Experimental Autoimmune Encephalomyelitis Influences GH-Axis in Female Rats
Abstract
:1. Introduction
2. Results
2.1. Exacerbation of EAE Severity Coincides with Body Weight Loss
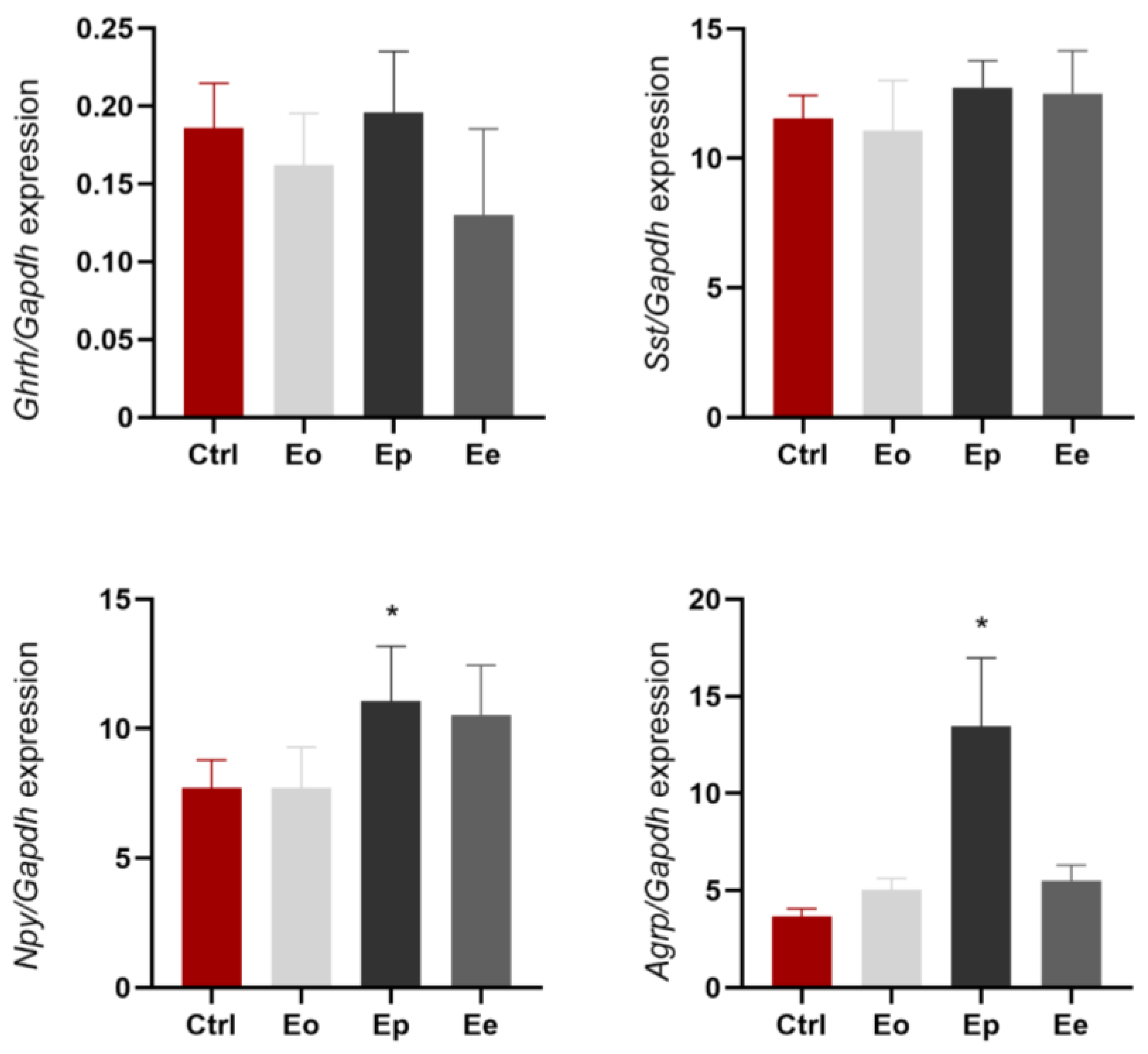
2.2. Hypothalamic Expression of Genes Associated with GH Axis
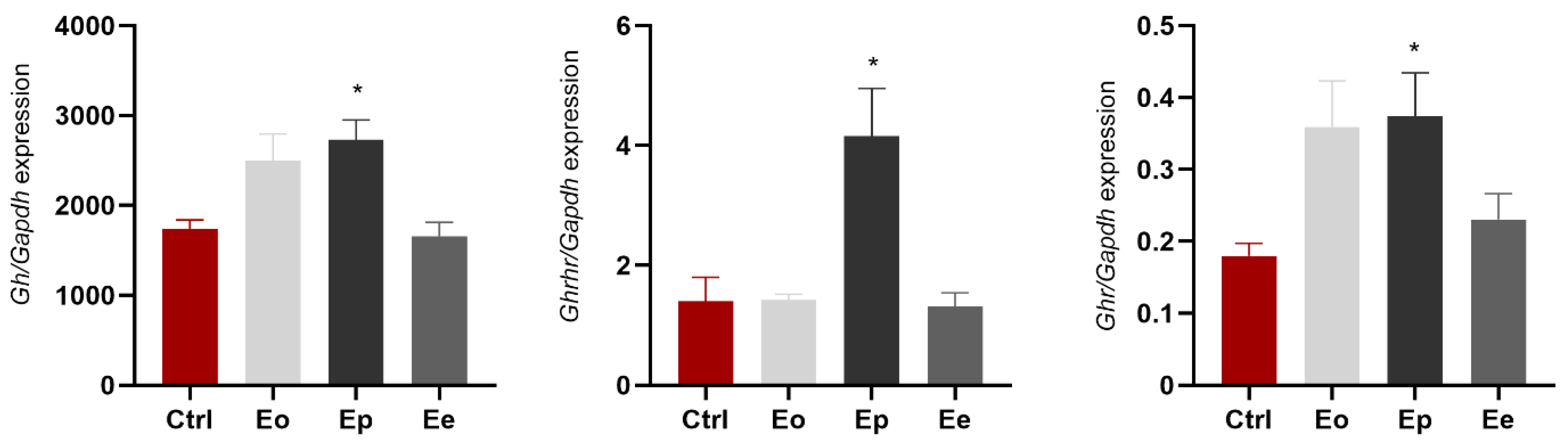
2.3. EAE Affects Expression of Gh, Ghrhr, and Ghr in Pituitary
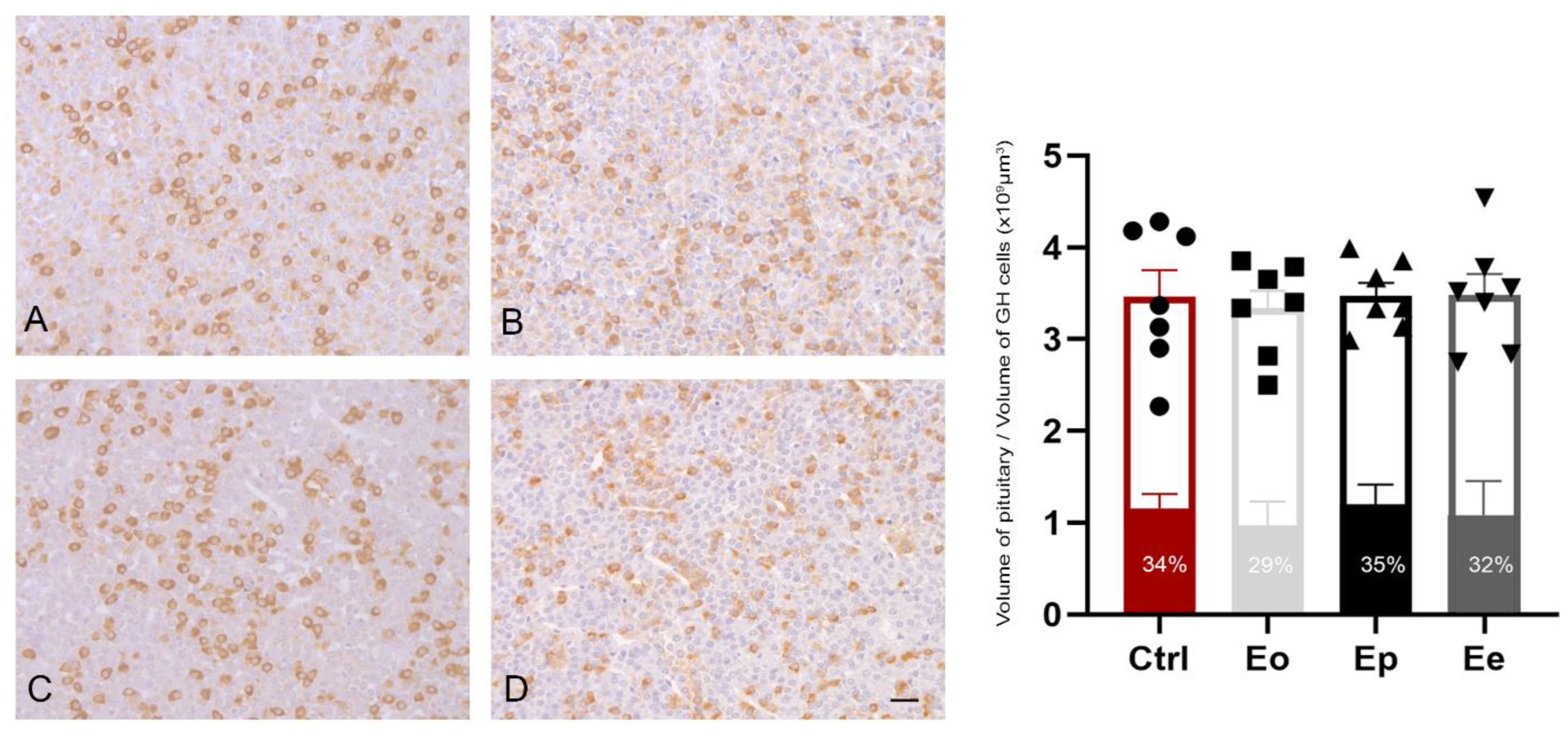
2.4. EAE Does Not Alter Stereological Parameters in Somatotrophs during EAE
2.5. Increased GH and Decreased IGF-1 Circulatory Levels Associate with Neuroinflammation
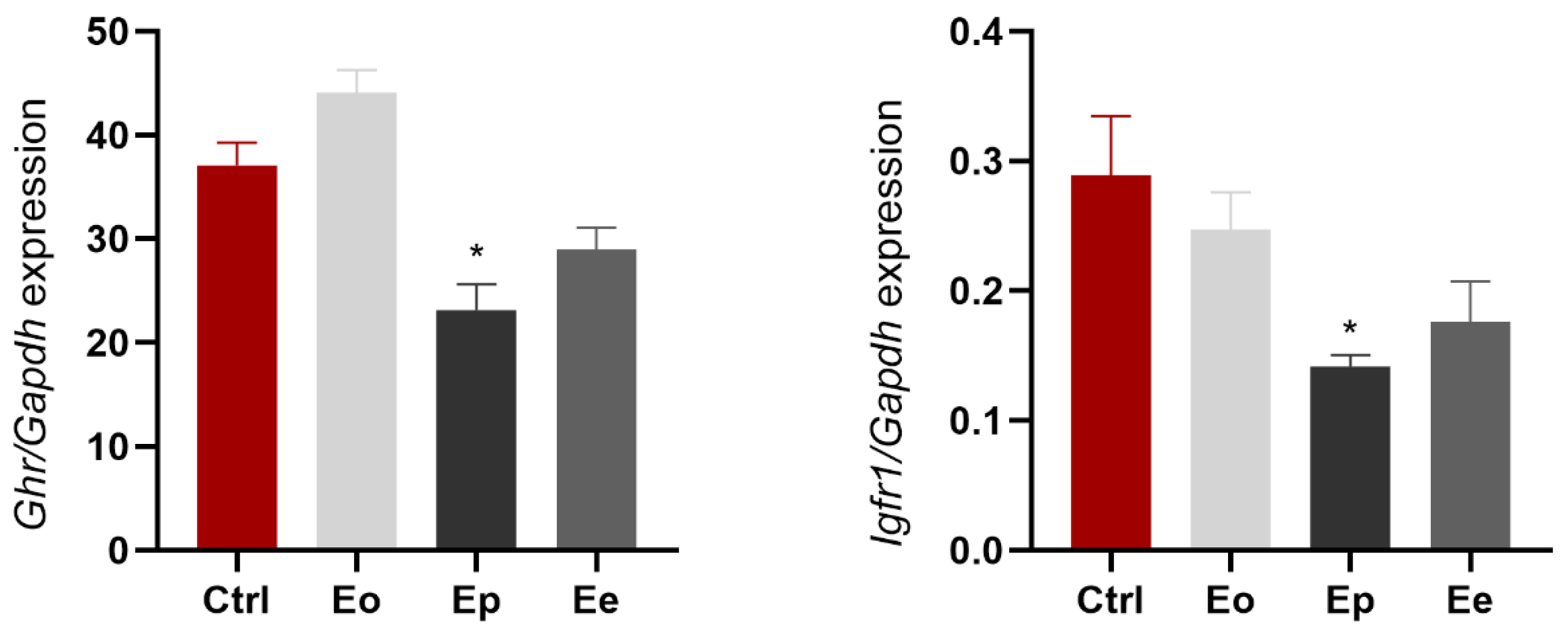
2.6. EAE Alters Ghr and Igf1r Expression in Liver
3. Discussion
4. Material and Methods
4.1. Animals
4.2. EAE Induction
4.3. Tissue Preparation
4.4. Immunohistochemistry
4.5. ELISA
4.6. Quantitative Real-Time PCR
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple Sclerosis and Neuroinflammation: The Overview of Current and Prospective Therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Begovic-Kupresanin, V.; Pekovic, S.; Lavrnja, I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2018, 96, 1021–1042. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Mace, J.W.; Calabresi, P.A. Animal models to investigate the effects of inflammation on remyelination in multiple sclerosis. Front. Mol. Neurosci. 2022, 15, 995477. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.; Nyamoya, S.; Hochstrasser, T.; Amor, S. Multiple sclerosis animal models: A clinical and histopathological perspective. Brain Pathol. 2017, 27, 123–137. [Google Scholar] [CrossRef]
