The Known, the Unknown and the Future of the Pathophysiology of Endometriosis
Abstract
1. Introduction
2. Postulated Origins
2.1. Sampson’s Theory: Retrograde Menstruation
2.2. Coelomic Metaplasia
2.3. Embryonic Rest Theory
2.4. Vascular and Lymphatic Metastasis
2.5. Tissue Injury and Repair Theory (TIAR)
2.6. Quinn’s “Denervation–Reinnervation” Theory
2.7. Stem Cell Theory
2.8. Genetic/Epigenetic Theory
3. Pathophysiology of Endometriosis
3.1. Hormonal Dysregulation
3.1.1. Estrogen Dominance
3.1.2. Progesterone Resistance
3.2. Inflammatory Response and Immune Dysregulation in Endometriosis
3.2.1. Proinflammatory Environment: Immune Cells
- T cells
- Macrophages
- Dendritic cells (DCs)
- Uterine natural killer (uNK) cells
3.2.2. Cytokines and Growth Factors
- Interleukin-6 (IL-6)
- Interleukin-8 (IL-8)
- Interleukin-1 (IL-1)
- Tumor necrosis factor alpha (TNF-α)
- Transforming growth factor-β (TGF-β)
3.3. The Fibrotic Component during Endometriosis
Fibrosis in Endometriotic Lesions
3.4. Infertility
4. Role of MicroRNAs in the Pathophysiology of Endometriosis
4.1. Biogenesis of miRNAs
4.2. Relevant miRNAs in Endometriosis
5. Management and Treatment in Current Clinical Practice
5.1. Management of Pain
5.2. Management of Infertility
6. Animal Models to Study the Disease
6.1. Baboon Model
6.2. Mouse Models
Induction Methods for the Development of Endometriosis in Rodents
- Suture method
- Injection method
- Menstruating mouse model
7. Importance of the Study of Endometriosis and Considerations
8. Outlook for Research on Endometriosis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018, 33, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Tu, F.F.; Agarwal, S.K.; Chapron, C.; Soliman, A.M.; Chiuve, S.; Eichner, S.; Flores-Caldera, I.; Horne, A.W.; Kimball, A.B.; et al. Impact of Endometriosis on Life-Course Potential: A Narrative Review. Int. J. Gen. Med. 2021, 14, 9–25. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of Endometriosis. Obstet. Gynecol. 2010, 116, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [CrossRef] [PubMed]
- Adamson, G.D. Endometriosis classification: An update. Curr. Opin. Obs. Gynecol. 2011, 23, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Falcone, T.; Lebovic, D.I. Clinical Management of Endometriosis. Obstet. Gynecol. 2011, 118, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, X.; Lin, L.; Xu, K.; Xu, M.; Ye, J.; Shen, X. Sexual function in patients with endometriosis: A prospective case-control study in China. J. Int. Med. Res. 2021, 49, 3000605211004388. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Wenger, J.-M.; Petignat, P.; Tal, R.; Bolmont, M.; Taylor, H.S.; Bianchi-Demicheli, F. Sexual function in endometriosis patients and their partners: Effect of the disease and consequences of treatment. Hum. Reprod. Update 2016, 22, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Filby, C.E.; Rombauts, L.; Montgomery, G.W.; Giudice, L.C.; Gargett, C.E. Cellular Origins of Endometriosis: Towards Novel Diagnostics and Therapeutics. Semin. Reprod. Med. 2020, 38, 201–215. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar] [PubMed]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obs. Gynecol. 1984, 64, 151–154. [Google Scholar]
- Dorien, F.O.; Roskams, T.; Van den Eynde, K.; Vanhie, A.; Peterse, D.P.; Meuleman, C.; Tomassetti, C.; Peeraer, K.; D’Hooghe, T.M.; Fassbender, A. The Presence of Endometrial Cells in Peritoneal Fluid of Women with and without Endometriosis. Reprod. Sci. 2017, 24, 242–251. [Google Scholar] [CrossRef]
- Vinatier, D.; Orazi, G.; Cosson, M.; Dufour, P. Theories of endometriosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2001, 96, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Oosterlynck, D.J.; Cornillie, F.J.; Waer, M.; Vandeputte, M.; Koninckx, P.R. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil. Steril. 1991, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Debrock, S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum. Reprod. Update 2002, 8, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Yoshimura, Y. Stem cell theory for the pathogenesis of endometriosis. Front. Biosci. 2012, 4, 2754–2763. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Berkkanoglu, M.; Arici, A. Pathogenesis of endometriosis. Obs. Gynecol. Clin. N. Am. 2003, 30, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Schrodt, G.R.; Alcorn, M.O.; Ibanez, J. Endometriosis of the male urinary system: A case report. J. Urol. 1980, 124, 722–723. [Google Scholar] [CrossRef] [PubMed]
- Schifrin, B.S.; Erez, S.; Moore, J.G. Teen-age endometriosis. Am. J. Obs. Gynecol. 1973, 116, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Troncon, J.K.; Zani, A.C.; Vieira, A.D.; Poli-Neto, O.B.; Nogueira, A.A.; Rosa, E.S.J.C. Endometriosis in a patient with mayer-rokitansky-kuster-hauser syndrome. Case Rep. Obs. Gynecol. 2014, 2014, 376231. [Google Scholar] [CrossRef] [PubMed]
- Konrad, L.; Dietze, R.; Kudipudi, P.K.; Horné, F.; Meinhold-Heerlein, I. Endometriosis in MRKH cases as a proof for the coelomic metaplasia hypothesis? Reproduction 2019, 158, R41–R47. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.D. Classic pages in obstetrics and gynecology. Aberrant portions of the müllerian duct found in an ovary: William Wood Russell Johns Hopkins Hospital Bulletin, vol. 10, pp. 8–10, 1899. Am. J. Obs. Gynecol. 1979, 134, 225–226. [Google Scholar]
- Oliker, A.J.; Harris, A.E. Endometriosis of the bladder in a male patient. J. Urol. 1971, 106, 858–859. [Google Scholar] [CrossRef] [PubMed]
- Jerman, L.F.; Hey-Cunningham, A.J. The role of the lymphatic system in endometriosis: A comprehensive review of the literature. Biol. Reprod. 2015, 92, 64. [Google Scholar] [CrossRef] [PubMed]
- Beavis, A.L.; Matsuo, K.; Grubbs, B.H.; Srivastava, S.A.; Truong, C.M.; Moffitt, M.N.; Maliglig, A.M.; Lin, Y.G. Endometriosis in para-aortic lymph nodes during pregnancy: Case report and review of literature. Fertil. Steril. 2011, 95, 2429.e9–2429.e13. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L. A new concept of endometriosis and adenomyosis: Tissue injury and repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011, 5, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Kunz, G.; Wildt, L.; Beil, D.; Deininger, H. Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility. Hum. Reprod. 1996, 11, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obs. 2009, 280, 529–538. [Google Scholar] [CrossRef]
- Quinn, M. Injuries to the levator ani in unexplained, chronic pelvic pain. J. Obs. Gynaecol. 2007, 27, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.J. Endometriosis: The consequence of uterine denervation-reinnervation. Arch. Gynecol. Obs. 2011, 284, 1423–1429. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, L.; Wang, D.; Guo, Q.; He, Y.; Liang, T.; Sun, L.; Wang, X.; Cheng, Y.; Zhang, G. Characteristics of Human Endometrium-Derived Mesenchymal Stem Cells and Their Tropism to Endometriosis. Stem Cells Int. 2017, 2017, 4794827. [Google Scholar] [CrossRef]
- Gargett, C.E.; Chan, R.W.; Schwab, K.E. Endometrial stem cells. Curr. Opin. Obstet. Gynecol. 2007, 19, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S. Endometrial Cells Derived from Donor Stem Cells in Bone Marrow Transplant Recipients. JAMA 2004, 292, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Barlow, D.; Kennedy, S. Implantation versus infiltration: The Sampson versus the endometriotic disease theory. Gynecol. Obs. Investig. 1999, 47 (Suppl. S1), 3–9, discussion 9–10. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, D.C.; Plotkin, J.B. Redundancy, antiredundancy, and the robustness of genomes. Proc. Natl. Acad. Sci. USA 2002, 99, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Nasu, K.; Li, H.; Tsuno, A.; Abe, W.; Takai, N.; Narahara, H. Application of the histone deacetylase inhibitors for the treatment of endometriosis: Histone modifications as pathogenesis and novel therapeutic target. Hum. Reprod. 2011, 26, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Xiaomeng, X.; Ming, Z.; Jiezhi, M.; Xiaoling, F. Aberrant histone acetylation and methylation levels in woman with endometriosis. Arch. Gynecol. Obstet. 2013, 287, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.B.; Colón-Díaz, M.; García, M.; Gutierrez, S.; Colón, M.; Seto, E.; Laboy, J.; Flores, I. Endometriosis Is Characterized by a Distinct Pattern of Histone 3 and Histone 4 Lysine Modifications. Reprod. Sci. 2014, 21, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.; Mehraein, F.; Shahhoseini, M.; Karimian, L.; Nikmard, F.; Ashrafi, M.; Afsharian, P.; Aflatoonian, R. Epigenetic alterations of CYP19A1 gene in Cumulus cells and its relevance to infertility in endometriosis. J. Assist. Reprod. Genet. 2016, 33, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Bedrick, B.S.; Courtright, L.; Zhang, J.; Snow, M.; Amendola, I.L.S.; Nylander, E.; Cayton-Vaught, K.; Segars, J.; Singh, B. A Systematic Review of Epigenetics of Endometriosis. F S Rev. 2024, 5, 100070. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Cheng, Y.H.; Pavone, M.E.; Xue, Q.; Attar, E.; Trukhacheva, E.; Tokunaga, H.; Utsunomiya, H.; Yin, P.; Luo, X.; et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin. Reprod. Med. 2010, 28, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2022, 23, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: From bench to treatment. Pharmacol. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Fukaya, T.; Suzuki, T.; Murakami, T.; Sasano, H.; Yajima, A. Oestrogen receptor alpha and beta mRNA expression in human endometrium throughout the menstrual cycle. Mol. Hum. Reprod. 1999, 5, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Lecce, G.; Meduri, G.; Ancelin, M.; Bergeron, C.; Perrot-Applanat, M. Presence of estrogen receptor beta in the human endometrium through the cycle: Expression in glandular, stromal, and vascular cells. J. Clin. Endocrinol. Metab. 2001, 86, 1379–1386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chantalat, E.; Valera, M.C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of estrogen receptor-β in endometriosis. Semin. Reprod. Med. 2012, 30, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.M.; Fazleabas, A.T. A baboon model for endometriosis: Implications for fertility. Reprod. Biol. Endocrinol. 2006, 4 (Suppl. S1), S7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Magon, N. Hormones in pregnancy. Niger. Med. J. 2012, 53, 179–183. [Google Scholar] [CrossRef]
- Vallve-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Lessey, B.A. Progesterone function in human endometrium: Clinical perspectives. Semin. Reprod. Med. 2010, 28, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Young, S.L. Homeostasis imbalance in the endometrium of women with implantation defects: The role of estrogen and progesterone. Semin. Reprod. Med. 2014, 32, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.R.; Miyadahira, E.H.; Afshar, Y.; Jeong, J.W.; Young, S.L.; Lessey, B.A.; Serafini, P.C.; Fazleabas, A.T. Progesterone Resistance in Endometriosis Is Modulated by the Altered Expression of MicroRNA-29c and FKBP4. J. Clin. Endocrinol. Metab. 2017, 102, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obs. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Iwase, A.; Ishida, C.; Nagai, T.; Mori, M.; Bayasula; Nakamura, T.; Osuka, S.; Ganiyeva, U.; Qin, Y.; et al. Upregulation of Fibroblast Growth Factors Caused by Heart and Neural Crest Derivatives Expressed 2 Suppression in Endometriotic Cells: A Possible Therapeutic Target in Endometriosis. Reprod. Sci. 2019, 26, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Talbi, S.; Hamilton, A.E.; Vo, K.C.; Nyegaard, M.; Nezhat, C.R.; Lessey, B.A.; Giudice, L.C. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007, 148, 3814–3826. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabbagh, M.; Lam, E.W.; Brosens, J.J. Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol. 2012, 358, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Afshar, Y.; Hastings, J.; Roqueiro, D.; Jeong, J.W.; Giudice, L.C.; Fazleabas, A.T. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol. Reprod. 2013, 88, 44. [Google Scholar] [CrossRef]
- Fazleabas, A.T. Progesterone resistance in a baboon model of endometriosis. Semin. Reprod. Med. 2010, 28, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Cheng, Y.H.; Yin, P.; Imir, G.; Utsunomiya, H.; Attar, E.; Innes, J.; Julie Kim, J. Progesterone resistance in endometriosis: Link to failure to metabolize estradiol. Mol. Cell. Endocrinol. 2006, 248, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone Receptor Isoform A But Not B Is Expressed in Endometriosis1. J. Clin. Endocrinol. Metab. 2000, 85, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Liu, C.; Liu, T.; Xiao, L.; Luo, B.; Tan, J.; Li, X.; Zhou, G.; Duan, C.; Huang, W. miR-194-3p Represses the Progesterone Receptor and Decidualization in Eutopic Endometrium from Women with Endometriosis. Endocrinology 2018, 159, 2554–2562. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fu, J.; Xiao, L.; Yang, S.; Song, Y.; Zhang, X.; Feng, X.; Sun, H.; Xu, W.; Huang, W. miR-196a overexpression activates the MEK/ERK signal and represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Hum. Reprod. 2016, 31, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006, 1, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Junior, C.V.; Da Broi, M.G.; Miranda-Furtado, C.L.; Navarro, P.A.; Ferriani, R.A.; Meola, J. Progesterone Receptor B (PGR-B) Is Partially Methylated in Eutopic Endometrium from Infertile Women with Endometriosis. Reprod. Sci. 2019, 26, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Oosterlynck, D.J.; Meuleman, C.; Lacquet, F.A.; Waer, M.; Koninckx, P.R. Flow cytometry analysis of lymphocyte subpopulations in peritoneal fluid of women with endometriosis. Am. J. Reprod. Immunol. 1994, 31, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Giudice, L.C. Chapter 11—Immune phenotypes and mediators affecting endometrial function in women with endometriosis. In Immunology of Endometriosis; Koga, K., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 169–191. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Givan, A.L.; White, H.D.; Stern, J.E.; Colby, E.; Gosselin, E.J.; Guyre, P.M.; Wira, C.R. Flow cytometric analysis of leukocytes in the human female reproductive tract: Comparison of fallopian tube, uterus, cervix, and vagina. Am. J. Reprod. Immunol. 1997, 38, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.P.; Gebel, H.; House, R.; Rana, N.; Dmowski, W.P. Spontaneous and induced synthesis of cytokines by peripheral blood monocytes in patients with endometriosis**Supported in part by Public Health Service Grants CA 58922, Bethesda, Maryland and a grant from Sterling International, New York, New York.††Presented at the 50th Annual Meeting of The American Fertility Society, San Antonio, Texas, November 5 to 10, 1994. Fertil. Steril. 1996, 65, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Berbic, M.; Fraser, I.S. Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J. Reprod. Immunol. 2011, 88, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A.; Lathbury, L.J. Endometrial leukocytes and menstruation. Hum. Reprod. Update 2000, 6, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yan, Y.; Liu, Z.; Wang, Y. Inflammation and endometriosis. FBL 2016, 21, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.A.; Pérgola, G.M.; Rao-Tekmal, R.; Dey, T.D.; Schenken, R.S. Enhanced expression of resident leukocyte interferon gamma mRNA in endometriosis. Am. J. Reprod. Immunol. 1993, 30, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. Molecular Mechanisms of T Helper Cell Differentiation and Functional Specialization. Immune Netw. 2023, 23, e4. [Google Scholar] [CrossRef] [PubMed]
- Podgaec, S.; Dias Junior, J.A.; Chapron, C.; Oliveira, R.M.; Baracat, E.C.; Abrão, M.S. Th1 and Th2 ummune responses related to pelvic endometriosis. Rev. Assoc. Med. Bras 2010, 56, 92–98. [Google Scholar] [CrossRef] [PubMed]
- de Barros, I.B.L.; Malvezzi, H.; Gueuvoghlanian-Silva, B.Y.; Piccinato, C.A.; Rizzo, L.V.; Podgaec, S. What do we know about regulatory T cells and endometriosis? A systematic review. J. Reprod. Immunol. 2017, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Cavani, A.; Girolomoni, G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: Synergistic or antagonist effects with IFN-gamma and TNF-alpha. J. Immunol. 1999, 162, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Gogacz, M.; Winkler, I.; Bojarska-Junak, A.; Tabarkiewicz, J.; Semczuk, A.; Rechberger, T.; Adamiak, A. Increased percentage of Th17 cells in peritoneal fluid is associated with severity of endometriosis. J. Reprod. Immunol. 2016, 117, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Edwards, A.K.; Singh, S.S.; Young, S.L.; Lessey, B.A.; Tayade, C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J. Immunol. 2015, 195, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Osuga, Y.; Takamura, M.; Saito, A.; Hasegawa, A.; Koga, K.; Yoshino, O.; Hirota, Y.; Harada, M.; Taketani, Y. Interleukin-17F increases the secretion of interleukin-8 and the expression of cyclooxygenase 2 in endometriosis. Fertil. Steril. 2011, 96, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Podgaec, S.; Barbeiro, D.F.; Gueuvoghlanian-Silva, B.Y.; Bellelis, P.; Abrao, M.S.; Baracat, E.C. Foxp3 expression in deep rectosigmoid endometriosis lesions and its association with chronic pelvic pain. J. Reprod. Immunol. 2014, 104–105, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Fazleabas, A.T.; Braundmeier, A.; Parkin, K. Endometriosis-induced changes in regulatory T cells—Insights towards developing permanent contraception. Contraception 2015, 92, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Wang, Y.; Chang, K.K.; Meng, Y.H.; Liu, L.B.; Mei, J.; Wang, Y.; Wang, X.Q.; Jin, L.P.; Li, D.J. CD4+Foxp3+ regulatory T cell differentiation mediated by endometrial stromal cell-derived TECK promotes the growth and invasion of endometriotic lesions. Cell Death Dis. 2014, 5, e1436. [Google Scholar] [CrossRef] [PubMed]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Li, X.; Li, P.; Zhang, H.; Xu, T.; Wang, H.; Wang, B.; Ma, X.; Jiang, X.; Zhang, Z. Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum. Reprod. 2019, 34, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pavez, T.N.; Martínez-Esparza, M.; Ruiz-Alcaraz, A.J.; Marín-Sánchez, P.; Machado-Linde, F.; García-Peñarrubia, P. The Role of Peritoneal Macrophages in Endometriosis. Int. J. Mol. Sci. 2021, 22, 10792. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Di Puppo, F.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages Are Alternatively Activated in Patients with Endometriosis and Required for Growth and Vascularization of Lesions in a Mouse Model of Disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Nagorsen, D.; Marincola, F.M.; Panelli, M.C. Cytokine and chemokine expression profiles of maturing dendritic cells using multiprotein platform arrays. Cytokine 2004, 25, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Poli-Neto, O.B.; Meola, J.; Rosa, E.S.J.C.; Tiezzi, D. Transcriptome meta-analysis reveals differences of immune profile between eutopic endometrium from stage I-II and III-IV endometriosis independently of hormonal milieu. Sci. Rep. 2020, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, E.; Parkin, K.L.; Lessey, B.A.; Young, S.L.; Fazleabas, A.T. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am. J. Reprod. Immunol. 2014, 72, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.N.; Chao, K.H.; Chen, H.F.; Wu, M.Y.; Yang, Y.S.; Lee, T.Y. Peritoneal natural killer cytotoxicity and CD25+ CD3+ lymphocyte subpopulation are decreased in women with stage III-IV endometriosis. Hum. Reprod. 1995, 10, 2671–2675. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed. Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Ma, Z.Y.; Song, N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fu, X.; Wu, T.; Yang, L.; Hu, C.; Wu, R. Role of Interleukin-6 and Its Receptor in Endometriosis. Med. Sci. Monit. 2017, 23, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Su, R.W.; Joshi, N.R.; Kim, T.H.; Lessey, B.A.; Jeong, J.W.; Fazleabas, A.T. Interleukin-6 (IL-6) Activates the NOTCH1 Signaling Pathway through E-Proteins in Endometriotic Lesions. J. Clin. Endocrinol. Metab. 2020, 105, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Ho, H.N. The role of cytokines in endometriosis. Am. J. Reprod. Immunol. 2003, 49, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Evers, J.L.H.; Healy, D.L. (Eds.) Endometriosis: Science and Practice; Wiley-Blackwell, Ed.: Hoboken, NJ, USA, 2012; p. 600. [Google Scholar]
- Burns, K.A.; Thomas, S.Y.; Hamilton, K.J.; Young, S.L.; Cook, D.N.; Korach, K.S. Early Endometriosis in Females Is Directed by Immune-Mediated Estrogen Receptor α and IL-6 Cross-Talk. Endocrinology 2018, 159, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Othman Eel, D.; Hornung, D.; Salem, H.T.; Khalifa, E.A.; El-Metwally, T.H.; Al-Hendy, A. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2008, 137, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Witz, C.A. Interleukin-6: Another piece of the endometriosis-cytokine puzzle11The opinions and commentary expressed in Editor’s Corner articles are solely those of the author. Publication does not imply endorsement by the Editor or American Society for Reproductive Medicine. Fertil. Steril. 2000, 73, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Arici, A. Local cytokines in endometrial tissue: The role of interleukin-8 in the pathogenesis of endometriosis. Ann. N. Y Acad. Sci. 2002, 955, 101–109, discussion 118, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Kondera-Anasz, Z.; Sikora, J.; Mielczarek-Palacz, A.; Jońca, M. Concentrations of interleukin (IL)-1alpha, IL-1 soluble receptor type II (IL-1 sRII) and IL-1 receptor antagonist (IL-1 Ra) in the peritoneal fluid and serum of infertile women with endometriosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2005, 123, 198–203. [Google Scholar] [CrossRef]
- Keller, N.R.; Sierra-Rivera, E.; Eisenberg, E.; Osteen, K.G. Progesterone exposure prevents matrix metalloproteinase-3 (MMP-3) stimulation by interleukin-1alpha in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 2000, 85, 1611–1619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Mbarik, M.; Kaabachi, W.; Henidi, B.; Sassi, F.H.; Hamzaoui, K. Soluble ST2 and IL-33: Potential markers of endometriosis in the Tunisian population. Immunol. Lett. 2015, 166, 1–5. [Google Scholar] [CrossRef]
- Miller, J.E.; Monsanto, S.P.; Ahn, S.H.; Khalaj, K.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Interleukin-33 modulates inflammation in endometriosis. Sci. Rep. 2017, 7, 17903. [Google Scholar] [CrossRef] [PubMed]
- Philippeaux, M.M.; Piguet, P.F. Expression of tumor necrosis factor-alpha and its mRNA in the endometrial mucosa during the menstrual cycle. Am. J. Pathol. 1993, 143, 480–486. [Google Scholar] [PubMed]
- Pizzo, A.; Salmeri, F.M.; Ardita, F.V.; Sofo, V.; Tripepi, M.; Marsico, S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol. Obs. Investig. 2002, 54, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tarokh, M.; Ghaffari Novin, M.; Poordast, T.; Tavana, Z.; Nazarian, H.; Norouzian, M.; Gharesi-Fard, B. Serum and Peritoneal Fluid Cytokine Profiles in Infertile Women with Endometriosis. Iran. J. Immunol. 2019, 16, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Smycz-Kubańska, M.; Mielczarek-Palacz, A.; Bednarek, I.; Kondera-Anasz, Z. The involvement of multifunctional TGF-β and related cytokines in pathogenesis of endometriosis. Immunol. Lett. 2018, 201, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Ahmad, S.F.; Duncan, W.C.; Horne, A.W. The role of TGF-β in the pathophysiology of peritoneal endometriosis. Hum. Reprod. Update 2017, 23, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Klemmt, P.A.B.; Starzinski-Powitz, A. Molecular and Cellular Pathogenesis of Endometriosis. Curr. Womens Health Rev. 2018, 14, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O. Fibrosis as a molecular hallmark of endometriosis pathophysiology. Fertil. Steril. 2022, 118, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Konzack, A.; Pihlajaniemi, T.; Heljasvaara, R.; Kietzmann, T. Redox-fibrosis: Impact of TGFβ1 on ROS generators, mediators and functional consequences. Redox Biol. 2015, 6, 344–352. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Research 2016, 5, F1000 Faculty Rev-752. [Google Scholar] [CrossRef]
- Young, V.J.; Brown, J.K.; Maybin, J.; Saunders, P.T.; Duncan, W.C.; Horne, A.W. Transforming growth factor-beta induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, 3450–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Duan, J.; Olson, M.; Fazleabas, A.; Guo, S.W. Cellular Changes Consistent with Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Progression of Experimental Endometriosis in Baboons. Reprod. Sci. 2016, 23, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Bianco, B.; Andre, G.M.; Vilarino, F.L.; Peluso, C.; Mafra, F.A.; Christofolini, D.M.; Barbosa, C.P. The possible role of genetic variants in autoimmune-related genes in the development of endometriosis. Hum. Immunol. 2012, 73, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Lebovic, D.I.; Taylor, R.N. Eutopic endometrium in women with endometriosis: Ground zero for the study of implantation defects. Semin. Reprod. Med. 2013, 31, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Clifton, V.L.; Fraser, I.S.; Taylor, H.S.; Critchley, H.; Giudice, L.C.; Petraglia, F. Infertility and reproductive disorders: Impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum. Reprod. Update 2016, 22, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obs. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.J.; Hull, M.G. Pituitary-ovarian dysfunction and endometriosis. Hum. Reprod. Update 2000, 6, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, A.; Oliveira, N.; Ruiz, A.; Remohí, J.; Simón, C. Exploring the mechanism(s) of endometriosis-related infertility: An analysis of embryo development and implantation in assisted reproduction. Hum. Reprod. 1995, 10 (Suppl. S2), 91–97. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D.; Pasta, D.J. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil. Steril. 2010, 94, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Olive, D.L.; Stohs, G.F.; Metzger, D.A.; Franklin, R.R. Expectant management and hydrotubations in the treatment of endometriosis-associated infertility. Fertil. Steril. 1985, 44, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.W.; Carraher, R.P.; Foldesy, R.G.; McGuire, J.L. Experimental evidence for failure to implant as a mechanism of infertility associated with endometriosis. Am. J. Obstet. Gynecol. 1986, 155, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Illera, M.J.; Juan, L.; Stewart, C.L.; Cullinan, E.; Ruman, J.; Lessey, B.A. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil. Steril. 2000, 74, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-Y.; Kim, T.H.; Shin, J.-H.; Marquardt, R.M.; Müller, U.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Yoon, H.-G.; Jeong, J.-W. Loss of MIG-6 results in endometrial progesterone resistance via ERBB2. Nat. Commun. 2022, 13, 1101. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Kayisli, U.A.; Taylor, H.S. The role of growth factors and cytokines during implantation: Endocrine and paracrine interactions. Semin. Reprod. Med. 2009, 27, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.D.; Bagchi, I.; Dey, S.K.; Enders, A.C.; Fazleabas, A.T.; Lessey, B.A.; Yoshinaga, K. Embryo implantation. Dev. Biol. 2000, 223, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A. Implantation defects in infertile women with endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 265–280, discussion 293–295, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Transforming growth factor β signaling in uterine development and function. J. Anim. Sci. Biotechnol. 2014, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Brown, J.K.; Saunders, P.T.; Duncan, W.C.; Horne, A.W. The peritoneum is both a source and target of TGF-beta in women with endometriosis. PLoS ONE 2014, 9, e106773. [Google Scholar] [CrossRef] [PubMed]
