High Overexpression of SiAAP9 Leads to Growth Inhibition and Protein Ectopic Localization in Transgenic Arabidopsis
Abstract
1. Introduction
2. Results
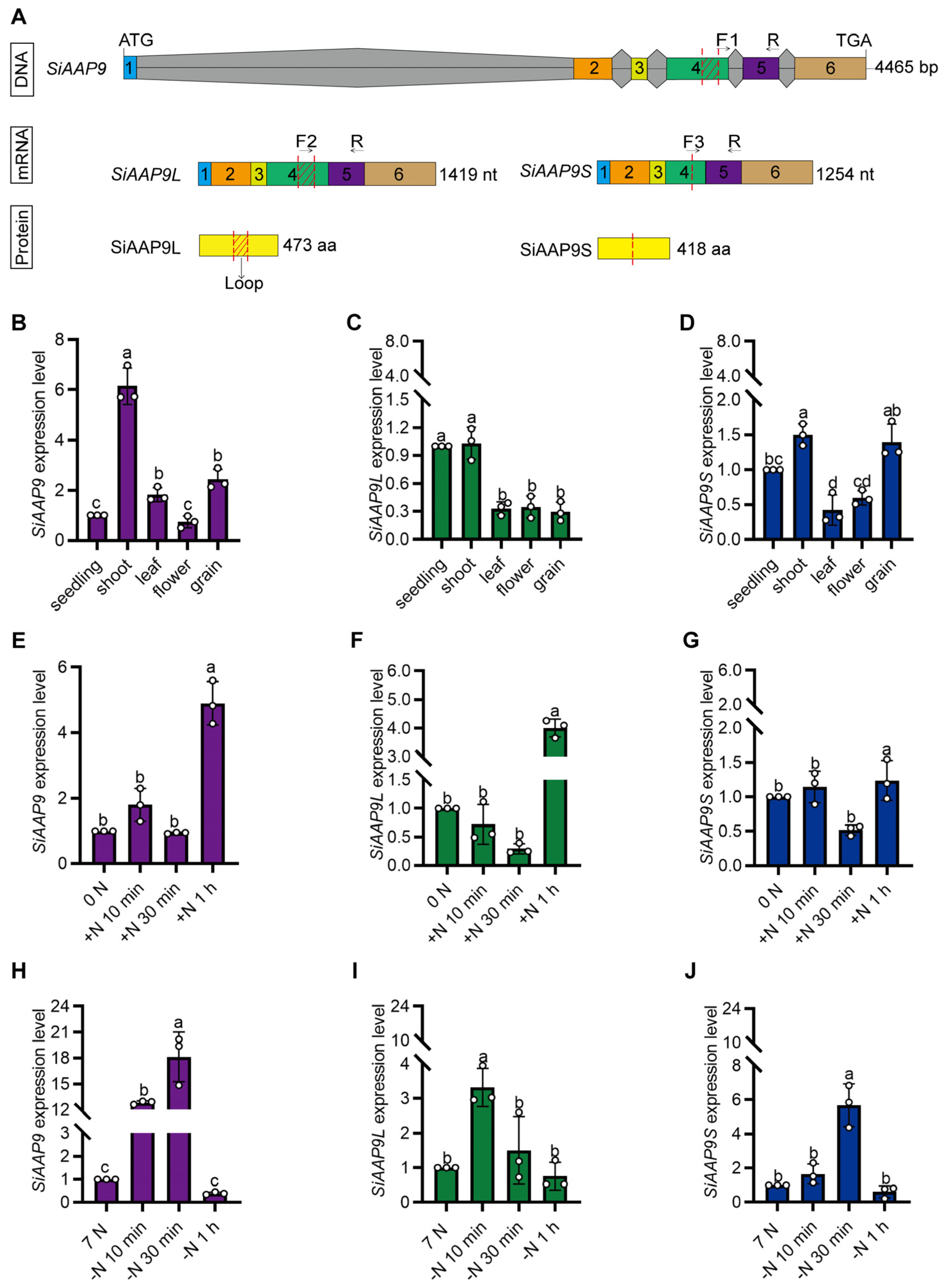
2.1. SiAAP9 Exhibits Two Alternative Splicing (AS) Events
2.2. Expression Pattern Analysis of SiAAP9
2.3. Overexpressing of SiAAP9 Inhibits Growth and Development in Transgenic Arabidopsis
2.4. Effect of SiAAP9 on Primary Root Growth Is Determined by Its Overexpression Level, Regardless of Nitrogen Availability
2.5. SiAAP9-OX Transgenic Arabidopsis Lines Exhibit Tolerance to High Concentrations of Glu and His
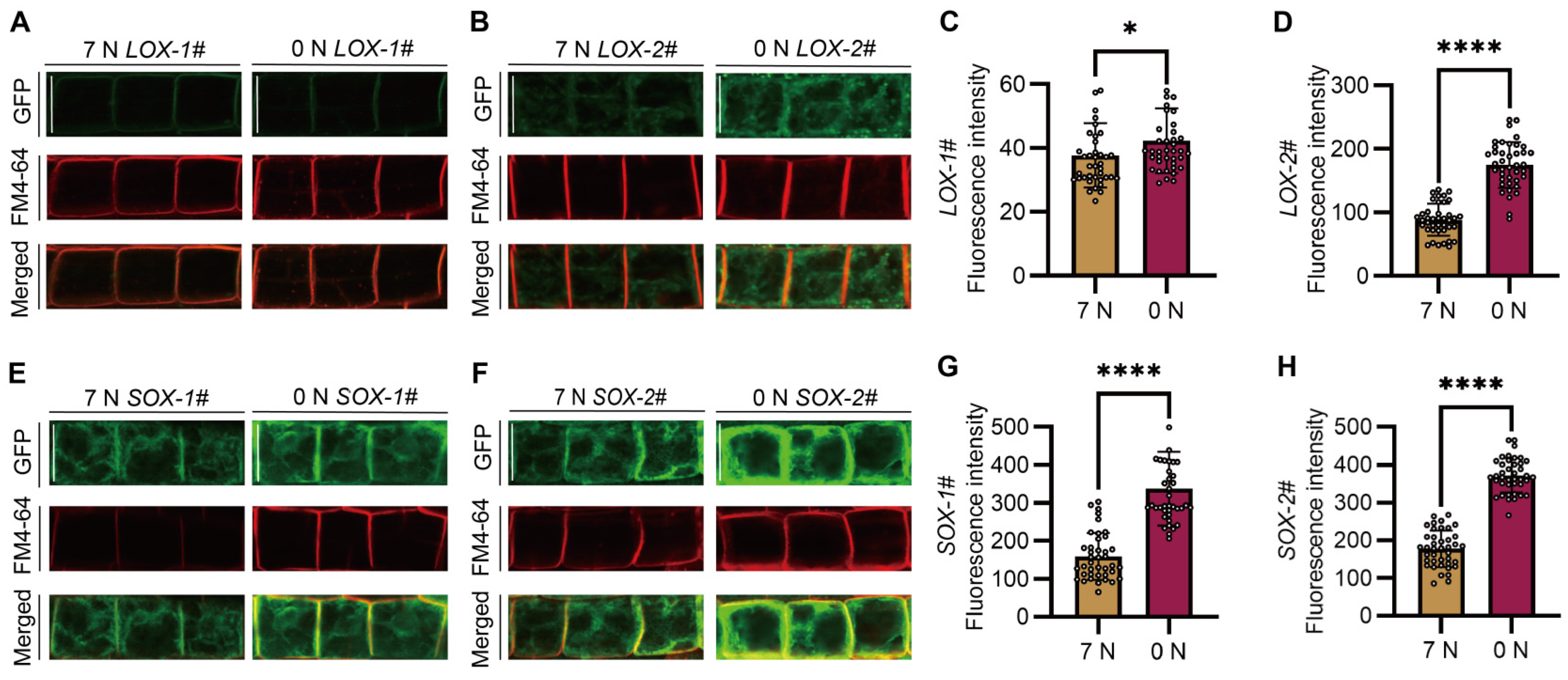
2.6. Two Proteins Encoded by SiAAP9L and SiAAP9S Have Different Subcellular Localization
2.7. SiAAP9 May Promote Glu Uptake in Jingu 21 Protoplast
3. Discussion
3.1. Higher Concentration of Amino Acids Transported to Seeds by SiAAP9 Transporter May Inhibit the Growth and Development of Arabidopsis Shoots and Roots
3.2. In the Absence of Inorganic Nitrogen, SiAAP9-OX Transgenic Arabidopsis Lines Were Intolerant to High Concentrations of Glu
3.3. Two Transcripts of SiAAP9 Perform Identical Functions but Exhibit Different Subcellular Localizations
3.4. High Overexpression of SiAAP9 May Lead to Ectopic Localization, Which Is Detrimental to Plant Growth and Development
4. Materials and Methods
4.1. Plasmid Construction and Plant Transformation
4.2. Plant Materials and Growth Conditions
4.3. Histochemical GUS Staining Assay
4.4. RNA Isolation and RT-qPCR Analysis
4.5. Nitrogen and Amino Acid Treatment Assay
4.6. Subcellular Localization under Different NO3− Conditions
4.7. Observation of SiAAP9 Localization by Reagent Treatment
4.8. Western Blot Analysis
4.9. Protoplast Preparation and Transformation
4.10. Protoplast Amino Acid Uptake Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, J.; Li, Z.; Mao, Y.; Struik, P.C.; Zhang, H.; Liu, L.; Wang, Z.; Yang, J. Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci. 2018, 274, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Marmagne, A.; Masclaux-Daubresse, C.; Chardon, F. Modulation of plant nitrogen remobilization and postflowering nitrogen uptake under environmental stresses. J. Plant Physiol. 2022, 77, 153781. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.N.; André, B.; Rentsch, D.; Krolkiewicz, S.; Tegeder, M.; Breitkreuz, K.; Frommer, W.B. Amino acid transport in plants. Trends Plant Sci. 1998, 3, 188–195. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Dinkeloo, K.; Boyd, S.; Pilot, G. Update on amino acid transporter functions and on possible amino acid sensing mechanisms in plants. Semin. Cell Dev. Biol. 2018, 74, 105–113. [Google Scholar] [CrossRef]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino acid transporters in plants: Identification and function. Plants 2020, 9, 972. [Google Scholar] [CrossRef]
