Nrf2 Signaling Pathway as a Key to Treatment for Diabetic Dyslipidemia and Atherosclerosis
Abstract
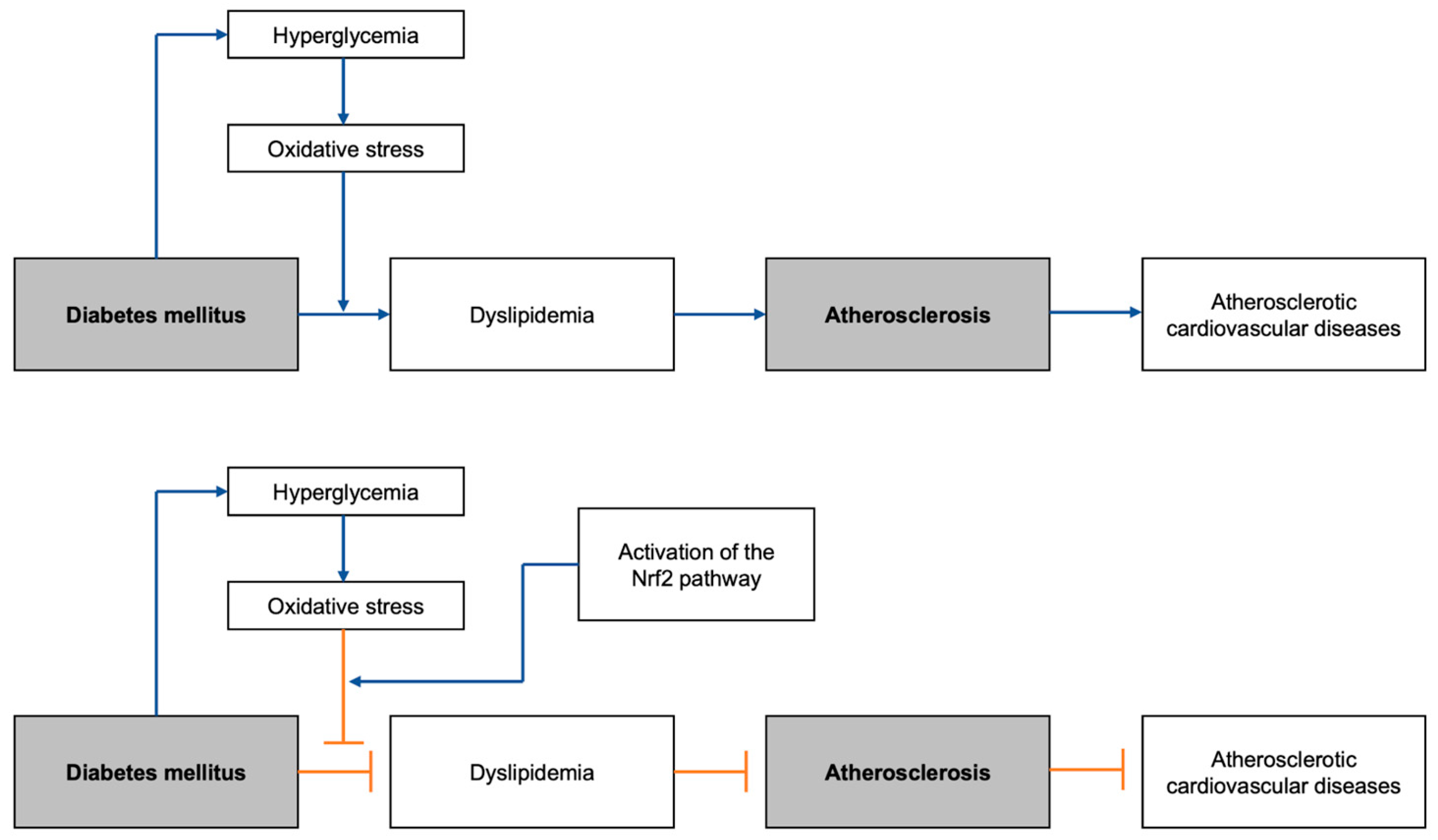
1. Introduction
2. Diabetic Dyslipidemia and Atherosclerosis
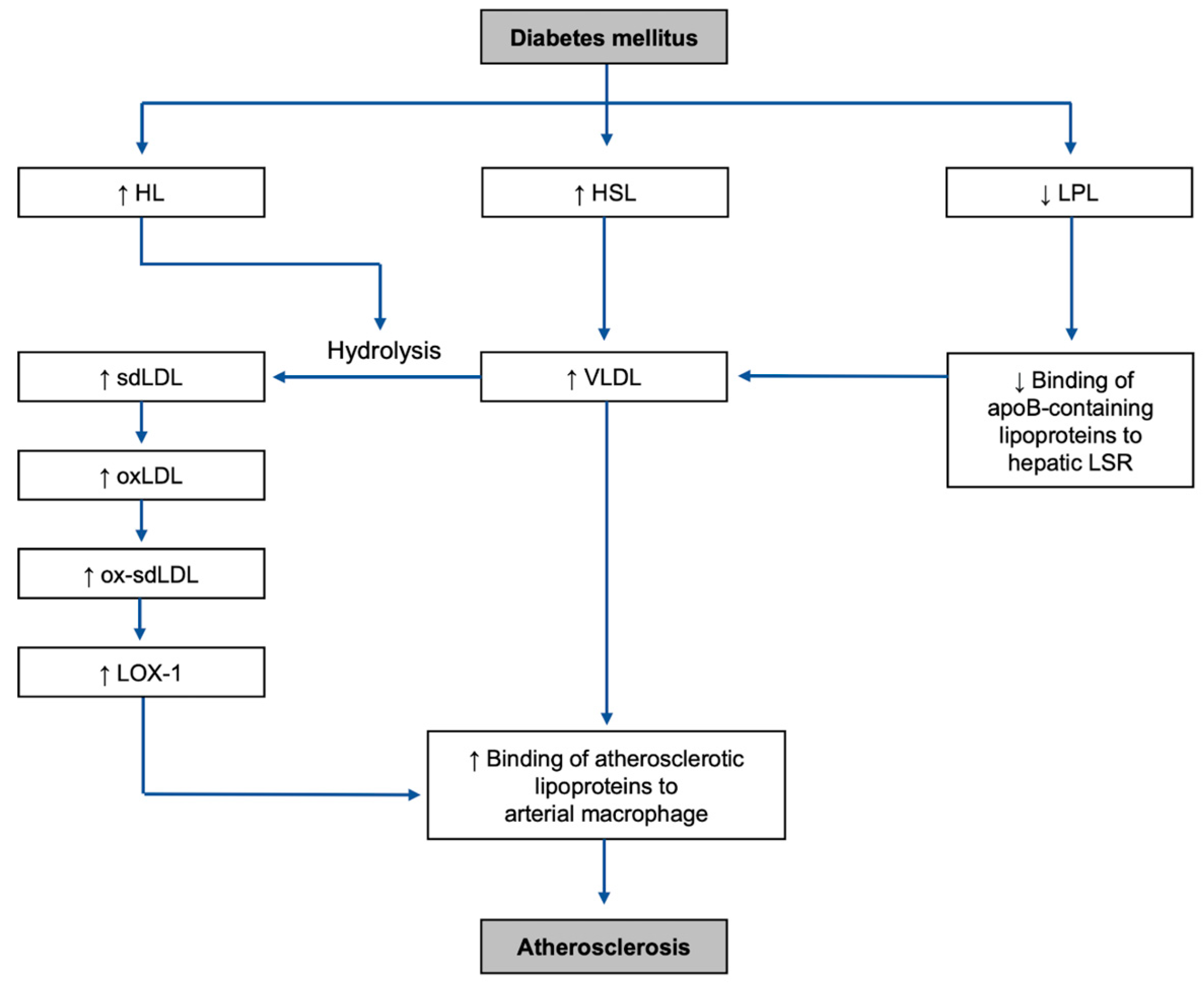
2.1. Lipase Upregulation
2.2. LPL Downregulation
3. Highlighted Risk Factors of Diabetic Dyslipidemia and Atherosclerosis
3.1. VLDL
3.1.1. How VLDL Is Formed in DM Environment
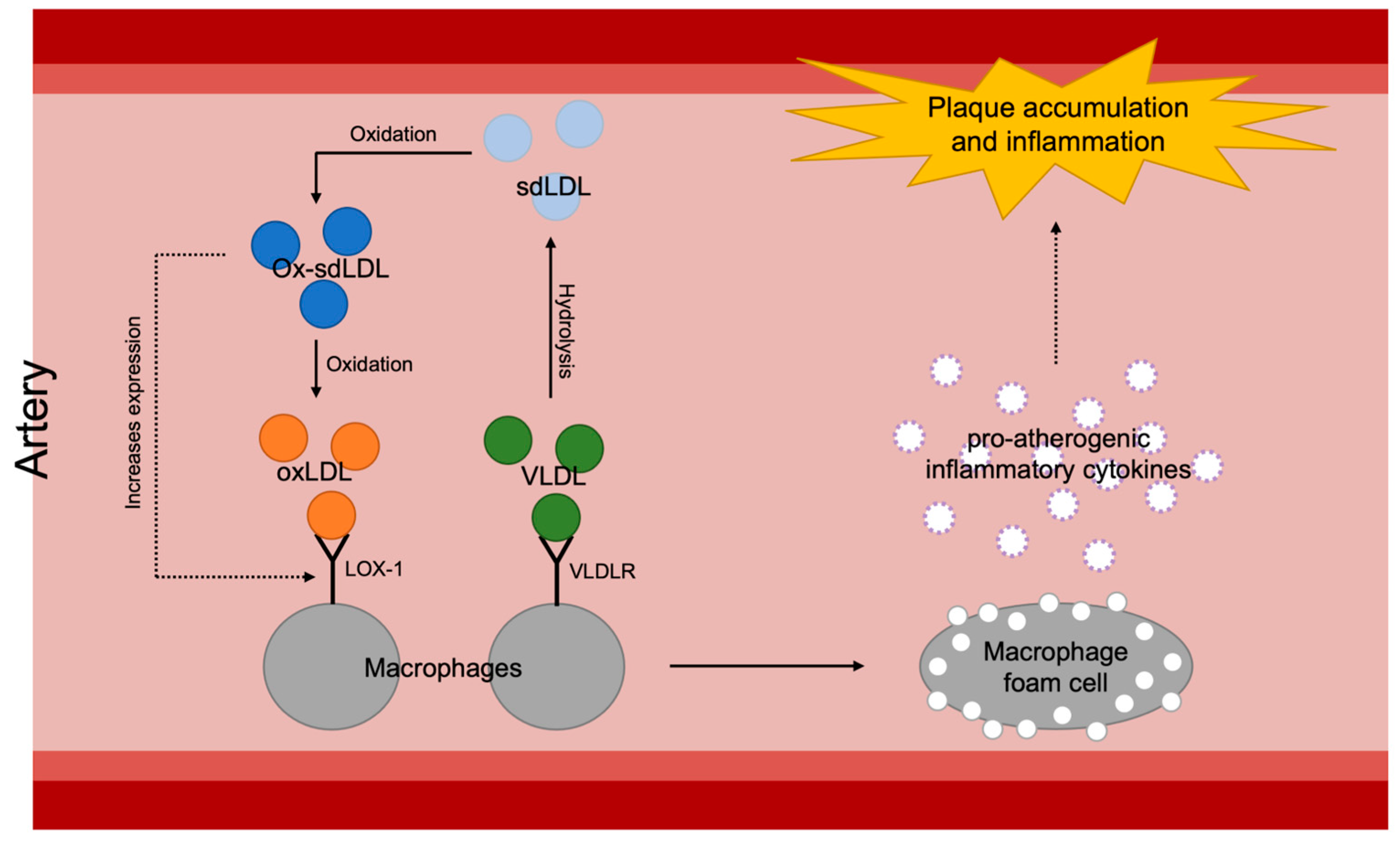
3.1.2. Atherosclerosis and VLDL
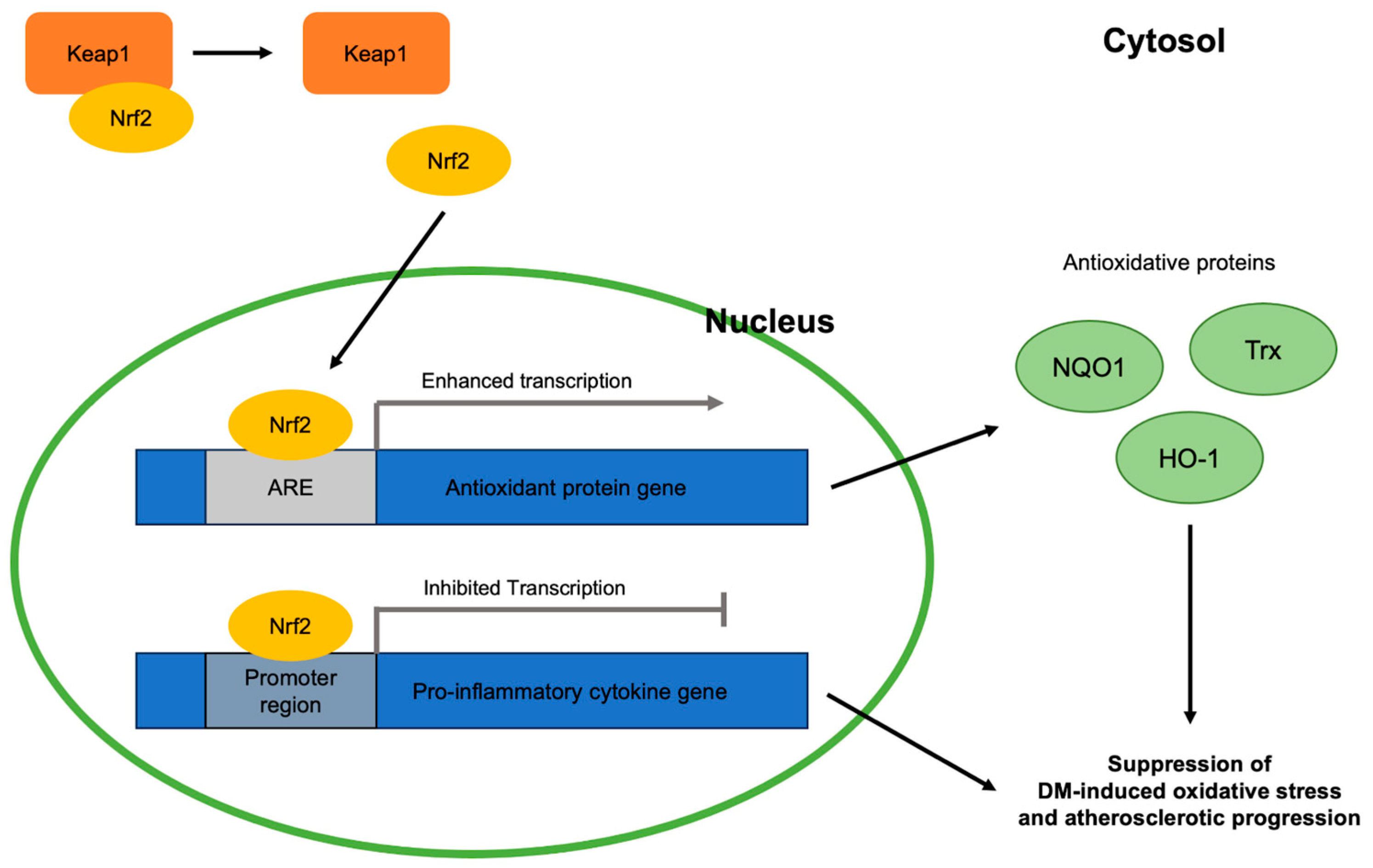
3.1.3. Nrf2-Targeting Treatments against VLDL-Dependent Oxidative Stress
| Target Risk Factor | Drug/Substance | Action in the Nrf2 Pathway and Other Antioxidative Pathways | References |
|---|---|---|---|
| VLDL | TUDCA | Increases the expressions of Nrf2 and its stabilizer DJ-1 | [87,88] |
| Antagonizes with Keap1 to prevent Nrf2 degradation | |||
| Activates the Nrf2/ARE pathway | |||
| Alleviates lipid peroxidation and inflammatory response | |||
| EP | Activates Nrf2 | [89] | |
| Activates Nrf2 translocation | |||
| Acacetin, curcumin | Enhances the Nrf2/Keap1 pathway | [97,98] | |
| Enhances PON1 concentration | |||
| sdLDL | Metformin | Activates the Nrf2 pathway | [99] |
| Prevents ROS generation | [100] | ||
| CDDO-Me | Increases Nrf2 transcription and translation | [101] | |
| Binds to Keap1 and activates the Nrf2/Keap1 pathway | [102] | ||
| Hepatic LSR impairment | Calcitriol | Activates the Nrf2 pathway | [103] |
| Impairs NF-κB |
3.2. Small Dense Low-Density Lipoprotein (sdLDL)
3.2.1. How sdLDL Is Formed in DM Environment
3.2.2. Atherosclerosis and sdLDL
3.2.3. Nrf2-Targeting Treatments against sdLDL-Dependent Oxidative Stress
3.3. Hepatic Lipolysis-Stimulated Lipoprotein Receptor Impairment
3.3.1. How It Is Caused in DM Environment
3.3.2. Atherosclerosis and Hepatic LSR Impairment
3.3.3. Nrf2-Targeting Treatments against LSR-Impairment-Dependent Oxidative Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The atherosclerosis risk in communities (aric) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, J.; Wang, W.; Wang, M.; Zhao, F.; Sun, J.; Liu, J.; Deng, Q.; Zhao, D. High sdldl cholesterol can be used to reclassify individuals with low cardiovascular risk for early intervention: Findings from the chinese multi-provincial cohort study. J. Atheroscler. Thromb. 2020, 27, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Kastelein, J.J.P.; Ray, K.K.; Ginsberg, H.N.; Chapman, M.J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; et al. Association of triglyceride-lowering lpl variants and ldl-c-lowering ldlr variants with risk of coronary heart disease. JAMA 2019, 321, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.J.; Costacou, T.; Kretowski, A.; Nesto, R.W. Type 1 diabetes and coronary artery disease. Diabetes Care 2006, 29, 2528–2538. [Google Scholar] [CrossRef]
- Yang, H.; Jin, X.; Kei Lam, C.W.; Yan, S.K. Oxidative stress and diabetes mellitus. Clin. Chem. Lab. Med. 2011, 49, 1773–1782. [Google Scholar] [CrossRef]
- Okdahl, T.; Wegeberg, A.M.; Pociot, F.; Brock, B.; Storling, J.; Brock, C. Low-grade inflammation in type 2 diabetes: A cross-sectional study from a danish diabetes outpatient clinic. BMJ Open 2022, 12, e062188. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and inflammation: Insights from the theory of general pathological processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Brooks, G.C.; Anderson, M.G.; Campbell, A.M.; Jacobs, L. Environmental complexity and reduced stocking density promote positive behavioral outcomes in broiler chickens. Animals 2023, 13, 2074. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.R.; Ann, S.H.; Won, K.B.; Park, G.M.; Kim, Y.G.; Yang, D.H.; Kang, J.W.; Lim, T.H.; Kim, H.K.; Choe, J.; et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci. Rep. 2019, 9, 6129. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and inflammation in atherosclerosis. Circ Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Selvarajah, D.; McDowell, D.; Jehangir, S.; Smith, G. Unnecessary ultrasound imaging in the management of undescended testis. Med. J. Aust. 2021, 215, 528. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, L.; Mauriello, A.; Sbarigia, E.; Spagnoli, L.G.; Violi, F. Radiolabeled native low-density lipoprotein injected into patients with carotid stenosis accumulates in macrophages of atherosclerotic plaque: Effect of vitamin e supplementation. Circulation 2000, 101, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Ebesunun, M.O.; Bankole, O.L.; Oduwole, O. Plasma oxidized low density lipoprotein cholesterol correlates inversely with testosterone in young adult male smokers. Pan Afr. Med. J. 2014, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.L.; Rizzo, M.; Chait, A.; Lewis, D.M.; Blanche, P.J.; Krauss, R.M. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am. J. Med. 2001, 110, 103–110. [Google Scholar] [CrossRef]
- Welty, F.K. How do elevated triglycerides and low hdl-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 2013, 15, 400. [Google Scholar] [CrossRef]
