Chem. 2010, 20, 53–56. (In Chinese) [Google Scholar]
- Liu, X.Q.; Zhang, X.D.; Zhu, Y.L.; Shin, B.Y.; Wu, S.X. Structrue identification of biflavones and determination of Taxol from Taxus Madia. Zhong Yao Cai 2008, 31, 1498–1501. (In Chinese) [Google Scholar] [PubMed]
- Cai, Q.; Song, Q.; Jiang, K.; Lin, Y.; Zhang, Y.; Zhang, J.; Lin, S.; Huang, L.; Xue, Q.; Huang, Z.; et al. Quality evaluation of compounds in leaves of six Taxus species based on UPLC-MS/MS and chemometrics. Front. Chem. 2023, 11, 1193188. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M. Flavonoids from the genus Taxus. Z. Fur Naturforschung Sect. C—A J. Biosci. 2004, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Fang, J.M.; Cheng, Y.S. Lignans, Flavonoids and Phenolic Derivatives from Taxus mairei. J. Chin. Chem. Soc. 1999, 46, 811–818. [Google Scholar] [CrossRef]
- Ye, B.; Hua, C.; Liang, S.; Wang, Y. Analysis of taxane accumulation in different parts of Taxus chinensis. Shanxi J. Traditinal Chin. Med. 2020, 41, 1162–1164. (In Chinese) [Google Scholar]
- Yu, C.; Luo, X.; Zhang, C.; Xu, X.; Huang, J.; Chen, Y.; Feng, S.; Zhan, X.; Zhang, L.; Yuan, H.; et al. Tissue-specific study across the stem of Taxus media identifies a phloem-specific TmMYB3 involved in the transcriptional regulation of paclitaxel biosynthesis. Plant J. 2020, 103, 95–110. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Qian, H.; Zhao, Y.; Liu, B.; Fu, C. Polyprenols from the needles of Taxus chinensis var. mairei. Fitoterapia 2012, 83, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Wang, C.L.; Gu, J.S.; Shi, Q.W. Studies on chemical constituents in seeds of Taxus mairei. China J. Chin. Mater. Medica 2005, 30, 1260–1263. (In Chinese) [Google Scholar]
- Liu, J.; Ma, S.; Ma, L.; Cao, X.; Liang, S. Three Effective Components of Taxanes from Taxus madia Rehd.: Comparative Study of Different Growth Period and of Different Origin. Inf. Tradit. Chin. Med. 2017, 34, 9–13. (In Chinese) [Google Scholar]
- Xu, X.; Wei, H.; Jia, X.; Xiat, T.A.; Ma, X.; Chen, G. The contents comparison of taxol and cephalomannine between Taxus wallichiana Zucc. and Taxus meadia. Northwest Pharm. J. 2015, 30, 682–684. (In Chinese) [Google Scholar]
- Kutne, J.; Gao, S. Contents of paclitaxel and brevis in seven Taxus plants from East Asia and North America. J. China Pharm. Univ. 1995, 26, 8–10. (In Chinese) [Google Scholar]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alkahtani, H.M.; AlQuadeib, B.T. Paclitaxel. Profiles Drug Subst. Excip. Relat. Methodol. 2019, 44, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.M.S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J. Control. Release 2003, 86, 33–48. [Google Scholar] [CrossRef]
- Huang, X.; Shi, Y. Advances in structural modification of anti-tumor drug paclitaxel. Shandong Med. J. 2019, 59, 95–100. (In Chinese) [Google Scholar]
- Liebmann, J.E.; Cook, J.A.; Lipschultz, C.; Teague, D.; Fisher, J.; Mitchell, J.B. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br. J. Cancer 1993, 68, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, J.; Zhang, Y.; Li, W. Anticancer Drugs (IV)—New Derivatives of Podophyllotoxin. Chem. Res. Coll. Univ. 1993, 2, 5. [Google Scholar]
- Wang, H.; Zhang, B.Y.; Gong, T.; Chen, T.J.; Chen, J.J.; Yang, J.L.; Zhu, P. Construction of acetyl-CoA and DBAT hybrid metabolic pathway for acetylation of 10-deacetylbaccatin III to baccatin III. Acta Pharm. Sin. B 2021, 11, 3322–3334. [Google Scholar] [CrossRef] [PubMed]
