Nutraceuticals and Functional Foods: A Comprehensive Review of Their Role in Bone Health
Abstract
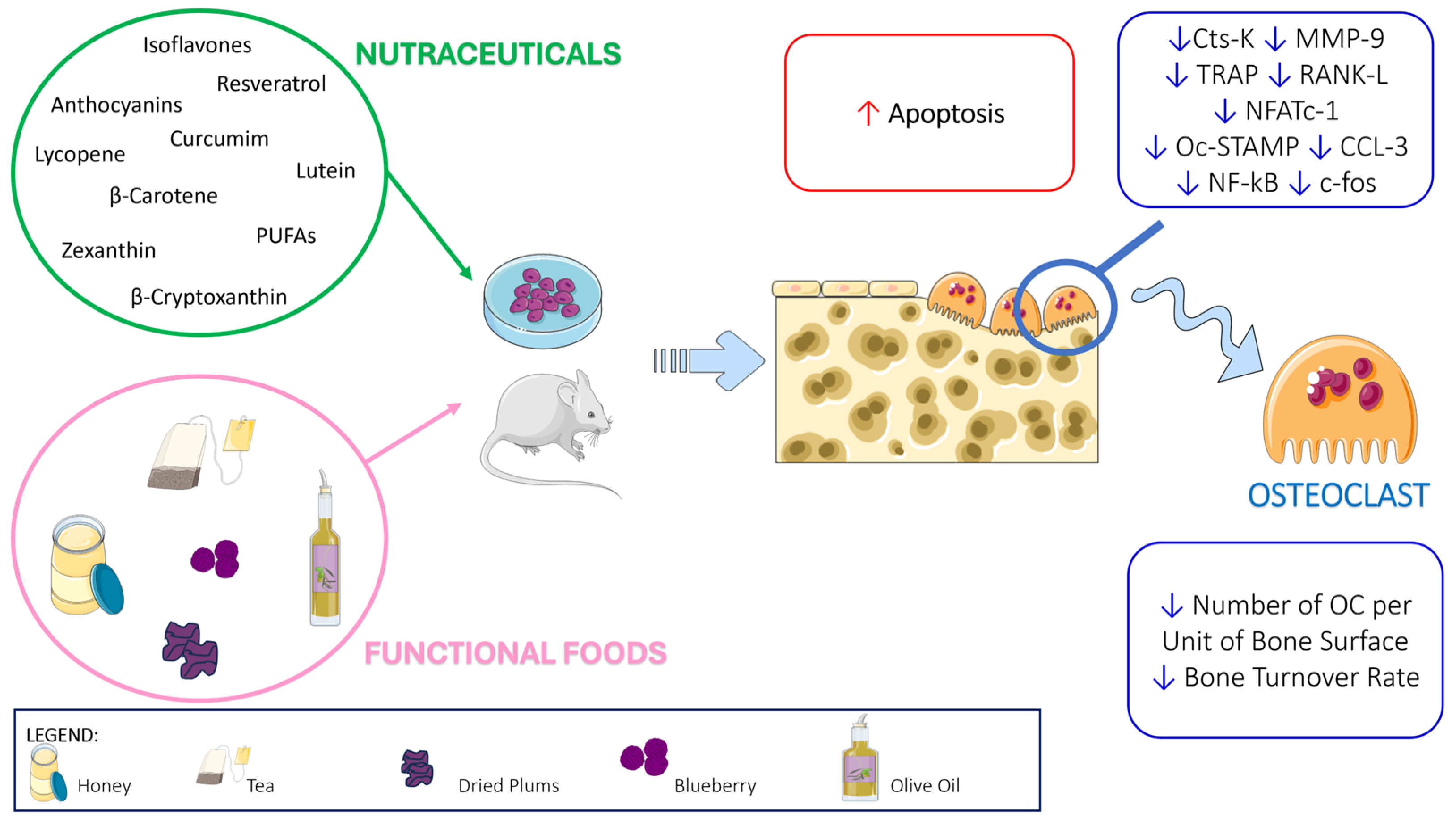
:1. Introduction
2. Nutraceuticals
2.1. Polyphenols
2.1.1. Flavonoids
In Vitro and In Vivo Animal Studies
In Vivo Human Studies
2.1.2. Anthocyanins (Cyanidin)
In Vitro and In Vivo Animal Studies
In Vivo Human Studies
2.1.3. Stilbenes
In Vitro and In Vivo Animal Studies
In Vivo Human Studies
2.1.4. Curcumin
In Vitro and In Vivo Animal Studies
In Vivo Human Studies
2.2. Carotenoids
2.2.1. Lycopene
In Vitro and In Vivo Animal Studies
In Vivo Human Studies
2.2.2. β-Carotene
In Vitro and In Vivo Animal Studies
In Vivo Human Studies
2.2.3. Lutein and Zeaxanthin
In Vitro and In Vivo Animal Studies
In Vivo Human Studies
2.2.4. β-Cryptoxanthin
In Vitro and In Vivo Animal Studies
In Vivo Human Studies
2.3. Polyunsaturated Fatty Acids (PUFAs)
2.3.1. In Vitro and In Vivo Animal Studies
2.3.2. In Vivo Human Studies
| Nutraceutical | Main Source | Effective Dosage | Reference |
|---|---|---|---|
| Isoflavones | Legumes (including soybeans, chickpeas, fava beans), nuts (pistachios, peanuts) | 106 mg/day | [26] |
| Cyanidin | Red berries, apples, plums, red cabbage, and red onion | Not available | [32,33,34,35] |
| Resveratrol | Skin of red grapes, mulberries, peanuts, and pines | 75 mg twice/day | [41] |
| Curcumin | Turmeric (curcuma longa) | 110 mg/day | [67,68] |
| Lycopene | Tomatoes, red carrots, watermelons, grapefruits, and papayas | 12.66 mg/day | [81] |
| β-carotene | Pumpkin, spinach, sweet potatoes | 30 mg/day | [88] |
| Lutein and zexanthin | Green leafy vegetables (kale, spinach, broccoli, peas, and lettuce), egg yolks | Not available | [93,94] |
| β-cryptoxanthin | Pumpkins, persimmons, chili peppers, tangerines, papaya | 2880 μg/day | [109] |
| PUFAs | Fish, algae, plant oils (walnuts, edible seeds, flaxseeds, hempseed oil) | Not available | [122,123] |
3. Functional Foods with Impact on Health
3.1. Honey
3.1.1. In Vitro and In Vivo Animal Studies
3.1.2. In Vivo Human Studies
3.2. Tea
3.2.1. In Vitro and In Vivo Animal Studies
3.2.2. In Vivo Human Studies
3.3. Dried Plums
3.3.1. In Vitro and In Vivo Animal Studies
3.3.2. In Vivo Human Studies
3.4. Blueberry
3.4.1. In Vitro and In Vivo Animal Studies
3.4.2. In Vivo Human Studies
3.5. Olive Oil
3.5.1. In Vitro and In Vivo Animal Models
3.5.2. In Vivo Human Studies
| Functional Food | Components | Effective Dosage | References |
|---|---|---|---|
| Honey | Flavonoids, phenolic acids, vitamin D3 | 20 g/day + aerobic exercise | [153,154,155] |
| Tea | Flavonoids | Not available | [178] |
| Dried plums | Chlorogenic acids, phenolic acids, and flavonoids | 100 g/day | [156,201,202,203,204,205,206] |
| Blueberry | Anthocyanins | 35 g/day | [200] |
| Extra virgin olive oil | Unsaturated fatty acid and phenolic compounds | 50 mL/day + Mediterranean Diet | [230] |
| Nutraceutical/Functional Food | Ref. | Study Design/Methods | Study Population | Test Report | Intervention Duration | Effect of Nutraceuticals/Functional Foods on Bone Health | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active Groups | Control Groups | |||||||||||
| Resveratrol | [39] | Randomized double-blind placebo-controlled trial RSVlow (n = 23) RSVhigh (n = 25) Placebo (n = 26) | 74 M obese men with metabolic syndrome, mean age 49.3 ± 6.3 y, mean BMI 33.7 ± 3.6 kg/m | RSVhigh: 1.000 mg/day RSV | RSVlow: 150 mg/day RSV | Placebo | 16 weeks | ↑ BALP and LS vBMDtrab dose-dependently with RSV, positive correlation in BALP and LS vBMDtrab | ||||
| Resveratrol, Isoflavones aglycones, and Equol | [40] | Randomized, placebo-controlled trial Active (n = 38) Control (n = 38) | 76 F healthy postmenopausal, 50–55 y, ≥ 12 months of cessation of menses | Dietary supplement containing 200 mg of fermented soy (including 80 mg of isoflavone aglycones and 10 mg of equol) and 25 mg of resveratrol from Vitis vinifera | Placebo | 12 months | ↓ DPD TRACP-5b, ↑ osteocalcin, BALP compared to placebo | |||||
| Resveratrol | [41] | Randomized, double-blind, placebo-controlled, two-period crossover intervention Active (n = 63) Control (n = 66) | 125 F healthy postmenopausal, age 45–85 y, ≥12 months of cessation of menses | 75 mg twice/day (total 150 mg) RSV | Placebo | 12 months | ↑ BMD lumbar spine and neck of femur, ↓ CTX, compared with placebo | |||||