- Yang, G.; Wei, Q.; Huang, H.; Xia, J. Amino acid transporters in plant cells: A brief review. Plants 2020, 9, 967. [Google Scholar] [CrossRef]
- Zhang, L.; Garneau, M.G.; Majumdar, R.; Grant, J.; Tegeder, M. Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J. 2015, 81, 134–146. [Google Scholar] [CrossRef]
- Peng, B.; Kong, H.; Li, Y.; Wang, L.; Zhong, M.; Sun, L.; Gao, G.; Zhang, Q.; Luo, L.; Wang, G.; et al. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat. Commun. 2014, 5, 4847. [Google Scholar] [CrossRef]
- Schmidt, R.; Stransky, H.; Koch, W. The amino acid permease AAP8 is important for early seed development in Arabidopsis thaliana. Planta 2007, 226, 805–813. [Google Scholar] [CrossRef]
- Grant, J.E.; Ninan, A.; Cripps-Guazzone, N.; Shaw, M.; Song, J.; Pet Ík, I.; Novák, O.E.; Tegeder, M.; Jameson, P.E. Concurrent overexpression of amino acid permease AAP1(3a) and SUT1 sucrose transporter in pea resulted in increased seed number and changed cytokinin and protein levels. Funct. Plant Biol. 2021, 48, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Yang, Y.; Chen, M. Genome-wide survey of the amino acid transporter gene family in wheat (Triticum aestivum L.): Identification, expression analysis and response to abiotic stress. Int. J. Biol. Macromol. 2020, 162, 1372–1387. [Google Scholar] [CrossRef]
- Ma, H.; Cao, X.; Shi, S.; Li, S.; Gao, J.; Ma, Y.; Zhao, Q.; Chen, Q. Genome-wide survey and expression analysis of the amino acid transporter superfamily in potato (Solanum tuberosum L.). Plant Physiol. Biochem. 2016, 107, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, S.; Chen, Z.; Dong, Q.; Yan, H.; Xiang, Y. Genome-wide survey and expression analysis of the amino acid transporter gene family in poplar. Tree Genet. Genomes 2015, 11, 83. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zhang, J.; Fan, B.; Zhou, Y.; Cui, X. Expression of AtAAP gene family and endosperm-specific expression of AtAAP1 gene promotes amino acid absorption in Arabidopsis thaliana and maize. Agronomy 2021, 11, 1668. [Google Scholar] [CrossRef]
- Sanders, A.; Collier, R.; Trethewy, A.; Gould, G.; Sieker, R.; Tegeder, M. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009, 59, 540–552. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Leaf amino acid supply affects photosynthetic and plant nitrogen use efficiency under nitrogen stress. Plant Physiol. 2018, 178, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Sonawala, U.; Dinkeloo, K.; Danna, C.H.; McDowell, J.M.; Pilot, G. Review: Functional linkages between amino acid transporters and plant responses to pathogens. Plant Sci. 2018, 277, 79–88. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, B.; Ji, Y. The amino acid transporter OsAAP4 contributes to rice tillering and grain yield by regulating neutral amino acid allocation through two splicing variants. Rice 2021, 14, 2. [Google Scholar] [CrossRef]
