Towards Enhanced Tunability of Aqueous Biphasic Systems: Furthering the Grasp of Fluorinated Ionic Liquids in the Purification of Proteins
Abstract
1. Introduction
2. Results and Discussion
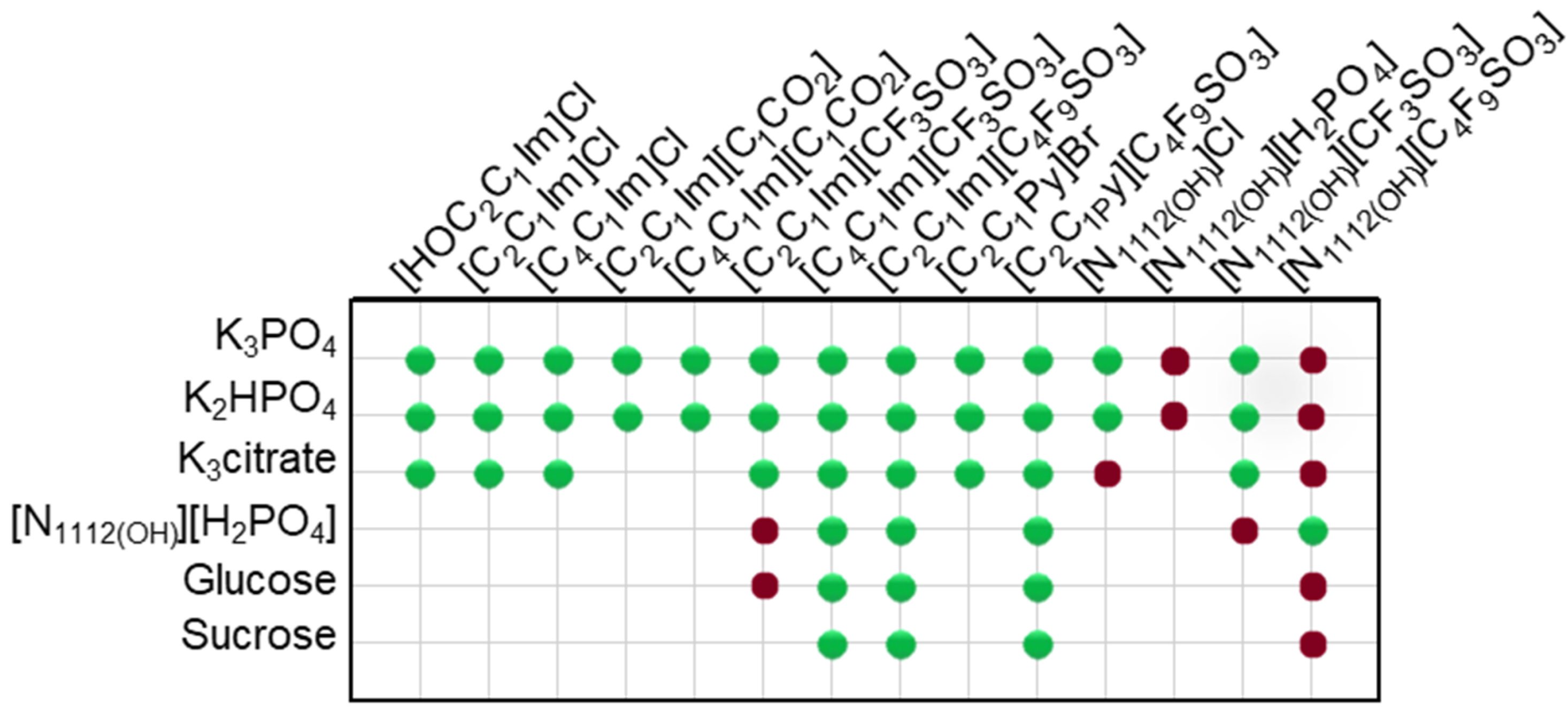
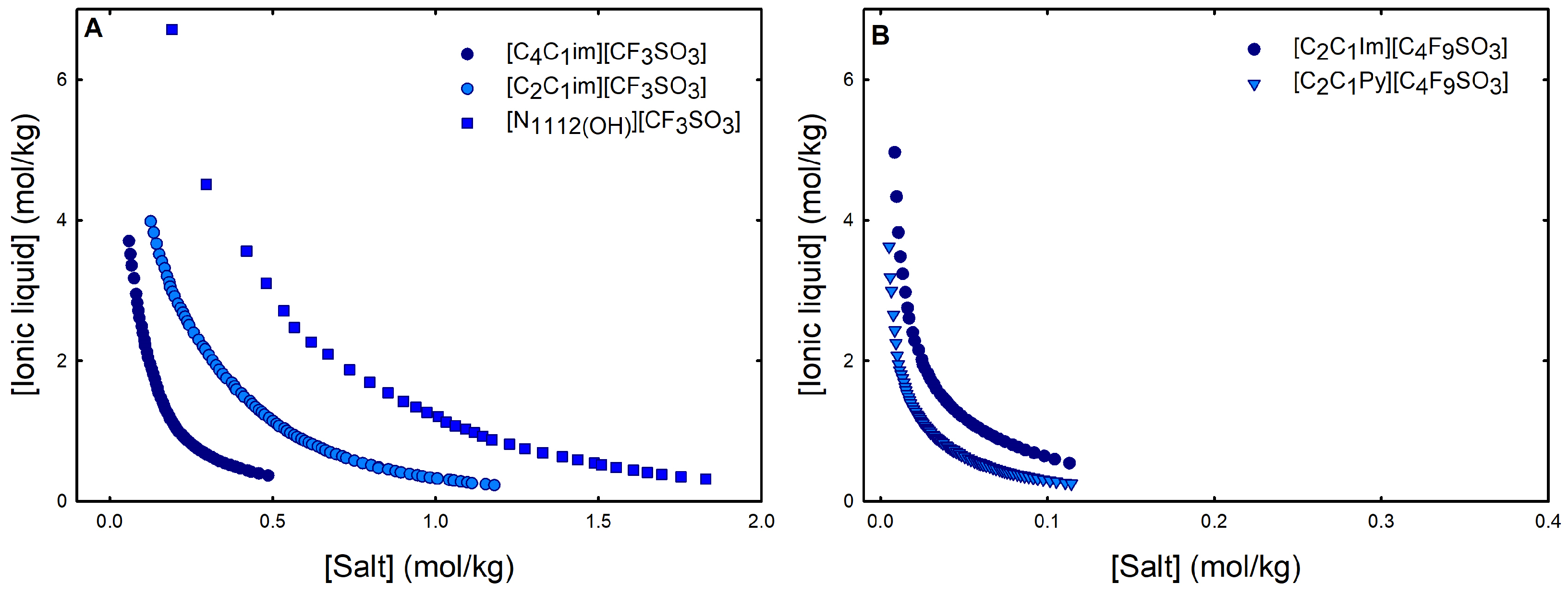
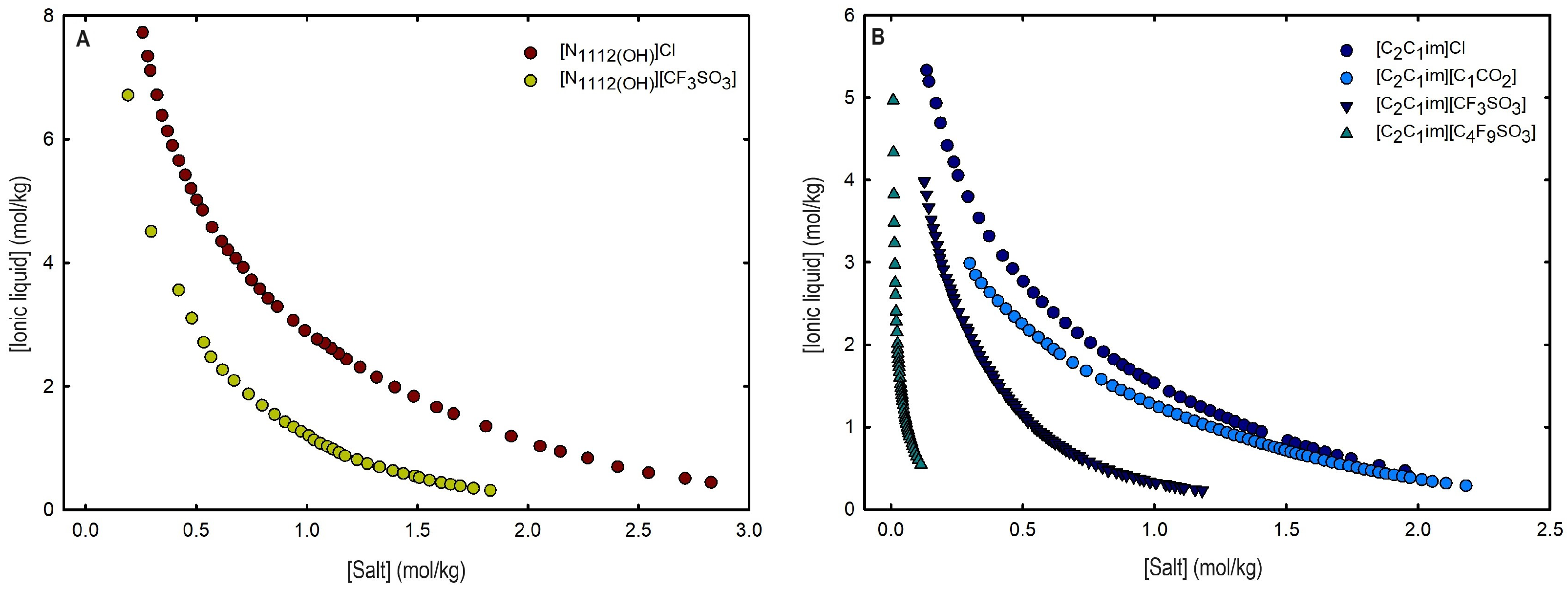
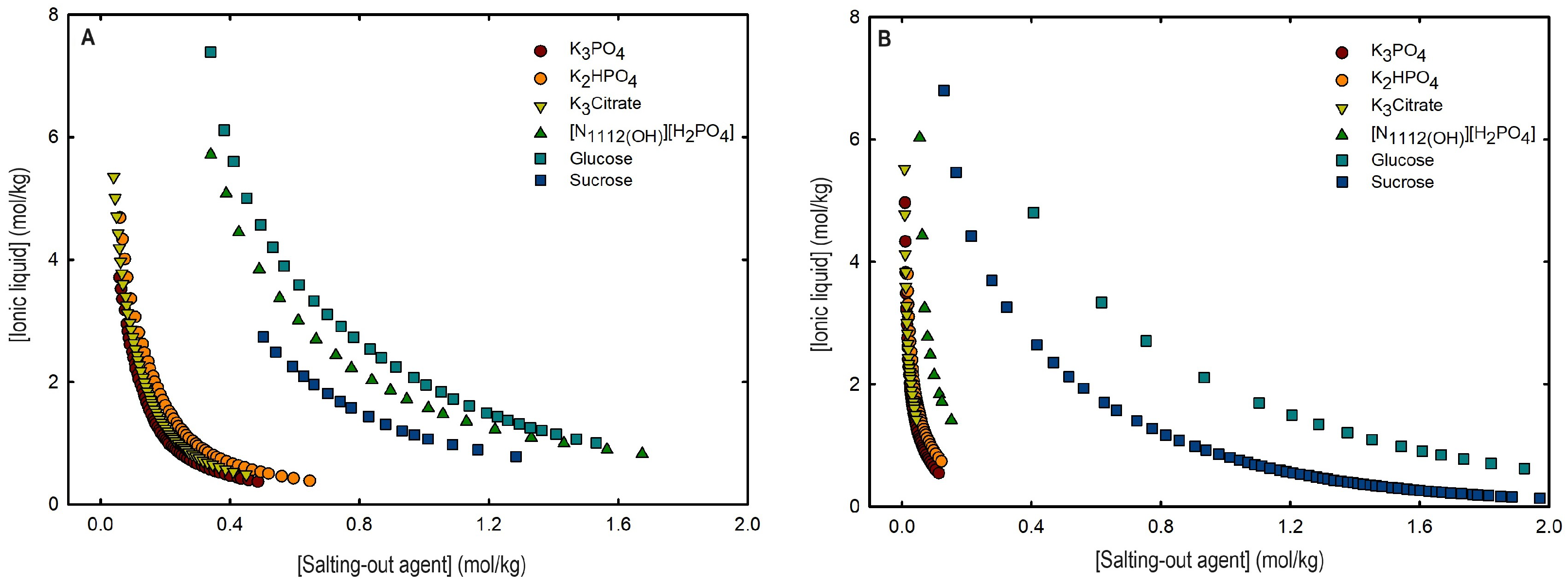
2.1. Phase Diagrams for Functionalized Aqueous Biphasic Systems
2.2. ABS Phase-Forming Component Effect on the Structure, Stability, and Function of Lysozyme
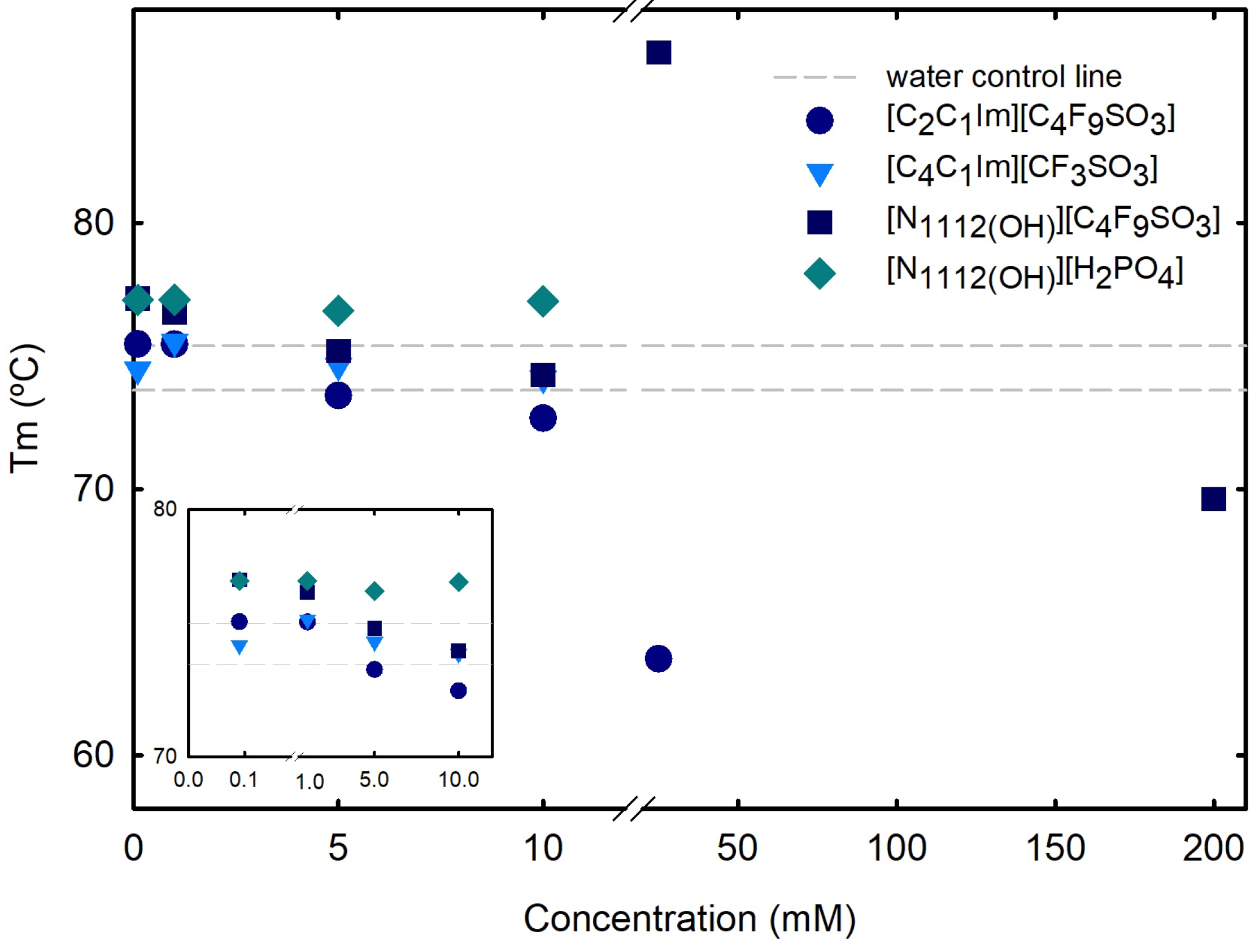
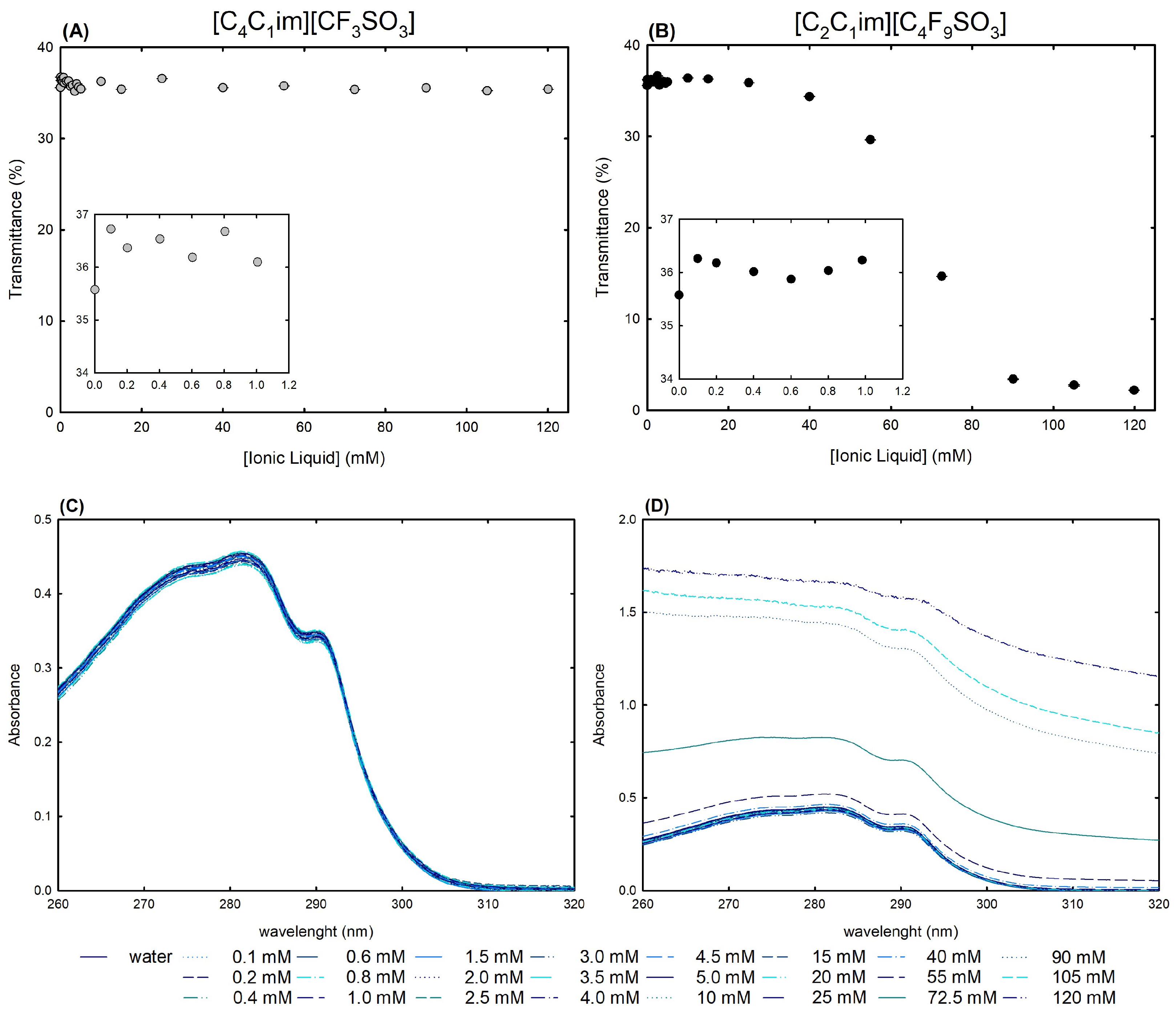
2.2.1. Stability and Function of Lysozyme
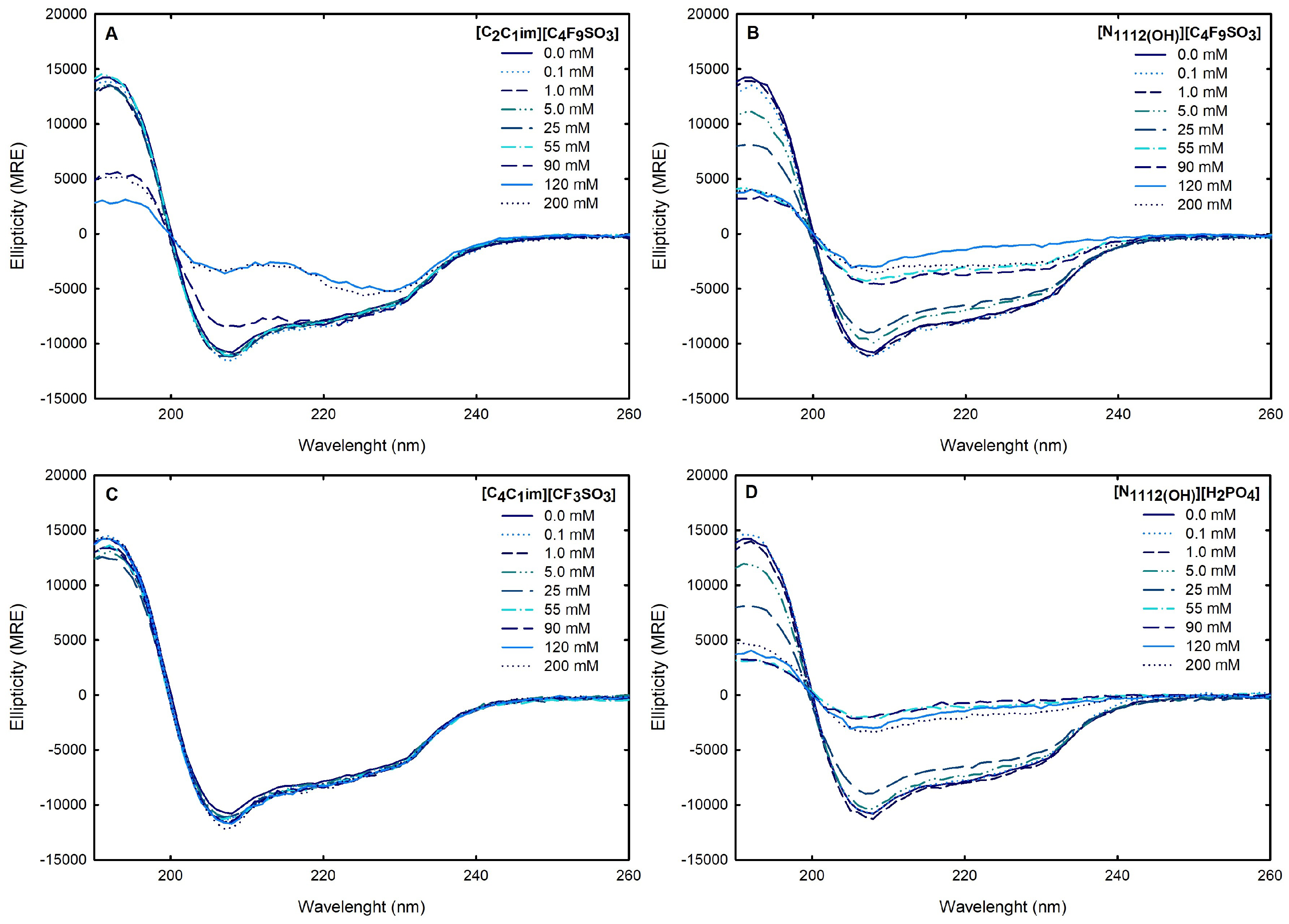
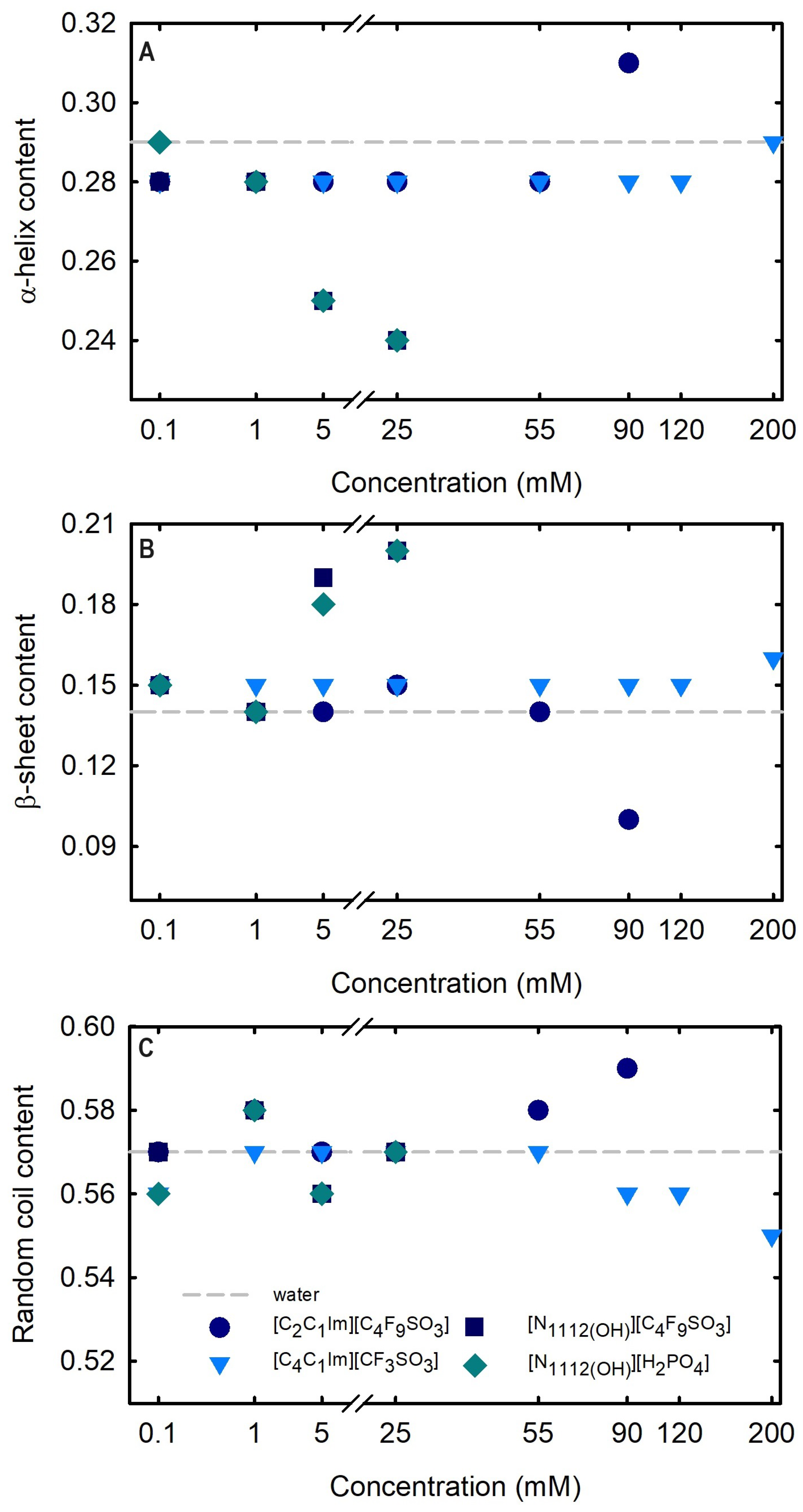
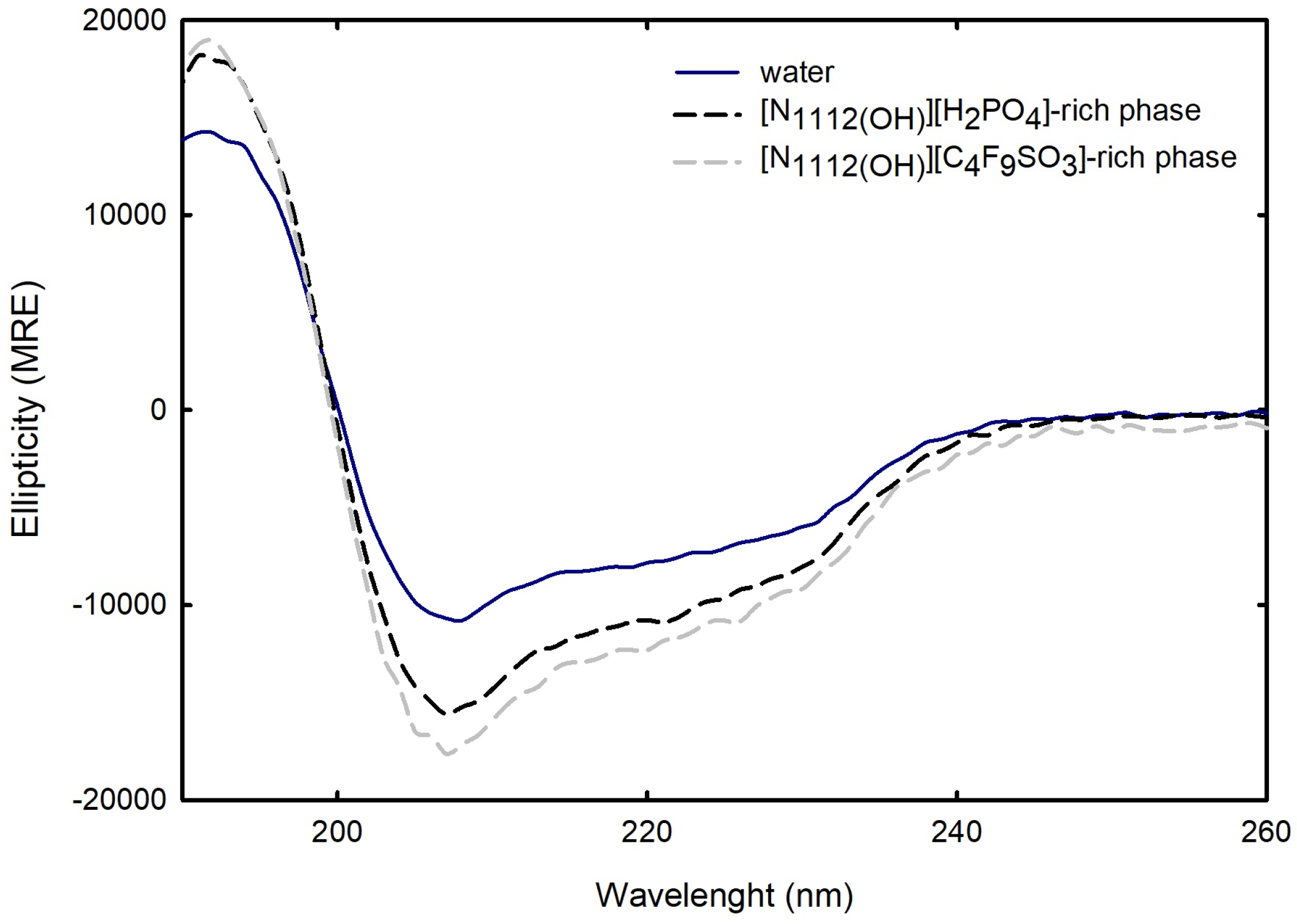
2.2.2. Structural Properties of Lysozyme
2.3. Lysozyme Partition in Functionalized ABS
2.3.1. Extraction Efficiency (%EE)
2.3.2. Structure, Stability, and Function of Lysozyme in ABS Phases
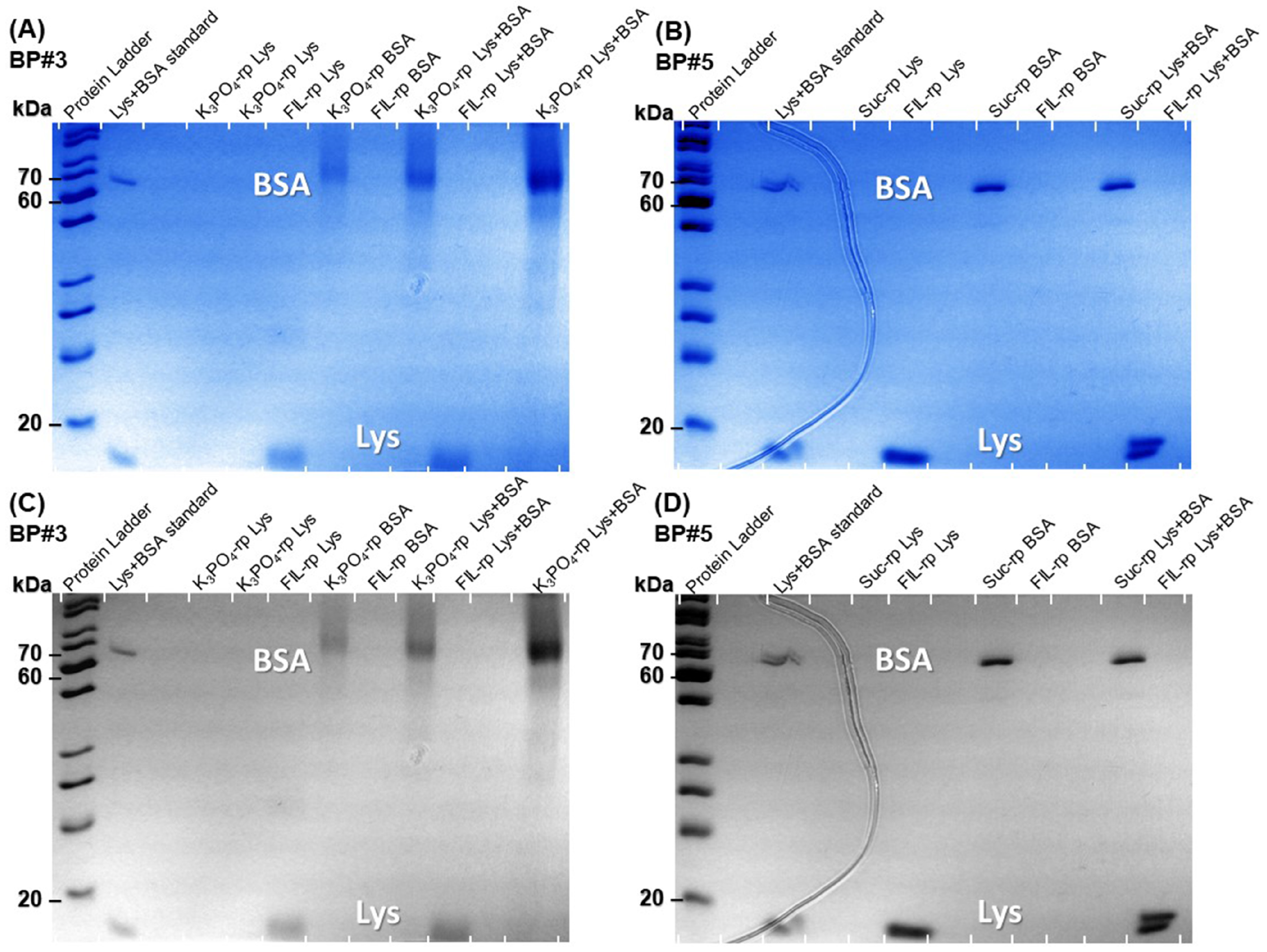
2.3.3. Lys and BSA Simultaneous Partition
2.3.4. Lysozyme Interaction with ABS Phase-Forming Components
3. Materials and Methods
3.1. Reagents
3.2. ABS Phase Diagrams, Merchuck Fitting, and Tie-Lines
3.3. Phase Characterization and Partition
3.4. Protein Quantification
3.5. Lysozyme Enzymatic Activity
3.6. Turbidimetry and UV-Vis Absorbance
3.7. Intrinsic Fluorescence
3.8. DSC
3.9. CD
3.10. SDS-PAGE
3.11. MST
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albertsson, P.A. Partition of Cell Particles and Macromolecules; Wiley: New York, NY, USA, 1986. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.; Coutinho, J.A.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Esteves, P.D.O.; Boal-Palheiros, I.; Pereiro, A.B.; Rebelo, L.P.N.; Freire, M.G. Enhanced tunability afforded by aqueous biphasic systems formed by fluorinated ionic liquids and carbohydrates. Green Chem. 2016, 18, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Zimmermann, I.; Berensmeier, S. Current research approaches in downstream processing of pharmaceutically relevant proteins. Curr. Opin. Biotechnol. 2022, 77, 102768. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.C.F.; Almeida, M.; Faria, J.; Silva, C.; Neves, M.; Freire, M.; Tavares, A. Overview on Protein Extraction and Purification Using Ionic-Liquid-Based Processes. J. Solut. Chem. 2022, 51, 243–278. [Google Scholar] [CrossRef]
- Przybycien, T.M.; Pujar, N.S.; Steele, L.M. Alternative bioseparation operations: Life beyond packed-bed chromatography. Curr. Opin. Biotechnol. 2004, 15, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Thömmes, J.; Etzel, M. Alternatives to Chromatographic Separations. Biotechnol. Prog. 2007, 23, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.M.; Yang, Y.Z.; Zhao, X.D.; Xia, C.B. Bovine serum albumin partitioning in polyethylene glycol (PEG)/potassium citrate aqueous two-phase systems. Food Bioprod. Process. 2010, 88, 40–46. [Google Scholar] [CrossRef]
- Madhusudhan, M.C.; Raghavarao, K.; Nene, S. Integrated process for extraction and purification of alcohol dehydrogenase from Baker’s yeast involving precipitation and aqueous two phase extraction. Biochem. Eng. J. 2008, 38, 414–420. [Google Scholar] [CrossRef]