| Curcumin | [67] | Randomized, double-blind trial. Alendronate (n = 20) Alendronate + curcumin (n = 20) Control (n = 20) | 60 F postmenopausal with osteoporosis, age 55–65 y, ≥5 y cessation of menses | Alendronate 5 mg/day + Calcium carbonate 1.000–1.500 mg/day | Alendronate 5 mg/day + Curcumin 110 mg/day + Calcium carbonate 1.000–1.500 mg/day | Calcium carbonate 1.000–1.500 mg/day | 12 months | Curcumin + alendronate group ↓ BALP and CTX; ↑ BMD in four areas compared to the control and alendronate groups | ||||
| Curcumin | [68] | Randomized, blind, placebo-controlled trial. Active (n = 50) Control (n = 50) | 100 adult patients with spinal cord injury trauma within the previous 6 months and paraplegia or quadriplegia, age 19–65 y | 110/mg/kg/day Curcumin | Placebo | 6 months | In curcumin group ↑ BMD compared with the beginning and compared to controls | |||||
| β-carotene | [88] | Intervention study to test the efficacy of high-dose retinol and BC supplements for reducing the risk of mesothelioma and lung cancer in a risk group | 2322 adults previously exposed to crocidolite (blue asbestos) | 30 mg/day BC for 6 y; after that, 7.5 mg RE/day | 0.75 mg/day BC for 6 y; after that, 7.5 mg RE/day | 7.5 mg/day RE as retinyl palmitate | 1–16 years | In M, cumulative dose of BC was associated with ↓ risk of any fracture and osteoporotic fracture | ||||
| β-cryptoxanthin | [109] | Interventional trial | 21 adults (10 M, 11 F), 23–47 y | 192 mL/day of reinforced juice prepared from Satuma mandarin containing 2880 μg of β-cryptoxanthin | 192 mL/day of juice prepared from Satuma mandarin containing 1540 μg of β-cryptoxanthin | 56 days | Juice consumption ↑ γ-carboxylated OC; reinforced juice ↓ TRAP and NTx | |||||
| β-cryptoxanthin | [110] | Interventional trial | 90 healthy adults, 19 M and 71 F (35 premenopausal and 36 postmenopausal) | Satsuma mandarin juice at concentration of 1.5 mg/200 mL | Satsuma mandarin juice at concentration of 3 mg/200 mL | Satsuma mandarin juice at concentration of 6 mg/200 mL | ↑ ALP and γ-carboxylated osteocalcin and ↓ TRAP and NTx | |||||
| Honey | [152] | Randomized controlled trial Honey group (n = 40) HRT (n = 39) | 79 healthy F postmenopausal, ≥12 months of cessation of menses, age 45–60 y | 20 g/day of Tualang honey | HRT (Femoston®) contain 1 mg Estradiol valerate and 5 mg Dydrogesterone | 4 months | No differences | |||||
| Honey | [153] | Interventional trial Ex (n = 10) H (n = 10) HEx (n = 10) C (n = 10) | 40 healthy F, 19–28 y | Ex: aerobic dance exercise * (1 h per session, 3 sessions per week) | H: 20 g/day Malaysian local Gelam honey diluted in 300 mL of plain water | HEx: aerobic dance exercise * +20 g/day Malaysian local Gelam honey diluted in 300 mL of plain water (30 min before exercise) | C: Sedentary and no honey supplementation | 6 weeks | In H and HEx groups: ↑ALP HEx group exhibited the highest percentage ↑ALP. | |||
| Honey | [154] | Randomized controlled trial 8Ex8S (n = 12) 8H8S (n = 12) 8ExH8S (n = 12) 16S (n = 12) | 48 healthy F, 19–25 y | 8Ex8S: aerobic dance exercise * (1 h per session, 3 sessions per week) | 8H8S: 20 g Tualang honey diluted in 300 mL of plain water | 8ExH8S: aerobic dance exercise * +20 g/day Tualang honey diluted in 300 mL of plain water (30 min before exercise) | 16S: 16 weeks of sedentary and no honey supplementation | 8 weeks of intervention followed by 8 weeks of sedentary lifestyle | After intervention (8 w) in 8E×H8S: ↑ ALP, osteocalcin and ↓1CTP. After 8 weeks of cessation of intervention in 8E×H8S: ↑ serum total calcium, ALP, TAS, GSH | |||
| Honey | [155] | Interventional trial Ex (n = 11) H (n = 11) HEx (n = 11) C (n = 11) | 44 healthy F, 25–40 y | Ex: aerobic dance exercise * (1 h per session, 3 sessions per week) | H: 20 g/day Malaysian local Gelam honey diluted in 300 mL of plain water | HEx: aerobic dance exercise * +20 g/day Malaysian local Gelam honey diluted in 300 mL of plain water (30 min before exercise) | C: sedentary and no honey supplementation | 8 weeks | H: ↑ serum total calcium level Ex: ↑ 1CTP HEx: ↓ 1CTP (bone resorption is mitigated by honey supplementation) | |||