- Deckx, N.; Lee, W.P.; Berneman, Z.N.; Cools, N. Neuroendocrine immunoregulation in multiple sclerosis. Clin. Dev. Immunol. 2013, 2013, 705232. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, S.; Stevanovic, I.; Milosevic, A.; Ristic, N.; Janjic, M.; Bjelobaba, I.; Savic, D.; Bozic, I.; Jakovljevic, M.; Tesovic, K.; et al. The Function of the Hypothalamic-Pituitary-Adrenal Axis during Experimental Autoimmune Encephalomyelitis: Involvement of Oxidative Stress Mediators. Front. Neurosci. 2021, 15, 649485. [Google Scholar] [CrossRef] [PubMed]
- Huitinga, I.; Erkut, Z.A.; van Beurden, D.; Swaab, D.F. The hypothalamo-pituitary-adrenal axis in multiple sclerosis. Ann. N. Y. Acad. Sci. 2003, 992, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Melief, J.; de Wit, S.J.; van Eden, C.G.; Teunissen, C.; Hamann, J.; Uitdehaag, B.M.; Swaab, D.; Huitinga, I. HPA axis activity in multiple sclerosis correlates with disease severity, lesion type and gene expression in normal-appearing white matter. Acta Neuropathol. 2013, 126, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Ysrraelit, M.C.; Gaitán, M.I.; Lopez, A.S.; Correale, J. Impaired hypothalamic-pituitary-adrenal axis activity in patients with multiple sclerosis. Neurology 2008, 71, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Ysrraelit, M.C.; Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Gironi, M.; Solaro, C.; Meazza, C.; Vaghi, M.; Montagna, L.; Rovaris, M.; Batocchi, A.P.; Nemni, R.; Albertini, R.; Zaffaroni, M.; et al. Growth hormone and disease severity in early stage of multiple sclerosis. Mult. Scler. Int. 2013, 2013, 836486. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J. The growth hormone receptor. Growth Horm. IGF Res. 2016, 28, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B.; Wit, J.M. Growth hormone—Past, present and future. Nat. Rev. Endocrinol. 