- Louwrier, A. Model isolations of nucleic acids from prokaryotic and eukaryotic sources using an organic/aqueous biphasic system. Biotechnol. Tech. 1999, 13, 329–330. [Google Scholar] [CrossRef]
- Zhi, W.; Deng, Q. Purification of salvianolic acid B from the crude extract of Salvia miltiorrhiza with hydrophilic organic/salt-containing aqueous two-phase system by counter-current chromatography. J. Chromatogr. A 2006, 1116, 149–152. [Google Scholar] [CrossRef]
- Jorge, A.M.; Coutinho, J.A.; Pereira, J.F. Hydrodynamics of cholinium chloride-based aqueous biphasic systems (ABS): A key study for their industrial implementation. Sep. Purif. Technol. 2023, 320, 124183. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Rebelo, L.P.N.; Rogers, R.D.; Coutinho, J.A.P.; Freire, M.G. Combining ionic liquids and polyethylene glycols to boost the hydrophobic–hydrophilic range of aqueous biphasic systems. Phys. Chem. Chem. Phys. 2013, 15, 19580–19583. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.A.J.; Azevedo, A.M.; Ferreira, I.F.; de Vries, J.; Korporaal, R.; Verhoef, H.J.; Visser, T.J.; Aires-Barros, M.R. Affinity partitioning of human antibodies in aqueous two-phase systems. J. Chromatogr. A 2007, 1162, 103–113. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Rosa, P.A.J.; Ferreira, I.F.; Aires-Barros, M.R. Chromatography-free recovery of biopharmaceuticals through aqueous two-phase processing. Trends Biotechnol. 2009, 27, 240–247. [Google Scholar] [CrossRef]
- Santos, J.H.P.M.; Flores-Santos, J.C.; Meneguetti, G.P.; Rangel-Yagui, C.O.; Coutinho, J.A.P.; Vitolo, M.; Ventura, S.P.M.; Pessoa, A., Jr. In situ purification of periplasmatic L-asparaginase by aqueous two phase systems with ionic liquids (ILs) as adjuvants. J. Chem. Technol. Biotechnol. 2018, 93, 1871–1880. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef]
- Shahriari, S.; Tomé, L.C.; Araújo, J.M.; Rebelo, L.P.N.; Coutinho, J.A.; Marrucho, I.M.; Freire, M.G. Aqueous biphasic systems: A benign route using cholinium-based ionic liquids. RSC Adv. 2013, 3, 1835–1843. [Google Scholar] [CrossRef]
- Carvalho, S.F.; Pereiro, A.B.; Araújo, J.M.M. Simultaneous Purification of Human Interferon Alpha-2b and Serum Albumin Using Bioprivileged Fluorinated Ionic Liquid-Based Aqueous Biphasic Systems. Int. J. Mol. Sci. 2024, 25, 2751. [Google Scholar] [CrossRef] [PubMed]
- Bastos, J.C.; Carvalho, S.F.; Welton, T.; Lopes, J.N.C.; Rebelo, L.P.N.; Shimizu, K.; Araújo, J.M.M.; Pereiro, A.B. Design of task-specific fluorinated ionic liquids: Nanosegregation versus hydrogen-bonding ability in aqueous solutions. Chem. Commun. 2018, 54, 3524–3527. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Araújo, J.M.; Teixeira, F.S.; Marrucho, I.M.; Piñeiro, M.M.; Rebelo, L.P.N. Aggregation behavior and total miscibility of fluorinated ionic liquids in water. Langmuir 2015, 31, 1283–1295. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Bastos, J.C.; Rebelo, L.P.N.; Matias, A.; Araújo, J.M.M.; Pereiro, A.B. Human cytotoxicity and octanol/water partition coefficients of fluorinated ionic liquids. Chemosphere 2019, 216, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Vieira, N.S.M.; Stolte, S.; Araújo, J.M.M.; Rebelo, L.P.N.; Pereiro, A.B.; Markiewicz, M. Acute Aquatic Toxicity and Biodegradability of Fluorinated Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 3733–3741. [Google Scholar] [CrossRef]