- Ji, Y.; Huang, W.; Wu, B.; Fang, Z.; Wang, X. The amino acid transporter AAP1 mediates growth and grain yield by regulating neutral amino acid uptake and reallocation in Oryza sativa. J. Exp. Bot. 2020, 71, 4763–4777. [Google Scholar] [CrossRef]
- Yang, X.; Yang, G.; Wei, X.; Huang, W.; Fang, Z. OsAAP15, an amino acid transporter in response to nitrogen concentration, mediates panicle branching and grain yield in rice. Plant Sci. 2023, 330, 111640. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.; Qian, J.; Huang, W.; Fang, Z. Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, B.; Lu, K.; Wei, Q.; Qian, J.; Chen, Y.; Fang, Z. The Amino acid permease 5 (OsAAP5) regulates tiller number and grain yield in rice. Plant Physiol. 2019, 180, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Guo, M.; Zhong, C.; Yan, C.; Sun, S. Targeted mutagenesis of amino acid transporter genes for rice quality improvement using the CRISPR/Cas9 system. Crop J. 2020, 8, 457–464. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Huang, Y.; Zhao, X.; Dong, Z.; Jin, W.; Huang, W. Amino acid permease 6 regulates grain protein content in maize. Crop J. 2022, 10, 1536–1544. [Google Scholar] [CrossRef]
- Liu, S.; Wang, D.; Mei, Y.; Xia, T.; Xu, W.; Zhang, Y.; You, X.; Zhang, X.; Li, L.; Wang, N. Overexpression of GmAAP6a enhances tolerance to low nitrogen and improves seed nitrogen status by optimizing amino acid partitioning in soybean. Plant Biotechnol. J. 2020, 18, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kwart, M.; Laubner, M.; Heineke, D.; Stransky, H.; Frommer, W.B.; Tegeder, M. Reduced amino acid content in transgenic potato tubers due to antisense inhibition of the leaf H+/amino acid symporter StAAP1. Plant J. 2003, 33, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chai, Y.; Liu, J.; Zheng, J.; Zhao, Z.; Amo, A.; Cui, C.; Lu, Q.; Chen, L.; Hu, Y.G. Amino acid transporter (AAT) gene family in foxtail millet (Setaria italica L.): Widespread family expansion, functional differentiation, roles in quality formation and response to abiotic stresses. BMC Genomics 2021, 22, 519. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kong, F.; Zhang, H.; Zhang, W.; Wu, H.; Chen, G. First report of root rot caused by Fusarium nygamai on foxtail millet (Setaria italica) in China. Plant Dis. 2023, 107, 2222. [Google Scholar] [CrossRef]
- Sharma, N.; Niranjan, K. Foxtail millet: Properties, processing, health benefits, and uses. Food Rev. Int. 2018, 34, 329–363. [Google Scholar] [CrossRef]
- Sachdev, N.; Goomer, S.; Singh, L.R. Foxtail millet: A potential crop to meet future demand scenario for alternative sustainable protein. J. Sci. Food Agric. 2021, 101, 831–842. [Google Scholar] [CrossRef]