2018, 14, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Gelato, M.C. Growth hormone-insulinlike growth factor I and immune function. Trends Endocrinol. Metab. 1993, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, V.H.; Daemen, M.A.; Buurman, W.A. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev. 1999, 10, 5–14. [Google Scholar] [CrossRef] [PubMed]
- van Buul-Offers, S.C.; Kooijman, R. The role of growth hormone and insulin-like growth factors in the immune system. Cell Mol. Life Sci. 1998, 54, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Kanaoka, M.; Kojima, S. Cutting edge: Requirement for growth hormone-releasing hormone in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2003, 171, 2769–2772. [Google Scholar] [CrossRef] [PubMed]
- Shohreh, R.; Pardo, C.A.; Guaraldi, F.; Schally, A.V.; Salvatori, R. GH, but not GHRH, plays a role in the development of experimental autoimmune encephalomyelitis. Endocrinology 2011, 152, 3803–3810. [Google Scholar] [CrossRef]
- Ivan, D.C.; Berve, K.C.; Walthert, S.; Monaco, G.; Borst, K.; Bouillet, E.; Ferreira, F.; Lee, H.; Steudler, J.; Buch, T.; et al. Insulin-like growth factor-1 receptor controls the function of CNS-resident macrophages and their contribution to neuroinflammation. Acta Neuropathol. Commun. 2023, 11, 35. [Google Scholar] [CrossRef]
- Lovett-Racke, A.E.; Bittner, P.; Cross, A.H.; Carlino, J.A.; Racke, M.K. Regulation of experimental autoimmune encephalomyelitis with insulin-like growth factor (IGF-1) and IGF-1/IGF-binding protein-3 complex (IGF-1/IGFBP3). J. Clin. Investig. 