Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability
Abstract
1. Introduction
2. Results
2.1. Membrane-Bound Hemoglobin Subunits
2.2. Correlations between the Levels of Membrane-Bound Hb Subunits
2.3. Variability in the Level of the Membrane-Bound Hb Subunits
2.4. Deformability of RBCs
2.5. Correlations between the Content of the Membrane-Bound Hb Subunits and the RBC Deformability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. RBC Sample Sources
- Nine blood samples were collected from nonsmoker healthy male donors (18–37 years, group O+, Hb > 13 mg/dL) without known disorders;
- Blood was collected from six nonsmoker healthy donors (group O+) per the blood bank routine and stored in sterile bags in SAGM at 4 °C in the Hadassah Hospital Blood Bank until their expiration date (42 days).
4.2.2. Isolation of RBCs from Freshly Collected Blood
4.2.3. Packed RBCs (PRBC)
4.2.4. Preparation of RBC Membranes
4.2.5. Determination of MBHb Composition
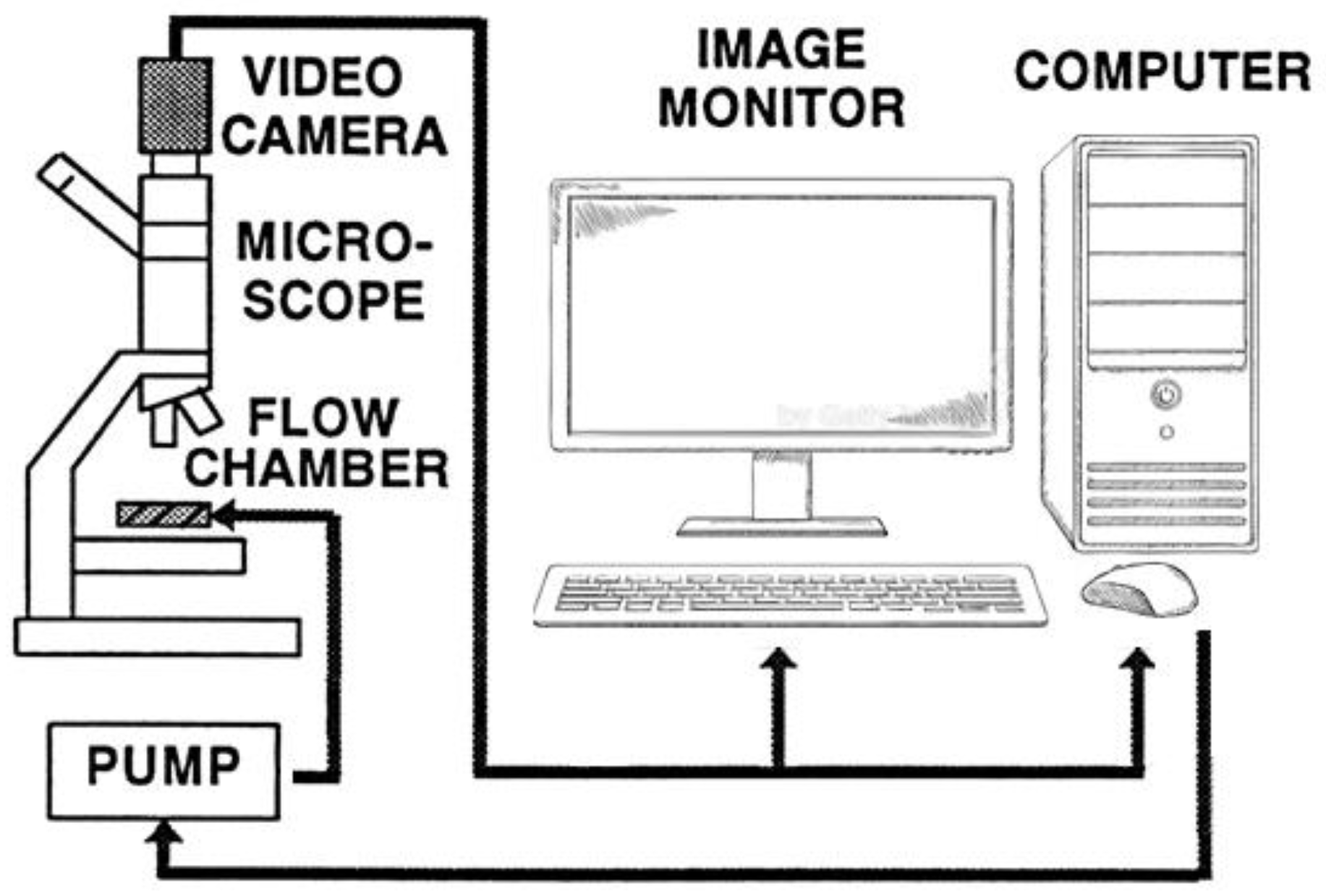
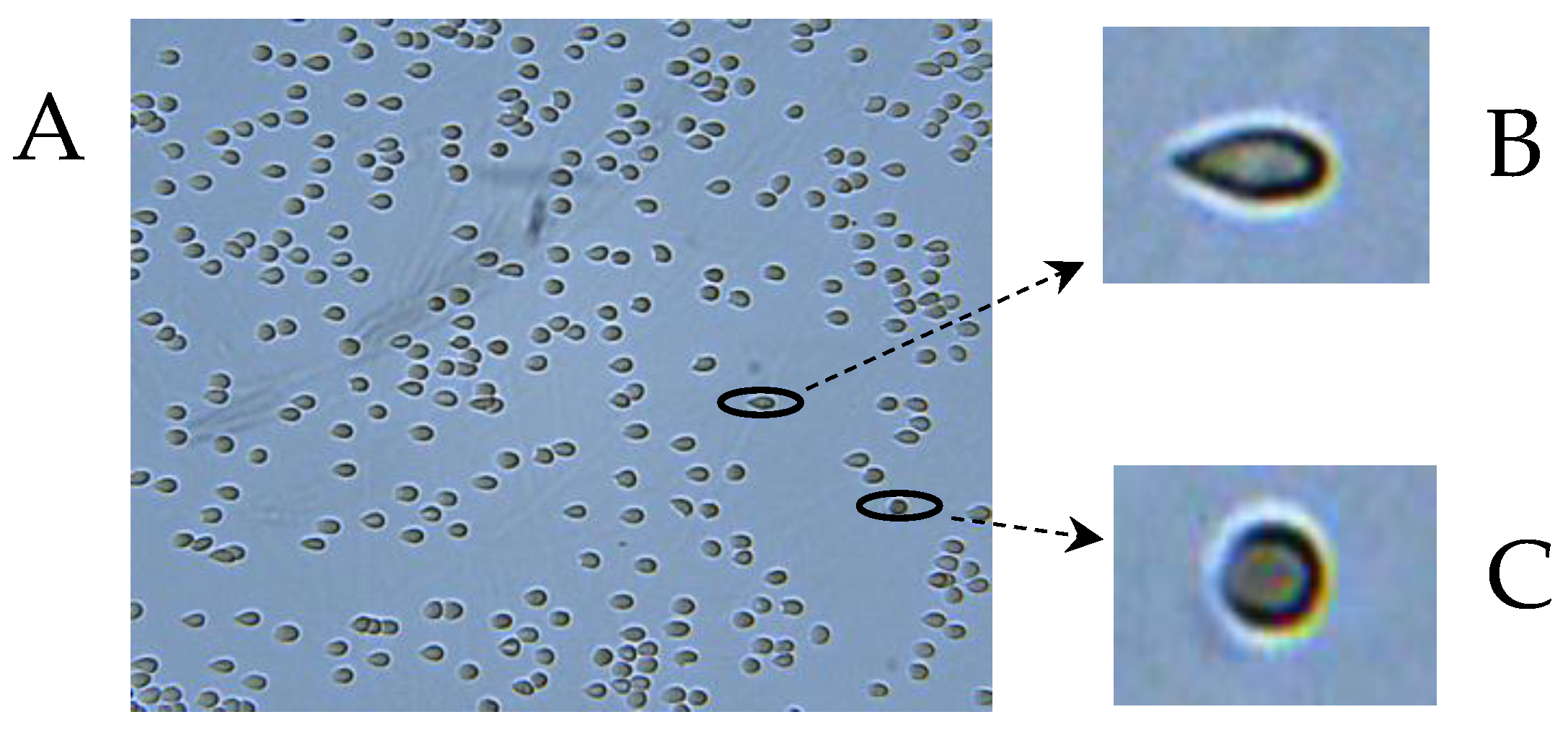
4.2.6. Determination of RBC Deformability
4.2.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guizouarn, H.; Barshtein, G. Editorial: Red Blood Cell Vascular Adhesion and Deformability, Volume II. Front. Physiol. 2022, 13, 849608. [Google Scholar] [CrossRef] [PubMed]
- Orbach, A.; Zelig, O.; Yedgar, S.; Barshtein, G. Biophysical and Biochemical Markers of Red Blood Cell Fragility. Transfus. Med. Hemotherapy 2017, 44, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Ii, S.; Sugiyama, K.; Noda, S.; Jing, P.; Liu, D.Y.; Che, X.J.; Gong, X.B. Effect of mechanical properties of red blood cells on their equilibrium states in microchannels. Phys. Fluids 2023, 35, 031910. [Google Scholar] [CrossRef]
- Renoux, C.; Faivre, M.; Bessaa, A.; Da Costa, L.; Joly, P.; Gauthier, A.; Connes, P. Impact of surface-area-to-volume ratio, internal viscosity and membrane viscoelasticity on red blood cell deformability measured in isotonic condition. Sci. Rep. 2019, 9, 6771. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, N.; Gallagher, P.G. Red cell membrane: Past, present, and future. Blood 2008, 112, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Chien, W.; Gompper, G.; Fedosov, D.A. Effect of cytosol viscosity on the flow behavior of red blood cell suspensions in microvessels. Microcirculation 2021, 28, e12668. [Google Scholar] [CrossRef] [PubMed]