- Chen, J.; Duan, W.; Ren, X.; Wang, C.; Pan, Z.; Diao, X.; Shen, Q. Effect of foxtail millet protein hydrolysates on lowering blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2017, 56, 2129–2138. [Google Scholar] [CrossRef]
- Wang, H.; Shen, Q.; Zhang, F.; Fu, Y.; Zhu, Y.; Zhao, L.; Wang, C.; Zhao, Q. Heat-treated foxtail millet protein delayed the development of pre-diabetes to diabetes in mice by altering gut microbiota and metabolomic profiles. Food Funct. 2023, 14, 4866–4880. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.P.; Tegeder, M. Connecting source with sink: The role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol. 2016, 171, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Hayer, K.E.; Pizarro, A.; Lahens, N.F.; Hogenesch, J.B.; Grant, G.R. Benchmark analysis of algorithms for determining and quantifying full-length mRNA splice forms from RNA-seq data. Bioinformatics 2015, 31, 3938–3945. [Google Scholar] [CrossRef]
- Goldstein, L.D.; Cao, Y.; Pau, G.; Lawrence, M.; Wu, T.D.; Seshagiri, S.; Gentleman, R. Prediction and quantification of splice events from RNA-seq data. PLoS ONE 2016, 11, e0156132. [Google Scholar] [CrossRef] [PubMed]
- Mapleson, D.; Venturini, L.; Kaithakottil, G.; Swarbreck, D. Efficient and accurate detection of splice junctions from RNA-seq with Portcullis. GigaScience 2018, 7, giy131. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Sun, J. Rice Grain Size and Quality. Rice 2022, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, Y.; Lu, J.; Zhang, C.; Liu, Q.; Li, Q. Genes and their molecular functions determining seed structure, components, and quality of rice. Rice 2022, 15, 18. [Google Scholar] [CrossRef]
- Pimpl, P.; Hanton, S.L.; Taylor, J.P.; Pinto-daSilva, L.L.; Denecke, J. The GTPase ARFlp controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell 2003, 15, 1242–1256. [Google Scholar] [CrossRef]
- Wang, J.; Cai, Y.; Miao, Y.; Lam, S.K.; Jiang, L. Wortmannin induces homotypic fusion of plant prevacuolar compartments. J. Exp. Bot. 2009, 60, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, S.; Kumar, S.; Ramtekey, V.; Kar, P.; Meena, R.S.; Jangir, C.K. Current status and potential of biofortification to enhance crop nutritional quality: An overview. Sustainability 2022, 14, 3301. [Google Scholar] [CrossRef]
- Liao, H.S.; Chung, Y.H.; Hsieh, M.H. Glutamate: A multifunctional amino acid in plants. Plant Sci. 2022, 318, 111238. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Liu, L.H.; Remans, T.; Tester, M.; Forde, B.G. Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Forde, B.G. Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises L-glutamate-induced changes in root architecture. Plant J. 2008, 54, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.F.; Feijão, C.V.; Duque, P. On the physiological significance of alternative splicing events in higher plants. Protoplasma 2013, 250, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Jabre, I.; Reddy, A.S.N.; Staiger, D.; Syed, N.H. Perspective on alternative splicing and proteome complexity in plants. Trends Plant Sci. 2019, 24, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Graveley, B.R. Alternative splicing: Increasing diversity in the proteomic world. Trends Genet. 