- Takahashi, S. Triglyceride rich lipoprotein -lpl-vldl receptor and lp(a)-vldl receptor pathways for macrophage foam cell formation. J. Atheroscler. Thromb. 2017, 24, 552–559. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The forgotten lipids: Triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef]
- Posadas-Sanchez, R.; Posadas-Romero, C.; Mendoza-Perez, E.; Caracas-Portilla, N.A.; Cardoso-Saldana, G.; Medina-Urrutia, A.; Jorge-Galarza, E.; Juarez-Rojas, J.G. Cholesterol efflux and metabolic abnormalities associated with low high-density-lipoprotein-cholesterol and high triglycerides in statin-treated coronary men with low-density lipoprotein-cholesterol <70 mg/dL. Am. J. Cardiol. 2012, 109, 636–641. [Google Scholar]
- Owen, D.M.; Magenau, A.; Williamson, D.; Gaus, K. The lipid raft hypothesis revisited--new insights on raft composition and function from super-resolution fluorescence microscopy. Bioessays 2012, 34, 739–747. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Goteri, G.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D. The role of nqo1 in ovarian cancer. Int. J. Mol. Sci. 2023, 24, 7839. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Cellular modulators of the nrf2/keap1 signaling pathway in prostate cancer. Front. Biosci. 2023, 28, 143. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Piani, F.; Crescimanno, C.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Modulation of nrf2/keap1 signaling in preeclampsia. Cells 2023, 12, 1545. [Google Scholar] [CrossRef]
- Bukke, V.N.; Moola, A.; Serviddio, G.; Vendemiale, G.; Bellanti, F. Nuclear factor erythroid 2-related factor 2-mediated signaling and metabolic associated fatty liver disease. World J. Gastroenterol. 2022, 28, 6909–6921. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin ii-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef]
- Gonzalez, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, N.; Elks, C.M.; Sriramula, S.; Guggilam, A.; Liu, Z.; Borkhsenious, O.; Francis, J. Nf-kappab-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type ii diabetes. Cardiovasc. Res. 2010, 85, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, E.; Rezaei-Chianeh, Y.; Nabi-Afjadi, M. Molecular mechanisms involved in intrarenal renin-angiotensin and alternative pathways in diabetic nephropathy—A review. Rev. Diabet. Stud. 2021, 17, 1–10. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The protective role of nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Song, S.; Wang, Y.; Bian, Y.; Wu, M.; Wu, H.; Shi, Y.; Duan, H. Nad(p)h: Quinone oxidoreductase 1 attenuates oxidative stress and apoptosis by regulating sirt1 in diabetic nephropathy. J. Transl. Med. 2022, 20, 44. [Google Scholar] [CrossRef]
- Schulze, P.C.; Yoshioka, J.; Takahashi, T.; He, Z.; King, G.L.; Lee, R.T. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004, 279, 30369–30374. [Google Scholar] [CrossRef]
- Waldman, M.; Nudelman, V.; Shainberg, A.; Zemel, R.; Kornwoski, R.; Aravot, D.; Peterson, S.J.; Arad, M.; Hochhauser, E. The role of heme oxygenase 1 in the protective effect of caloric restriction against diabetic cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 2427. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J. Clinical review 124: Diabetic dyslipidemia: Causes and consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. Low-density lipoprotein triglycerides: Widening the atherogenic landscape in cvd risk assessment. J. Am. Coll. Cardiol. 2018, 72, 170–172. [Google Scholar] [CrossRef]
- Holm, C.; Osterlund, T.; Laurell, H.; Contreras, J.A. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu. Rev. Nutr. 2000, 20, 365–393. [Google Scholar] [CrossRef] [PubMed]
- Pavlic, M.; Valero, R.; Duez, H.; Xiao, C.; Szeto, L.; Patterson, B.W.; Lewis, G.F. Triglyceride-rich lipoprotein-associated apolipoprotein c-iii production is stimulated by plasma free fatty acids in humans. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1660–1665. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Hamm, J.K.; Verhagen, L.A.; Peroni, O.; Katic, M.; Flier, J.S. Adipose triglyceride lipase: Function, regulation by insulin, and comparison with adiponutrin. Diabetes 2006, 55, 148–157. [Google Scholar] [CrossRef]
- Lewis, G.F.; Uffelman, K.D.; Szeto, L.W.; Weller, B.; Steiner, G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J. Clin. Investig. 1995, 95, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Baynes, C.; Henderson, A.D.; Anyaoku, V.; Richmond, W.; Hughes, C.L.; Johnston, D.G.; Elkeles, R.S. The role of insulin insensitivity and hepatic lipase in the dyslipidaemia of type 2 diabetes. Diabet. Med. 1991, 8, 560–566. [Google Scholar] [CrossRef]
- Knauer, T.E.; Woods, J.A.; Lamb, R.G.; Fallon, H.J. Hepatic triacylglycerol lipase activities after induction of diabetes and administration of insulin or glucagon. J. Lipid Res. 1982, 23, 631–637. [Google Scholar] [CrossRef]