- Alves, M.; Vieira, N.S.M.; Rebelo, L.P.N.; Araújo, J.M.M.; Pereiro, A.B.; Archer, M. Fluorinated ionic liquids for protein drug delivery systems: Investigating their impact on the structure and function of lysozyme. Int. J. Pharm. 2017, 526, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; Vieira, N.S.M.; Araújo, J.M.M.; Pereiro, A.B. Unveiling the Influence of Non-Toxic Fluorinated Ionic Liquids Aqueous Solutions in the Encapsulation and Stability of Lysozyme. Sustain. Chem. 2021, 2, 149–166. [Google Scholar] [CrossRef]
- Alves, M.M.S.; Araújo, J.M.M.; Martins, I.C.; Pereiro, A.B.; Archer, M. Insights into the interaction of Bovine Serum Albumin with Surface-Active Ionic Liquids in aqueous solution. J. Mol. Liq. 2021, 322, 114537. [Google Scholar] [CrossRef]
- Alves, M.M.; Leandro, P.; Mertens, H.D.; Pereiro, A.B.; Archer, M. Impact of Fluorinated Ionic Liquids on Human Phenylalanine Hydroxylase—A Potential Drug Delivery System. Nanomaterials 2022, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; Vieira, N.S.; Oliveira, A.L.; Araújo, J.M.; Pereiro, A.B. Disclosing the Potential of Fluorinated Ionic Liquids as Interferon-Alpha 2b Delivery Systems. Nanomaterials 2022, 12, 1851. [Google Scholar] [CrossRef] [PubMed]
- Haria, M.; Benfield, P. Inlerferon-a-2a A Review of its Pharmacological Properties and Therapeutic Use in the Management of Viral Hepatitis. Drugs 1995, 50, 873–896. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Summary of recombinant human serum albumin development. Biologicals 2006, 34, 55–59. [Google Scholar] [CrossRef]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef]
- Ascoli, G.A.; Domenici, E.; Bertucci, C. Drug binding to human serum albumin: Abridged review of results obtained with high-performance liquid chromatography and circular dichroism. Chirality 2006, 18, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C. Proteins in Ionic Liquids: Current Status of Experiments and Simulations. Top. Curr. Chem. 2017, 375, 25. [Google Scholar] [CrossRef] [PubMed]
- Molodenskiy, D.; Shirshin, E.; Tikhonova, T.; Gruzinov, A.; Peters, G.; Spinozzi, F. Thermally induced conformational changes and protein–protein interactions of bovine serum albumin in aqueous solution under different pH and ionic strengths as revealed by SAXS measurements. Phys. Chem. Chem. Phys. 2017, 19, 17143–17155. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.V.S.; Benedetto, A. Ionic liquids in protein amyloidogenesis: A brief screenshot of the state-of-the-art. Biophys. Rev. 2018, 10, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Faustino, V.F.M.; Mondal, D.; Coutinho, J.A.P.; Freire, M.G. Improving the extraction and purification of immunoglobulin G by the use of ionic liquids as adjuvants in aqueous biphasic systems. J. Biotechnol. 2016, 236, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, A.; Kumar, S.; Venkatesu, P. Contemporary Advancement of Cholinium-Based Ionic Liquids for Protein Stability and Long-Term Storage: Past, Present, and Future Outlook. ACS Sustain. Chem. Eng. 2022, 10, 4323–4344. [Google Scholar] [CrossRef]