- Bryk, A.H.; Wisniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.R. Red Cell Indices. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Cokelet, G.R.; Meiselman, H.J. Rheological comparison of hemoglobin solutions and erythrocyte suspensions. Science 1968, 162, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Brazhe, N.A.; Abdali, S.; Brazhe, A.R.; Luneva, O.G.; Bryzgalova, N.Y.; Parshina, E.Y.; Sosnovtseva, O.V.; Maksimov, G.V. New insight into erythrocyte through in vivo surface-enhanced Raman spectroscopy. Biophys. J. 2009, 97, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, Y.X.; Kang, L.L.; Wu, Z.J.; Luo, M. Effect of pH on molecular constitution and distribution of hemoglobin in living erythrocyte. Biopolymers 2010, 93, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.L.; Huang, Y.X.; Liu, W.J.; Zheng, X.J.; Wu, Z.J.; Luo, M. Confocal Raman microscopy on single living young and old erythrocytes. Biopolymers 2008, 89, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.M.; Turner, J.C. Relation of hemoglobin to the red cell membrane. J. Clin. Investig. 1960, 39, 1–7. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Nasybullinaa, E.I.; Blindarb, V.N.; Topunova, A.F. Binding of Erythrocyte Hemoglobin to the Membrane to Realize Signal-Regulatory Function. Appl. Biochem. Microbiol. 2019, 55, 83–98. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Topunov, A.F. Alternate and Additional Functions of Erythrocyte Hemoglobin. Biochemistry 2018, 83, 1575–1593. [Google Scholar] [CrossRef]
- Nash, G.B.; Meiselman, H.J. Red cell and ghost viscoelasticity. Effects of hemoglobin concentration and in vivo aging. Biophys. J. 1983, 43, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Seal, P.; Sikdar, J.; Haldar, R. Oxidative degradation perturbs physico-chemical properties of hemoglobin in cigarette smokers: A threat to different biomolecules. Inhal. Toxicol. 2021, 33, 275–284. [Google Scholar] [CrossRef]
- Shaklai, N.; Yguerabide, J.; Ranney, H.M. Classification and localization of hemoglobin binding sites on the red blood cell membrane. Biochemistry 1977, 16, 5593–5597. [Google Scholar] [CrossRef]
- Rauenbuehler, P.B.; Cordes, K.A.; Salhany, J.M. Identification of the hemoglobin binding sites on the inner surface of the erythrocyte membrane. Biochim. Biophys. Acta 1982, 692, 361–370. [Google Scholar] [CrossRef]
