Exploring the Role of Extracellular Vesicles in Skeletal Muscle Regeneration
Abstract
1. Introduction
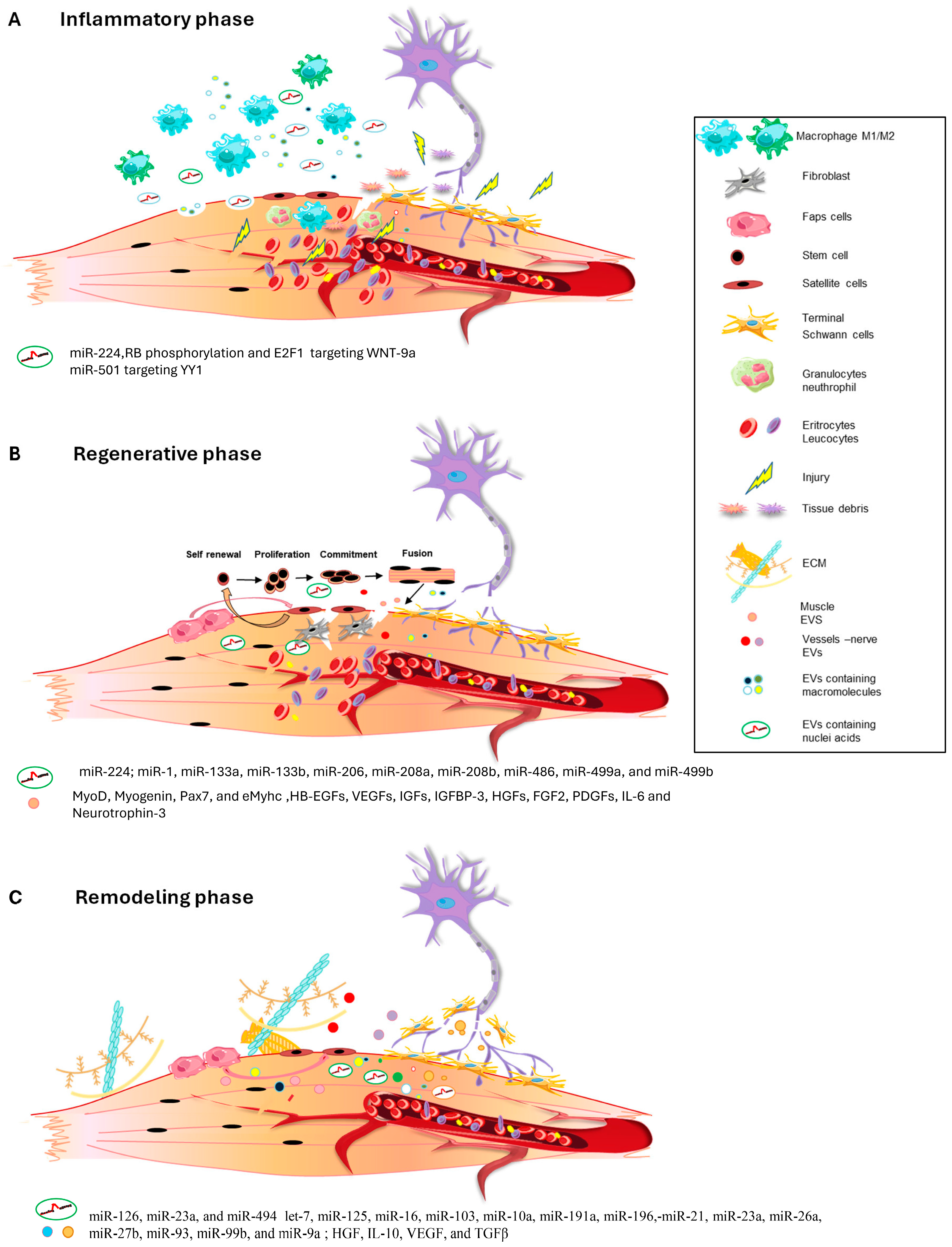
2. The Inflammatory Phase
3. The Regenerative Phase
4. The Remodeling Phase
5. Role of Extracellular Vesicles in Skeletal Muscle Pathologies
6. Extracellular Vesicle Delivery as a Therapeutic Tool for Tissue Repair and Regeneration
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Acronyms
| EVs | extracellular vesicles |
| miRNAs | microRNAs |
| SCs | satellite cells |
| lncRNAs | long non-coding RNAs |
| YY1 | Yin Yang 1 |
| RB | retinoblastoma |
| E2F1 | E2 promoter binding factor 1 |
| MSCs | mesenchymal stem cells |
| HSkMs | human skeletal muscle cells |
| Pax7 | paired box 7 |
| eMyhc | embryonal myosin heavy chain |
| EGF | epidermal growth factor |
| HB-EGFs | heparin-binding EGF-like growth factors |
| VEGFs | vascular endothelial growth factors |
| IGFs | insulin-like growth factors |
| IGFBP-3 | IGF-binding protein 3 |
| HGFs | hepatocyte growth factors |
| FGF2 | fibroblast growth factor-2 |
| PDGFs | platelet-derived growth factors |
| IL6 | interleukin 6 |
| RRBP1 | ribosome binding protein 1 |
| ECM | extracellular matrix |
| HGF | hepatocyte growth factor |
| VEGF | vascular endothelial growth factor |
| TGF-b | transforming growth factor b |
| FAPs | fibroadipogenic cells |
| PL-MSCs | placental MSCs |
| DMD | Duchenne muscular dystrophy |
| mTOR | mechanistic target of rapamycin |
| PEP | purified exosome product |
References
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Endo, T. Molecular mechanisms of skeletal muscle development, regeneration, and osteogenic conversion. Bone 2015, 80, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B.; Sonnet, C.; Lafuste, P.; Bassez, G.; Rimaniol, A.C.; Poron, F.; Authier, F.J.; Dreyfus, P.A.; Gherardi, R.K. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 2003, 163, 1133–1143. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Barberi, L.; Scicchitano, B.M.; De Rossi, M.; Bigot, A.; Duguez, S.; Wielgosik, A.; Stewart, C.; McPhee, J.; Conte, M.; Narici, M.; et al. Age-dependent alteration in muscle regeneration: The critical role of tissue niche. Biogerontology 2013, 14, 273–292. [Google Scholar] [CrossRef] [PubMed]
- MAURO, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Comai, G.; Tajbakhsh, S. Molecular and cellular regulation of skeletal myogenesis. Curr. Top. Dev. Biol. 2014, 110, 1–73. [Google Scholar] [PubMed]
- Chang, N.C.; Rudnicki, M.A. Satellite cells: The architects of skeletal muscle. Curr. Top. Dev. Biol. 2014, 107, 161–181. [Google Scholar]
- Konigsberg, U.R.; Lipton, B.H.; Konigsberg, I.R. The regenerative response of single mature muscle fibers isolated in vitro. Dev. Biol. 1975, 45, 260–275. [Google Scholar] [CrossRef]
- Bischoff, R. Regeneration of single skeletal muscle fibers in vitro. Anat. Rec. 1975, 182, 215–235. [Google Scholar] [CrossRef]
- Karpati, G.; Molnar, M.J. Muscle Fibre Regeneration in Human Skeletal Muscle Diseases. In Skeletal Muscle Repair and Regeneration; Advances in Muscle Research; Springer: Dordrecht, The Netherlands, 2008; Volume 3. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [PubMed]