- Swaminathan, R.; Ravi, V.K.; Kumar, S.; Kumar, M.V.S.; Chandra, N. Lysozyme: A model protein for amyloid research. Adv. Protein Chem. Struct. Biol. 2011, 84, 63–111. [Google Scholar] [CrossRef] [PubMed]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Imoto, T.; Forstert, L.S.; Rupley, J.A.; Tanaka, F. Fluorescence of Lysozyme: Emissions from Tryptophan Residues 62 and 108 and Energy Migration. Proc. Natl. Acad. Sci. USA 1972, 69, 1151–1155. [Google Scholar] [CrossRef]
- Neves, C.M.; Ventura, S.P.; Freire, M.G.; Marrucho, I.M.; Coutinho, J.A. Evaluation of cation influence on the formation and extraction capability of ionic-liquid-based aqueous biphasic systems. J. Phys. Chem. B 2009, 113, 5194–5199. [Google Scholar] [CrossRef]
- Ventura, S.P.; Sousa, S.G.; Serafim, L.S.; Lima, Á.S.; Freire, M.G.; Coutinho, J.A. Ionic liquid based aqueous biphasic systems with controlled pH: The ionic liquid cation effect. J. Chem. Eng. Data 2011, 56, 4253–4260. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Merchuk, J.C.; Andrews, B.A.; Asenjo, J.A. Aqueous two-phase systems for protein separation: Studies on phase inversion. J. Chromatogr. B Biomed. Sci. Appl. 1998, 711, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Bridges, N.J.; Gutowski, K.E.; Rogers, R.D. Investigation of aqueous biphasic systems formed from solutions of chaotropic salts with kosmotropic salts (salt–salt ABS). Green Chem. 2007, 9, 177–183. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Sousa, S.G.; Serafim, L.S.; Lima, Á.S.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Based Aqueous Biphasic Systems with Controlled pH: The Ionic Liquid Anion Effect. J. Chem. Eng. Data 2012, 57, 507–512. [Google Scholar] [CrossRef]
- Khan, I.; Kurnia, K.A.; Mutelet, F.; Pinho, S.P.; Coutinho, J.A.P. Probing the Interactions between Ionic Liquids and Water: Experimental and Quantum Chemical Approach. J. Phys. Chem. B 2014, 118, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Pei, Y.; Wang, J.; He, M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012, 14, 2941–2950. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Kurnia, K.A.; Freire, M.G.; Coutinho, J.A.P.; Rogers, R.D. Controlling the Formation of Ionic-Liquid-based Aqueous Biphasic Systems by Changing the Hydrogen-Bonding Ability of Polyethylene Glycol End Groups. ChemPhysChem 2015, 16, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Carralero, C.; Ruiz-Aceituno, L.; Ramos, L.; Moreno, F.J.; Sanz, M.L. Influence of Chemical Structure on the Solubility of Low Molecular Weight Carbohydrates in Room Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2014, 53, 13843–13850. [Google Scholar] [CrossRef]
- Paduszyński, K.; Okuniewski, M.; Domańska, U. “Sweet-in-Green” Systems Based on Sugars and Ionic Liquids: New Solubility Data and Thermodynamic Analysis. Ind. Eng. Chem. Res. 2013, 52, 18482–18491. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Kumari, M.; Dohare, N.; Maurya, N.; Dohare, R.; Patel, R. Effect of 1-methyl-3-octyleimmidazolium chloride on the stability and activity of lysozyme: A spectroscopic and molecular dynamics studies. J. Biomol. Struct. Dyn. 2017, 35, 2016–2030. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Singh, U.K.; Beg, I.; Alanazi, A.M.; Khan, A.A.; Patel, R. Effect of cations and anions of ionic liquids on the stability and activity of lysozyme: Concentration and temperature effect. J. Mol. Liq. 2018, 272, 253–263. [Google Scholar] [CrossRef]