- Bruner, K.L.; Rodgers, W.H.; Gold, L.I.; Korc, M.; Hargrove, J.T.; Matrisian, L.M.; Osteen, K.G. Transforming growth factor beta mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc. Natl. Acad. Sci. USA 1995, 92, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Creighton, C.J.; Han, D.Y.; Zariff, A.; Anderson, M.L.; Gunaratne, P.H.; Matzuk, M.M. Functional microRNA involved in endometriosis. Mol. Endocrinol. 2011, 25, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Hamilton, A.E.; Aghajanova, L.; Vo, K.C.; Nezhat, C.N.; Lessey, B.A.; Giudice, L.C. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 2009, 15, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Sen, C.K. MicroRNA Biogenesis in Regenerative Medicine. In MicroRNA in Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2015; pp. 3–46. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: Microrna biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Mari-Alexandre, J.; Sanchez-Izquierdo, D.; Gilabert-Estelles, J.; Barcelo-Molina, M.; Braza-Boils, A.; Sandoval, J. miRNAs Regulation and Its Role as Biomarkers in Endometriosis. Int. J. Mol. Sci. 2016, 17, 93. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Cannell, I.G.; Kong, Y.W.; Bushell, M. How do microRNAs regulate gene expression? Biochem. Soc. Trans. 2008, 36, 1224–1231. [Google Scholar] [CrossRef]
- Marí-Alexandre, J. MicroRNAs: New players in endometriosis. World J. Obstet. Gynecol. 2016, 5, 28–38. [Google Scholar] [CrossRef]
- Joshi, N.R.; Su, R.W.; Chandramouli, G.V.; Khoo, S.K.; Jeong, J.W.; Young, S.L.; Lessey, B.A.; Fazleabas, A.T. Altered expression of microRNA-451 in eutopic endometrium of baboons (Papio anubis) with endometriosis. Hum. Reprod. 2015, 30, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B.; Falcone, T.; Joshi, N.; Fazleabas, A.T.; Graham, A. Serum miR-451a Levels Are Significantly Elevated in Women with Endometriosis and Recapitulated in Baboons (Papio anubis) with Experimentally-Induced Disease. Reprod. Sci. 2017, 24, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obs. Gynecol. 2020, 223, 557.e1–557.e11. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Q.; Teng, H.; Xu, X.H.; Liu, S.Y.; Wang, Y.H.; Guo, F.J.; Liu, X.J. Microarray analysis of microRNA deregulation and angiogenesis-related proteins in endometriosis. Genet. Mol. Res. 2016, 15, gmr.15027826. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Joshi, N.R.; Burns, G.W.; Hrbek, S.M.; Vegter, E.L.; Ochoa-Bernal, M.A.; Song, Y.; Moldovan, G.E.; Sempere, L.F.; Miyadahira, E.H.; et al. MicroRNA-210-3p Regulates Endometriotic Lesion Development by Targeting IGFBP3 in Baboons and Women with Endometriosis. Reprod. Sci. 2023, 30, 2932–2944. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Koo, Y.J.; Lee, D.H. Classification of endometriosis. Yeungnam Univ. J. Med. 2021, 38, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Shebl, O.; Shamiyeh, A.; Oppelt, P. The rASRM score and the Enzian classification for endometriosis: Their strengths and weaknesses. Acta Obs. Gynecol. Scand. 2013, 92, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Harth, S.; Kaya, H.E.; Zeppernick, F.; Meinhold-Heerlein, I.; Keckstein, J.; Yildiz, S.M.; Nurkan, E.; Krombach, G.A.; Roller, F.C. Application of the #Enzian classification for endometriosis on MRI: Prospective evaluation of inter- and intraobserver agreement. Front. Med. 2023, 10, 1303593. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Fedorkow, D.; Collins, J.; Vandekerckhove, P. Ovulation suppression for endometriosis. Cochrane Database Syst. Rev. 2003, CD000155. [Google Scholar] [CrossRef] [PubMed]
- Decherney, A.Z.a.A.H. Surgery and Endometriosis. Clin. Obs. Gynecol. 2017, 60, 477–484. [Google Scholar]
- Berkley, K.J.; Rapkin, A.J.; Papka, R.E. The pains of endometriosis. Science 2005, 308, 1587–1589. [Google Scholar] [CrossRef] [PubMed]
- Olive, D.L. Medical therapy of endometriosis. Semin. Reprod. Med. 2003, 21, 209–222. [Google Scholar] [CrossRef]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 2010, Cd008475. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, K.; Bena, J.F.; McGill, K.M.; Minger, J.; Falcone, T. Surgical treatment of endometriosis: A 7-year follow-up on the requirement for further surgery. Obs. Gynecol. 2008, 111, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Mavrelos, D. Endometriosis-associated pain in women undergoing hysterectomy. Bjog 2021, 128, 856. [Google Scholar] [CrossRef] [PubMed]
- Sandström, A.; Bixo, M.; Johansson, M.; Bäckström, T.; Turkmen, S. Effect of hysterectomy on pain in women with endometriosis: A population-based registry study. Bjog 2020, 127, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, N.; Shafrir, A.L.; Wallace, B.M.; Vitonis, A.F.; Fraer, C.J.; Sadler Gallagher, J.; DePari, M.; Ghiasi, M.; Laufer, M.R.; Sieberg, C.B.; et al. Trends in pelvic pain symptoms over 2 years of follow-up among adolescents and young adults with and without endometriosis. Pain 2023, 164, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.E.; Rizzello, F.; Palagiano, A.; Scarselli, G. Long-term follow-up after laparoscopic treatment for endometriosis: Multivariate analysis of predictive factors for recurrence of endometriotic lesions and pain. Eur. J. Obs. Gynecol. Reprod. Biol. 2011, 157, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, N.; Ngo, L.; Vitonis, A.F.; Dillon, S.T.; Prasad, P.; Laufer, M.R.; As-Sanie, S.; Schrepf, A.; Missmer, S.A.; Libermann, T.A.; et al. Plasma proteins and persistent post-surgical pelvic pain among adolescents and young adults with endometriosis. Am. J. Obs. Gynecol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Brown, J.; Collins, J.J.; Farquhar, C.; Fedorkow, D.M.; Vandekerckhove, P. Ovulation suppression for endometriosis. Cochrane Database Syst. Rev. 2007, 2007, Cd000155. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J.; Hickey, M.; Maouris, P.; Buckett, W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst. Rev. 2008, CD004992. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Outley, J.; Botteman, M.; Spalding, J.; Simon, J.A.; Pashos, C.L. Economic burden of endometriosis. Fertil. Steril. 2006, 86, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Sharpe-Timms, K.L. (Ed.) Animal Models For Endometriosis, 1st ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Brandão, V.C.M.; Meola, J.; Garcia, S.B.; Candido-Dos-Reis, F.J.; Poli-Neto, O.B.; Nogueira, A.A.; Rosa, E.S.J.C. Increased Expression Levels of Metalloprotease, Tissue Inhibitor of Metalloprotease, Metallothionein, and p63 in Ectopic Endometrium: An Animal Experimental Study. Rev. Bras. Ginecol. Obs. 2018, 40, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Abreu, J.P.; Rebelatto, C.L.K.; Savari, C.A.; Capriglione, L.G.A.; Miyague, L.; Noronha, L.; Amaral, V.F.D. The Effect of Mesenchymal Stem Cells on Fertility in Experimental Retrocervical Endometriosis. Rev. Bras. Ginecol. Obs. 2017, 39, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Saad-Hossne, R.; Barretto, A.B.; Siqueira, J.M.; Denadai, R. Evaluation of peritoneal endometriosis treatment using intralesional acetylsalicylic acid injection in rabbits. Acta Cir. Bras. 2016, 31, 227–234. [Google Scholar] [CrossRef]
- Tapdıgova, R.; Bayrak, G.; Yılmaz, B.C.; Aytan, H. Antilipidemic ezetimibe induces regression of endometriotic explants in a rat model of endometriosis with its anti-inflammatory and anti-angiogenic effects. Naunyn Schmiedebergs Arch. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, C.; Ying, Y.; Lv, J.; Qu, X.; McGowan, E.; Lin, Y.; Zhu, X. Metformin Alleviates Endometriosis and Potentiates Endometrial Receptivity via Decreasing VEGF and MMP9 and Increasing Leukemia Inhibitor Factor and HOXA10. Front. Pharmacol. 2022, 13, 750208. [Google Scholar] [CrossRef] [PubMed]