| Tea | [178] | Randomized, double-blind, placebo-controlled clinical trial Active (n = 61) Control (n = 60) | 121 F postmenopausal, 50–70 y, BMI ≥ 25 kg/m2 | Decaffeinated green tea extract containing 843 mg (2)-epigallocatechin-3-gallate in 4 capsules/day | Placebo in 4 capsules/day | 12 months | No effects | |||||
| Dried plums | [201] | Randomized controlled clinical trial | 58 F postmenopausal | 100 g/day dried plums | 75 g dried apples/day | 3 months | ↑ IGF-I and BALP | |||||
| Dried plums | [202] | Comparative control randomized study Dried plums (n = 55) Dried apples (n = 45) | 110 F, 1–10 years postmenopausal with mild bone loss | 100 g/day dried plums + 500 mg/day Ca + 400 IU/day vitamin D | 75 g/day dried apples + 500 mg/day Ca + 400 IU/day vitamin D | 12 months | ↑ BMD of ulna and spine in comparison with dried apple; ↓ BALP, TRACP 5b in comparison with corresponding baseline values | |||||
| Dried plums | [203] | Randomized controlled trial Dried plums (n = 55) Dried apples (n = 45) | 110 F, 1–10 years postmenopausal with mild bone loss | 100 g/day dried plums + 500 mg/day Ca + 400 IU/day vitamin D | 75 g/day dried apples + 500 mg/day Ca + 400 IU/day vitamin D | 12 months | Dried plums: in comparison with corresponding baseline values, not statistically relevant ↑ RANKL, OPG, ↓ sclerostin | |||||
| Dried plums | [204] | Randomized controlled trial 100 gDP (n = 16) 50 gDP (n = 16) Control (n = 16) | 48 F postmenopausal with mild bone loss, 65–79 y | 100 g/day dried plums + 500 mg/day Ca + 400 IU/day vitamin D | 50 g/day dried plums + 500 mg/day Ca + 400 IU/day vitamin D | 75 g/day dried apples + 500 mg/day Ca + 400 IU/day vitamin D | 6 months | Both doses of dried plum: no changes in BMD (whereas control group ↓ BMD). ↓ TRAP-5b at 3 months | ||||
| Dried plums | [205] | Randomized controlled trial 100gDP (n = 78) 50gDP (n = 79) Control (n = 78) | 235 F postmenopausal, 62.1 ± 5.0 y with a BMD T-score of <0.0 and >−3.0 at any site | 100 g/day prunes + supplemented as necessary to meet the daily intake of 1200 mg calcium carbonate and 800 IU vitamin D3 (diet + supplements) | 50 g/day prunes + supplemented as necessary to meet the daily intake of 1200 mg calcium carbonate and 800 IU vitamin D3 (diet + supplements) | Supplemented as necessary to meet the daily intake of 1200 mg calcium carbonate and 800 IU vitamin D3 (diet + supplements) | 12 months | 50 g prune group: no changes in BMD (whereas control group ↓ BMD); in both prunes dosages no changes in FRAX (worsened in controls) | ||||
| Dried plums | [206] | Randomized trial Group A (n = 15) Group B (n = 12) Group C (n = 8) | 35 M, 55–80 y with a BMD t-score 0.1–2.5 SD | Group A: 100 g/day prunes + multivitamin containing 800 IU/day vitamin D and 450 mg/day calcium | Group B: 50 g/day prunes + multivitamin containing 800 IU/day vitamin D and 450 mg/day calcium | Multivitamin containing 800 IU/day vitamin D and 450 mg/day calcium | 3 months | 100 g prunes: ↓ osteocalcin; 50 g: ↓ OPG and osteocalcin, ↑ OPG:RANKL ratio | ||||
| Blueberries | [124] | Double-blind randomized crossover trial | 13 F, 45–70 y, >4 y post natural menopause or total hysterectomy | Randomly assigned to a sequence of 3 intervention periods, each corresponding to a low (17.5 g/d), medium (35 g/d), or high (70 g/d) dose of freeze-dried BB powder | 6 weeks | 17.5 g/d and 35 g/d: ↑ calcium retention; 35 g/d ↓ RANKL; all doses ↓ P1NP | ||||||
| Olive oil | [230] | Randomized trial MedDiet + VOO (n = 42) MedDiet+nuts (n = 51) Controls (n = 34) | 127 M 55–80 y with diagnosis of type 2 diabetes or at least three cardiovascular risk factors (hypertension/dyslipidemia/BMI ≥ 25 kg/m) or a family history of premature cardiovascular disease | MedDiet + VOO: Mediterrean Diet + at least 50 mL/day of extra virgin olive oil | MedDiet + nuts: Mediterrean Diet + 30 g/day mixed nuts (walnuts, almonds, and hazelnuts) | Advice on low-fat diet | 2 years | MedDiet+VOO: ↑ osteocalcin and P1NP ↓ CTX in all study groups | ||||
| Olive oil | [231] | Multicenter, randomized controlled parallel group trial | 870 participants, M 55–80 y and F 60–80 y with diagnosis of type 2 diabetes or at least three cardiovascular risk factors (hypertension/dyslipidemia /BMI ≥ 25 kg/m) | MedDiet + VOO: Mediterrean Diet + at least 50 mL/day of extra virgin olive oil | MedDiet + nuts: Mediterrean Diet + 30 g/day mixed nuts (walnuts, almonds, and hazelnuts) | Advice on low-fat diet | Median of 5.2 years of intervention | −51% in the risk of osteoporosis-related fractures in individuals with the highest EVOO consumption (mean 56.5 g/day) | ||||