- Rodrigues, J.V.; Prosinecki, V.; Marrucho, I.; Rebelo, L.P.N.; Gomes, C.M. Protein stability in an ionic liquid milieu: On the use of differential scanning fluorimetry. Phys. Chem. Chem. Phys. 2011, 13, 13614–13616. [Google Scholar] [CrossRef]
- Satish, L.; Rana, S.; Arakha, M.; Rout, L.; Ekka, B.; Jha, S.; Dash, P.; Sahoo, H. Impact of imidazolium-based ionic liquids on the structure and stability of lysozyme. Spectrosc. Lett. 2016, 49, 383–390. [Google Scholar] [CrossRef]
- Vrikkis, R.M.; Fraser, K.J.; Fujita, K.; MacFarlane, D.R.; Elliott, G.D. Biocompatible Ionic Liquids: A New Approach for Stabilizing Proteins in Liquid Formulation. J. Biomech. Eng. 2009, 131, 074514. [Google Scholar] [CrossRef]
- Pandit, S.; Kundu, S.; Abbas, S.; Aswal, V.K.; Kohlbrecher, J. Structures and interactions among lysozyme proteins below the isoelectric point in presence of divalent ions. Chem. Phys. Lett. 2018, 711, 8–14. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, T.A.; Singh, L.R.; Rather, G.M.; Bhat, M.A. Structural-functional integrity of lysozyme in imidazolium based surface active ionic liquids. Int. J. Biol. Macromol. 2020, 156, 271–279. [Google Scholar] [CrossRef]
- Mandal, B.; Mondal, S.; Pan, A.; Moulik, S.P.; Ghosh, S. Physicochemical study of the interaction of lysozyme with surface active ionic liquid 1-butyl-3-methylimidazolium octylsulfate [BMIM] [OS] in aqueous and buffer media. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 484, 345–353. [Google Scholar] [CrossRef]
- Mandal, B.; Ghosh, S.; Moulik, S.P. Detailed characterization of lysozyme (Lyz)–surfactant (SDDS) interaction and the structural transitions. New J. Chem. 2016, 40, 4617–4624. [Google Scholar] [CrossRef]
- Bisht, M.; Kumar, A.; Venkatesu, P. Analysis of the driving force that rule the stability of lysozyme in alkylammonium-based ionic liquids. Int. J. Biol. Macromol. 2015, 81, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Segawa, S.I.; Sugihara, M. Characterization of the transition state of lysozyme unfolding. II. Effects of the intrachain crosslinking and the inhibitor binding on the transition state. Biopolymers 1984, 23, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Chactfn, P.; Merelo, J.J.; Mordn, F. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. Des. Sel. 1993, 6, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Corin, K.; Baaske, P.; Ravel, D.B.; Song, J.; Brown, E.; Wang, X.; Wienken, C.J.; Jerabek-Willemsen, M.; Duhr, S.; Luo, Y.; et al. Designer Lipid-Like Peptides: A Class of Detergents for Studying Functional Olfactory Receptors Using Commercial Cell-Free Systems. PLoS ONE 2011, 6, e25067. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; Volume 1. [Google Scholar] [CrossRef]
- Weaver, K.D.; Vrikkis, R.M.; Vorst, M.P.V.; Trullinger, J.; Vijayaraghavan, R.; Foureau, D.M.; McKillop, I.H.; MacFarlane, D.R.; Krueger, J.K.; Elliott, G.D. Structure and function of proteins in hydrated choline dihydrogen phosphate ionic liquid. Phys. Chem. Chem. Phys. 2012, 14, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kukutla, P.; Devunuri, N.; Venkatesu, P. How does cholinium cation surpass tetraethylammonium cation in amino acid-based ionic liquids for thermal and structural stability of serum albumins? Int. J. Biol. Macromol. 2020, 148, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Forciniti, D. Studying the Influence of Salts on Partitioning of Proteins; Humana Press: Humana Totowa, NJ, USA, 2000; pp. 201–208. [Google Scholar] [CrossRef]