- Eisalou, M.Y.; Farahpour, M.R. Effectiveness of Gamma Oryzanol on prevention of surgical induced endometriosis development in rat model. Sci. Rep. 2022, 12, 2816. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Pearson, A.M.; Slack, J.L.; Por, E.D.; Scribner, A.N.; Eti, N.A.; Burney, R.O. Endometriosis in the Mouse: Challenges and Progress toward a ‘Best Fit’ Murine Model. Front. Physiol. 2022, 12, 806574. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Liu, X.; Guo, S.W. The establishment of a mouse model of deep endometriosis. Hum. Reprod. 2019, 34, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.S.; Taratula, O.R.; Lee, H.; Luo, F.; Grenz, T.; Korzun, T.; Lorenz, A.S.; Sabei, F.Y.; Bracha, S.; Alani, A.W.G.; et al. Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis. Small 2020, 16, e1906936. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.H.; Nowland, M.H.; Nemzek-Hamlin, J.A. Surgical treatment of spontaneous endometriosis in rhesus macaques (Macaca mulatta): 11 cases (2007–2011). J. Am. Vet. Med. Assoc. 2019, 254, 1454–1458. [Google Scholar] [CrossRef] [PubMed]
- Fazleabas, A.T.; Brudney, A.; Gurates, B.; Chai, D.; Bulun, S. A modified baboon model for endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 308–317, discussion 340–302, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Tirado-González, I.; Barrientos, G.; Tariverdian, N.; Arck, P.C.; García, M.G.; Klapp, B.F.; Blois, S.M. Endometriosis research: Animal models for the study of a complex disease. J. Reprod. Immunol. 2010, 86, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Braundmeier, A.G.; Fazleabas, A.T. The non-human primate model of endometriosis: Research and implications for fecundity. Mol. Hum. Reprod. 2009, 15, 577–586. [Google Scholar] [CrossRef]
- D’Hooghe, T.M. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil. Steril. 1997, 68, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.A. Spontaneous endometriosis in the Kenya baboon (Papio doguera). Am. J. Obstet. Gynecol. 1968, 101, 569–570. [Google Scholar] [CrossRef]
- DaRif, C.A.; Parker, R.F.; Schoeb, T.R. Endometriosis with bacterial peritonitis in a baboon. Lab. Anim. Sci. 1984, 34, 491–493. [Google Scholar] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Isahakia, M.; Koninckx, P.R. Evolution of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) over a 12-month period. Fertil. Steril. 1992, 58, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Cornillie, F.J.; D’Hooghe, T.M.; Bambra, C.S.; Lauweryns, J.M.; Isahakia, M.; Koninckx, P.R. Morphological characteristics of spontaneous endometriosis in the baboon (Papio anubis and Papio cynocephalus). Gynecol. Obstet. Investig. 1992, 34, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Shalev, M.; Ciurea, D.; Deligdisch, L. Endometriosis and stromal tumor in a baboon (Papio hamadryas). Lab. Anim. Sci. 1992, 42, 204–208. [Google Scholar] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Suleman, M.A.; Dunselman, G.A.; Evers, H.L.; Koninckx, P.R. Development of a model of retrograde menstruation in baboons (Papio anubis). Fertil. Steril. 1994, 62, 635–638. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Koninckx, P.R. Cycle fecundity in baboons of proven fertility with minimal endometriosis. Gynecol. Obstet. Investig. 1994, 37, 63–65. [Google Scholar] [CrossRef]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; Koninckx, P.R. Increased prevalence and recurrence of retrograde menstruation in baboons with spontaneous endometriosis. Hum. Reprod. 1996, 11, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; De Jonge, I.; Hill, J.A.; Koninckx, P.R. The effects of immunosuppression on development and progression of endometriosis in baboons (Papio anubis). Fertil. Steril. 1995, 64, 172–178. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; De Jonge, I.; Lauweryns, J.M.; Koninckx, P.R. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am. J. Obstet. Gynecol. 1995, 173, 125–134. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Kazungu, J.; Koninckx, P.R. Peritoneal fluid volume and steroid hormone concentrations in baboons with and without either spontaneous minimal/mild endometriosis or the luteinized unruptured follicle syndrome. Arch. Gynecol. Obstet. 1995, 256, 17–22. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; De Jonge, I.; Machai, P.N.; Korir, R.; Koninckx, P.R. A serial section study of visually normal posterior pelvic peritoneum from baboons (Papio cynocephalus, Papio anubis) with and without spontaneous minimal endometriosis. Fertil. Steril. 1995, 63, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Scheerlinck, J.P.; Koninckx, P.R.; Hill, J.A.; Bambra, C.S. Anti-endometrial lymphocytotoxicity and natural killer cell activity in baboons (Papio anubis and Papio cynocephalus) with endometriosis. Hum. Reprod. 1995, 10, 558–562. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Hill, J.A.; Oosterlynck, D.J.; Koninckx, P.R.; Bambra, C.S. Effect of endometriosis on white blood cell subpopulations in peripheral blood and peritoneal fluid of baboons. Hum. Reprod. 1996, 11, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; Koninckx, P.R. Development of spontaneous endometriosis in baboons. Obstet. Gynecol. 1996, 88, 462–466. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; Koninckx, P.R. Serial laparoscopies over 30 months show that endometriosis in captive baboons (Papio anubis, Papio cynocephalus) is a progressive disease. Fertil. Steril. 1996, 65, 645–649. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; Riday, A.M.; Suleman, M.A.; Koninckx, P.R. The cycle pregnancy rate is normal in baboons with stage I endometriosis but decreased in primates with stage II and stage III–IV disease. Fertil. Steril. 1996, 66, 809–813. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; Koninckx, P.R. Increased incidence and recurrence of recent corpus luteum without ovulation stigma (luteinized unruptured follicle syndrome?) in baboons with endometriosis. J. Soc. Gynecol. Investig. 1996, 3, 140–144. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; De Jonge, I.; Lauweryns, J.M.; Raeymaekers, B.M.; Koninckx, P.R. The effect of pregnancy on endometriosis in baboons (Papio anubis, Papio cynocephalus). Arch. Gynecol. Obstet. 1997, 261, 15–19. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; Hill, J.A. Pelvic inflammation induced by diagnostic laparoscopy in baboons. Fertil. Steril. 1999, 72, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Strakova, Z.; Srisuparp, S.; Fazleabas, A.T. Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology 2000, 141, 4664–4670. [Google Scholar] [CrossRef] [PubMed]
- Strakova, Z.; Szmidt, M.; Srisuparp, S.; Fazleabas, A.T. Inhibition of matrix metalloproteinases prevents the synthesis of insulin-like growth factor binding protein-1 during decidualization in the baboon. Endocrinology 2003, 144, 5339–5346. [Google Scholar] [CrossRef]
- Cameo, P.; Szmidt, M.; Strakova, Z.; Mavrogianis, P.; Sharpe-Timms, K.L.; Fazleabas, A.T. Decidualization regulates the expression of the endometrial chorionic gonadotropin receptor in the primate. Biol. Reprod. 2006, 75, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Xiao, L.; Peixe, K.; Hill, J.A. Effect of menstruation and intrapelvic injection of endometrium on inflammatory parameters of peritoneal fluid in the baboon (Papio anubis and Papio cynocephalus). Am. J. Obstet. Gynecol. 2001, 184, 917–925. [Google Scholar] [CrossRef]
- Fazleabas, A.T.; Brudney, A.; Chai, D.; Mwenda, J. Endometriosis in the baboon. Gynecol. Obstet. Investig. 2004, 57, 46–47. [Google Scholar]
- Fazleabas, A.T. A baboon model for inducing endometriosis. Methods Mol. Med. 2006, 121, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Kyama, C.M.; Mihalyi, A.; Chai, D.; Simsa, P.; Mwenda, J.M.; D’Hooghe, T.M. Baboon model for the study of endometriosis. Womens Health 2007, 3, 637–646. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Kyama, C.M.; Chai, D.; Fassbender, A.; Vodolazkaia, A.; Bokor, A.; Mwenda, J.M. Nonhuman primate models for translational research in endometriosis. Reprod. Sci. 2009, 16, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.B.; Baker, R.; Owston, M.A.; Escalona, R.; Dick, E.J.; VandeBerg, J.L.; Nickisch, K.J. An efficient model of human endometriosis by induced unopposed estrogenicity in baboons. Oncotarget 2016, 7, 10857–10869. [Google Scholar] [CrossRef] [PubMed]
- Fazleabas, A.T.; Strakova, Z. Endometrial function: Cell specific changes in the uterine environment. Mol. Cell. Endocrinol. 2002, 186, 143–147. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Debrock, S.; Hill, J.A.; Meuleman, C. Endometriosis and subfertility: Is the relationship resolved? Semin. Reprod. Med. 2003, 21, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Fazleabas, A.T.; Brudney, A.; Chai, D.; Langoi, D.; Bulun, S.E. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil. Steril. 2003, 80 (Suppl. S2), 820–827. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Hubbard, G.B.; Leland, M.M.; Dunn, B.G.; Best, R.G. Spontaneous ovarian tumors in twelve baboons: A review of ovarian neoplasms in non-human primates. J. Med. Primatol. 2003, 32, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Barrier, B.F.; Malinowski, M.J.; Dick, E.J., Jr.; Hubbard, G.B.; Bates, G.W. Adenomyosis in the baboon is associated with primary infertility. Fertil. Steril. 2004, 82 (Suppl. S3), 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Barrier, B.F.; Bates, G.W.; Leland, M.M.; Leach, D.A.; Robinson, R.D.; Propst, A.M. Efficacy of anti-tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertil. Steril. 2004, 81 (Suppl. S1), 775–779. [Google Scholar] [CrossRef] [PubMed]