- Lewis, G.F.; Murdoch, S.; Uffelman, K.; Naples, M.; Szeto, L.; Albers, A.; Adeli, K.; Brunzell, J.D. Hepatic lipase mrna, protein, and plasma enzyme activity is increased in the insulin-resistant, fructose-fed syrian golden hamster and is partially normalized by the insulin sensitizer rosiglitazone. Diabetes 2004, 53, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Rapp, R.J. Hypertriglyceridemia: A review beyond low-density lipoprotein. Cardiol. Rev. 2002, 10, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Welty, F.K.; Lichtenstein, A.H.; Barrett, P.H.; Dolnikowski, G.G.; Schaefer, E.J. Human apolipoprotein (apo) b-48 and apob-100 kinetics with stable isotopes. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2966–2974. [Google Scholar] [CrossRef]
- De Man, F.H.; Cabezas, M.C.; Van Barlingen, H.H.; Erkelens, D.W.; de Bruin, T.W. Triglyceride-rich lipoproteins in non-insulin-dependent diabetes mellitus: Post-prandial metabolism and relation to premature atherosclerosis. Eur. J. Clin. Investig. 1996, 26, 89–108. [Google Scholar] [CrossRef]
- Taskinen, M.R. Lipoprotein lipase in diabetes. Diabetes Metab. Rev. 1987, 3, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; McConathy, W.J.; Kloer, H.U.; Alaupovic, P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein c-iii. J. Clin. Investig. 1985, 75, 384–390. [Google Scholar] [CrossRef]
- Whitacre, B.E.; Howles, P.; Street, S.; Morris, J.; Swertfeger, D.; Davidson, W.S. Apolipoprotein e content of vldl limits lpl-mediated triglyceride hydrolysis. J. Lipid Res. 2022, 63, 100157. [Google Scholar] [CrossRef]
- Verges, B. Lipid disorders in type 1 diabetes. Diabetes Metab. 2009, 35, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Boulouiz, S.; Kossir, A.; Mouedder, F.; Miri, C.; Ismaili, N.; El Ouafi, N. Shone syndrome revealed by treatment-resistant hypertension. Ann. Med. Surg. 2021, 71, 102955. [Google Scholar] [CrossRef] [PubMed]
- Carneheim, C.M.; Alexson, S.E. Refeeding and insulin increase lipoprotein lipase activity in rat brown adipose tissue. Am. J. Physiol. 1989, 256, E645–E650. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Nikkila, E.A.; Nousiainen, R.; Gordin, A. Lipoprotein lipase activity in adipose tissue and skeletal muscle of human diabetics during insulin deprivation and restoration. Scand. J. Clin. Lab. Investig. 1981, 41, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Rosato, E.F.; Vemulapalli, P.; Lang, C.H.; Lanza-Jacoby, S. Insulin stimulates lipoprotein lipase activity and synthesis in adipocytes from septic rats. J. Surg. Res. 1997, 73, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Nikkila, E.A. Lipoprotein lipase activity of adipose tissue and skeletal muscle in insulin-deficient human diabetes. Relation to high-density and very-low-density lipoproteins and response to treatment. Diabetologia 1979, 17, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Amor, A.J.; Castelblanco, E.; Hernandez, M.; Gimenez, M.; Granado-Casas, M.; Blanco, J.; Soldevila, B.; Esmatjes, E.; Conget, I.; Alonso, N.; et al. Advanced lipoprotein profile disturbances in type 1 diabetes mellitus: A focus on ldl particles. Cardiovasc. Diabetol. 2020, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, A.C.; Feitosa-Filho, G.S.; Freitas, F.R.; Wajchenberg, B.L.; Maranhao, R.C. Lipoprotein metabolism in patients with type 1 diabetes under intensive insulin treatment. Lipids Health Dis. 2013, 12, 15. [Google Scholar] [CrossRef]
- Kalmar, T.; Seres, I.; Balogh, Z.; Kaplar, M.; Winkler, G.; Paragh, G. Correlation between the activities of lipoprotein lipase and paraoxonase in type 2 diabetes mellitus. Diabetes Metab. 2005, 31, 574–580. [Google Scholar] [CrossRef]
- Tsuda, K.; Akiba, T.; Kimura, F.; Ishibashi, M.; Moriya, C.; Nakagawa, K.; Kurata, N.; Ito, Y. Onion2 fatty acid elongase is required for shoot development in rice. Plant Cell Physiol. 2013, 54, 209–217. [Google Scholar] [CrossRef]
- An, K.; Guo, P.; Zhang, H.; Zhu, W.; Cao, W.; Shi, J.; Wang, S. Decreased plasma level of lipoprotein lipase predicted verbal disfluency in chinese type 2 diabetes mellitus patients with early cognitive deficits. Curr. Alzheimer Res. 2021, 18, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Miyamoto, W.; Inoue, J.; Terada, T.; Imanaka, T.; Maeda, M. Sterol regulatory element-binding protein negatively regulates microsomal triglyceride transfer protein gene transcription. J. Biol. Chem. 1999, 274, 24714–24720. [Google Scholar] [CrossRef] [PubMed]
- Julius, U. Influence of plasma free fatty acids on lipoprotein synthesis and diabetic dyslipidemia. Exp. Clin. Endocrinol. Diabetes 2003, 111, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Fisher, E.A. The ever-expanding role of degradation in the regulation of apolipoprotein b metabolism. J. Lipid Res. 2009, 50, S162–S166. [Google Scholar] [CrossRef]