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musarò, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 2011, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; I Goberdhan, D.C.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; P Driedonks, T.A.; Erdbrügger, U.; Falcon-Perez, J.M.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Jose Alcaraz, M.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Bach, M.; Bachurski, D.; Baharvand, H.; et al. (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles Minimal Inf. Stud. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.J.; Cavallini, L.; Ciardiello, C.; Sobreiro, M.R.; Morello, M.; et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327–11341. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Rodrigues, R.M.; Wang, X.; Seo, W.; Ma, J.; Hwang, S.; Fu, Y.; Trojnár, E.; Mátyás, C.; Zhao, S.; et al. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J. Clin. Invest. 2021, 131, e141513. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zheng, H.; Luo, Y.; Kong, Y.; An, M.; Li, Y.; He, W.; Gao, B.; Zhao, Y.; Huang, H.; et al. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J. Clin. Invest. 2021, 131, e146431. [Google Scholar] [CrossRef] [PubMed]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.T.; Lee, W.T.; Abedini, M.R.; Wang, P.W.; Shieh, D.-B.; Dilworth, F.J.; Carmona, E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2020, 39, 1600–1616. [Google Scholar] [CrossRef]
- Torralba, D.; Baixauli, F.; Villarroya-Beltri, C.; Fernández-Delgado, I.; Latorre-Pellicer, A.; Acín-Pérez, R.; Martín-Cófreces, N.B.; Jaso-Tamame, Á.L.; Iborra, S.; Jorge, I.; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018, 9, 2658. [Google Scholar] [CrossRef]
- Tavakoli Dargani, Z.; Singla, R.; Johnson, T.; Kukreja, R.; Singla, D.K. Exosomes derived from embryonic stem cells inhibit doxorubicin and inflammation-induced pyroptosis in muscle cells. Can. J. Physiol. Pharmacol. 2018, 96, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Malgundkar, S.H.; Tamimi, Y. Exosomes as crucial emerging tools for intercellular communication with therapeutic potential in ovarian cancer. Futur. Sci. OA 2023, 9, FSO833. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 2001, 294, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Diniz, G.P.; Wang, D.Z. Regulation of Skeletal Muscle by microRNAs. Compr. Physiol. 2016, 6, 1279–1294. [Google Scholar] [PubMed]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef]
- van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm, R.J.; Olson, E.N. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 2009, 17, 662–673. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, J. MicroRNAs in skeletal myogenesis. Cell Cycle 2011, 10, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, R.J.O.; Lindgren Niss, M.H.L.; Krook, A. Skeletal Muscle microRNAs: Roles in Differentiation, Disease and Exercise. Res. Perspect. Endocr. Interact. 2017, 67–81. [Google Scholar]
- O’Rourke, J.R.; Georges, S.A.; Seay, H.R.; Tapscott, S.J.; McManus, M.T.; Goldhamer, D.J.; Swanson, M.S.; Harfe, B.D. Essential role for Dicer during skeletal muscle development. Dev. Biol. 2007, 311, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.T.; Gartz, M.; Thomas, L.; Haberman, M.; Strande, J.L. Influence of microRNAs and exosomes in muscle health and diseases. J. Muscle Res. Cell Motil. 2020, 41, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef] [PubMed]