- Falconer, H.; Bambra, C.S.; Chai, D.; Cornillie, F.J.; Hill, J.A.; D’Hooghe, T.M. The effect of endometriosis, cycle stage, lymphocyte suppression and pregnancy on CA-125 levels in peritoneal fluid and serum in baboons. Hum. Reprod. 2005, 20, 3033–3038. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Debrock, S.; Kyama, C.M.; Chai, D.C.; Cuneo, S.; Hill, J.A.; Mwenda, J.M. Baboon model for fundamental and preclinical research in endometriosis. Gynecol. Obstet. Investig. 2004, 57, 43–46. [Google Scholar]
- Gashaw, I.; Hastings, J.M.; Jackson, K.S.; Winterhager, E.; Fazleabas, A.T. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol. Reprod. 2006, 74, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.M.; Jackson, K.S.; Mavrogianis, P.A.; Fazleabas, A.T. The estrogen early response gene FOS is altered in a baboon model of endometriosis. Biol. Reprod. 2006, 75, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Falconer, H.; Mwenda, J.M.; Chai, D.C.; Wagner, C.; Song, X.Y.; Mihalyi, A.; Simsa, P.; Kyama, C.; Cornillie, F.J.; Bergqvist, A.; et al. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum. Reprod. 2006, 21, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Denton, J.; Fazleabas, A.T. Morphological and glycosylation changes associated with the endometrium and ectopic lesions in a baboon model of endometriosis. Hum. Reprod. 2006, 21, 3068–3080. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Nugent, N.P.; Cuneo, S.; Chai, D.C.; Deer, F.; Debrock, S.; Kyama, C.M.; Mihalyi, A.; Mwenda, J.M. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: A prospective, randomized, placebo- and drug-controlled study. Biol. Reprod. 2006, 74, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Kyama, C.M.; Overbergh, L.; Mihalyi, A.; Cuneo, S.; Chai, D.; Debrock, S.; Mwenda, J.M.; Mathieu, C.; Nugent, N.P.; D’Hooghe, T.M. Effect of recombinant human TNF-binding protein-1 and GnRH antagonist on mRNA expression of inflammatory cytokines and adhesion and growth factors in endometrium and endometriosis tissues in baboons. Fertil. Steril. 2008, 89, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Afshar, Y.; Stanculescu, A.; Miele, L.; Fazleabas, A.T. The role of chorionic gonadotropin and Notch1 in implantation. J. Assist. Reprod. Genet. 2007, 24, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Afshar, Y.; Miele, L.; Fazleabas, A.T. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology 2012, 153, 2884–2896. [Google Scholar] [CrossRef] [PubMed]
- Su, R.W.; Strug, M.R.; Joshi, N.R.; Jeong, J.W.; Miele, L.; Lessey, B.A.; Young, S.L.; Fazleabas, A.T. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J. Clin. Endocrinol. Metab. 2015, 100, E433–E442. [Google Scholar] [CrossRef] [PubMed]
- Barrier, B.F.; Dick, E.J., Jr.; Butler, S.D.; Hubbard, G.B. Endometriosis involving the ileocaecal junction with regional lymph node involvement in the baboon--striking pathological finding identical between the human and the baboon: A case report. Hum. Reprod. 2007, 22, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Taylor, H.S.; Lu, Z.; Ladhani, O.; Hastings, J.M.; Jackson, K.S.; Wu, Y.; Guo, S.W.; Fazleabas, A.T. Altered expression of HOXA10 in endometriosis: Potential role in decidualization. Mol. Hum. Reprod. 2007, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lebovic, D.I.; Mwenda, J.M.; Chai, D.C.; Mueller, M.D.; Santi, A.; Fisseha, S.; D’Hooghe, T. PPAR-gamma receptor ligand induces regression of endometrial explants in baboons: A prospective, randomized, placebo- and drug-controlled study. Fertil. Steril. 2007, 88, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Falconer, H.; Mwenda, J.M.; Chai, D.C.; Song, X.Y.; Cornillie, F.J.; Bergqvist, A.; Fried, G.; D’Hooghe, T.M. Effects of anti-TNF-mAb treatment on pregnancy in baboons with induced endometriosis. Fertil. Steril. 2008, 89, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.W.; Dick, E.J., Jr.; Schlabritz-Loutsevitch, N.E.; Lopez-Alvarenga, J.C.; Williams, P.C.; Sharp, R.M.; Hubbard, G.B. Endometrial and cervical polyps in 22 baboons (Papio sp.), 5 cynomolgus macaques (Macaca fascicularis) and one marmoset (Callithrix jacchus). J. Med. Primatol. 2009, 38, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kyama, C.M.; Mihalyi, A.; Simsa, P.; Falconer, H.; Fulop, V.; Mwenda, J.M.; Peeraer, K.; Tomassetti, C.; Meuleman, C.; D’Hooghe, T.M. Role of cytokines in the endometrial-peritoneal cross-talk and development of endometriosis. Front. Biosci. 2009, 1, 444–454. [Google Scholar] [CrossRef]
- Jones, C.J.; Nardo, L.G.; Litta, P.; Fazleabas, A.T. Ultrastructure of ectopic peritoneal lesions from women with endometriosis, including observations on the contribution of coelomic mesothelium. Reprod. Sci. 2009, 16, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Winterhager, E.; Grümmer, R.; Mavrogianis, P.A.; Jones, C.J.; Hastings, J.M.; Fazleabas, A.T. Connexin expression pattern in the endometrium of baboons is influenced by hormonal changes and the presence of endometriotic lesions. Mol. Hum. Reprod. 2009, 15, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Fazleabas, A.T. Endometrial responses to embryonic signals in the primate. Int. J. Dev. Biol. 2010, 54, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, J.R.; Hastings, J.M.; Jackson, K.S.; Mavrogianis, P.A.; Sharkey, A.M.; Fazleabas, A.T. The endometrial response to chorionic gonadotropin is blunted in a baboon model of endometriosis. Endocrinology 2010, 151, 4982–4993. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.K.; Turner, M.A.; Drury, J.; Heathcote, L.; Afshar, Y.; Mavrogianis, P.A.; Fazleabas, A.T. Aberrant expression of regulators of cell-fate found in eutopic endometrium is found in matched ectopic endometrium among women and in a baboon model of endometriosis. Hum. Reprod. 2010, 25, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Ilad, R.S.; Fleming, S.D.; Murphy, C.R.; Fazleabas, A.T. Immunohistochemical study of the ubiquitin-nuclear factor-kB pathway in the endometrium of the baboon (Papio anubis) with and without endometriosis. Reprod. Fertil. Dev. 2010, 22, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Braundmeier, A.G.; Fazleabas, A.T.; Nowak, R.A. Extracellular matrix metalloproteinase inducer expression in the baboon endometrium: Menstrual cycle and endometriosis. Reproduction 2010, 140, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.J.; Hodgetts, A.; Feroze-Zaidi, F.; Sherwin, J.R.; Fusi, L.; Salker, M.S.; Higham, J.; Rose, G.L.; Kajihara, T.; Young, S.L.; et al. Proteomic analysis of endometrium from fertile and infertile patients suggests a role for apolipoprotein A-I in embryo implantation failure and endometriosis. Mol. Hum. Reprod. 2010, 16, 273–285. [Google Scholar] [CrossRef]
- Lebovic, D.I.; Mwenda, J.M.; Chai, D.C.; Santi, A.; Xu, X.; D’Hooghe, T. Peroxisome proliferator-activated receptor-(gamma) receptor ligand partially prevents the development of endometrial explants in baboons: A prospective, randomized, placebo-controlled study. Endocrinology 2010, 151, 1846–1852. [Google Scholar] [CrossRef]
- Hey-Cunningham, A.J.; Fazleabas, A.T.; Braundmeier, A.G.; Markham, R.; Fraser, I.S.; Berbic, M. Endometrial stromal cells and immune cell populations within lymph nodes in a nonhuman primate model of endometriosis. Reprod. Sci. 2011, 18, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; Ihnatovych, I.; Ionetz, E.; Reed, J.; Braundmeier, A.; Strakova, Z. Cofilin and slingshot localization in the epithelium of uterine endometrium changes during the menstrual cycle and in endometriosis. Reprod. Sci. 2011, 18, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Kemnitz, J.W. Calorie restriction and aging in nonhuman primates. Ilar J. 2011, 52, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Harirchian, P.; Gashaw, I.; Lipskind, S.T.; Braundmeier, A.G.; Hastings, J.M.; Olson, M.R.; Fazleabas, A.T. Lesion kinetics in a non-human primate model of endometriosis. Hum. Reprod. 2012, 27, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Campo, V.; Benagiano, G. Infertility and adenomyosis. Obstet. Gynecol. Int. 2012, 2012, 786132. [Google Scholar] [CrossRef] [PubMed]
- Langoi, D.; Pavone, M.E.; Gurates, B.; Chai, D.; Fazleabas, A.; Bulun, S.E. Aromatase inhibitor treatment limits progression of peritoneal endometriosis in baboons. Fertil. Steril. 2013, 99, 656–662.e653. [Google Scholar] [CrossRef] [PubMed]