- Sztalryd, C.; Kraemer, F.B. Regulation of hormone-sensitive lipase in streptozotocin-induced diabetic rats. Metabolism 1995, 44, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Carey, A.L.; Wolsk-Petersen, E.; Kraemer, F.B.; Pedersen, B.K.; Febbraio, M.A. Hormone-sensitive lipase is reduced in the adipose tissue of patients with type 2 diabetes mellitus: Influence of il-6 infusion. Diabetologia 2005, 48, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Reynisdottir, S.; Angelin, B.; Langin, D.; Lithell, H.; Eriksson, M.; Holm, C.; Arner, P. Adipose tissue lipoprotein lipase and hormone-sensitive lipase. Contrasting findings in familial combined hyperlipidemia and insulin resistance syndrome. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Steiner, G. Acute effects of insulin in the control of vldl production in humans. Implications for the insulin-resistant state. Diabetes Care 1996, 19, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Talayero, B.G.; Sacks, F.M. The role of triglycerides in atherosclerosis. Curr. Cardiol. Rep. 2011, 13, 544–552. [Google Scholar] [CrossRef]
- Teramoto, R.; Tada, H.; Kawashiri, M.A.; Nohara, A.; Nakahashi, T.; Konno, T.; Inazu, A.; Mabuchi, H.; Yamagishi, M.; Hayashi, K. Molecular and functional characterization of familial chylomicronemia syndrome. Atherosclerosis 2018, 269, 272–278. [Google Scholar] [CrossRef]
- Raposeiras-Roubin, S.; Rossello, X.; Oliva, B.; Fernandez-Friera, L.; Mendiguren, J.M.; Andres, V.; Bueno, H.; Sanz, J.; Martinez de Vega, V.; Abu-Assi, E.; et al. Triglycerides and residual atherosclerotic risk. J. Am. Coll. Cardiol. 2021, 77, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The role of lipids and lipoproteins in atherosclerosis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; Mdtext.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Moore, K.J.; Kunjathoor, V.V.; Koehn, S.L.; Manning, J.J.; Tseng, A.A.; Silver, J.M.; McKee, M.; Freeman, M.W. Loss of receptor-mediated lipid uptake via scavenger receptor a or cd36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Investig. 2005, 115, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Manning-Tobin, J.J.; Moore, K.J.; Seimon, T.A.; Bell, S.A.; Sharuk, M.; Alvarez-Leite, J.I.; de Winther, M.P.; Tabas, I.; Freeman, M.W. Loss of sr-a and cd36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Freeman, M.W. Scavenger receptors in atherosclerosis: Beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Ito, T.; Zenimaru, Y.; Suzuki, J.; Miyamori, I.; Takahashi, M.; Takahashi, M.; Ishida, T.; Ishida, T.; Hirata, K.; et al. Species differences of macrophage very low-density-lipoprotein (vldl) receptor protein expression. Biochem. Biophys. Res. Commun. 2011, 407, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Eck, M.V.; Oost, J.; Goudriaan, J.R.; Hoekstra, M.; Hildebrand, R.B.; Bos, I.S.; van Dijk, K.W.; Van Berkel, T.J. Role of the macrophage very-low-density lipoprotein receptor in atherosclerotic lesion development. Atherosclerosis 2005, 183, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Yagyu, H.; Lutz, E.P.; Kako, Y.; Marks, S.; Hu, Y.; Choi, S.Y.; Bensadoun, A.; Goldberg, I.J. Very low density lipoprotein (vldl) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with vldl receptor deficiency. J. Biol. Chem. 2002, 277, 10037–10043. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Millns, H.; Neil, H.A.; Stratton, I.M.; Manley, S.E.; Matthews, D.R.; Holman, R.R. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United kingdom prospective diabetes study (ukpds: 23). BMJ 1998, 316, 823–828. [Google Scholar] [CrossRef]
- Group, A.S.; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar]
- Pinto, C.S.; Lana, J.M.; Gabbay, M.A.; de Sa, J.R.; Dib, S.A. Hdl cholesterol levels and weight are the main determinants of subclinical atherosclerosis in the young with type 1 diabetes and suitable glycaemic control. Diabetes Vasc. Dis. Res. 2014, 11, 125–128. [Google Scholar] [CrossRef]
- Zhong, S.; Goldberg, I.J.; Bruce, C.; Rubin, E.; Breslow, J.L.; Tall, A. Human apoa-ii inhibits the hydrolysis of hdl triglyceride and the decrease of hdl size induced by hypertriglyceridemia and cholesteryl ester transfer protein in transgenic mice. J. Clin. Investig. 1994, 94, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.D.; Cartolano, F.C.; Freitas, M.C.P.; Santa-Helena, E.; Markus, M.R.P.; Santos, R.D.; Damasceno, N.R.T. Adiponectin predicts the antioxidant capacity and size of high-density lipoprotein (hdl) in individuals with diabetes mellitus. J. Diabetes Complicat. 2021, 35, 107856. [Google Scholar] [CrossRef]
- Sokooti, S.; Flores-Guerrero, J.L.; Kieneker, L.M.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Hdl particle subspecies and their association with incident type 2 diabetes: The prevend study. J. Clin. Endocrinol. Metab. 2021, 106, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Gourgari, E.; Ma, J.; Playford, M.P.; Mehta, N.N.; Goldman, R.; Remaley, A.T.; Gordon, S.M. Proteomic alterations of hdl in youth with type 1 diabetes and their associations with glycemic control: A case-control study. Cardiovasc. Diabetol. 2019, 18, 43. [Google Scholar] [CrossRef]
- Moren, X.; Lhomme, M.; Bulla, A.; Sanchez, J.C.; Kontush, A.; James, R.W. Proteomic and lipidomic analyses of paraoxonase defined high density lipoprotein particles: Association of paraoxonase with the anti-coagulant, protein s. Proteom. Clin. Appl. 2016, 10, 230–238. [Google Scholar] [CrossRef]