- De Santa, F.; Vitiello, L.; Torcinaro, A.; Ferraro, E. The Role of Metabolic Remodeling in Macrophage Polarization and Its Effect on Skeletal Muscle Regeneration. Antioxidants Redox Signal. 2019, 30, 1553–1598. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, D.; Saederup, N.; Charo, I.F.; Ransohoff, R.M.; Zhou, L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011, 25, 358–369. [Google Scholar] [CrossRef]
- Lu, H.; Huang, D.; Ransohoff, R.M.; Zhou, L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011, 25, 3344–3355. [Google Scholar] [CrossRef]
- Sun, D.; Martinez, C.O.; Ochoa, O.; Ruiz-Willhite, L.; Bonilla, J.R.; Centonze, V.E.; Waite, L.L.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 2009, 23, 382–395. [Google Scholar] [CrossRef]
- Contreras-Shannon, V.; Ochoa, O.; Reyes-Reyna, S.M.; Sun, D.; Michalek, J.E.; Kuziel, W.A.; McManus, L.M.; Shireman, P.K. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2-/- mice following ischemic injury. Am. J. Physiol. Cell Physiol. 2007, 292, C953–C967. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Shi, H.; Zhao, H.; Gao, R.; Weng, X.; Liu, R.; Li, X.; Zou, Y.; Hu, K.; et al. Exosomes derived from M1 macrophages aggravate neointimal hyperplasia following carotid artery injuries in mice through miR-222/CDKN1B/CDKN1C pathway. Cell Death Dis. 2019, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, B.; Liu, C.; Hu, M.; Tang, J.; Min, J.; Cheng, J.; Hong, L. M2 Macrophage-derived exosomal miR-501 contributes to pubococcygeal muscle regeneration. Int. Immunopharmacol. 2021, 101, 108223. [Google Scholar] [CrossRef]
- Luo, Z.; Qi, B.; Sun, Y.; Chen, Y.; Lin, J.; Qin, H.; Wang, N.; Shi, R.; Shang, X.; Chen, S.; et al. Engineering Bioactive M2 Macrophage-Polarized, Anti-inflammatory, miRNA-Based Liposomes for Functional Muscle Repair: From Exosomal Mechanisms to Biomaterials. Small 2022, 18, 2201957. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef]
- Youssef El Baradie, K.B.; Hamrick, M.W. Therapeutic application of extracellular vesicles for musculoskeletal repair & regeneration. Connect. Tissue Res. 2021, 62, 99–114. [Google Scholar] [PubMed]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Mellows, B.; Mitchell, R.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Denecke, B.; Musante, L.; Ramachandra, D.L.; Debacq-Chainiaux, F.; et al. Protein and Molecular Characterization of a Clinically Compliant Amniotic Fluid Stem Cell-Derived Extracellular Vesicle Fraction Capable of Accelerating Muscle Regeneration Through Enhancement of Angiogenesis. Stem Cells Dev. 2017, 26, 1316–1333. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolny, G.; Martone, J.; Lepore, E.; Casola, I.; Petrucci, A.; Inghilleri, M.; Morlando, M.; Colantoni, A.; Scicchitano, B.M.; Calvo, A.; et al. A longitudinal study defined circulating microRNAs as reliable biomarkers for disease prognosis and progression in ALS human patients. Cell death Discov. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Casola, I.; Scicchitano, B.M.; Lepore, E.; Mandillo, S.; Golini, E.; Nicoletti, C.; Barberi, L.; Dobrowolny, G.; Musarò, A. Circulating myomiRs in Muscle Denervation: From Surgical to ALS Pathological Condition. Cells 2021, 10, 2043. [Google Scholar] [CrossRef]