- Jagirdar, J.; Sirohi, D.; Dick, E.J., Jr.; Hubbard, G. Pleuro-pulmonary endometriosis in baboons (Papio spp.): Insights into pathogenesis. J. Med. Primatol. 2013, 42, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Van Langendonckt, A.; Defrère, S.; Colette, S.; Van Kerk, O.; Dehoux, J.P.; Squifflet, J.; Donnez, J. Induction of endometriotic nodules in an experimental baboon model mimicking human deep nodular lesions. Fertil. Steril. 2013, 99, 783–789.e3. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Soares, M.; Defrère, S.; Dehoux, J.P.; van Langendonckt, A.; Donnez, J.; Dolmans, M.M.; Colette, S. Nerve fiber density in deep nodular endometriotic lesions induced in a baboon experimental model. Fertil. Steril. 2013, 100, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Orellana, R.; García-Solares, J.; Donnez, J.; van Kerk, O.; Dolmans, M.M.; Donnez, O. Important role of collective cell migration and nerve fiber density in the development of deep nodular endometriosis. Fertil. Steril. 2017, 107, 987–995.e5. [Google Scholar] [CrossRef] [PubMed]
- Kyama, C.M.; Falconer, H.; Cuneo, S.; Chai, D.; Mihalyi, A.; Mwenda, J.; D’Hooghe, T. Menstrual endometrial supernatant may induce stromal endometriosis in baboons. Front Biosci. 2014, 6, 16–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugihara, K.; Kobayashi, Y.; Suzuki, A.; Tamura, N.; Motamedchaboki, K.; Huang, C.T.; Akama, T.O.; Pecotte, J.; Frost, P.; Bauer, C.; et al. Development of pro-apoptotic peptides as potential therapy for peritoneal endometriosis. Nat. Commun. 2014, 5, 4478. [Google Scholar] [CrossRef] [PubMed]
- Braundmeier, A.; Jackson, K.; Hastings, J.; Koehler, J.; Nowak, R.; Fazleabas, A. Induction of endometriosis alters the peripheral and endometrial regulatory T cell population in the non-human primate. Hum. Reprod. 2012, 27, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Yoo, J.Y.; Kim, T.H.; Shin, J.H.; Langenheim, J.F.; Ferguson, S.D.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Jeong, J.W. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum. Reprod. 2015, 30, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Jeong, J.W.; Fazleabas, A.T.; Tayade, C.; Young, S.L.; Lessey, B.A. Protein Inhibitor of Activated STAT3 (PIAS3) Is Down-Regulated in Eutopic Endometrium of Women with Endometriosis. Biol. Reprod. 2016, 95, 11. [Google Scholar] [CrossRef] [PubMed]
- Su, R.W.; Fazleabas, A.T. Implantation and Establishment of Pregnancy in Human and Nonhuman Primates. Adv. Anat. Embryol. Cell Biol. 2015, 216, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Olson, M.; Wang, K.; Fazleabas, A.; De La Fuente, R. Arginine methyltransferases mediate an epigenetic ovarian response to endometriosis. Reproduction 2015, 150, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Parkin, K.L.; Fazleabas, A.T. Uterine Leukocyte Function and Dysfunction: A Hypothesis on the Impact of Endometriosis. Am. J. Reprod. Immunol. 2016, 75, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Slayden, O.D. Translational In Vivo Models for Women’s Health: The Nonhuman Primate Endometrium--A Predictive Model for Assessing Steroid Receptor Modulators. Handb. Exp. Pharmacol. 2016, 232, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Chai, D.C.; Kyama, C.M.; Mwenda, J.M.; Palmer, S.S.; Gotteland, J.P.; D’Hooghe, T.M. c-Jun NH2-terminal kinase inhibitor bentamapimod reduces induced endometriosis in baboons: An assessor-blind placebo-controlled randomized study. Fertil. Steril. 2016, 105, 815–824.e5. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Alderman Iii, M.; D’Hooghe, T.M.; Fazleabas, A.T.; Duleba, A.J. Effect of simvastatin on baboon endometriosis. Biol. Reprod. 2017, 97, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Cosar, E.; Mamillapalli, R.; Moridi, I.; Duleba, A.; Taylor, H.S. Serum MicroRNA Biomarkers Regulated by Simvastatin in a Primate Model of Endometriosis. Reprod. Sci. 2019, 26, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, T.H.; Fazleabas, A.T.; Palomino, W.A.; Ahn, S.H.; Tayade, C.; Schammel, D.P.; Young, S.L.; Jeong, J.W.; Lessey, B.A. KRAS Activation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci. Rep. 2017, 7, 6765. [Google Scholar] [CrossRef] [PubMed]
- Stouffer, R.L.; Woodruff, T.K. Nonhuman Primates: A Vital Model for Basic and Applied Research on Female Reproduction, Prenatal Development, and Women’s Health. Ilar J. 2017, 58, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.A.; Parkin, K.L.; Coyne, L.; Giuliani, E.; Fazleabas, A.T.; Hapangama, D.K. The dynamic changes in the number of uterine natural killer cells are specific to the eutopic but not to the ectopic endometrium in women and in a baboon model of endometriosis. Reprod. Biol. Endocrinol. 2018, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Yoo, J.Y.; Kim, T.H.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Jeong, J.W. Overexpression of Four Joint Box-1 Protein (FJX1) in Eutopic Endometrium From Women with Endometriosis. Reprod. Sci. 2018, 25, 207–213. [Google Scholar] [CrossRef]
- Hufnagel, D.; Goetz, T.G.; Hu, Z.; Nyachieo, A.; D’Hooghe, T.; Fazleabas, A.; Duleba, A.; Krikun, G.; Taylor, H.S.; Lockwood, C.J. Icon immunoconjugate treatment results in regression of red lesions in a non-human primate (Papio anubis) model of endometriosis. Reprod. Biol. 2018, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B.; Falcone, T.; Olson, M.R.; Fazleabas, A.T.; Tawfik, O.W.; Graham, A. Macrophage Migration Inhibitory Factor Receptor, CD74, is Overexpressed in Human and Baboon (Papio anubis) Endometriotic Lesions and Modulates Endometriotic Epithelial Cell Survival and Interleukin 8 Expression. Reprod. Sci. 2018, 25, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yoo, J.Y.; Choi, K.C.; Shin, J.H.; Leach, R.E.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Yoon, H.G.; Jeong, J.W. Loss of HDAC3 results in nonreceptive endometrium and female infertility. Sci. Transl. Med. 2019, 11, eaaf7533. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.K.; Drury, J.; Da Silva, L.; Al-Lamee, H.; Earp, A.; Valentijn, A.J.; Edirisinghe, D.P.; Murray, P.A.; Fazleabas, A.T.; Gargett, C.E. Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Hum. Reprod. 2019, 34, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Kirejczyk, S.; Pinelli, C.; Gonzalez, O.; Kumar, S.; Dick, E., Jr.; Gumber, S. Urogenital Lesions in Nonhuman Primates at 2 National Primate Research Centers. Vet. Pathol. 2021, 58, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Cregger, M.; Fazleabas, A.; Braundmeier-Fleming, A. Effects of endometriosis on immunity and mucosal microbial community dynamics in female olive baboons. Sci. Rep. 2022, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Poirier, D.; Nyachieo, A.; Romano, A.; Roy, J.; Maltais, R.; Chai, D.; Delvoux, B.; Tomassetti, C.; Vanhie, A. An irreversible inhibitor of 17β-hydroxysteroid dehydrogenase type 1 inhibits estradiol synthesis in human endometriosis lesions and induces regression of the non-human primate endometriosis. J. Steroid Biochem. Mol. Biol. 2022, 222, 106136. [Google Scholar] [CrossRef] [PubMed]
- Strug, M.R.; Su, R.; Young, J.E.; Dodds, W.G.; Shavell, V.I.; Diaz-Gimeno, P.; Ruiz-Alonso, M.; Simon, C.; Lessey, B.A.; Leach, R.E.; et al. Intrauterine human chorionic gonadotropin infusion in oocyte donors promotes endometrial synchrony and induction of early decidual markers for stromal survival: A randomized clinical trial. Hum. Reprod. 2016, 31, 1552–1561. [Google Scholar] [CrossRef]
- Su, R.W.; Strug, M.R.; Jeong, J.W.; Miele, L.; Fazleabas, A.T. Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc. Natl. Acad. Sci. USA 2016, 113, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.G.; Peterson, P.E. Perspectives on the use of the baboon in embryology and teratology research. Hum. Reprod. Update 1997, 3, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Cummings, A.M.; Metcalf, J.L. Induction of endometriosis in mice: A new model sensitive to estrogen. Reprod. Toxicol. 1995, 9, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Cousins, F.L.; Murray, A.; Esnal-Zufiaurre, A.; Fassbender, A.; Horne, A.W.; Saunders, P.T. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am. J. Pathol. 2014, 184, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Xu, H.; Man, G.C.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef]
- Dabrosin, C.; Gyorffy, S.; Margetts, P.; Ross, C.; Gauldie, J. Therapeutic effect of angiostatin gene transfer in a murine model of endometriosis. Am. J. Pathol. 2002, 161, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Rodriguez, K.F.; Hewitt, S.C.; Janardhan, K.S.; Young, S.L.; Korach, K.S. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology 2012, 153, 3960–3971. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Quattrone, F.; Pannese, M.; Ulisse, A.; Candiani, M.; Diaz-Alonso, J.; Velasco, G.; Panina-Bordignon, P. The cannabinoid receptor CB1 contributes to the development of ectopic lesions in a mouse model of endometriosis. Hum. Reprod. 2017, 32, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simón, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef] [PubMed]