4. Methods
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.F.; Tobias, J.H.; Duncan, E.; Evans, D.M.; Eriksson, J.; Paternoster, L.; Yerges-Armstrong, L.M.; Lehtimaki, T.; Bergstrom, U.; Kahonen, M.; et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012, 8, e1002745. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Urbano, F.; Chiarito, M.; Lassandro, G.; Giordano, P. Musculoskeletal health in children and adolescents. Front Pediatr 2023, 11, 1226524. [Google Scholar] [CrossRef] [PubMed]
- Ramchand, S.K.; Leder, B.Z. Sequential Therapy for the Long-Term Treatment of Postmenopausal Osteoporosis. J. Clin. Endocrinol. Metab. 2024, 109, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Edelstein, S.; Barrett-Connor, E. Endogenous sex steroids and bone mineral density in older women and men: The Rancho Bernardo Study. J. Bone Min. Res. 1997, 12, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Bodine, P.V.; Billiard, J.; Moran, R.A.; Ponce-de-Leon, H.; McLarney, S.; Mangine, A.; Scrimo, M.J.; Bhat, R.A.; Stauffer, B.; Green, J.; et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J. Cell Biochem. 2005, 96, 1212–1230. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Clevers, H. Wnt signalling in stem cells and cancer. Nature 2005, 434, 843–850. [Google Scholar] [CrossRef]
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007, 13, 156–163. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Ventura, A.; Colucci, S.; Cavallo, L.; Grano, M.; Brunetti, G. Bone Fragility in Turner Syndrome: Mechanisms and Prevention Strategies. Front. Endocrinol. 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; D’Amato, G.; Chiarito, M.; Tullo, A.; Colaianni, G.; Colucci, S.; Grano, M.; Faienza, M.F. An update on the role of RANKL-RANK/osteoprotegerin and WNT-ss-catenin signaling pathways in pediatric diseases. World J. Pediatr. 2019, 15, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; D’Amato, G.; Chiarito, M.; Colaianni, G.; Colucci, S.; Grano, M.; Corbo, F.; Brunetti, G. Mechanisms Involved in Childhood Obesity-Related Bone Fragility. Front. Endocrinol. 2019, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Abioja, M.O.; Ogundimu, K.B.; Akibo, T.E.; Odukoya, K.E.; Ajiboye, O.O.; Abiona, J.A.; Williams, T.J.; Oke, E.O.; Osinowo, O.A. Growth, Mineral Deposition, and Physiological Responses of Broiler Chickens Offered Honey in Drinking Water during Hot-Dry Season. Int. J. Zool. 2012, 2012, 403502. [Google Scholar] [CrossRef]
- Kim, B.; Cho, Y.; Lim, W. Osteoporosis therapies and their mechanisms of action (Review). Exp. Ther. Med. 2021, 22, 1379. [Google Scholar] [CrossRef] [PubMed]
- Karpouzos, A.; Diamantis, E.; Farmaki, P.; Savvanis, S.; Troupis, T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017, 2017, 4218472. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Arihara, K. Functional Foods. In Encyclopedia of Meat Sciences; Elsevier: Amsterdam, The Netherlands, 2014; pp. 32–36. [Google Scholar]
- Stefania, D.S.; Clodoveo, M.L.; Cariello, M.; D’Amato, G.; Franchini, C.; Faienza, M.F.; Corbo, F. Polyphenols and obesity prevention: Critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit. Rev. Food Sci. Nutr. 2020, 61, 1804–1826. [Google Scholar] [CrossRef]
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of Sweet Cherry Polyphenols on Enhanced Osteoclastogenesis Associated With Childhood Obesity. Front. Immunol. 2019, 10, 1001. [Google Scholar] [CrossRef]
- Sharma, A.R.; Lee, Y.H.; Bat-Ulzii, A.; Chatterjee, S.; Bhattacharya, M.; Chakraborty, C.; Lee, S.S. Bioactivity, Molecular Mechanism, and Targeted Delivery of Flavonoids for Bone Loss. Nutrients 2023, 15, 919. [Google Scholar] [CrossRef] [PubMed]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy Protein. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–45. [Google Scholar]
- Huang, J.; Zheng, J.; Dadihanc, T.; Gao, Y.; Zhang, Y.; Li, Z.; Wang, X.; Yu, L.; Mijiti, W.; Xie, Z.; et al. Isoflavones isolated from chickpea sprouts alleviate ovariectomy-induced osteoporosis in rats by dual regulation of bone remodeling. Biomed. Pharmacother. 2024, 171, 116214. [Google Scholar] [CrossRef] [PubMed]