- Sandonà, M.; Consalvi, S.; Tucciarone, L.; De Bardi, M.; Scimeca, M.; Angelini, D.F.; Buffa, V.; D’Amico, A.; Bertini, E.S.; Cazzaniga, S.; et al. HDAC inhibitors tune miRNAs in extracellular vesicles of dystrophic muscle-resident mesenchymal cells. EMBO Rep. 2020, 21, e50863. [Google Scholar] [CrossRef]
- Yue, B.; Yang, H.; Wang, J.; Ru, W.; Wu, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020, 53, e12857. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Berenstein, P.; Kronfeld, N.; Morgoulis, D.; Ziv-Av, A.; Goldstein, H.; Kazimirsky, G.; Cazacu, S.; Meir, R.; Popovtzer, R.; et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials 2018, 174, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Gong, W.; Wang, Q.; Wang, P.; Shi, T.; Mahmut, A.; Qin, J.; Yao, Y.; Yan, W.; Chen, D.; et al. Atrophic skeletal musle fiber-derived small extracellular vesicle miR-690 inhibits satellite cell differentiation during ageing. J. Cachexia Sarcopenia Muscle 2022, 13, 3163–3180. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Miano, C.; Pelosi, L.; Musarò, A. An Overview about the Biology of Skeletal Muscle Satellite Cells. Curr. Genomics 2019, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Bittel, D.C.; Jaiswal, J.K. Contribution of Extracellular Vesicles in Rebuilding Injured Muscles. Front. Physiol. 2019, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, X.; Xiang, Y.; Fan, D.; An, Q.; Yue, G.; Jin, Z.; Ding, J.; Hu, Y.; Du, Q.; et al. Exosome-mediated effects and applications in inflammatory diseases of the digestive system. Eur. J. Med. Res. 2022, 27, 163. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Aswad, H.; Jalabert, A.; Rome, S. Depleting extracellular vesicles from fetal bovine serum alters proliferation and differentiation of skeletal muscle cells in vitro. BMC Biotechnol. 2016, 16, 32. [Google Scholar] [CrossRef]
- Ji, S.; Ma, P.; Cao, X.; Wang, J.; Yu, X.; Luo, X.; Lu, J.; Hou, W.; Zhang, Z.; Yan, Y.; et al. Myoblast-derived exosomes promote the repair and regeneration of injured skeletal muscle in mice. FEBS Open Bio 2022, 12, 2213–2226. [Google Scholar] [CrossRef]
- Choi, J.S.; Yoon, H.I.; Lee, K.S.; Choi, Y.C.; Yang, S.H.; Kim, I.S.; Cho, Y.W. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Control. Release 2016, 222, 107–115. [Google Scholar] [CrossRef]
- Nakasa, T.; Ishikawa, M.; Shi, M.; Shibuya, H.; Adachi, N.; Ochi, M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J. Cell. Mol. Med. 2010, 14, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Mytidou, C.; Koutsoulidou, A.; Katsioloudi, A.; Prokopi, M.; Kapnisis, K.; Michailidou, K.; Anayiotos, A.; Phylactou, L.A. Muscle-derived exosomes encapsulate myomiRs and are involved in local skeletal muscle tissue communication. FASEB J. 2021, 35, e21279. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Budnik, V.; Ruiz-Cañada, C.; Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Korkut, C.; Li, Y.; Koles, K.; Brewer, C.; Ashley, J.; Yoshihara, M.; Budnik, V. Regulation of Postsynaptic Retrograde Signaling by Presynaptic Exosome Release. Neuron 2013, 77, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Madison, R.D.; Robinson, G.A. Muscle-Derived Extracellular Vesicles Influence Motor Neuron Regeneration Accuracy. Neuroscience 2019, 419, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Ma, K.; Zhang, C.; Fu, X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: An emerging approach for treatment of ischemic diseases. Stem Cell Res. Ther. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, H.S.; Hong, I.S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chan, P. Skeletal Muscle Metabolic Alternation Develops Sarcopenia. Aging Dis. 2022, 13, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.P.; Thaden, J.J.; ten Have, G.A.M.; Walker, D.K.; Engelen, M.P.K.J. Metabolic phenotyping using kinetic measurements in young and older healthy adults. Metabolism 2018, 78, 167–178. [Google Scholar] [CrossRef]
- Aswad, H.; Forterre, A.; Wiklander, O.P.B.; Vial, G.; Danty-Berger, E.; Jalabert, A.; Lamazière, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Chemello, F.; Bean, C.; Cancellara, P.; Laveder, P.; Reggiani, C.; Lanfranchi, G. Microgenomic analysis in skeletal muscle: Expression signatures of individual fast and slow myofibers. PLoS ONE 2011, 6, e16807. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ruegg, U.T.; Takeda, S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int. J. Mol. Sci. 2018, 19, 2804. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, J.H.; Lee, M.J. Docosahexaenoic Acid, a Potential Treatment for Sarcopenia, Modulates the Ubiquitin–Proteasome and the Autophagy–Lysosome Systems. Nutrients 2020, 12, 2597. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, J.; Song, E.J. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch. Pharm. Res. 2020, 43, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Q.; Huang, Z.; Zhu, J.; Qiu, J.; Ma, W.; Yang, X.; Ding, F.; Sun, H. Isoquercitrin Delays Denervated Soleus Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation. Front. Physiol. 2020, 11, 553095. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Zaldivar, F.; Cooper, D.M.; Adams, G.R. IL-6-induced skeletal muscle atrophy. J. Appl. Physiol. 2005, 98, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Shen, Y.; Kim, I.M.; Weintraub, N.L.; Hamrick, M.; Tang, Y. Extracellular Vesicles for Muscle Atrophy Treatment. Adv. Exp. Med. Biol. 2023, 1418, 119–126. [Google Scholar]
- Wan, R.; Hussain, A.; Behfar, A.; Moran, S.L.; Zhao, C. The Therapeutic Potential of Exosomes in Soft Tissue Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 3869. [Google Scholar] [CrossRef]