- Bellofiore, N.; Ellery, S.J.; Mamrot, J.; Walker, D.W.; Temple-Smith, P.; Dickinson, H. First evidence of a menstruating rodent: The spiny mouse (Acomys cahirinus). Am. J. Obs. Gynecol. 2017, 216, 40.e1–40.e11. [Google Scholar] [CrossRef]
- Cousins, F.L.; Murray, A.; Esnal, A.; Gibson, D.A.; Critchley, H.O.; Saunders, P.T. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS ONE 2014, 9, e86378. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Horne, A.W.; Jerina, H.; Mikolajczak, M.; Hilferty, L.; Mitchell, R.; Fleetwood-Walker, S.M.; Saunders, P.T.K. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci. Rep. 2017, 7, 44169. [Google Scholar] [CrossRef] [PubMed]
- Dorning, A.; Dhami, P.; Panir, K.; Hogg, C.; Park, E.; Ferguson, G.D.; Hargrove, D.; Karras, J.; Horne, A.W.; Greaves, E. Bioluminescent imaging in induced mouse models of endometriosis reveals differences in four model variations. Dis. Model. Mech. 2021, 14, dmm049070. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.; Lowton, K.; Wright, J. What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil. Steril. 2006, 86, 1296–1301. [Google Scholar] [CrossRef]
- Pašalić, E.; Tambuwala, M.M.; Hromić-Jahjefendić, A. Endometriosis: Classification, pathophysiology, and treatment options. Pathol. Res. Pract. 2023, 251, 154847. [Google Scholar] [CrossRef] [PubMed]
- Oală, I.E.; Mitranovici, M.I.; Chiorean, D.M.; Irimia, T.; Crișan, A.I.; Melinte, I.M.; Cotruș, T.; Tudorache, V.; Moraru, L.; Moraru, R.; et al. Endometriosis and the Role of Pro-Inflammatory and Anti-Inflammatory Cytokines in Pathophysiology: A Narrative Review of the Literature. Diagnostics 2024, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-J.; Yang, H.-L.; Shao, J.; Mei, J.; Chang, K.-K.; Zhu, R.; Li, M.-Q. Anti-inflammatory cytokines in endometriosis. Cell. Mol. Life Sci. 2019, 76, 2111–2132. [Google Scholar] [CrossRef] [PubMed]
- Luisi, S.; Pinzauti, S.; Regini, C.; Petraglia, F. Serum Markers for the Noninvasive Diagnosis of Endometriosis. Women’s Health 2015, 11, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Mihalyi, A.; Gevaert, O.; Kyama, C.M.; Simsa, P.; Pochet, N.; De Smet, F.; De Moor, B.; Meuleman, C.; Billen, J.; Blanckaert, N.; et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum. Reprod. 2009, 25, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Donnez, J. Emerging Drug Targets for Endometriosis. Biomolecules 2022, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- El-Zayadi, A.A.; Mohamed, S.A.; Arafa, M.; Mohammed, S.M.; Zayed, A.; Abdelhafez, M.S.; Badawy, A.M. Anti-IL-6 receptor monoclonal antibody as a new treatment of endometriosis. Immunol. Res. 2020, 68, 389–397. [Google Scholar] [CrossRef]
- Rocha, A.L.; Vieira, E.L.; Maia, L.M.; Teixeira, A.L.; Reis, F.M. Prospective Evaluation of a Panel of Plasma Cytokines and Chemokines as Potential Markers of Pelvic Endometriosis in Symptomatic Women. Gynecol. Obstet. Investig. 2016, 81, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Hogg, C.; Horne, A.W.; Greaves, E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front. Endocrinol. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Talebloo, N.; Bernal, M.A.O.; Kenyon, E.; Mallett, C.L.; Fazleabas, A.; Moore, A. Detection of Endometriosis Lesions Using Gd-Based Collagen I Targeting Probe in Murine Models of Endometriosis. Mol. Imaging Biol. 2023, 25, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Rangi, S.; Hur, C.; Richards, E.; Falcone, T. Fertility Preservation in Women with Endometriosis. J. Clin. Med. 2023, 12, 4331. [Google Scholar] [CrossRef]
- Becker, C.M.; Laufer, M.R.; Stratton, P.; Hummelshoj, L.; Missmer, S.A.; Zondervan, K.T.; Adamson, G.D.; Group, W.E.W. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil. Steril. 2014, 102, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhai, L.; Wang, J.; Yu, Q.; Li, T.; Xu, X.; Guo, X.; Mao, X.; Zhou, J.; Zhang, X. Comprehensive Analysis of RNA-Seq in Endometriosis Reveals Competing Endogenous RNA Network Composed of circRNA, lncRNA and mRNA. Front. Genet. 2022, 13, 828238. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; Garcia-Moreno, E.; Aghajanova, L.; Salumets, A.; Horcajadas, J.A.; Esteban, F.J.; Altmae, S. The mid-secretory endometrial transcriptomic landscape in endometriosis: A meta-analysis. Hum. Reprod. Open 2022, 2022, hoac016. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, L.; Zhan, H.; Mo, Y.; Ren, Z.; Shao, A.; Lin, J. Single-cell transcriptomic analysis of endometriosis provides insights into fibroblast fates and immune cell heterogeneity. Cell Biosci. 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.A.S.; Haro, M.; Wright, K.N.; Lin, X.; Abbasi, F.; Sun, J.; Hernandez, L.; Orr, N.L.; Hong, J.; Choi-Kuaea, Y.; et al. Single-cell transcriptomic analysis of endometriosis. Nat. Genet. 2023, 55, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, J.; Yi, D.; Li, X.; Gu, Z.; Yan, H.; Leng, J. The pathogenesis of endometriosis and adenomyosis: Insights from single-cell RNA sequencing. Biol. Reprod. 2024, 110, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, L.; Liu, M.; Zhu, H.; Zhang, X.; Cai, E.; Xu, X.; Chen, T.; Cheng, H.; Liu, J.; et al. Single-cell analysis reveals insights into epithelial abnormalities in ovarian endometriosis. Cell Rep. 2024, 43, 113716. [Google Scholar] [CrossRef] [PubMed]
- Au, E.D.; Farkas, M.H. Chapter 4—RNA sequencing and transcriptome analysis. In Genetics and Genomics of Eye Disease; Gao, X.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 41–53. [Google Scholar] [CrossRef]
- Giudice, L.C. Genomics’ role in understanding the pathogenesis of endometriosis. Semin. Reprod. Med. 2003, 21, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Altmae, S.; Esteban, F.J.; Stavreus-Evers, A.; Simon, C.; Giudice, L.; Lessey, B.A.; Horcajadas, J.A.; Macklon, N.S.; D’Hooghe, T.; Campoy, C.; et al. Guidelines for the design, analysis and interpretation of ‘omics’ data: Focus on human endometrium. Hum. Reprod. Update 2014, 20, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.J.; Adelson, R.P.; Vashistha, H.; Khalili, H.; Nayyar, A.; Puran, R.; Herrera, R.; Chatterjee, P.K.; Lee, A.T.; Truskinovsky, A.M.; et al. Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis. BMC Med. 2022, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Chan, R.W.S.; Ng, E.H.Y.; Gemzell-Danielsson, K.; Yeung, W.S.B. Single-cell RNA sequencing of cultured human endometrial CD140b(+)CD146(+) perivascular cells highlights the importance of in vivo microenvironment. Stem Cell Res. Ther. 2021, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, L.; Handfield, L.F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef] [PubMed]
- Cousins, F.L.; McKinnon, B.D.; Mortlock, S.; Fitzgerald, H.C.; Zhang, C.; Montgomery, G.W.; Gargett, C.E. New concepts on the etiology of endometriosis. J. Obs. Gynaecol. Res. 2023, 49, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, F.; Favaedi, R.; Heidari-Khoei, H.; Chitsazian, F.; Yari, S.; Piryaei, A.; Ghafari, F.; Baharvand, H.; Shahhoseini, M. Insight into epigenetics of human endometriosis organoids: DNA methylation analysis of HOX genes and their cofactors. Fertil. Steril. 2021, 115, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Martin, D.C. How organoids from endometrium and endometriosis could help to understand the pathogenesis of endometriosis. Fertil. Steril. 2021, 115, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, F.; Heidari Khoei, H.; Saber, M.; Favaedi, R.; Piryaei, A.; Moini, A.; Shahhoseini, M.; Ramezanali, F.; Ghaffari, F.; Baharvand, H. Disturbed progesterone signalling in an advanced preclinical model of endometriosis. Reprod. BioMed. Online 2021, 43, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Burns, G.W.; Joshi, N.R.; Arora, R.; Kim, J.J.; Fazleabas, A.T. Spheroids as a model for endometriotic lesions. JCI Insight 2023, 8, e160815. [Google Scholar] [CrossRef]
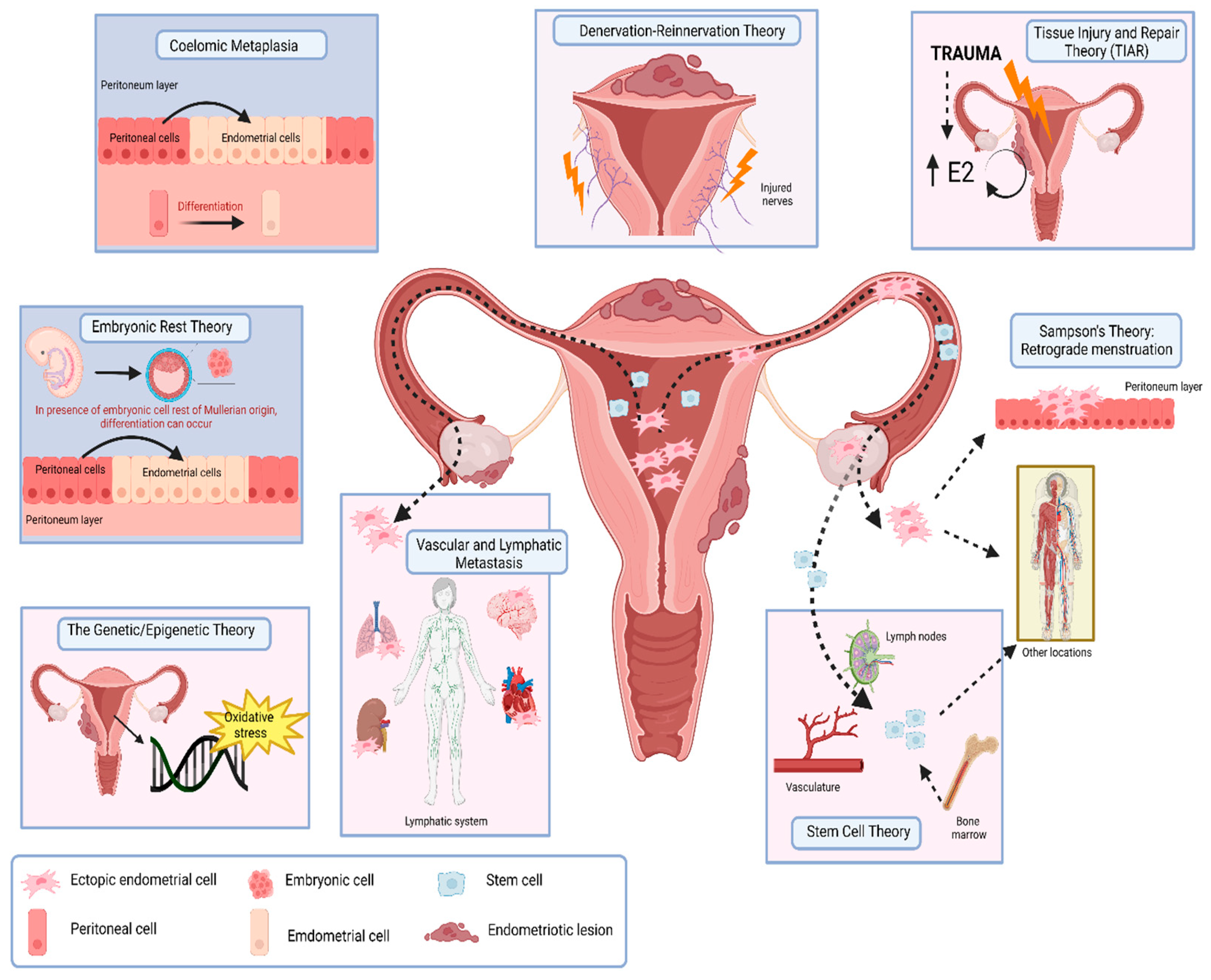
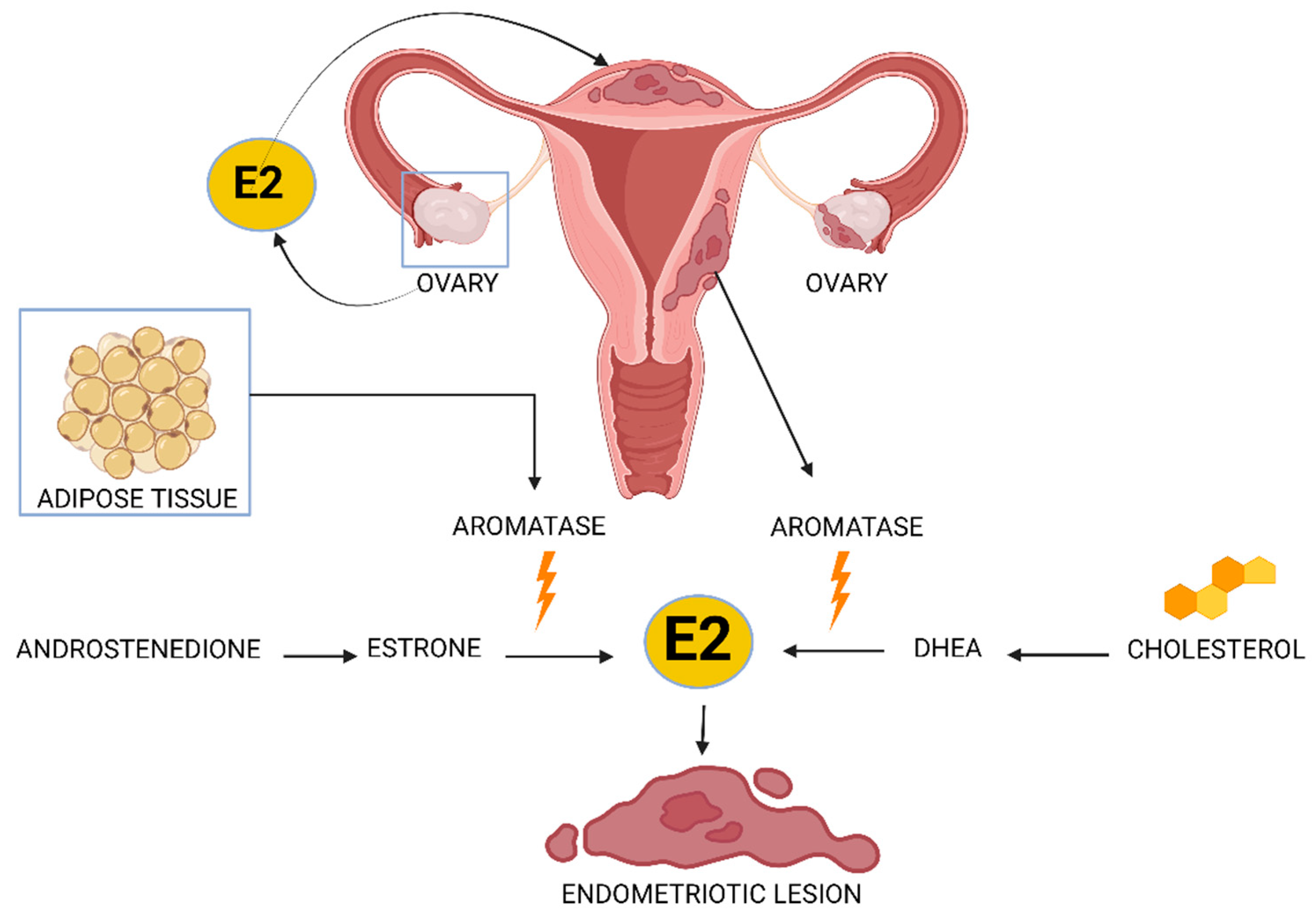

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa Bernal, M.A.; Fazleabas, A.T. The Known, the Unknown and the Future of the Pathophysiology of Endometriosis. Int. J. Mol. Sci. 2024, 25, 5815. https://doi.org/10.3390/ijms25115815
Ochoa Bernal MA, Fazleabas AT. The Known, the Unknown and the Future of the Pathophysiology of Endometriosis. International Journal of Molecular Sciences. 2024; 25(11):5815. https://doi.org/10.3390/ijms25115815
Chicago/Turabian StyleOchoa Bernal, Maria Ariadna, and Asgerally T. Fazleabas. 2024. "The Known, the Unknown and the Future of the Pathophysiology of Endometriosis" International Journal of Molecular Sciences 25, no. 11: 5815. https://doi.org/10.3390/ijms25115815
APA StyleOchoa Bernal, M. A., & Fazleabas, A. T. (2024). The Known, the Unknown and the Future of the Pathophysiology of Endometriosis. International Journal of Molecular Sciences, 25(11), 5815. https://doi.org/10.3390/ijms25115815