- Escrich, A.; Almagro, L.; Moyano, E.; Cusido, R.M.; Bonfill, M.; Hosseini, B.; Palazon, J. Improved biotechnological production of paclitaxel in Taxus media cell cultures by the combined action of coronatine and calix[8]arenes. Plant Physiol. Biochem. 2021, 163, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Chen, Y.; Guo, Y.Q.; Qiu, J.; Zhu, C.G.; Jin, J.; Tang, G.H.; Bu, X.Z.; Yin, S. Isolation and cytotoxicity evaluation of taxanes from the barks of Taxus wallichiana var. mairei. Bioorganic Med. Chem. Lett. 2015, 25, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, F.; Wang, S.M.; Guo, R.X.; Zhang, Y.F.; Gu, Y.C.; Shi, Q.W. Chemical studies on Taxus canadensis. Chem. Biodivers. 2013, 10, 1729–1753. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Peng, S.; Lu, J.; Zhou, T.; Hong, X.; Chen, S.; Liu, G.; Li, H.; Huang, J.; Chen, X.; et al. UBE2S interacting with TRIM21 mediates the K11-linked ubiquitination of LPP to promote the lymphatic metastasis of bladder cancer. Cell Death Dis. 2023, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Leong, S.W.; Wang, J.; Wu, Q.; Ghauri, M.A.; Sarwar, A.; Su, Q.; Zhang, Y. Cephalomannine inhibits hypoxia-induced cellular function via the suppression of APEX1/HIF-1α interaction in lung cancer. Cell Death Dis. 2021, 12, 490. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, T. Review on Anti-tumor Components from Taxus and Their Derivatives. Res. Dev. Nat. Prod. 2016, 28, 1664–1675. (In Chinese) [Google Scholar]
- Xie, C. Synthesis and Antitumor Activity of Multi-Region Modified 1-Deoxypaclitaxel Analogues. Master’s Thesis, Shanghai University, Shanghai, China, 2019. [Google Scholar]
- Li, J.; Li, A.; Li, M.; Qiao, Y.; Zhang, H. Synthesis and Cytotoxicity of Two Active Metabolites of Larotaxel. Anti-Cancer Agents Med. Chem. 2016, 16, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.; Kumari, M.; Subban, K.; Chelliah, J. Evaluation of the anticancer activity of enzymatically synthesized Baccatin III: An intermediate precursor of Taxol®. Agric. Biol. Sci.-Agric. Biol. Sci. 2020, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Kui, Q.; Cong-Mei, C.; Wei, W. Effect of the Compounds from Taxus chinensis var. mairei and T. cuspidata on the Proliferation of Human Breast Cancer Cells. Nat. Prod. Res. Dev. 2007, 19, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, E.; Luczak, M.; Kniotek, M.; Nowaczyk, M. Cytotoxic, antiviral (in-vitro and in-vivo), immunomodulatory activity and influence on mitotic divisions of three taxol derivatives: 10-deacetyl-baccatin III, methyl (N-benzoyl-(2’R,3’S)-3’-phenylisoserinate) and N-benzoyl-(2’R,3’S)-3’-phenylisoserine. J. Pharm. Pharmacol. 2005, 57, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Frapolli, R.; Marangon, E.; Zaffaroni, M.; Colombo, T.; Falcioni, C.; Bagnati, R.; Simone, M.; D’Incalci, M.; Manzotti, C.; Fontana, G.; et al. Pharmacokinetics and metabolism in mice of IDN 5390 (13-(N-Boc-3-i-butylisoserinoyl)-C-7,8-seco-10-deacetylbaccatin III), a new oral c-seco-taxane derivative with antiangiogenic property effective on paclitaxel-resistant tumors. Drug Metab. Dispos. 2006, 34, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; Micheletti, G.; Rieppi, M.; Poli, M.; Turatto, M.; Rossi, C.; Borsotti, P.; Roccabianca, P.; Scanziani, E.; Nicoletti, M.I.; et al. Antiangiogenic and antitumor activity of IDN 5390, a new taxane derivative. Clin. Cancer Res. 2002, 8, 1182–1188. [Google Scholar]