1998, 101, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.V.; Christensen, S.E.; Dupont, E.; Hansen, A.P.; Pedersen, E.; Orskov, H. Low somatostatin content in cerebrospinal fluid in multiple sclerosis. An indicator of disease activity? Acta Neurol. Scand. 1980, 61, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Kowalski, D.; Kowalska, K.; Błaszczyk, B.; Banasinska, E. Growth hormone levels in the blood serum and cerebrospinal fluid of patients with multiple sclerosis. Neurol. Neurochir. Pol. 1987, 21, 315–318. [Google Scholar] [PubMed]
- Poljakovic, Z.; Zurak, N.; Brinar, V.; Korsic, M.; Basic, S.; Hajnsek, S. Growth hormone and insulin growth factor-I levels in plasma and cerebrospinal fluid of patients with multiple sclerosis. Clin. Neurol. Neurosurg. 2006, 108, 255–258. [Google Scholar] [CrossRef]
- Shahbazi, M.; Abdolmohammadi, R.; Ebadi, H.; Farazmandfar, T. Novel functional polymorphism in IGF-1 gene associated with multiple sclerosis: A new insight to MS. Mult. Scler. Relat. Disord. 2017, 13, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Akcali, A.; Bal, B.; Erbagci, B. Circulating IGF-1, IGFB-3, GH and TSH levels in multiple sclerosis and their relationship with treatment. Neurol. Res. 2017, 39, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Feifel, D.; Vaccarino, F.J. Growth hormone-regulatory peptides (GHRH and somatostatin) and feeding: A model for the integration of central and peripheral function. Neurosci. Biobehav. Rev. 1994, 18, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Klibanski, A. Determinants of GH resistance in malnutrition. J. Endocrinol. 2014, 220, R57–R65. [Google Scholar] [CrossRef] [PubMed]
- Soendergaard, C.; Young, J.A.; Kopchick, J.J. Growth Hormone Resistance-Special Focus on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1019. [Google Scholar] [CrossRef] [PubMed]