- Salhany, J.M.; Cordes, K.A.; Gaines, E.D. Light-scattering measurements of hemoglobin binding to the erythrocyte membrane. Evidence for transmembrane effects related to a disulfonic stilbene binding to band 3. Biochemistry 1980, 19, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Nagel, R.L.; Bookchin, R.M.; Roth, E.F., Jr.; Tellez-Nagel, I. The binding of hemoglobin to membranes of normal and sickle erythrocytes. Biochim. Biophys. Acta 1975, 375, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Sears, D.A.; Luthra, M.G. Membrane-bound hemoglobin in the erythrocytes of sickle cell anemia. J. Lab. Clin. Med. 1983, 102, 694–698. [Google Scholar] [PubMed]
- Wang, K.; Li, Z.; Egini, O.; Wadgaonkar, R.; Jiang, X.C.; Chen, Y. Atomic force microscopy reveals involvement of the cell envelope in biomechanical properties of sickle erythrocytes. BMC Biol. 2023, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Ghanashyam, C.; Shetty, S.; Bharati, S.; Chidangil, S.; Bankapur, A. Optical Trapping and Micro-Raman Spectroscopy of Functional Red Blood Cells Using Vortex Beam for Cell Membrane Studies. Anal. Chem. 2021, 93, 5484–5493. [Google Scholar] [CrossRef] [PubMed]
- Lane, P.A.; Galili, U.; Iarocci, T.A.; Shew, R.L.; Mentzer, W.C. Cellular dehydration and immunoglobulin binding in senescent neonatal erythrocytes. Pediatr. Res. 1988, 23, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P.; Schubert, D. Band 3-hemoglobin associations. The band 3 tetramer is the oxyhemoglobin binding site. FEBS Lett. 1991, 293, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Sega, M.F.; Chu, H.; Christian, J.A.; Low, P.S. Fluorescence assay of the interaction between hemoglobin and the cytoplasmic domain of erythrocyte membrane band 3. Blood Cells Mol. Dis. 2015, 55, 266–271. [Google Scholar] [CrossRef]
- Stefanovic, M.; Puchulu-Campanella, E.; Kodippili, G.; Low, P.S. Oxygen regulates the band 3-ankyrin bridge in the human erythrocyte membrane. Biochem. J. 2013, 449, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; McKenna, M.M.; Krump, N.A.; Zheng, S.; Mendelsohn, L.; Thein, S.L.; Garrett, L.J.; Bodine, D.M.; Low, P.S. Reversible binding of hemoglobin to band 3 constitutes the molecular switch that mediates O2 regulation of erythrocyte properties. Blood 2016, 128, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Breite, A.; Ciraolo, P.; Franco, R.S.; Low, P.S. Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: Implications for O2 regulation of erythrocyte properties. Blood 2008, 111, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Arashiki, N.; Kimata, N.; Manno, S.; Mohandas, N.; Takakuwa, Y. Membrane peroxidation and methemoglobin formation are both necessary for band 3 clustering: Mechanistic insights into human erythrocyte senescence. Biochemistry 2013, 52, 5760–5769. [Google Scholar] [CrossRef] [PubMed]