- Shih, D.M.; Gu, L.; Xia, Y.R.; Navab, M.; Li, W.F.; Hama, S.; Castellani, L.W.; Furlong, C.E.; Costa, L.G.; Fogelman, A.M.; et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998, 394, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Tward, A.; Xia, Y.R.; Wang, X.P.; Shi, Y.S.; Park, C.; Castellani, L.W.; Lusis, A.J.; Shih, D.M. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 2002, 106, 484–490. [Google Scholar] [CrossRef]
- Zarei, M.; Barroso, E.; Palomer, X.; Dai, J.; Rada, P.; Quesada-Lopez, T.; Escola-Gil, J.C.; Cedo, L.; Zali, M.R.; Molaei, M.; et al. Hepatic regulation of vldl receptor by pparbeta/delta and fgf21 modulates non-alcoholic fatty liver disease. Mol. Metab. 2018, 8, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Fonseca, I.; Nunes, M.J.; Rosa, A.; Lemos, L.; Rodrigues, E.; Carvalho, A.N.; Outeiro, T.F.; Rodrigues, C.M.P.; Gama, M.J.; et al. Nrf2 activation by tauroursodeoxycholic acid in experimental models of parkinson’s disease. Exp. Neurol. 2017, 295, 77–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Liu, J.; Zuo, J.; Yan, L.; Thring, R.W.; Ba, X.; Qi, D.; Wu, M.; Gao, Y.; et al. Tauroursodeoxycholic acid functions as a critical effector mediating insulin sensitization of metformin in obese mice. Redox Biol. 2022, 57, 102481. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, S.W.; Jin, Y.; Kim, I.D.; Lee, J.K. Ethyl pyruvate-mediated nrf2 activation and hemeoxygenase 1 induction in astrocytes confer protective effects via autocrine and paracrine mechanisms. Neurochem. Int. 2012, 61, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Yilmaz, E.; Ozcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Gorgun, C.Z.; Hotamisligil, G.S. Chemical chaperones reduce er stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Hong, S.H.; Lee, Y.J.; Chung, S.S.; Jung, H.S.; Park, S.G.; Park, K.S. Tauroursodeoxycholate (tudca), chemical chaperone, enhances function of islets by reducing er stress. Biochem. Biophys. Res. Commun. 2010, 397, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Cadavez, L.; Montane, J.; Alcarraz-Vizan, G.; Visa, M.; Vidal-Fabrega, L.; Servitja, J.M.; Novials, A. Chaperones ameliorate beta cell dysfunction associated with human islet amyloid polypeptide overexpression. PLoS ONE 2014, 9, e101797. [Google Scholar] [CrossRef] [PubMed]
- Bronczek, G.A.; Vettorazzi, J.F.; Soares, G.M.; Kurauti, M.A.; Santos, C.; Bonfim, M.F.; Carneiro, E.M.; Balbo, S.L.; Boschero, A.C.; Costa Junior, J.M. The bile acid tudca improves beta-cell mass and reduces insulin degradation in mice with early-stage of type-1 diabetes. Front. Physiol. 2019, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Koprivica, I.; Vujicic, M.; Gajic, D.; Saksida, T.; Stojanovic, I. Ethyl pyruvate stimulates regulatory t cells and ameliorates type 1 diabetes development in mice. Front. Immunol. 2018, 9, 3130. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, H.; Kelle, I.; Tunik, S.; Bahceci, S.; Sencar, L.; Ayaz, E.; Nergiz, Y.; Erdinc, L.; Erdinc, M. Protective effect of ethyl pyruvate on liver injury in streptozotocin-induced diabetic rats. Acta Gastroenterol. Belg. 2012, 75, 336–341. [Google Scholar] [PubMed]
- Ju, K.D.; Shin, E.K.; Cho, E.J.; Yoon, H.B.; Kim, H.S.; Kim, H.; Yang, J.; Hwang, Y.H.; Ahn, C.; Oh, K.H. Ethyl pyruvate ameliorates albuminuria and glomerular injury in the animal model of diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2012, 302, F606–F613. [Google Scholar] [CrossRef]
- Gatbonton-Schwager, T.; Yagishita, Y.; Joshi, T.; Wakabayashi, N.; Srinivasan, H.; Suzuki, T.; Yamamoto, M.; Kensler, T.W. A point mutation at c151 of keap1 of mice abrogates nrf2 signaling, cytoprotection in vitro, and hepatoprotection in vivo by bardoxolone methyl (cddo-me). Mol. Pharmacol. 2023, 104, 51–61. [Google Scholar] [CrossRef]
- Lewis, J.H.; Jadoul, M.; Block, G.A.; Chin, M.P.; Ferguson, D.A.; Goldsberry, A.; Meyer, C.J.; O’Grady, M.; Pergola, P.E.; Reisman, S.A.; et al. Effects of bardoxolone methyl on hepatic enzymes in patients with type 2 diabetes mellitus and stage 4 ckd. Clin. Transl. Sci. 2021, 14, 299–309. [Google Scholar] [CrossRef]
- Chokshi, N.P.; Messerli, F.H.; Sutin, D.; Supariwala, A.A.; Shah, N.R. Appropriateness of statins in patients aged >/=80 years and comparison to other age groups. Am. J. Cardiol. 2012, 110, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Z.; Han, W.; Yuan, Y.; Li, Y.; Zhou, K.; Wang, Q.; Xie, L.; Xu, K.; Zhang, H.; et al. Metformin promotes axon regeneration after spinal cord injury through inhibiting oxidative stress and stabilizing microtubule. Oxid. Med. Cell. Longev. 2020, 2020, 9741369. [Google Scholar] [CrossRef]
- Wei, Y.; Jing, J.; Peng, Z.; Liu, X.; Wang, X. Acacetin ameliorates insulin resistance in obesity mice through regulating treg/th17 balance via mir-23b-3p/neu1 axis. BMC Endocr. Disord. 2021, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.B.; Kang, M.J.; Ryu, H.W.; Lee, S.; Lee, J.W.; Lee, M.K.; Lee, H.S.; Lee, S.U.; Oh, S.R.; Kim, M.O. Acacetin enhances glucose uptake through insulin-independent glut4 translocation in l6 myotubes. Phytomedicine 2020, 68, 153178. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ji, F.; Liu, Y.; Zhang, W.; Ma, X. Calcitriol plays a protective role in diabetic nephropathy through anti-inflammatory effects. Int. J. Clin. Exp. Med. 2014, 7, 5437–5444. [Google Scholar] [PubMed]