- Yumet, G.; Shumate, M.L.; Bryant, D.P.; Lang, C.H.; Cooney, R.N. Hepatic growth hormone resistance during sepsis is associated with increased suppressors of cytokine signaling expression and impaired growth hormone signaling. Crit. Care Med. 2006, 34, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- de Groof, F.; Joosten, K.F.; Janssen, J.A.; de Kleijn, E.D.; Hazelzet, J.A.; Hop, W.C.; Uitterlinden, P.; van Doorn, J.; Hokken-Koelega, A.C. Acute stress response in children with meningococcal sepsis: Important differences in the growth hormone/insulin-like growth factor I axis between nonsurvivors and survivors. J. Clin. Endocrinol. Metab. 2002, 87, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, X.; Frank, S.J.; Lin, H.Y.; Xia, Y. Distinct mechanisms of induction of hepatic growth hormone resistance by endogenous IL-6, TNF-α, and IL-1β. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E186–E198. [Google Scholar] [CrossRef] [PubMed]
- Scacchi, M.; Ida Pincelli, A.; Cavagnini, F. Nutritional status in the neuroendocrine control of growth hormone secretion: The model of anorexia nervosa. Front. Neuroendocr. 2003, 24, 200–224. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, M.E.; Gusmao, D.O.; Dos Santos, W.O.; Moriya, H.T.; de Lima, F.F.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Fasting and prolonged food restriction differentially affect GH secretion independently of GH receptor signaling in AgRP neurons. J. Neuroendocr. 2023, e13254. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Sędek, E.; Pyrżak, B. Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Cent. Eur. J. Immunol. 2020, 45, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.S.; Takemoto, C.D. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol. Endocrinol. 1990, 4, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Beauloye, V.; Willems, B.; de Coninck, V.; Frank, S.J.; Edery, M.; Thissen, J.P. Impairment of liver GH receptor signaling by fasting. Endocrinology 2002, 143, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, A.; Janjic, M.M.; Lavrnja, I.; Savic, D.; Bozic, I.D.; Tesovic, K.; Jakovljevic, M.; Pekovic, S.; Stojilkovic, S.S.; Bjelobaba, I. The sex-specific patterns of changes in hypothalamic-pituitary-gonadal axis during experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2020, 89, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Saito, R.; Sanada, K.; Nishimura, H.; Nishimura, K.; Sonoda, S.; Ueno, H.; Motojima, Y.; Matsuura, T.; Yoshimura, M.; et al. Expression of hypothalamic feeding-related peptide genes and neuroendocrine responses in an experimental allergic encephalomyelitis rat model. Peptides 2020, 129, 170313. [Google Scholar] [CrossRef] [PubMed]
- Murao, S.; Sato, M.; Tamaki, M.; Niimi, M.; Ishida, T.; Takahara, J. Gene expression of hypothalamic growth hormone (GH)-releasing hormone and somatostatin does not correlate with pulsatile secretion of GH in the adult mouse. Res. Commun. Mol. Pathol. Pharmacol. 1995, 89, 269–277. [Google Scholar] [PubMed]
- Petrescu, A.D.; An, S.Y.; Venter, J.; McMillin, M.; DeMorrow, S. The Role of Hypothalamic Neuropeptides in Regulation of Liver Functions in Health and Disease. Endocrines 2023, 4, 457–487. [Google Scholar] [CrossRef]
- Fernandez-Perez, L.; Borja-Guerra, C.; Diaz-Chico, J.C.; Flores-Morales, A. Estrogens Regulate the Hepatic Effects of Growth Hormone, a Hormonal Interplay with Multiple Fates. Front. Endocrinol. 2013, 4, 51600. [Google Scholar] [CrossRef] [PubMed]