- Jenni, S.; Ludwig-Peisker, O.; Jagannathan, V.; Lapsina, S.; Stirn, M.; Hofmann-Lehmann, R.; Bogdanov, N.; Schetle, N.; Giger, U.; Leeb, T.; et al. Methemoglobinemia, Increased Deformability and Reduced Membrane Stability of Red Blood Cells in a Cat with a CYB5R3 Splice Defect. Cells 2023, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Welbourn, E.M.; Wilson, M.T.; Yusof, A.; Metodiev, M.V.; Cooper, C.E. The mechanism of formation, structure and physiological relevance of covalent hemoglobin attachment to the erythrocyte membrane. Free Radic. Biol. Med. 2017, 103, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; Bianchi, P.; Andolfo, I.; Russo, R.; Barcellini, W.; Fermo, E.; Toldi, G.; Ghirardello, S.; Rees, D.; Van Wijk, R.; et al. Recommendations for diagnosis and treatment of methemoglobinemia. Am. J. Hematol. 2021, 96, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E.; Voelter, W.; Fago, A.; Echner, H.; Campanella, E.; Low, P.S. Modulation of red cell glycolysis: Interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R454–R464. [Google Scholar] [CrossRef] [PubMed]
- Issaian, A.; Hay, A.; Dzieciatkowska, M.; Roberti, D.; Perrotta, S.; Darula, Z.; Redzic, J.; Busch, M.P.; Page, G.P.; Rogers, S.C.; et al. The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica 2021, 106, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Nikitaev, V.G.; Pronichev, A.N.; Polyakov, E.V.; Topunov, A.F. Hemoglobin as substantial object for biomedical studies and diagnostics. J. Phys. Conf. Ser. 2019, 1189, 012048. [Google Scholar] [CrossRef]
- Friederichs, E.; Meiselman, H.J.; Linderkamp, O. Calcium regulated membrane-attached hemoglobin and mechanical properties of density fractionated erythrocytes hembranes: Implications for hemolytic diseases and rbc ageing. Pediatr. Res. 1990, 28, 299. [Google Scholar] [CrossRef]
- Friederichs, E.; Farley, R.A.; Meiselman, H.J. Influence of calcium permeabilization and membrane-attached hemoglobin on erythrocyte deformability. Am. J. Hematol. 1992, 41, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Gural, A.; Arbell, D.; Barkan, R.; Livshits, L.; Pajic-Lijakovic, I.; Yedgar, S. Red Blood Cell Deformability Is Expressed by a Set of Interrelated Membrane Proteins. Int. J. Mol. Sci. 2023, 24, 12755. [Google Scholar] [CrossRef] [PubMed]