- Hong, J.; Zhang, Y.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; Zhao, J.; Cui, L.; et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013, 36, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W.; et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.M.; da Costa, D.C.; Resende, A.C.; Soulage, C.O.; Bezerra, F.F.; Daleprane, J.B. Activation of nrf2-antioxidant signaling by 1,25-dihydroxycholecalciferol prevents leptin-induced oxidative stress and inflammation in human endothelial cells. J. Nutr. 2017, 147, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Boesch-Saadatmandi, C.; Breckwoldt, D.; Schrader, C.; Schmelzer, C.; Doring, F.; Hashida, K.; Hori, O.; Matsugo, S.; Rimbach, G. Ascorbic acid partly antagonizes resveratrol mediated heme oxygenase-1 but not paraoxonase-1 induction in cultured hepatocytes—Role of the redox-regulated transcription factor nrf2. BMC Complement. Altern. Med. 2011, 11, 1. [Google Scholar] [CrossRef]
- Wu, B.; Wang, F.; Zhou, J.; Hou, Y.; Hong, G.; Zhao, G.; Ge, Y.; Liu, Y.; Qiu, Q.; Lu, Z. Effect of pon1 overexpression on mouse diaphragmatic muscle cells injury caused by acute dichlorvos poisoning. Zhonghua Yi Xue Za Zhi 2015, 95, 2955–2959. [Google Scholar]
- Wu, Y.; Song, F.; Li, Y.; Li, J.; Cui, Y.; Hong, Y.; Han, W.; Wu, W.; Lakhani, I.; Li, G.; et al. Acacetin exerts antioxidant potential against atherosclerosis through nrf2 pathway in apoE−/− mice. J. Cell. Mol. Med. 2021, 25, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015, 6, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Angeletti, P.M.; Marziliano, C.; Mastrodomenico, M.; Giuliani, A.R.; Petrocelli, R. Potential therapeutic effects of curcumin on glycemic and lipid profile in uncomplicated type 2 diabetes-a meta-analysis of randomized controlled trial. Nutrients 2021, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via sirt1-foxo1 and pi3k-akt signalling pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Park, H.D.; Kim, S.W.; Bae, J.C.; Tan, A.H.; Chung, H.S.; Hur, K.Y.; Kim, J.H.; Kim, K.W.; Lee, M.K. Smaller mean ldl particle size and higher proportion of small dense ldl in korean type 2 diabetic patients. Diabetes Metab. J. 2011, 35, 536–542. [Google Scholar] [CrossRef]
- Resnick, H.E.; Foster, G.L.; Bardsley, J.; Ratner, R.E. Achievement of american diabetes association clinical practice recommendations among u.S. Adults with diabetes, 1999–2002: The national health and nutrition examination survey. Diabetes Care 2006, 29, 531–537. [Google Scholar] [CrossRef]
- Huang, J.; Gu, J.X.; Bao, H.Z.; Li, S.S.; Yao, X.Q.; Yang, M.; Li, Y.; Zhang, A.M.; Yin, Y.; Zhang, N.; et al. Elevated serum small dense low-density lipoprotein cholesterol may increase the risk and severity of coronary heart disease and predict cardiovascular events in patients with type 2 diabetes mellitus. Dis. Markers 2021, 2021, 5597028. [Google Scholar] [CrossRef]
- Berneis, K.; Jeanneret, C.; Muser, J.; Felix, B.; Miserez, A.R. Low-density lipoprotein size and subclasses are markers of clinically apparent and non-apparent atherosclerosis in type 2 diabetes. Metabolism 2005, 54, 227–234. [Google Scholar] [CrossRef]
- Liu, M.L.; Ylitalo, K.; Vakkilainen, J.; Nuotio, I.; Valkonen, M.; Lahdenpera, S.; Viikari, J.; Taskinen, M.R. Susceptibility of ldl to oxidation in vitro and antioxidant capacity in familial combined hyperlipidemia: Comparison of patients with different lipid phenotypes. Ann. Med. 2002, 34, 48–54. [Google Scholar] [CrossRef]
- Tani, M.; Kawakami, A.; Mizuno, Y.; Imase, R.; Ito, Y.; Kondo, K.; Ishii, H.; Yoshida, M. Small dense ldl enhances thp-1 macrophage foam cell formation. J. Atheroscler. Thromb. 2011, 18, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.N.; Soran, H.; Pemberton, P.; Charlton-Menys, V.; Elseweidy, M.M.; Durrington, P.N. Small dense ldl is more susceptible to glycation than more buoyant ldl in type 2 diabetes. Clin. Sci. 2013, 124, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil, H.A.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; Fuller, J.H.; et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (cards): Multicentre randomised placebo-controlled trial. Lancet 2004, 364, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Mansi, I.A.; Chansard, M.; Lingvay, I.; Zhang, S.; Halm, E.A.; Alvarez, C.A. Association of statin therapy initiation with diabetes progression: A retrospective matched-cohort study. JAMA Intern. Med. 2021, 181, 1562–1574. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, M.; Torres, G.; Wu, S.; Ouyang, C.; Xie, Z.; Zou, M.H. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of drp1-mediated mitochondrial fission. Diabetes 2017, 66, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Gao, L.; Zhang, A.; Hackfort, B.T.; Zucker, I.H. Therapeutic effects of nrf2 activation by bardoxolone methyl in chronic heart failure. J. Pharmacol. Exp. Ther. 2019, 371, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Yang, Y.X.; Zhe, H.; He, Z.X.; Zhou, S.F. Bardoxolone methyl (cddo-me) as a therapeutic agent: An update on its pharmacokinetic and pharmacodynamic properties. Drug Des. Dev. Ther. 2014, 8, 2075–2088. [Google Scholar]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef]