- Wasinski, F.; Frazão, R.; Donato, J., Jr. Effects of growth hormone in the central nervous system. Arch. Endocrinol. Metab. 2019, 63, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J. The Complex World of Regulation of Pituitary Growth Hormone Secretion: The Role of Ghrelin, Klotho, and Nesfatins in It. Front. Endocrinol. 2021, 12, 636403. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Wang, Q.; Zigman, J.M. The central nervous system sites mediating the orexigenic actions of ghrelin. Annu. Rev. Physiol. 2014, 76, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Lewiński, A.; Karbownik-Lewińska, M.; Wieczorek-Szukała, K.; Stasiak, M.; Stawerska, R. Contribution of Ghrelin to the Pathogenesis of Growth Hormone Deficiency. Int. J. Mol. Sci. 2021, 22, 9066. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147 Pt 3, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Dorph-Petersen, K.A.; Nyengaard, J.R.; Gundersen, H.J. Tissue shrinkage and unbiased stereological estimation of particle number and size. J. Microsc. 2001, 204 Pt 3, 232–246. [Google Scholar] [CrossRef] [PubMed]
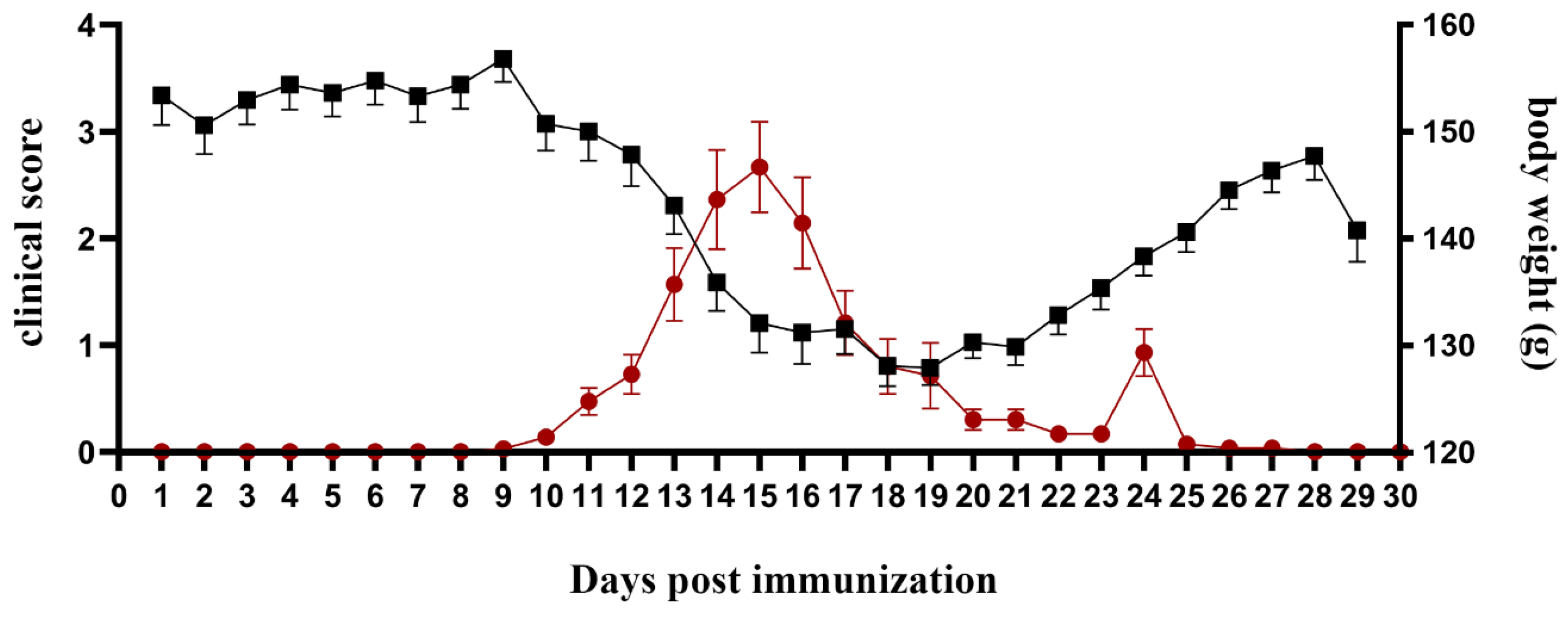
| Duration of Disease (Days) | Duration of Paralysis (Days) | Maximal Clinical Score | Cumulative Disease Index |
|---|---|---|---|
| 8.3 ± 1.3 | 2.2 ± 0.4 | 2.9 ± 0.1 | 11.7 ± 1.5 |
| Ctrl | Eo | Ep | Ee | |
|---|---|---|---|---|
| GH (ng/mL) | 4.2 ± 3.53 | 4.8 ± 4.03 | 15 ± 6.56 * | 6.7 ± 2.52 |
| IGF-1 (ng/mL) | 1357 ± 346.30 | 842.67 ± 111.60 | 731.11 ± 95.34 * | 2418.2 ± 337.46 * |
| Gene Symbol | Forward Primer Sequence | Reverse Primer Sequence | Accession Number |