2001, 17, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kriventseva, E.V.; Koch, I.; Apweiler, R.; Vingron, M.; Bork, P.; Gelfand, M.S.; Sunyaev, S. Increase of functional diversity by alternative splicing. Trends Genet. 2003, 19, 124–128. [Google Scholar] [CrossRef]
- Filichkin, S.; Priest, H.D.; Megraw, M.; Mockler, T.C. Alternative splicing in plants: Directing traffic at the cross roads of adaptation and environmental stress. Curr. Opin. Plant Biol. 2015, 24, 125–135. [Google Scholar] [CrossRef]
- Staiger, D.; Brown, J.W. Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell 2013, 25, 3640–3656. [Google Scholar] [CrossRef]
- Szakonyi, D.; Duque, P. Alternative splicing as a regulator of early plant development. Front. Plant Sci. 2018, 9, 382146. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.; Hu, K.; Zheng, H.; Zhang, S.; Liu, X.; Ma, M.; Zhao, H. Two alternative splicing variants of a wheat gene TaNAK1, TaNAK1.1 andTaNAK1.2, differentially regulate flowering time and plant architecture leading to differences in seed yield of transgenic Arabidopsis. Front. Plant Sci. 2022, 13, 1014176. [Google Scholar] [CrossRef]
- Kashkan, I.; Hrtyan, M.; Retzer, K.; Humpolíčková, J.; Jayasree, A.; Filepová, R.; Vo-ndráková, Z.; Simon, S.; Rombaut, D.; Jacobs, T.B.; et al. Mutually opposing activity of PIN7 splicing isoforms is required for auxin-mediated tropic responses in Arabidopsis thaliana. New Phytol. 2022, 233, 329–343. [Google Scholar] [CrossRef]
- Fávero Peixoto-Junior, R.; Mara de Andrade, L.; Dos Santos Brito, M.; Macedo Nobile, P.; Palma Boer Martins, A.; Domingues Carlin, S.; Vasconcelos Ribeiro, R.; de Souza Goldman, M.H.; Nebó Carlos de Oliveira, J.F.; Vargas de Oliveira Figueira, A.; et al. Overexpression of ScMYBAS1 alternative splicing transcripts differentially impacts biomass accumulation and drought tolerance in rice transgenic plants. PLoS ONE 2018, 13, e0207534. [Google Scholar] [CrossRef]
- Lu, F.; Li, W.; Peng, Y.; Cao, Y.; Qu, J.; Sun, F.; Yang, Q.; Lu, Y.; Zhang, X.; Zheng, L.; et al. ZmPP2C26 alternative splicing variants negatively regulate drought tolerance in maize. Front. Plant Sci. 2022, 13, 851531. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.H.; Guo, Q.H.; Liu, P.; Li, Y.; Wu, C.A.; Yang, G.D.; Huang, J.G.; Zhang, S.Z.; Zheng, C.C.; et al. Salt responsive alternative splicing of a RING finger E3 ligase modulates the salt stress tolerance by fine-tuning the balance of COP9 signalosome subunit 5A. PLoS Genet. 2021, 17, e1009898. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Zhang, H.; Wang, L.; Zhu, Z.; Gao, J.; Li, C.; Zhu, Y. High temperature inhibits the accumulation of storage materials by inducing alternative splicing of OsbZIP58 during filling stage in rice. Plant Cell Environ. 2020, 43, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhi, L.; Liu, L.; Meng, D.; Su, Q.; Batool, A.; Ji, J.; Song, L.; Zhang, N.; Guo, L.; et al. Alternative splicing of TaGS3 differentially regulates grain weight and size in bread wheat. Int. J. Mol. Sci. 2021, 22, 11692. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wu, Y.; Zhang, J.; Zhang, J.; Zhou, H.; Zeng, R.; Zheng, W.; Qiu, M.; Zhou, J.; Xie, Z.; et al. A bZIP transcription factor (CiFD) regulates drought and low-temperature-induced flowering by alternative splicing in citrus. J. Integr. Plant Biol. 2023, 65, 674–691. [Google Scholar] [CrossRef]
- Garneau, M.G.; Tan, Q.; Tegeder, M. Function of pea amino acid permease AAP6 in nodule nitrogen metabolism and export, and plant nutrition. J. Exp. Bot. 2018, 69, 5205–5219. [Google Scholar] [CrossRef] [PubMed]
- Okumoto, S.; Koch, W.; Tegeder, M.; Fischer, W.N.; Biehl, A.; Leister, D.; Stierhof, Y.D.; Frommer, W.B. Root phloem-specific expression of the plasma membrane amino acid proton co-transporter AAP3. J. Exp. Bot. 2004, 55, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R.; Reinders, A.; Ward, J.M. Transport Function of Rice Amino Acid Permeases (AAPs). Plant Cell Physiol. 2015, 56, 1355–1363. [Google Scholar] [CrossRef]
- Yao, X.H.; Li, H.J.; Nie, J.; Liu, H.; Guo, Y.C.; Lv, L.J.; Yang, Z.; Sui, X.L. Disruption of the amino acid transporter CsAAP2 inhibits auxin-mediated root development in cucumber. New Phytol. 2023, 239, 639–659. [Google Scholar] [CrossRef]
- Chung, K.P.; Zeng, Y.; Jiang, L. COPII paralogs in plants: Functional redundancy or diversity? Trends Plant Sci. 2016, 21, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Cantizano, N.; Ko, D.K.; Angelos, E.; Pu, Y.; Brandizzi, F. Functional diversification of ER stress responses in Arabidopsis. Trends Biochem. Sci. 2020, 45, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Poór, P.; Czékus, Z.; Tari, I.; Ördög, A. The multifaceted roles of plant hormone salicylic acid in endoplasmic reticulum stress and unfolded protein response. Int. J. Mol. Sci. 2019, 20, 5842. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.B.; Lohani, N.; Bhalla, P.L. The role of endoplasmic reticulum stress response in pollen development and heat stress tolerance. Front. Plant Sci. 2021, 12, 661062. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Nie, J.; Chen, Z.; Wang, X.; Hu, H.; Xu, J.; Lu, J.; Ma, L.; Ji, H.; Yuan, J.; et al. Cold exposure-induced endoplasmic reticulum stress regulates autophagy through the SIRT2/FoxO1 signaling pathway. J. Cell Physiol. 2022, 237, 3960–3970. [Google Scholar] [CrossRef]