- Tan, S.M.; Sharma, A.; Stefanovic, N.; Yuen, D.Y.; Karagiannis, T.C.; Meyer, C.; Ward, K.W.; Cooper, M.E.; de Haan, J.B. Derivative of bardoxolone methyl, dh404, in an inverse dose-dependent manner lessens diabetes-associated atherosclerosis and improves diabetic kidney disease. Diabetes 2014, 63, 3091–3103. [Google Scholar] [CrossRef]
- Narvekar, P.; Berriel Diaz, M.; Krones-Herzig, A.; Hardeland, U.; Strzoda, D.; Stohr, S.; Frohme, M.; Herzig, S. Liver-specific loss of lipolysis-stimulated lipoprotein receptor triggers systemic hyperlipidemia in mice. Diabetes 2009, 58, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Sugase, T.; Takahashi, T.; Serada, S.; Fujimoto, M.; Ohkawara, T.; Hiramatsu, K.; Koh, M.; Saito, Y.; Tanaka, K.; Miyazaki, Y.; et al. Lipolysis-stimulated lipoprotein receptor overexpression is a novel predictor of poor clinical prognosis and a potential therapeutic target in gastric cancer. Oncotarget 2018, 9, 32917–32928. [Google Scholar] [CrossRef]
- Jun, J.Y.; Ma, Z.; Segar, L. Spontaneously diabetic ins2(+/akita):Apoe-deficient mice exhibit exaggerated hypercholesterolemia and atherosclerosis. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E145–E154. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. Diabetic dyslipidemia: Basic mechanisms underlying the common hypertriglyceridemia and low hdl cholesterol levels. Diabetes 1996, 45 (Suppl. 3), S27–S30. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.; Plutzky, J.; Sobel, B.E. A review of metabolic and cardiovascular effects of oral antidiabetic agents: Beyond glucose-level lowering. J. Cardiovasc. Risk 1999, 6, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Zhang, Y.L.; Hernandez-Ono, A. Metabolic syndrome: Focus on dyslipidemia. Obesity 2006, 14 (Suppl. 1), 41S–49S. [Google Scholar] [CrossRef] [PubMed]
- Stenger, C.; Hanse, M.; Pratte, D.; Mbala, M.L.; Akbar, S.; Koziel, V.; Escanye, M.C.; Kriem, B.; Malaplate-Armand, C.; Olivier, J.L.; et al. Up-regulation of hepatic lipolysis stimulated lipoprotein receptor by leptin: A potential lever for controlling lipid clearance during the postprandial phase. FASEB J. 2010, 24, 4218–4228. [Google Scholar] [CrossRef]
- Sparks, J.D.; Sparks, C.E. Insulin modulation of hepatic synthesis and secretion of apolipoprotein b by rat hepatocytes. J. Biol. Chem. 1990, 265, 8854–8862. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Watanabe, T.; Sakaue, T.; Lewis, G.F. Mechanisms of hdl lowering in insulin resistant, hypertriglyceridemic states: The combined effect of hdl triglyceride enrichment and elevated hepatic lipase activity. Clin. Biochem. 2003, 36, 421–429. [Google Scholar] [CrossRef]
- Watts, G.F.; Barrett, P.H.; Ji, J.; Serone, A.P.; Chan, D.C.; Croft, K.D.; Loehrer, F.; Johnson, A.G. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes 2003, 52, 803–811. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; Fuller, J.H.; Mulnier, H.E.; Raleigh, V.S.; Lawrenson, R.A.; Colhoun, H.M. High risk of cardiovascular disease in patients with type 1 diabetes in the u.K.: A cohort study using the general practice research database. Diabetes Care 2006, 29, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Rayfield, E.J. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc. Diabetol. 2002, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Zgibor, J.C.; Piatt, G.A.; Ruppert, K.; Orchard, T.J.; Roberts, M.S. Deficiencies of cardiovascular risk prediction models for type 1 diabetes. Diabetes Care 2006, 29, 1860–1865. [Google Scholar] [CrossRef]
- Yen, F.T.; Roitel, O.; Bonnard, L.; Notet, V.; Pratte, D.; Stenger, C.; Magueur, E.; Bihain, B.E. Lipolysis stimulated lipoprotein receptor: A novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J. Biol. Chem. 2008, 283, 25650–25659. [Google Scholar] [CrossRef] [PubMed]
- Klempfner, R.; Erez, A.; Sagit, B.Z.; Goldenberg, I.; Fisman, E.; Kopel, E.; Shlomo, N.; Israel, A.; Tenenbaum, A. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: Twenty-two-year follow-up of the bezafibrate infarction prevention study and registry. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bihain, B.E.; Yen, F.T. Free fatty acids activate a high-affinity saturable pathway for degradation of low-density lipoproteins in fibroblasts from a subject homozygous for familial hypercholesterolemia. Biochemistry 1992, 31, 4628–4636. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.T.; Mann, C.J.; Guermani, L.M.; Hannouche, N.F.; Hubert, N.; Hornick, C.A.; Bordeau, V.N.; Agnani, G.; Bihain, B.E. Identification of a lipolysis-stimulated receptor that is distinct from the ldl receptor and the ldl receptor-related protein. Biochemistry 1994, 33, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J.; Khallou, J.; Chevreuil, O.; Troussard, A.A.; Guermani, L.M.; Launay, K.; Delplanque, B.; Yen, F.T.; Bihain, B.E. Mechanism of activation and functional significance of the lipolysis-stimulated receptor. Evidence for a role as chylomicron remnant receptor. Biochemistry 1995, 34, 10421–10431. [Google Scholar] [CrossRef]