- Chien, S. Red cell deformability and its relevance to blood flow. Annu. Rev. Physiol. 1987, 49, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.H.; Werre, J.M.; Schipper, L.; Roerdinkholder-Stoelwinder, B.; Huls, T.; Willekens, F.L.; Wichers, G.; Halie, M.R. Determinants of red blood cell deformability in relation to cell age. Eur. J. Haematol. 1994, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.R. Mean corpuscular hemoglobin concentration and cell deformability. Ann. N. Y. Acad. Sci. 1989, 565, 284–294. [Google Scholar] [CrossRef] [PubMed]
- von Tempelhoff, G.F.; Schelkunov, O.; Demirhan, A.; Tsikouras, P.; Rath, W.; Velten, E.; Csorba, R. Correlation between blood rheological properties and red blood cell indices(MCH, MCV, MCHC) in healthy women. Clin. Hemorheol. Microcirc. 2016, 62, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, Y.; Xiong, Y.; Zhao, Y.; Tang, F.; Wang, X. Cluster of erythrocyte band 3: A potential molecular target of exhaustive exercise-induced dysfunction of erythrocyte deformability. Can. J. Physiol. Pharmacol. 2013, 91, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Pajic-Lijakovic, I.; Milivojevic, M. Role of Band 3 in the Erythrocyte Membrane Structural Changes Under Isotonic and Hypotonic Conditions. In Cytoskeleton: Structure, Dynamics, Function and Disease; Jimenez-Lopez, J.C., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Koshkaryev, A.; Yedgar, S.; Relevy, H.; Fibach, E.; Barshtein, G. Acridine orange induces translocation of phosphatidylserine to red blood cell surface. Am. J. Physiol. Cell Physiol. 2003, 285, C720–C722. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.F. Physiological characteristics of human red blood cell ghosts. J. Gen. Physiol. 1958, 42, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Livshits, L.; Peretz, S.; Bogdanova, A.; Zoabi, H.; Eitam, H.; Barshtein, G.; Galindo, C.; Feldman, Y.; Pajic-Lijakovic, I.; Koren, A.; et al. The Impact of Ca(2+) on Intracellular Distribution of Hemoglobin in Human Erythrocytes. Cells 2023, 12, 2280. [Google Scholar] [CrossRef]
- Datta, P.; Chakrabarty, S.; Chakrabarty, A.; Chakrabarti, A. Membrane interactions of hemoglobin variants, HbA, HbE, HbF and globin subunits of HbA: Effects of aminophospholipids and cholesterol. Biochim. Biophys. Acta 2008, 1778, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bennett, V. Spectrin-based membrane skeleton: A multipotential adaptor between plasma membrane and cytoplasm. Physiol. Rev. 1990, 70, 1029–1065. [Google Scholar] [CrossRef] [PubMed]