- Baranska, A.; Kanadys, W.; Bogdan, M.; Stepien, E.; Barczynski, B.; Klak, A.; Augustynowicz, A.; Szajnik, M.; Religioni, U. The Role of Soy Isoflavones in the Prevention of Bone Loss in Postmenopausal Women: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2022, 11, 4676. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Xu, K.; Chen, X. Intake of dietary flavonoids in relation to bone loss among U.S. adults: A promising strategy for improving bone health. Food Funct. 2024, 15, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Maeda, T.; Chatani, M.; Handa, K.; Yamakawa, T.; Kiyohara, S.; Negishi-Koga, T.; Kato, Y.; Takami, M.; Niida, S.; et al. A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models. Antioxidants 2019, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Saulite, L.; Jekabsons, K.; Klavins, M.; Muceniece, R.; Riekstina, U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine 2019, 53, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Suzuki, K.; Muramatsu, M.; Nomura, A.; Inoue, F.; Into, T.; Yoshiko, Y.; Niida, S. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS ONE 2014, 9, e97177. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Maeda, T.; Moriwaki, S.; Nomura, A.; Kato, Y.; Niida, S.; Kruger, M.C.; Suzuki, K. Petunidin, a B-ring 5’-O-Methylated Derivative of Delphinidin, Stimulates Osteoblastogenesis and Reduces sRANKL-Induced Bone Loss. Int. J. Mol. Sci. 2019, 20, 2795. [Google Scholar] [CrossRef]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; Macdonald, H.M. Associations between dietary flavonoid intakes and bone health in a Scottish population. J. Bone Min. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Min. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; He, L.P.; Liu, Y.H.; Liu, J.; Su, Y.X.; Chen, Y.M. Association between dietary intake of flavonoid and bone mineral density in middle aged and elderly Chinese women and men. Osteoporos. Int. 2014, 25, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Liang, S.J.; Huang, L.; Yu, H.R.; Wu, Y.L.; Wei, Q.Z.; Zhang, Z.Q. Associations of Dietary Anthocyanidins Intake with Bone Health in Children: A Cross-Sectional Study. Calcif. Tissue Int. 2023, 113, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Hairi, H.; Jayusman, P.A.; Shuid, A.N. Revisiting Resveratrol as an Osteoprotective Agent: Molecular Evidence from In Vivo and In Vitro Studies. Biomedicines 2023, 11, 1453. [Google Scholar] [CrossRef]
- Feng, Y.L.; Jiang, X.T.; Ma, F.F.; Han, J.; Tang, X.L. Resveratrol prevents osteoporosis by upregulating FoxO1 transcriptional activity. Int. J. Mol. Med. 2018, 41, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Martins, G.; Cancela, M.L.; Gavaia, P.J. Resveratrol-Mediated Reversal of Doxorubicin-Induced Osteoclast Differentiation. Int. J. Mol. Sci. 2022, 23, 15160. [Google Scholar] [CrossRef] [PubMed]
- Ornstrup, M.J.; Harsløf, T.; Kjær, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol Increases Bone Mineral Density and Bone Alkaline Phosphatase in Obese Men: A Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Nobile, V.; Conti, V.; Cannavo, A.; Sorrenti, V.; Medoro, A.; Scapagnini, G.; Davinelli, S. Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial. Int. J. Mol. Sci. 2023, 24, 12063. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation With Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Min. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Kapilevich, L.; Zhang, X.; Huang, Y. Protective effects of curcumin against osteoporosis and its molecular mechanisms: A recent review in preclinical trials. Front. Pharmacol. 2023, 14, 1249418. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Bu, J.; Zhu, Y.; Xiao, X.; Liang, Z.; Zhang, R. Curcumin improves bone microarchitecture in glucocorticoid-induced secondary osteoporosis mice through the activation of microRNA-365 via regulating MMP-9. Int. J. Clin. Exp. Pathol. 2015, 8, 15684–15695. [Google Scholar]
- Bukhari, S.N.A.; Hussain, F.; Thu, H.E.; Hussain, Z. Synergistic effects of combined therapy of curcumin and Fructus Ligustri Lucidi for treatment of osteoporosis: Cellular and molecular evidence of enhanced bone formation. J. Integr. Med. 2019, 17, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xue, J.; Shen, T.; Mu, S.; Fu, Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int. J. Mol. Med. 2016, 37, 329–338. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Yang, Y.; Zhang, D.; Wang, J.; Chen, S.; Zhou, D. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporos. Int. 2015, 26, 2665–2676. [Google Scholar] [CrossRef]