- Guan, Y.; Lilley, T.H.; Treffry, T.E.; Zhou, C.L.; Wilkinson, P.B. Use of aqueous two-phase systems in the purification of human interferon-α1 from recombinant Escherichia coli. Enzym. Microb. Technol. 1996, 19, 446–455. [Google Scholar] [CrossRef]
- Vieira, N.S.; Castro, P.J.; Marques, D.F.; Araújo, J.M.; Pereiro, A.B. Tailor-made fluorinated ionic liquids for protein delivery. Nanomaterials 2020, 10, 1594. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.R.; Tomé, C.S.; Russo, R.; Paterna, R.; Leandro, J.; Candeias, N.R.; Gonçalves, L.M.; Teixeira, M.; Sousa, P.M.; Guedes, R.C.; et al. Modulation of human phenylalanine hydroxylase by 3-hydroxyquinolin-2(1h)-one derivatives. Biomolecules 2021, 11, 462. [Google Scholar] [CrossRef]
- Toro, T.B.; Nguyen, T.P.; Watt, T.J. An improved 96-well turbidity assay for T4 lysozyme activity. Methods X 2015, 2, 256–262. [Google Scholar] [CrossRef]
- Johnston, M.J.W.; Nemr, K.; Hefford, M.A. Influence of bovine serum albumin on the secondary structure of interferon alpha 2b as determined by far UV circular dichroism spectropolarimetry. Biologicals 2010, 38, 314–320. [Google Scholar] [CrossRef]
- Johnson, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Shugar, D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta 1952, 8, 302–309. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Ferreira, A.S.D.; Araújo, J.M.M.; Cabrita, E.J.; Pereiro, A.B. The impact of fluorinated ionic liquids aggregation in the interactions with proteins. Fluid Phase Equilibria 2022, 559, 113488. [Google Scholar] [CrossRef]
- Li, R.; Wu, Z.; Wangb, Y.; Ding, L.; Wang, Y. Role of pH-induced structural change in protein aggregation in foam fractionation of bovine serum albumin. Biotechnol. Rep. 2016, 9, 46–52. [Google Scholar] [CrossRef]
- Hédoux, A.; Willart, J.F.; Paccou, L.; Guinet, Y.; Affouard, F.; Lerbret, A.; Descamps, M. Thermostabilization Mechanism of Bovine Serum Albumin by Trehalose. J. Phys. Chem. B 2009, 113, 6119–6126. [Google Scholar] [CrossRef] [PubMed]
- Banipal, T.S.; Kaur, A.; Banipal, P.K. Physicochemical aspects of the energetics of binding of sulphanilic acid with bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 170, 214–225. [Google Scholar] [CrossRef]
- Russell, B.A.; Kubiak-Ossowska, K.; Mulheran, P.A.; Birch, D.J.S.; Chen, Y. Locating the nucleation sites for protein encapsulated gold nanoclusters: A molecular dynamics and fluorescence study. Phys. Chem. Chem. Phys. 2015, 17, 21935–21941. [Google Scholar] [CrossRef]
- Rezwan, K.; Studart, A.R.; Vörös, J.; Gauckler, L.J. Change of ζ Potential of Biocompatible Colloidal Oxide Particles upon Adsorption of Bovine Serum Albumin and Lysozyme. J. Phys. Chem. B 2005, 109, 14469–14474. [Google Scholar] [CrossRef]
- Andrews, B.A.; Schmidt, A.S.; Asenjo, J.A. Correlation for the partition behavior of proteins in aqueous two-phase systems: Effect of surface hydrophobicity and charge. Biotechnol. Bioeng. 2005, 90, 380–390. [Google Scholar] [CrossRef]
- Moon, Y.U.; Curtis, R.A.; Anderson, C.O.; Blanch, H.W.; Prausnitz, J.M. Protein—Protein Interactions in Aqueous Ammonium Sulfate Solutions. Lysozyme and Bovine Serum Albumin (BSA). J. Solut. Chem. 2000, 29, 699–718. [Google Scholar] [CrossRef]