- Korsgren, C.; Cohen, C.M. Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3. J. Biol. Chem. 1988, 263, 10212–10218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Avsievich, T.; Su, X.; Bykov, A.; Popov, A.; Meglinski, I. Hemorheological alterations of red blood cells induced by 450-nm and 520-nm laser radiation. J. Photochem. Photobiol. B 2022, 230, 112438. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.H.; Belding, T.K.; Margolet, L.; Noyes, A.N. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science 1975, 188, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Dover, G.J.; Boyer, S.H.; Zinkham, W.H. Production of erythrocytes that contain fetal hemoglobin in anemia. Transient in vivo changes. J. Clin. Investig. 1979, 63, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.H.; Rodgers, G.P. HbA2: Biology, clinical relevance and a possible target for ameliorating sickle cell disease. Br. J. Haematol. 2015, 170, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Shaklai, N.; Ranney, H.R. Interaction of hemoglobin with membrane lipids: A source of pathological phenomena. Isr. J. Med. Sci. 1978, 14, 1152–1156. [Google Scholar] [PubMed]
- Basu, A.; Chakrabarti, A. Hemoglobin interacting proteins and implications of spectrin hemoglobin interaction. J. Proteom. 2015, 128, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Boulter, J.; Harding, S.E.; Colfen, H.; Watts, A. Hydrodynamic properties of human erythrocyte band 3 solubilized in reduced Triton X-100. Biophys. J. 1999, 76, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Antonelou, M.H.; Kriebardis, A.G.; Stamoulis, K.E.; Economou-Petersen, E.; Margaritis, L.H.; Papassideri, I.S. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion 2010, 50, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Bosman, G.J.; Lasonder, E.; Groenen-Dopp, Y.A.; Willekens, F.L.; Werre, J.M.; Novotny, V.M. Comparative proteomics of erythrocyte aging in vivo and in vitro. J. Proteom. 2010, 73, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Waugh, S.M.; Zinke, K.; Drenckhahn, D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science 1985, 227, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.R.; Turner, M.S.; Sens, P. Interactions between proteins bound to biomembranes. Phys. Rev. E 2003, 67, 041907. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.; Ipsen, J.H.; Mouritsen, O.G.; Sabra, M.C.; Sperotto, M.M.; Zuckermann, M.J. Theoretical analysis of protein organization in lipid membranes. Biochim. Biophys. Acta 1998, 1376, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Gov, N.S. Less is more: Removing membrane attachments stiffens the RBC cytoskeleton. New J. Phys. 2007, 9, 429. [Google Scholar] [CrossRef]