- Wang, B.; Du, H.; Zhang, Z.; Xu, W.; Deng, X. BhbZIP60 from resurrection plant boea hygrometrica is an mRNA splicing-activated endoplasmic reticulum stress regulator involved in drought tolerance. Front. Plant Sci. 2017, 8, 251571. [Google Scholar] [CrossRef]
- Ozgur, R.; Turkan, I.; Uzilday, B.; Sekmen, A.H. Endoplasmic reticulum stress triggers ROS signalling, changes the redox state, and regulates the anti-oxidant defence of Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Zang, X.; Li, H.; Liu, Z.; Zhao, A.; Liu, J.; Peng, H.; Yao, Y.; Hu, Z.; Ni, Z.; et al. Unconventional splicing of wheat TabZIP60 confers heat tolerance in transgenic Arabidopsis. Plant Sci. 2018, 274, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, S.; Wang, M.; Bi, D.; Sun, L.; Zhou, S.; Song, Z.; Liu, J. A plasma membrane-tethered transcription factor, NAC062/ANAC062/NTL6, mediates the unfolded protein response in Arabidopsis. Plant J. 2014, 79, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, M.; Innes, R.W. Loss of the acetyltransferase NAA50 induces endoplasmic reticulum stress and immune responses and suppresses growth. Plant Physiol. 2020, 183, 1838–1854. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, L.; Liu, Y.; Zhao, Y.; Han, T.; Miao, X.; Zhang, A. Calcineurin B-like protein 5 (SiCBL5) in Setaria italica enhances salt tolerance by regulating Na+ homeostasis. Crop J. 2022, 10, 234–242. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014, 9, 2395–2410. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, R.; Li, Z.; Kang, X.; Zhang, Y.; Wang, Y.; Ma, Y.; Wu, Y.; Dong, S.; Li, X.; Gao, L.; et al. High Overexpression of SiAAP9 Leads to Growth Inhibition and Protein Ectopic Localization in Transgenic Arabidopsis. Int. J. Mol. Sci. 2024, 25, 5840. https://doi.org/10.3390/ijms25115840
Meng R, Li Z, Kang X, Zhang Y, Wang Y, Ma Y, Wu Y, Dong S, Li X, Gao L, et al. High Overexpression of SiAAP9 Leads to Growth Inhibition and Protein Ectopic Localization in Transgenic Arabidopsis. International Journal of Molecular Sciences. 2024; 25(11):5840. https://doi.org/10.3390/ijms25115840
Chicago/Turabian StyleMeng, Ru, Zhipeng Li, Xueting Kang, Yujia Zhang, Yiru Wang, Yuchao Ma, Yanfeng Wu, Shuqi Dong, Xiaorui Li, Lulu Gao, and et al. 2024. "High Overexpression of SiAAP9 Leads to Growth Inhibition and Protein Ectopic Localization in Transgenic Arabidopsis" International Journal of Molecular Sciences 25, no. 11: 5840. https://doi.org/10.3390/ijms25115840
APA StyleMeng, R., Li, Z., Kang, X., Zhang, Y., Wang, Y., Ma, Y., Wu, Y., Dong, S., Li, X., Gao, L., Chu, X., Yang, G., Yuan, X., & Wang, J. (2024). High Overexpression of SiAAP9 Leads to Growth Inhibition and Protein Ectopic Localization in Transgenic Arabidopsis. International Journal of Molecular Sciences, 25(11), 5840. https://doi.org/10.3390/ijms25115840