- Townsend, R.J.; Hill, M.; Harris, N.R.; White, N.M. Modelling of particle paths passing through an ultrasonic standing wave. Ultrasonics 2004, 42, 319–324. [Google Scholar] [CrossRef]
- Santos, M.B.; de Carvalho, C.W.P.; Garcia-Rojas, E.E. Heteroprotein complex formation of bovine serum albumin and lysozyme: Structure and thermal stability. Food Hydrocoll. 2018, 74, 267–274. [Google Scholar] [CrossRef]
- Sarmah, R.J.; Kundu, S. Structure and morphology of bovine serum albumin–lysozyme (BSA–Lys) complex films at air–water interface. Food Hydrocoll. 2022, 131, 107788. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, L.; Yang, R.; Qu, L.B.; Harrington, P.D.B. A novel method for the study of molecular interaction by using microscale thermophoresis. Talanta 2015, 132, 894–901. [Google Scholar] [CrossRef]
- Jerabek-Willemsen, M.; André, T.; Wanner, R.; Roth, H.M.; Duhr, S.; Baaske, P.; Breitsprecher, D. MicroScale Thermophoresis: Interaction analysis and beyond. J. Mol. Struct. 2014, 1077, 101–113. [Google Scholar] [CrossRef]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 2010, 1, 100. [Google Scholar] [CrossRef]
- Khavrutskii, L.A.; Yeh, J.A.; Timofeeva, O.A.; Tarasov, S.G.A.; Pritt, S.A.; Stefanisko, K.A.; Tarasova, N.A. Protein Purification-free Method of Binding Affinity Determination by Microscale Thermophoresis. JoVE 2013, e50541. [Google Scholar] [CrossRef]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef]
- Zahradník, J.; Kolářová, L.; Pařízková, H.; Kolenko, P.; Schneider, B. Interferons type II and their receptors R1 and R2 in fish species: Evolution, structure, and function. Fish Shellfish Immunol. 2018, 79, 140–152. [Google Scholar] [CrossRef]
- Tosstorff, A.; Svilenov, H.; Peters, G.H.J.; Harris, P.; Winter, G. Structure-based discovery of a new protein-aggregation breaking excipient. Eur. J. Pharm. Biopharm. 2019, 144, 207–216. [Google Scholar] [CrossRef]
- Corrêa, D.; Henrique, C.; Ramos, I.; Corrêa, D.H.A.; Ramos, C.H.I. The use of circular dichroism spectroscopy to study protein folding, form and function Structure and function of molecular chaperones View project Protein stability, folding and misfolding View project The use of circular dichroism spectroscopy to study protein folding, form and function. Afr. J. Biochem. Res. 2009, 3, 164–173. [Google Scholar]
- Sundaram, V.; Ramanan, R.N.; Selvaraj, M.; Vijayaraghavan, R.; MacFarlane, D.R.; Ooi, C.W. Enhanced structural stability of insulin aspart in cholinium aminoate ionic liquids. Int. J. Biol. Macromol. 2022, 208, 544–552. [Google Scholar] [CrossRef]
- MacKenzie, R.; Honig, P.; Sewards, J.; Goodwin, R.; Hellio, M.P. COVID-19 must catalyse changes to clinical development. Nat. Rev. Drug Discov. 2020, 19, 653–654. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef]


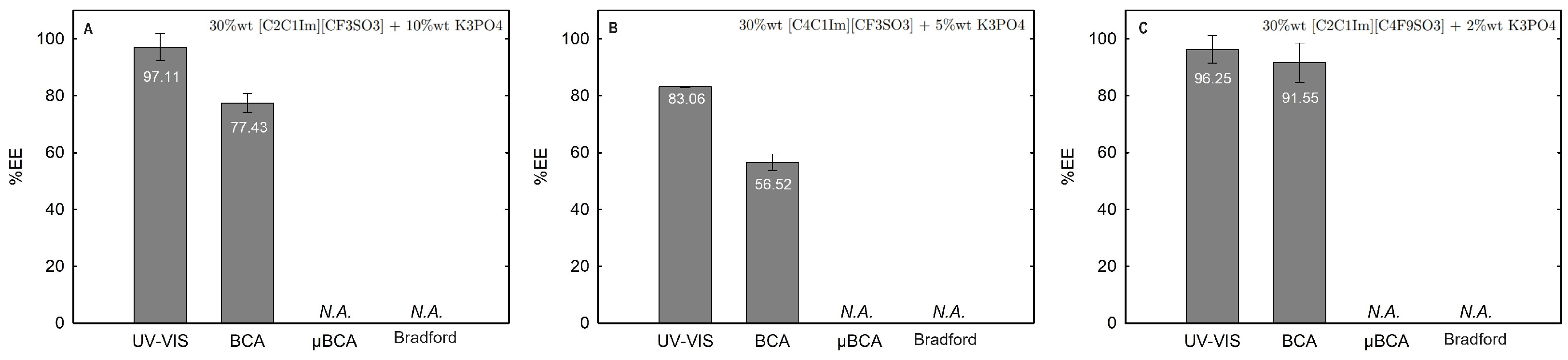

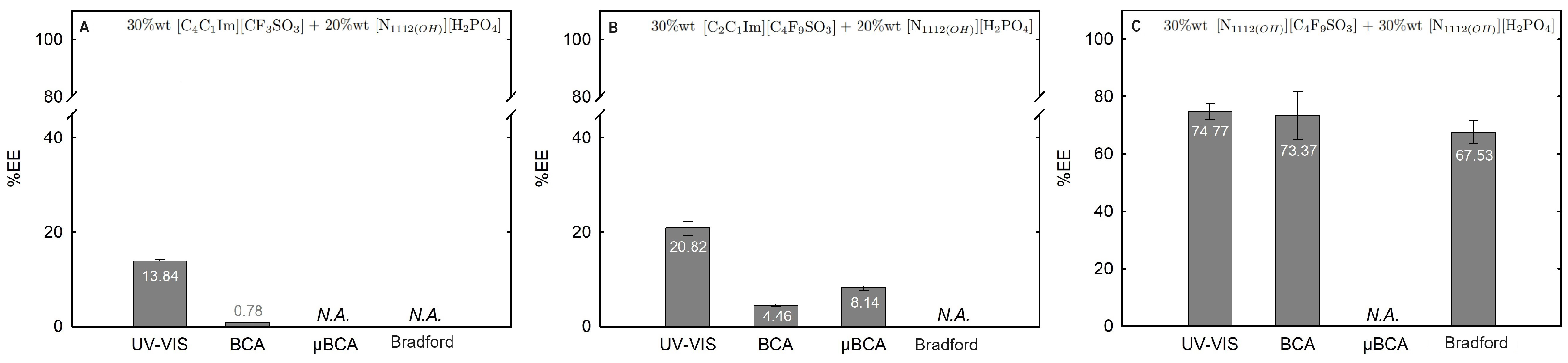
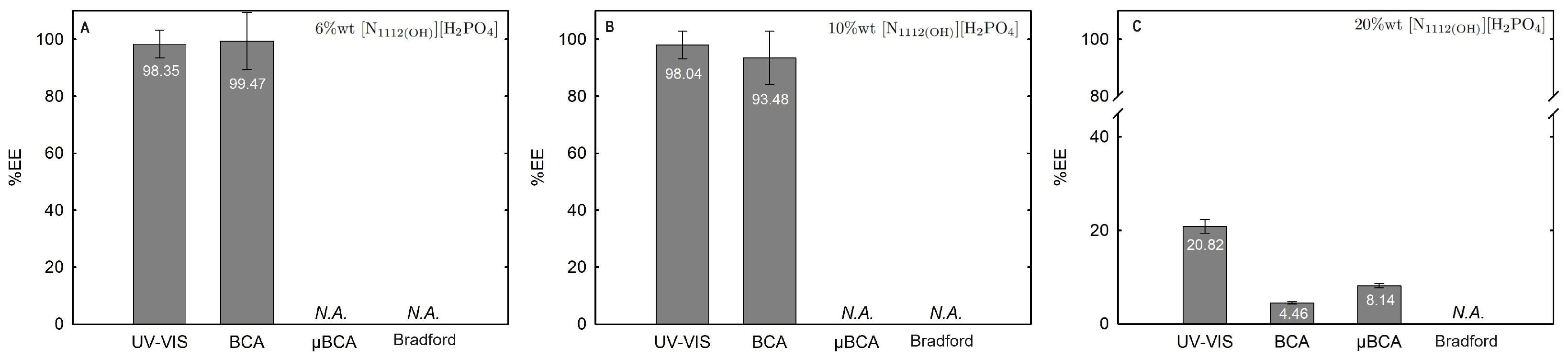
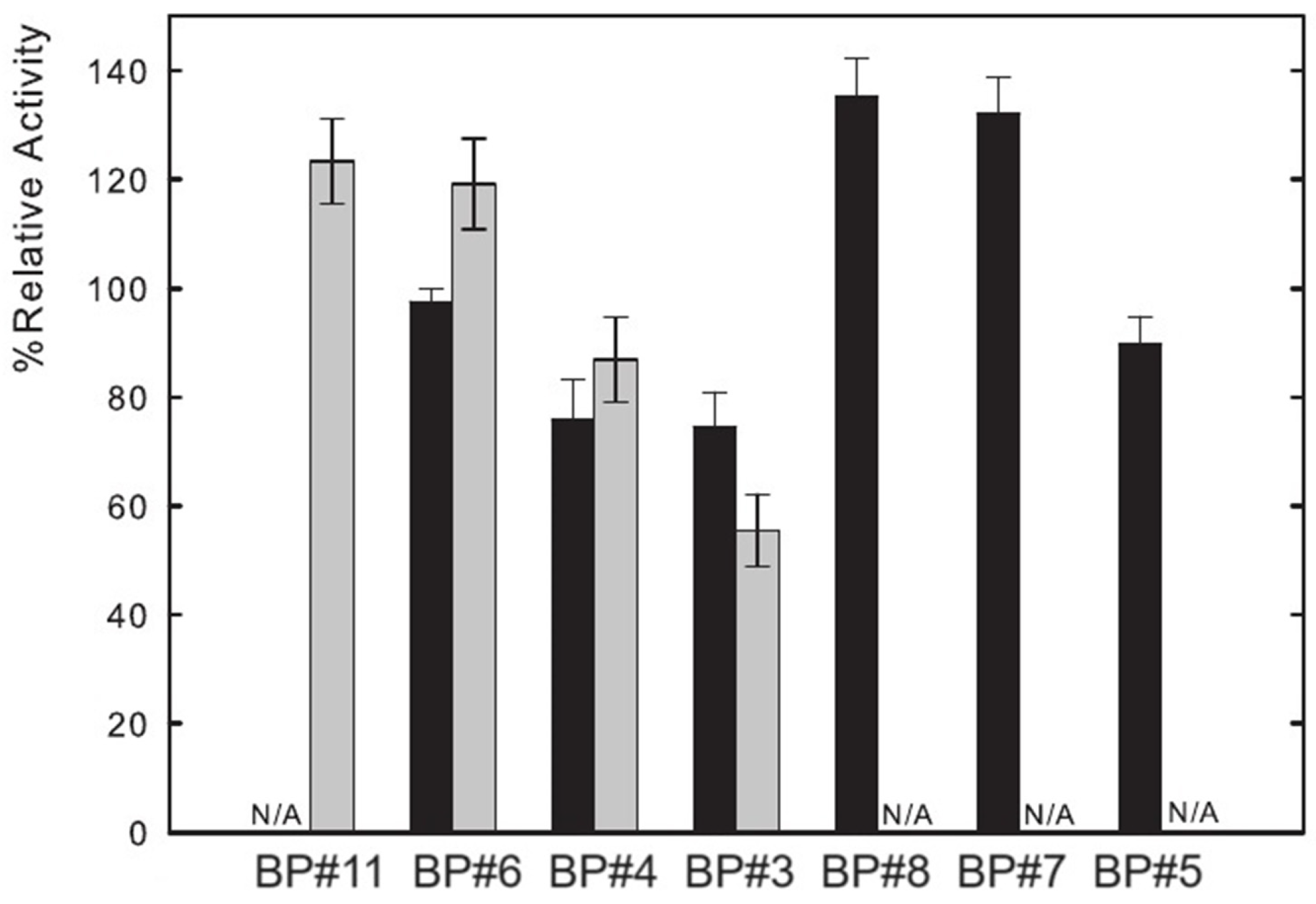

| Ionic-Liquid-Rich Phase (Bottom Phase) | Non-Ionic-Liquid-Rich Phase (Top Phase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BP# | ABS Composition (%wt) | Volume Ratio † | pH | %wt H2O | %wt IL | %wt non-IL | pH | %wt H2O | %wt IL | %wt non-IL |