|---|---|---|---|
| Gapdh | CAACTCCCTCAAGATTGTCAGCAA | GGCATGGACTGTGGTCATGA | NM_017008.4 |
| Gh | GCACAAGGCAGAGACCTACC | CAAAGTGTAGGGGTGGCAGT | NM_001034848.2 |
| Ghr | CCAACTCCCCTCTACACCAA | GGGCTAACCCTGCCTTAATC | NM_017094.1 |
| Ghrh | GGGTGTTCTTTGTGCTCCTC | GCAGTTTGCGGGCATATAAT | NM_031577.1 |
| Ghrhr | CTCTGCTTGCTGAACCTGTG | GACGAGTTGTTGGTCCCCTC | NM_012850.2 |
| Igf1r | TCCCAAGCTGTGTGTCTCTG | CTCCGTTGTTCCTGGTGTTT | NM_052807.2 |
| Sst | GATAGCGGCTGAAGGAGACG | CAAAGCCAGGACGATGCAGA | NM_012659.2 |
| Npy | TACTACTCCGCTCTGCGACA | GGGCATTTTCTGTGCTTTCT | NM_012614.2 |
| Agrp | TGTGTAAGGCTGCACGAGTC | AGTACCTAGCTTGCGGCAGT | NM_033650.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivkovic, A.; Trifunovic, S.; Savic, D.; Milosevic, K.; Lavrnja, I. Experimental Autoimmune Encephalomyelitis Influences GH-Axis in Female Rats. Int. J. Mol. Sci. 2024, 25, 5837. https://doi.org/10.3390/ijms25115837
Zivkovic A, Trifunovic S, Savic D, Milosevic K, Lavrnja I. Experimental Autoimmune Encephalomyelitis Influences GH-Axis in Female Rats. International Journal of Molecular Sciences. 2024; 25(11):5837. https://doi.org/10.3390/ijms25115837
Chicago/Turabian StyleZivkovic, Anica, Svetlana Trifunovic, Danijela Savic, Katarina Milosevic, and Irena Lavrnja. 2024. "Experimental Autoimmune Encephalomyelitis Influences GH-Axis in Female Rats" International Journal of Molecular Sciences 25, no. 11: 5837. https://doi.org/10.3390/ijms25115837
APA StyleZivkovic, A., Trifunovic, S., Savic, D., Milosevic, K., & Lavrnja, I. (2024). Experimental Autoimmune Encephalomyelitis Influences GH-Axis in Female Rats. International Journal of Molecular Sciences, 25(11), 5837. https://doi.org/10.3390/ijms25115837