- Clement, K.; Vaisse, C.; Lahlou, N.; Cabrol, S.; Pelloux, V.; Cassuto, D.; Gourmelen, M.; Dina, C.; Chambaz, J.; Lacorte, J.M.; et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998, 392, 398–401. [Google Scholar] [CrossRef]
- Mazen, I.; El-Gammal, M.; Abdel-Hamid, M.; Amr, K. A novel homozygous missense mutation of the leptin gene (n103k) in an obese egyptian patient. Mol. Genet. Metab. 2009, 97, 305–308. [Google Scholar] [CrossRef]
- Yupanqui-Lozno, H.; Bastarrachea, R.A.; Yupanqui-Velazco, M.E.; Alvarez-Jaramillo, M.; Medina-Mendez, E.; Giraldo-Pena, A.P.; Arias-Serrano, A.; Torres-Forero, C.; Garcia-Ordonez, A.M.; Mastronardi, C.A.; et al. Congenital leptin deficiency and leptin gene missense mutation found in two colombian sisters with severe obesity. Genes 2019, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Goldsmith, R.; Bloomfield, D.; Magnano, A.; Weimer, L.; Heymsfield, S.; Gallagher, D.; Mayer, L.; Murphy, E.; Leibel, R.L. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Investig. 2005, 115, 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.N.; Buchanich, J.M.; Youk, A.; Brooks, M.M.; Barinas-Mitchell, E.; Conroy, M.B.; Sutton-Tyrrell, K. Reductions in arterial stiffness with weight loss in overweight and obese young adults: Potential mechanisms. Atherosclerosis 2012, 223, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Redman, L.M.; Heilbronn, L.K.; Smith, J.V.; Martin, C.K.; Rood, J.C.; Greenway, F.L.; Williamson, D.A.; Smith, S.R.; Ravussin, E.; et al. Caloric restriction alone and with exercise improves cvd risk in healthy non-obese individuals. Atherosclerosis 2009, 203, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kveiborg, M.; Mosekilde, L.; Eriksen, E.F.; Kassem, M.S. Biological action mechanisms and effects of calcitriol. Ugeskr. Laeger 1999, 161, 5669–5674. [Google Scholar]
- Eftekhari, M.H.; Akbarzadeh, M.; Dabbaghmanesh, M.H.; Hasanzadeh, J. Impact of treatment with oral calcitriol on glucose indices in type 2 diabetes mellitus patients. Asia Pac. J. Clin. Nutr. 2011, 20, 521–526. [Google Scholar] [PubMed]
- Bouloumie, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999, 13, 1231–1238. [Google Scholar] [CrossRef]
- Berger, S.; Polotsky, V.Y. Leptin and leptin resistance in the pathogenesis of obstructive sleep apnea: A possible link to oxidative stress and cardiovascular complications. Oxid. Med. Cell. Longev. 2018, 2018, 5137947. [Google Scholar] [CrossRef]
| Treatment | Subject | Study Results | References |
|---|---|---|---|
| Leptin | Human, obese | Significant weight loss | [150] |
| Mean weight loss of 7%, reduced heart rate and heart inflammation | [151] | ||
| Human, non-obese | 10% (approximated) weight loss, positive changes in lipid profile and blood pressure, limitation of inflammation | [152] | |
| Mouse | Upregulation of hepatic LSR, increase of LSR protein and mRNA levels, lipid clearance during postprandial phase | [134] | |
| Human endothelial cells | Increased oxidative stress | [153] | |
| Calcitriol | Human, T2DM | Increased insulin secretion under vitamin D deficiency without change in insulin resistance | [154] |
| Human, T1DM | Alleviation of renal inflammation, delay of diabetic nephropathy, no significant alteration in the blood glucose levels or glucose metabolism | [155] | |
| Leptin + Calcitriol | Human endothelial cells | Calcitriol-induced increase of vitamin D led to a decline in leptin-induced superoxide anion production (oxidative stress), improvement of Nrf2 translocation, impairment of NF-κB | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, M.; Toribio, A.J.; Salem, Y.M.; Alexander, M.; Ferrey, A.; Swentek, L.; Tantisattamo, E.; Ichii, H. Nrf2 Signaling Pathway as a Key to Treatment for Diabetic Dyslipidemia and Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 5831. https://doi.org/10.3390/ijms25115831
Yi M, Toribio AJ, Salem YM, Alexander M, Ferrey A, Swentek L, Tantisattamo E, Ichii H. Nrf2 Signaling Pathway as a Key to Treatment for Diabetic Dyslipidemia and Atherosclerosis. International Journal of Molecular Sciences. 2024; 25(11):5831. https://doi.org/10.3390/ijms25115831
Chicago/Turabian StyleYi, Michelle, Arvin John Toribio, Yusuf Muhammad Salem, Michael Alexander, Antoney Ferrey, Lourdes Swentek, Ekamol Tantisattamo, and Hirohito Ichii. 2024. "Nrf2 Signaling Pathway as a Key to Treatment for Diabetic Dyslipidemia and Atherosclerosis" International Journal of Molecular Sciences 25, no. 11: 5831. https://doi.org/10.3390/ijms25115831
APA StyleYi, M., Toribio, A. J., Salem, Y. M., Alexander, M., Ferrey, A., Swentek, L., Tantisattamo, E., & Ichii, H. (2024). Nrf2 Signaling Pathway as a Key to Treatment for Diabetic Dyslipidemia and Atherosclerosis. International Journal of Molecular Sciences, 25(11), 5831. https://doi.org/10.3390/ijms25115831