| BP#1 | 30% [C2C1Im][CF3SO3] + 10% K3PO4 | 0.60 | 13.44 | 40.8785 | 56.5614 | 2.0652 | 13.18 | 73.4861 | 8.7068 | 16.8167 |
| BP#2 | 30% [C4C1Im][CF3SO3] + 5% K3PO4 | 0.40 | 12.87 | 34.7684 | 64.4549 | 0.8176 | 13.25 | 78.6973 | 14.3401 | 6.7798 |
| BP#3 | 30% [C2C1Im][C4F9SO3] + 2% K3PO4 | 0.94 | 12.78 | 43.6779 | 54.0350 | 0.3349 | 12.70 | 88.5482 | 4.2344 | 6.7525 |
| BP#4 | 30% [C4C1Im][CF3SO3] + 25% sucrose | 0.39 | 5.00 | 31.1747 | 57.6533 | 10.3701 | 5.25 | 47.1635 | 17.7208 | 32.0208 |
| BP#5 | 30% [C2C1Im][C4F9SO3] + 25% sucrose | 0.33 | 6.75 | 27.6055 | 66.8372 | 6.2338 | 6.75 | 53.5971 | 15.5558 | 31.8248 |
| BP#6 | 30% [C4C1Im][CF3SO3] + 25% glucose | 0.42 | 5.25 | 24.8533 | 72.0629 | 4.7980 | 5.25 | 53.2700 | 11.9669 | 32.7273 |
| BP#7 | 30% [C2C1Im][C4F9SO3] + 25% glucose | 0.43 | 6.50 | 20.5454 | 76.1935 | 4.1664 | 6.50 | 58.9178 | 7.7100 | 34.4053 |
| BP#8 | 30% [C2C1Im][C4F9SO3] + 6% [][H2PO4] | 0.89 | 3.37 | 41.3607 | 55.5876 | 1.5481 | 3.41 | 84.2108 | 4.0618 | 12.1251 |
| BP#9 | 30% [C2C1Im][C4F9SO3] + 10% [][H2PO4] | 0.59 | 3.65 | 17.7743 | 83.7801 | 0.6989 | 3.56 | 67.3516 | 5.1015 | 28.3455 |
| BP#10 | 30% [C2C1Im][C4F9SO3] + 20% [][H2PO4] | 0.42 | 3.99 | 30.1971 | 69.0974 | 1.0569 | 4.03 | 83.6562 | 2.3820 | 15.7034 |
| BP#11 | 30% [C4C1Im][CF3SO3] + 20% [][H2PO4] | 0.25 | 3.87 | 27.6891 | 66.5303 | 5.7394 | 3.78 | 54.8263 | 21.0133 | 23.5764 |
| BP#12 | 30% [][C4F9SO3] + 30% [][H2PO4] | 0.60 | 4.15 | 25.3343 | 63.9061 | 10.1689 | 4.16 | 45.8046 | 7.6946 | 43.9659 |
| Ligand | Max. Conc. (mM) (First Capillary) | (mM) |
|---|---|---|
| [C2C1Im][C4F9SO3] | ]0, 14.40[ (monomer) | no binding detected |
| [14.4, 34.48[ (1st CAC) | 1.27 ± 0.64 | |
| [34.48, 76.54[ (2nd CAC) | 0.87 ± 0.36 | |
| [][C4F9SO3] | ]0, 16.02[ (monomer) | no binding detected |
| [16.02, 35.17[ (1st CAC) | 6.05 ± 1.67 | |
| [35.17, 185.65[ (2nd CAC) | 6.25 ± 1.47 | |
| [C4C1Im][CF3SO3] | ]0, 100] (monomer) | no binding detected |
| [][H2PO4] | ]0, 100] (monomer) | no binding detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, S.F.; Custódio, M.H.; Pereiro, A.B.; Araújo, J.M.M. Towards Enhanced Tunability of Aqueous Biphasic Systems: Furthering the Grasp of Fluorinated Ionic Liquids in the Purification of Proteins. Int. J. Mol. Sci. 2024, 25, 5766. https://doi.org/10.3390/ijms25115766
Carvalho SF, Custódio MH, Pereiro AB, Araújo JMM. Towards Enhanced Tunability of Aqueous Biphasic Systems: Furthering the Grasp of Fluorinated Ionic Liquids in the Purification of Proteins. International Journal of Molecular Sciences. 2024; 25(11):5766. https://doi.org/10.3390/ijms25115766
Chicago/Turabian StyleCarvalho, Sara F., Margarida H. Custódio, Ana B. Pereiro, and João M. M. Araújo. 2024. "Towards Enhanced Tunability of Aqueous Biphasic Systems: Furthering the Grasp of Fluorinated Ionic Liquids in the Purification of Proteins" International Journal of Molecular Sciences 25, no. 11: 5766. https://doi.org/10.3390/ijms25115766
APA StyleCarvalho, S. F., Custódio, M. H., Pereiro, A. B., & Araújo, J. M. M. (2024). Towards Enhanced Tunability of Aqueous Biphasic Systems: Furthering the Grasp of Fluorinated Ionic Liquids in the Purification of Proteins. International Journal of Molecular Sciences, 25(11), 5766. https://doi.org/10.3390/ijms25115766