- Dai, P.; Mao, Y.; Sun, X.; Li, X.; Muhammad, I.; Gu, W.; Zhang, D.; Zhou, Y.; Ni, Z.; Ma, J.; et al. Attenuation of Oxidative Stress-Induced Osteoblast Apoptosis by Curcumin is Associated with Preservation of Mitochondrial Functions and Increased Akt-GSK3beta Signaling. Cell Physiol. Biochem. 2017, 41, 661–677. [Google Scholar] [CrossRef]
- Zhuang, S.; Yu, R.; Zhong, J.; Liu, P.; Liu, Z. Rhein from Rheum rhabarbarum Inhibits Hydrogen-Peroxide-Induced Oxidative Stress in Intestinal Epithelial Cells Partly through PI3K/Akt-Mediated Nrf2/HO-1 Pathways. J. Agric. Food Chem. 2019, 67, 2519–2529. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Mao, Y.; Dai, P.; Sun, X.; Zhang, X.; Cheng, H.; Wang, Y.; Banda, I.; Wu, G.; et al. Curcumin Protects Osteoblasts From Oxidative Stress-Induced Dysfunction via GSK3beta-Nrf2 Signaling Pathway. Front. Bioeng. Biotechnol. 2020, 8, 625. [Google Scholar]
- Liang, Z.; Xue, Y.; Wang, T.; Xie, Q.; Lin, J.; Wang, Y. Curcumin inhibits the migration of osteoclast precursors and osteoclastogenesis by repressing CCL3 production. BMC Complement. Med. Ther. 2020, 20, 234. [Google Scholar] [CrossRef]
- Oh, S.; Kyung, T.-W.; Choi, H.-S. Curcumin Inhibits Osteoclastogenesis by Decreasing Receptor Activator of Nuclear Factor-κB Ligand (RANKL) in Bone Marrow Stromal Cells. Mol. Cells 2008, 26, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Oh, S.; Shin, H.K.; Kim, S.H.; Ham, J.; Song, J.S.; Lee, S. Synthesis of substituted triazolyl curcumin mimics that inhibit RANKL-induced osteoclastogenesis. Bioorg Med. Chem. Lett. 2011, 21, 3573–3577. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Wang, Y.; Yu, Y.; Wang, Y.; Zheng, W.; Fu, X.; Han, J.; Zhang, G.; Xu, J. Curcumin-activated autophagy plays a negative role in its anti-osteoclastogenic effect. Mol. Cell Endocrinol. 2020, 500, 110637. [Google Scholar] [CrossRef]
- Kim, W.K.; Ke, K.; Sul, O.J.; Kim, H.J.; Kim, S.H.; Lee, M.H.; Kim, H.J.; Kim, S.Y.; Chung, H.T.; Choi, H.S. Curcumin protects against ovariectomy-induced bone loss and decreases osteoclastogenesis. J. Cell Biochem. 2011, 112, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, K.; Yuan, X.; Zhang, X.; Qian, Y.; Cheng, T. Curcumin has immunomodulatory effects on RANKL-stimulated osteoclastogenesis in vitro and titanium nanoparticle-induced bone loss in vivo. J. Cell Mol. Med. 2020, 24, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- French, D.L.; Muir, J.M.; Webber, C.E. The ovariectomized, mature rat model of postmenopausal osteoporosis: An assessment of the bone sparing effects of curcumin. Phytomedicine 2008, 15, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.C.; Jung, H.S.; Kim, K.T.; Jeon, Y.; Sung, J.K.; Hwang, J.H. Therapeutic advantages of treatment of high-dose curcumin in the ovariectomized rat. J. Korean Neurosurg. Soc. 2013, 54, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jing, Y.; Zhang, W.; Fu, X.; Zhao, H.; Zhou, X.; Tao, Y.; Yang, H.; Zhang, Y.; Zen, K.; et al. Silencing miR-106b accelerates osteogenesis of mesenchymal stem cells and rescues against glucocorticoid-induced osteoporosis by targeting BMP2. Bone 2017, 97, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xue, J.; Shen, T.; Ba, G.; Yu, D.; Fu, Q. Curcumin alleviates glucocorticoid-induced osteoporosis by protecting osteoblasts from apoptosis in vivo and in vitro. Clin. Exp. Pharmacol. Physiol. 2016, 43, 268–276. [Google Scholar] [CrossRef]
- He, J.; Su, X.; Xie, W. MiR-582-3p alleviates osteoarthritis progression by targeting YAP1. Mol. Immunol. 2020, 128, 258–267. [Google Scholar] [CrossRef]
- Chen, S.; Liang, H.; Ji, Y.; Kou, H.; Zhang, C.; Shang, G.; Shang, C.; Song, Z.; Yang, L.; Liu, L.; et al. Curcumin Modulates the Crosstalk Between Macrophages and Bone Mesenchymal Stem Cells to Ameliorate Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 634650. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Lu, J.; Yu, N.; Xie, Y.; Zhen, L. Curcumin Prevents Diabetic Osteoporosis through Promoting Osteogenesis and Angiogenesis Coupling via NF-kappaB Signaling. Evid. Based Complement. Altern. Med. 2022, 2022, 4974343. [Google Scholar] [CrossRef] [PubMed]