- Wang, H.; Lu, M. Effect of Baccatin III in Components of Chinese Yew on the Airway Remodeling in Rats with COPD. J. Zhejiang Chin. Med. Univ. 2016, 50, 400–404. (In Chinese) [Google Scholar] [CrossRef]
- Bissery, M.C.; Guénard, D.; Guéritte-Voegelein, F.; Lavelle, F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res. 1991, 51, 4845–4852. [Google Scholar] [PubMed]
- Lee, Y.H.; Lee, Y.R.; Park, C.S.; Im, S.A.; Song, S.; Hong, J.T.; Whang, B.Y.; Kim, K.; Lee, C.K. Baccatin III, a precursor for the semisynthesis of paclitaxel, inhibits the accumulation and suppressive activity of myeloid-derived suppressor cells in tumor-bearing mice. Int. Immunopharmacol. 2014, 21, 487–493. [Google Scholar] [CrossRef]
- Cheng, H.L.; Zhao, R.Y.; Chen, T.J.; Yu, W.B.; Wang, F.; Cheng, K.D.; Zhu, P. Cloning and characterization of the glycoside hydrolases that remove xylosyl groups from 7-β-xylosyl-10-deacetyltaxol and its analogues. Mol. Cell. Proteom. 2013, 12, 2236–2248. [Google Scholar] [CrossRef]
- Cao, C.; Li, Z.; Shi, Q. Chemical Constituents in Taxus cuspidata and Their Bioactivities. Nat. Prod. Res. Dev. 2006, 18, 330–342. (In Chinese) [Google Scholar]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2021, 137, 111308. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.H.; Lin, Y.L.; Liu, Y.K.; Chen, C.H.; Lai, Y.K. 7,7′′-Dimethoxyagastisflavone-induced Apoptotic or Autophagic Cell Death in Different Cancer Cells. Phytother. Res. 2012, 26, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Lin, Y.L.; Ho, S.Y.; Chen, P.R.; Tsai, Y.H.; Chung, C.H.; Hwang, C.H.; Tsai, N.M.; Tzou, S.C.; Ke, C.Y.; et al. The inhibitory effect of 7,7″-dimethoxyagastisflavone on the metastasis of melanoma cells via the suppression of F-actin polymerization. Oncotarget 2017, 8, 60046–60059. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Nejad, M.Y.; Delshad, L. Synergistic Effects of Taxus baccata Extract Mixtures with Silver Nanoparticles against Bacteria and Fungal. Islam. Azad Univ.—Varamin Branch 2015, 4, 25–30. [Google Scholar]
- Nema, R.; Khare, S.; Jain, P.; Pradhan, A. Anticancer Activity of Withania Somnifera (Leaves) Flavonoids Compound. Int. J. Pharm. Sci. Rev. Res. 2013, 19, 103–106. [Google Scholar]
- Reddy, S.P.; Jamil, K.; Madhusudhan, P.; Anjani, G.; Das, B. Antibacterial Activity of Isolates from Piper longum and Taxus baccata. Pharm. Biol. 2001, 39, 236–238. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Li, G.; Zheng, Z.; Su, W. Antitumor and antifungal activities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. FEMS Immunol. Med. Microbiol. 2001, 31, 163–167. [Google Scholar] [CrossRef]
- Binwal, M.; Babu, V.; Israr, K.M.M.; Kashyap, P.K.; Maurya, A.K.; Padalia, R.C.; Tandon, S.; Bawankule, D.U. Taxoids-rich extract from Taxus wallichiana alleviates high-fat diet-induced insulin resistance in C57BL/6 mice through inhibition of low-grade inflammation. Inflammopharmacology 2023, 31, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Duan, D. Study on the Extraction, Separation and Determination of Sequoyitol from Taxus media. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2013. [Google Scholar]
- Dai, L. Protective Effect and Mechanism of Sequetaxol on Vascular Endothelium in Diabetic Rats. Master’s Thesis, Central South University, Changsha, China, 2012. [Google Scholar]