- Ehrig, J.; Petrov, E.P.; Schwille, P. Near-critical fluctuations and cytoskeleton-assisted phase separation lead to subdiffusion in cell membranes. Biophys. J. 2011, 100, 80–89. [Google Scholar] [CrossRef] [PubMed]
- de Meyer, F.J.; Rodgers, J.M.; Willems, T.F.; Smit, B. Molecular simulation of the effect of cholesterol on lipid-mediated protein-protein interactions. Biophys. J. 2010, 99, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, D.; Honigmann, A.; Koike, S.; Gottfert, F.; Pahler, G.; Junius, M.; Mullar, S.; Diederichsen, U.; Janshoff, A.; Grubmuller, H.; et al. Hydrophobic mismatch sorts SNARE proteins into distinct membrane domains. Nat. Commun. 2015, 6, 5984. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Esteban-Martin, S.; Ulrich, A.S.; Salgado, J. Hydrophobic mismatch of mobile transmembrane helices: Merging theory and experiments. Biochim. Biophys. Acta 2012, 1818, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Pajic-Lijakovic, I.; Gural, A. Deformability of Stored Red Blood Cells. Front. Physiol. 2021, 12, 722896. [Google Scholar] [CrossRef] [PubMed]
- Guillet, R.; Driss, F.; Perrotin, P.; Pautou, C.; Nalpas, B.; Boynard, M. Gender, menstrual cycle, oral contraceptives and red blood cell deformability in healthy adult subjects. Clin. Hemorheol. Microcirc. 1998, 19, 83–88. [Google Scholar] [PubMed]
- Kameneva, M.V.; Watach, M.J.; Borovetz, H.S. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin. Hemorheol. Microcirc. 1999, 21, 357–363. [Google Scholar] [PubMed]
- Salbas, K. Effect of acute smoking on red blood cell deformability in healthy young and elderly non-smokers, and effect of verapamil on age- and acute smoking-induced change in red blood cell deformability. Scand. J. Clin. Lab. Investig. 1994, 54, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Tomschi, F.; Bloch, W.; Grau, M. Impact of Type of Sport, Gender and Age on Red Blood Cell Deformability of Elite Athletes. Int. J. Sports Med. 2018, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Freitas Leal, J.K.; Lasonder, E.; Sharma, V.; Schiller, J.; Fanelli, G.; Rinalducci, S.; Brock, R.; Bosman, G. Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences. Proteomes 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Gautier, E.F.; Ducamp, S.; Leduc, M.; Salnot, V.; Guillonneau, F.; Dussiot, M.; Hale, J.; Giarratana, M.C.; Raimbault, A.; Douay, L.; et al. Comprehensive Proteomic Analysis of Human Erythropoiesis. Cell Rep. 2016, 16, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