- Hie, M.; Yamazaki, M.; Tsukamoto, I. Curcumin suppresses increased bone resorption by inhibiting osteoclastogenesis in rats with streptozotocin-induced diabetes. Eur. J. Pharmacol. 2009, 621, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, B.; Li, S.; Zhai, Y.; Yang, Y.; Bai, Z.; Zeng, Y.; Li, D. Curcumin protects bone biomechanical properties and microarchitecture in type 2 diabetic rats with osteoporosis via the TGFbeta/Smad2/3 pathway. Exp. Ther. Med. 2020, 20, 2200–2208. [Google Scholar] [PubMed]
- Khanizadeh, F.; Rahmani, A.; Asadollahi, K.; Ahmadi, M.R.H. Combination therapy of curcumin and alendronate modulates bone turnover markers and enhances bone mineral density in postmenopausal women with osteoporosis. Arch. Endocrinol. Metab. 2018, 62, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, M.; Ahmadi, M.R.H.; Rahmani, A.; Dastjerdi, M.M.; Asadollahi, K. Effects of Curcumin on Bone Loss and Biochemical Markers of Bone Turnover in Patients with Spinal Cord Injury. World Neurosurg. 2018, 114, e785–e791. [Google Scholar] [CrossRef]
- Rao, L.G.; Krishnadev, N.; Banasikowska, K.; Rao, A.V. Lycopene I—Effect on Osteoclasts: Lycopene Inhibits Basal and Parathyroid Hormone-Stimulated Osteoclast Formation and Mineral Resorption Mediated by Reactive Oxygen Species in Rat Bone Marrow Cultures. J. Med. Food 2003, 6, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and bone: An in vitro investigation and a pilot prospective clinical study. J. Transl. Med. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.S.M.; Badawoud, M.H.; Hassan, S.M.; Ardawi, A.M.S.; Rouzi, A.A.; Qari, M.H.; Mousa, S.A. Lycopene nanoparticles promotes osteoblastogenesis and inhibits adipogenesis of rat bone marrow mesenchymal stem cells. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6894–6907. [Google Scholar]
- Bengi, V.U.; Saygun, I.; Bal, V.; Ozcan, E.; Kose Ozkan, C.; Torun, D.; Avcu, F.; Kantarcı, A. Effect of antioxidant lycopene on human osteoblasts. Clin. Oral. Investig. 2022, 27, 1637–1643. [Google Scholar] [CrossRef]
- Yang, X.-z.; Ji, C.-j.; Wang, D.-c.; Chen, X.-l. Antiosteoporotic activity of lycopene in mature ovariectomized rats. Chin. J. Tissue Eng. Res. 2012, 16, 2750. [Google Scholar]
- Iimura, Y.; Agata, U.; Takeda, S.; Kobayashi, Y.; Yoshida, S.; Ezawa, I.; Omi, N. The protective effect of lycopene intake on bone loss in ovariectomized rats. J. Bone Miner. Metab. 2014, 33, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.R.; Vargas-Sanchez, P.K.; Fernandes, R.R.; Ricoldi, M.S.T.; Semeghini, M.S.; Pitol, D.L.; de Sousa, L.G.; Siessere, S.; Bombonato-Prado, K.F. Lycopene influences osteoblast functional activity and prevents femur bone loss in female rats submitted to an experimental model of osteoporosis. J. Bone Miner. Metab. 2018, 37, 658–667. [Google Scholar] [CrossRef]
- Ardawi, M.M.; Badawoud, M.H.; Hassan, S.M.; Rouzi, A.A.; Ardawi, J.M.S.; AlNosani, N.M.; Qari, M.H.; Mousa, S.A. Lycopene treatment against loss of bone mass, microarchitecture and strength in relation to regulatory mechanisms in a postmenopausal osteoporosis model. Bone 2016, 83, 127–140. [Google Scholar] [CrossRef]
- Xia, B.; Zhu, R.; Zhang, H.; Chen, B.; Liu, Y.; Dai, X.; Ye, Z.; Zhao, D.; Mo, F.; Gao, S.; et al. Lycopene Improves Bone Quality and Regulates AGE/RAGE/NF-кB Signaling Pathway in High-Fat Diet-Induced Obese Mice. Oxidative Med. Cell. Longev. 2022, 2022, 3697067. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yu, F.; Tong, Z.; Zeng, W. Lycopene Effects on Serum Mineral Elements and Bone Strength in Rats. Molecules 2012, 17, 7093–7102. [Google Scholar] [CrossRef] [PubMed]
- Iimura, Y.; Agata, U.; Takeda, S.; Kobayashi, Y.; Yoshida, S.; Ezawa, I.; Omi, N. Lycopene Intake Facilitates the Increase of Bone Mineral Density in Growing Female Rats. J. Nutr. Sci. Vitaminol. 2014, 60, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Semeghini, M.S.; Scalize, P.H.; Coelho, M.C.; Fernandes, R.R.; Pitol, D.L.; Tavares, M.S.; de Sousa, L.G.; Coppi, A.A.; Siessere, S.; Bombonato-Prado, K.F. Lycopene prevents bone loss in ovariectomized rats and increases the number of osteocytes and osteoblasts. J. Anat. 2022, 241, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Hannan, M.T.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: A 17-year follow-up from the Framingham Osteoporosis Study. J. Bone Min. Res. 2009, 24, 1086–1094. [Google Scholar] [CrossRef]