- Liu, B.; Jia, N.; Wei, H.L.; Lan, M.; Liu, J.M.; Xue, Y.Z. Knockdown of p66ShcA activates Nrf2 pathway to protect cardiomyocytes from oxidative stress and inflammation induced by H2O2. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6994–7001. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Baldari, C.T. Apoptosis and oxidative stress-related diseases: The p66Shc connection. Curr. Mol. Med. 2009, 9, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tian, J.; Chen, H.; Xu, X.; Zhu, W.; Wang, L. Intervention effect of bacatine III, active ingredient of Taxus, on pulmonary fibrosis in rats. Zhejiang J. Tradit. Chin. Med. 2009, 44, 193. (In Chinese) [Google Scholar]
- Qayum, M.; Nisar, M.; Shah, M.R.; Adhikari, A.; Kaleem, W.A.; Khan, I.; Khan, N.; Gul, F.; Khan, I.A.; Zia-Ul-Haq, M.; et al. Analgesic and antiinflammatory activities of taxoids from Taxus wallichiana Zucc. Phytother. Res. 2012, 26, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Nisar, M.; Shah, M.R.; Shah, H.; Gilani, S.N.; Gul, F.; Abdullah, S.M.; Ismail, M.; Khan, N.; Kaleem, W.A.; et al. Anti-inflammatory activities of Taxusabietane A isolated from Taxus wallichiana Zucc. Fitoterapia 2011, 82, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- White, J. The short course in toxinology: Training the trainers. Toxicon 2013, 69, 114–119. [Google Scholar] [CrossRef]
- Schultes, R.E. Common poisonous plants and mushrooms of North America. J. Ethnopharmacol. 1991, 35, 1948–1957. [Google Scholar] [CrossRef]


| Bioactive Constituents | Molecular Weight | CAS | Chemical Structure | Source | Content | Reference |
|---|---|---|---|---|---|---|
| Paclitaxel | 853.9 g/mol | 33069-62-4 | C47H51NO14 | The leaves, needles and barks of Taxus × media | 0.13–0.60 mg/g | [2,26,27] |
| Cephalomannine | 831.9 g/mol | 71610-00-9 | C5H53NO14 | The leaves, needles and barks of Taxus × media | 0. 053 mg/g | [2] |
| Baccatine III | 586.6 g/mol | 27548-93-2 | C31H38O11 | The needles of Taxus × media | 0.13–0.31 mg/g | [26] |
| 10-DAB | 544.6 g/mol | 32981-86-5 | C29H36O10 | The leaves and needles of Taxus × media | 0.24–0.78 mg/g | [28,29] |
| Taxinine | 606.7 g/mol | 3835-52-7 | C35H42O9 | The needles and bark of Taxus × media | [30] | |
| Taxinine A | 476.6 g/mol | 18530-09-1 | C26H36O8 | The seeds and needles of Taxus × media | [30,31] | |
| 9-Deacetyltaxinine E | 608.7 g/mol | 284672-78-2 | C35H44O9 | The seeds of Taxus × media | [32] | |
| 10-DAT | 544.6 g/mol | 32981-86-5 | C29H36O10 | The needles of Taxus × media | 0. 126 mg/g | [33] |
| 7-epi-10-DAT | 544.6 g/mol | 7162-920 | C29H36O10 | The needles of Taxus × media | 0.033 mg/g | [2] |
| 7-Xylosyl-10-deacetyltaxol | 944.0 g/mol | 90332-63-1 | C50H57NO17 | The needles of Taxus × media | 0.315 mg/g | [34,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Zhang, N.; Xie, W. Research on the Medicinal Chemistry and Pharmacology of Taxus × media. Int. J. Mol. Sci. 2024, 25, 5756. https://doi.org/10.3390/ijms25115756
Gao X, Zhang N, Xie W. Research on the Medicinal Chemistry and Pharmacology of Taxus × media. International Journal of Molecular Sciences. 2024; 25(11):5756. https://doi.org/10.3390/ijms25115756
Chicago/Turabian StyleGao, Xinyu, Ni Zhang, and Weidong Xie. 2024. "Research on the Medicinal Chemistry and Pharmacology of Taxus × media" International Journal of Molecular Sciences 25, no. 11: 5756. https://doi.org/10.3390/ijms25115756
APA StyleGao, X., Zhang, N., & Xie, W. (2024). Research on the Medicinal Chemistry and Pharmacology of Taxus × media. International Journal of Molecular Sciences, 25(11), 5756. https://doi.org/10.3390/ijms25115756