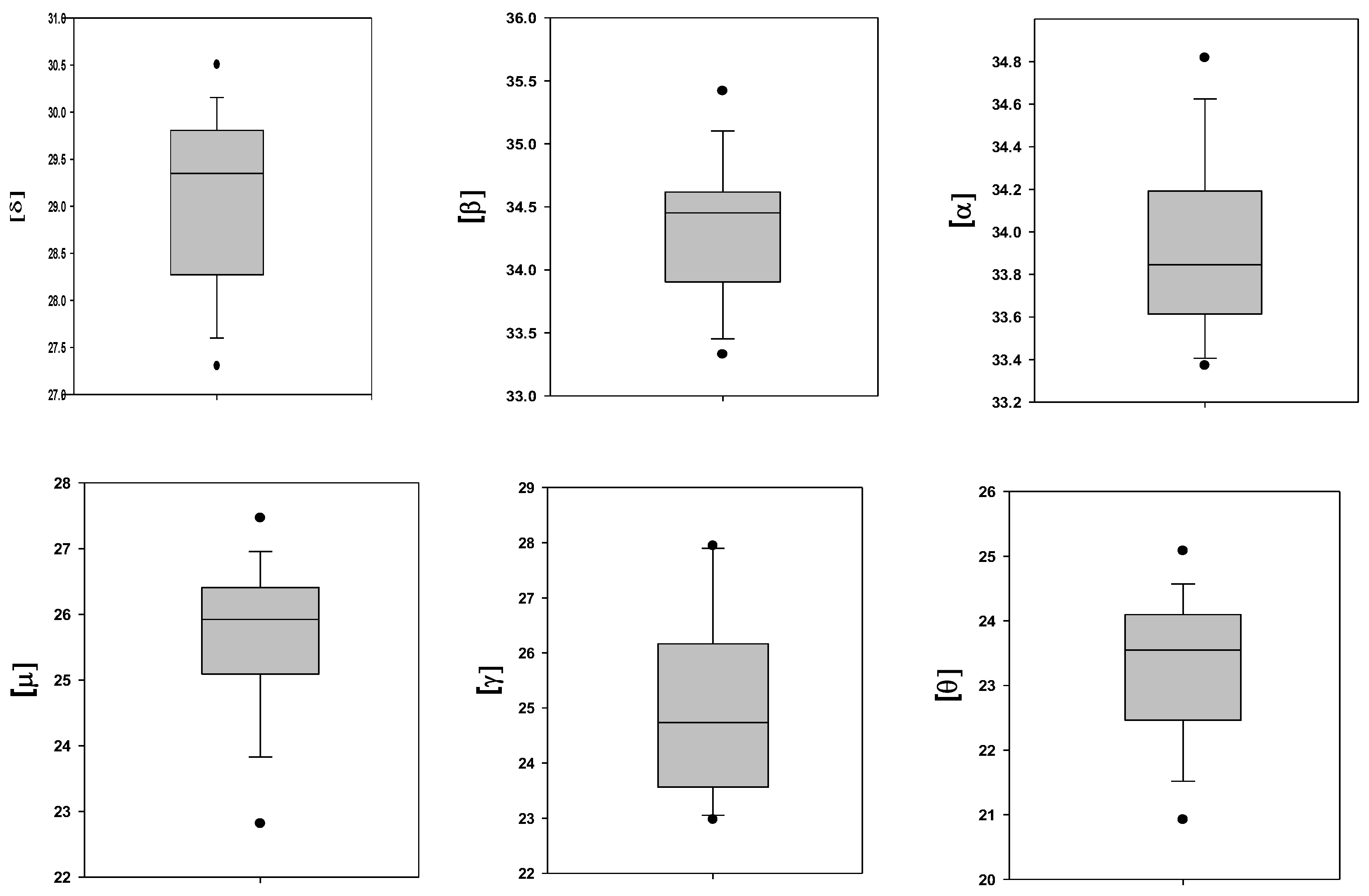
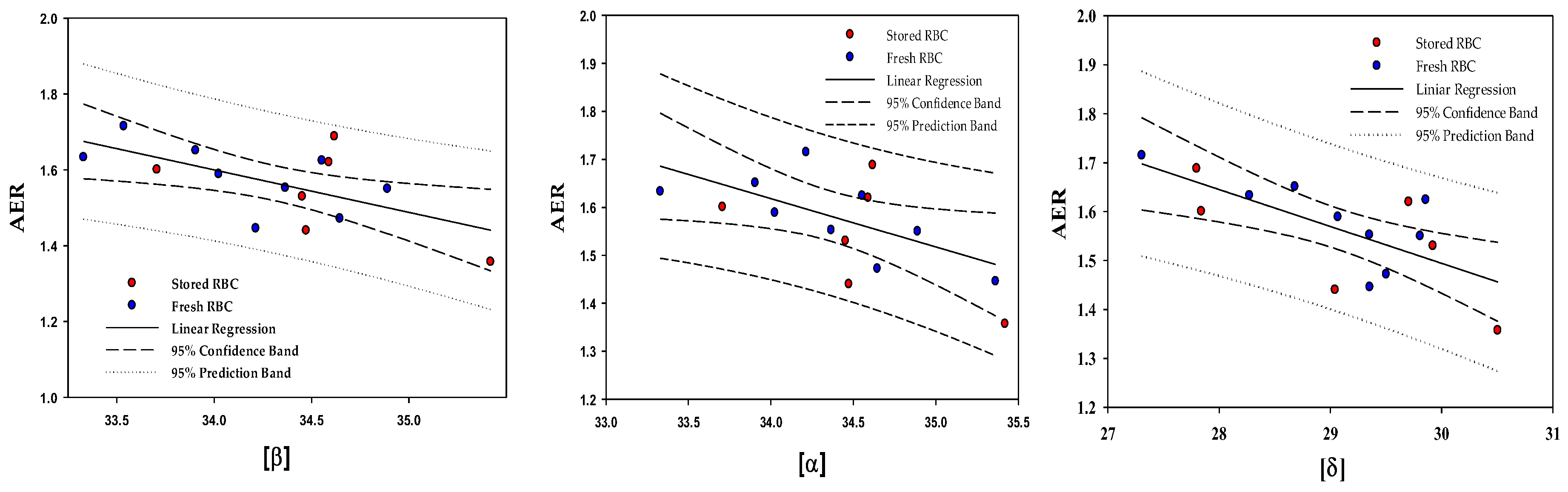
| Number | Hemoglobin Subunit | Gene | Content; Ln(LFQ) |
|---|---|---|---|
| 1 | β | HBB | 34.4 ± 0.5 |
| 2 | α | HBA1 | 34.0 ± 0.4 |
| 3 | δ | HBD | 29.1 ± 0.9 |
| 4 | µ | HBM | 25.7 ± 1.1 |
| 5 | γ | HBG2 | 25.1 ± 1.6 |
| 6 | θ | HBQ1 | 23.3 ± 1.1 |
| β | α | δ | μ | γ | θ | |
|---|---|---|---|---|---|---|
| β | 1.00 | |||||
| α | 0.82 ** | 1.00 | ||||
| δ | 0.76 ** | 0.76 ** | 1.00 | |||
| μ | 0.25 | 0.07 | 0.63 | 1.00 | ||
| γ | 0.37 | 0.08 | 0.43 | 0.21 | 1.00 | |
| θ | 0.42 | 0.46 | 0.39 | 0.54 * | 0.26 | 1.00 |
| № | Hemoglobin Subunit | Content; Ln(LFQ) | Significance, p | |
|---|---|---|---|---|
| Fresh | Stored | |||
| 1 | β | 34.2 ± 0.5 | 34.5 ± 0.6 | 0.20 |
| 2 | α | 34.1 ± 0.4 | 33.9 ± 0.2 | 0.31 |
| 3 | δ | 29.0 ± 0.8 | 29.1 ± 1.1 | 0.84 |
| 4 | μ | 26.0 ± 0.6 | 25.2 ± 1.4 | 0.09 |
| 5 | γ | 24.4 ± 0.5 | 26.7 ± 0.8 | 0.007 |
| 6 | θ | 23.4 ± 0.7 | 23.4 ± 1.5 | 0.45 |
| № | Hemoglobin Subunit | Significance, p | Pearson Coefficient, r |
|---|---|---|---|
| 1 | β | 0.017 | −0.606 |
| 2 | α | 0.0053 | −0.722 |
| 3 | δ | 0.0047 | −0.687 |
| 4 | γ | NS | - |
| 5 | μ | NS | - |
| 6 | θ | NS | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barshtein, G.; Livshits, L.; Gural, A.; Arbell, D.; Barkan, R.; Pajic-Lijakovic, I.; Yedgar, S. Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability. Int. J. Mol. Sci. 2024, 25, 5814. https://doi.org/10.3390/ijms25115814
Barshtein G, Livshits L, Gural A, Arbell D, Barkan R, Pajic-Lijakovic I, Yedgar S. Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability. International Journal of Molecular Sciences. 2024; 25(11):5814. https://doi.org/10.3390/ijms25115814
Chicago/Turabian StyleBarshtein, Gregory, Leonid Livshits, Alexander Gural, Dan Arbell, Refael Barkan, Ivana Pajic-Lijakovic, and Saul Yedgar. 2024. "Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability" International Journal of Molecular Sciences 25, no. 11: 5814. https://doi.org/10.3390/ijms25115814
APA StyleBarshtein, G., Livshits, L., Gural, A., Arbell, D., Barkan, R., Pajic-Lijakovic, I., & Yedgar, S. (2024). Hemoglobin Binding to the Red Blood Cell (RBC) Membrane Is Associated with Decreased Cell Deformability. International Journal of Molecular Sciences, 25(11), 5814. https://doi.org/10.3390/ijms25115814