- Campanella, C.; Bavisotto, C.C.; Logozzi, M.; Gammazza, A.M.; Mizzoni, D.; Cappello, F.; Fais, S. On the Choice of the Extracellular Vesicles for Therapeutic Purposes. Int. J. Mol. Sci. 2019, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, Y.; Han, S.; Zhang, W.; Zhou, Q.; Guan, H.; Liu, J.; Shi, J.; Su, L.; Hu, D. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J. Mol. Histol. 2017, 48, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W. Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Conceição, M.; Forcina, L.; Wiklander, O.P.B.; Gupta, D.; Nordin, J.Z.; Vrellaku, B.; McClorey, G.; Mäger, I.; Gӧrgens, A.; Lundin, P.; et al. Engineered extracellular vesicle decoy receptor-mediated modulation of the IL6 trans-signalling pathway in muscle. Biomaterials 2021, 266, 120435. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, L.; Zhang, J.; Chiang, C.L.; Pan, J.; Wang, X.; Kwak, K.J.; Li, H.; Zhao, R.; Rima, X.Y.; et al. Exosomal mRNAs for Angiogenic-Osteogenic Coupled Bone Repair. Adv. Sci. 2023, 10, E2302622. [Google Scholar] [CrossRef]
| Tissue/Source EVs | miRNAs Cargo | Physiological Effect | Reference |
|---|---|---|---|
| M2 macrophages | miR-501 | Decreased inflammation (Stress urinary incontinace/Pubococcygeal muscle) | [43] |
| Myogenic cells | miR224 | Increased inflammation indamaged muscle | [44] |
| Myogenic cells | miR-1, miR-133a, miR-133b, miR-206, miR-208a, miR-208b, miR-486, miR-499a, and miR-499b | Improved regeneration of damaged muscle | [45] |
| Satellite cells | miR-1, miR-133a, miR-133b, miR-206 | Muscle differentiation of adipose stem cells/inhibition of fibrotic tissue deposition | [46] |
| Mesenchymal stem cells | miR-126, miR-23a, miR-494 | Restoration of vessels integrity in damaged muscle | [47,48] |
| Schwann cells | let-7, miR-125, miR-16, miR-103, miR-10a, miR-191a, miR-196,-miR-21, miR-23a, miR-26a, miR-27b, miR-93, miR-99b, and miR-9a | Axonogenesis/guidance in damaged muscle | [49,50] |
| FAPS cells | miR-206 | Improvement of muscle regeneration in DMD | [51] |
| Adipocyte cells | miR27a | Insulin-resistance in Skeletal Muscle | [52] |
| Placental MSC | miR29c | Improvement muscle regeneration | [53] |
| Atrophic muscle fiber | miR690 | Sarcopenia | [54] |
| Clinical Trial Identifier | Status | Disease or Condition | Phase | Intervention |
|---|---|---|---|---|
| NCT04664738 | Active not recruiting | Skin graft, peripheral nerve regeneration, vaginal tissue regeneration | I | Purified Exosome Product |
| NCT04327635 | Enrolling by invitation | Myocardial infarction recovery | I | Purified Exosome Product |
| NCT06249412 | Not yet recruiting | Amyotrophic Lateral Sclerosis ALS7 | Not applicable | Purified Exosome Product |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcu, C.; Dobrowolny, G.; Scicchitano, B.M. Exploring the Role of Extracellular Vesicles in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2024, 25, 5811. https://doi.org/10.3390/ijms25115811
Porcu C, Dobrowolny G, Scicchitano BM. Exploring the Role of Extracellular Vesicles in Skeletal Muscle Regeneration. International Journal of Molecular Sciences. 2024; 25(11):5811. https://doi.org/10.3390/ijms25115811
Chicago/Turabian StylePorcu, Cristiana, Gabriella Dobrowolny, and Bianca Maria Scicchitano. 2024. "Exploring the Role of Extracellular Vesicles in Skeletal Muscle Regeneration" International Journal of Molecular Sciences 25, no. 11: 5811. https://doi.org/10.3390/ijms25115811
APA StylePorcu, C., Dobrowolny, G., & Scicchitano, B. M. (2024). Exploring the Role of Extracellular Vesicles in Skeletal Muscle Regeneration. International Journal of Molecular Sciences, 25(11), 5811. https://doi.org/10.3390/ijms25115811