- Rao, L.G.; Mackinnon, E.S.; Josse, R.G.; Murray, T.M.; Strauss, A.; Rao, A.V. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos. Int. 2006, 18, 109–115. [Google Scholar] [CrossRef]
- Wang, F.; Wang, N.; Gao, Y.; Zhou, Z.; Liu, W.; Pan, C.; Yin, P.; Yu, X.; Tang, M. beta-Carotene suppresses osteoclastogenesis and bone resorption by suppressing NF-kappaB signaling pathway. Life Sci. 2017, 174, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nishide, Y.; Tousen, Y.; Tadaishi, M.; Inada, M.; Miyaura, C.; Kruger, M.; Ishimi, Y. Combined Effects of Soy Isoflavones and β-Carotene on Osteoblast Differentiation. Int. J. Environ. Res. Public Health 2015, 12, 13750–13761. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Tousen, Y.; Ishimi, Y. β-Carotene prevents bone loss in hind limb unloading mice. J. Clin. Biochem. Nutr. 2018, 63, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Händel, M.N.; Moon, R.J.; Titcombe, P.; Abrahamsen, B.; Heitmann, B.L.; Calder, P.C.; Dennison, E.M.; Robinson, S.M.; Godfrey, K.M.; Inskip, H.M.; et al. Maternal serum retinol and β-carotene concentrations and neonatal bone mineralization: Results from the Southampton Women’s Survey cohort. Am. J. Clin. Nutr. 2016, 104, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Cho, S.H.; Park, H.M.; Chang, Y.K. Relationship between bone mineral density and dietary intake of β-carotene, vitamin C, zinc and vegetables in postmenopausal Korean women: A cross-sectional study. J. Int. Med. Res. 2016, 44, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.L.; Bremner, A.P.; Reid, A.; Mackerras, D.; Alfonso, H.; Olsen, N.J.; Musk, A.W.; de Klerk, N.H. No dose-dependent increase in fracture risk after long-term exposure to high doses of retinol or beta-carotene. Osteoporos. Int. 2012, 24, 1285–1293. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhao, Y. The effects of β-carotene on osteoporosis: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2022, 34, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Lutein, a carotenoid, suppresses osteoclastic bone resorption and stimulates bone formation in cultures. Biosci. Biotechnol. Biochem. 2017, 81, 302–306. [Google Scholar] [CrossRef]
- Takeda, H.; Tominari, T.; Hirata, M.; Watanabe, K.; Matsumoto, C.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Lutein Enhances Bone Mass by Stimulating Bone Formation and Suppressing Bone Resorption in Growing Mice. Biol. Pharm. Bull. 2017, 40, 716–721. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef]
- Niu, P.; Liu, Y.; Zhang, Y.; Li, L. Associations between blood antioxidant levels and femoral neck strength. BMC Musculoskelet. Disord. 2023, 24, 252. [Google Scholar]
- Murphy, C.H.; Duggan, E.; Davis, J.; O’Halloran, A.M.; Knight, S.P.; Kenny, R.A.; McCarthy, S.N.; Romero-Ortuno, R. Plasma lutein and zeaxanthin concentrations associated with musculoskeletal health and incident frailty in The Irish Longitudinal Study on Ageing (TILDA). Exp. Gerontol. 2023, 171, 112013. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Reuss, L.; Wang, Y. β-Cryptoxanthin: Chemistry, Occurrence, and Potential Health Benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar] [CrossRef]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of beta-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Sumida, T.; Yamaguchi, M. Oral Administration of.BETA.-Cryptoxanthin Induces Anabolic Effects on Bone Components in the Femoral Tissues of Rats in Vivo. Biol. Pharm. Bull. 2004, 27, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Yamaguchi, M. β-cryptoxanthin stimulates cell differentiation and mineralization in osteoblastic MC3T3-E1 cells. J. Cell. Biochem. 2005, 95, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Ishiyama, K.; Hashimoto, K.; Yamaguchi, M. Synergistic Effect of β-Cryptoxanthin and Zinc Sulfate on the Bone Component in Rat Femoral Tissues in Vitro: The Unique Anabolic Effect with Zinc. Biol. Pharm. Bull. 2005, 28, 2142–2145. [Google Scholar] [PubMed]
- Nishigaki, M.; Yamamoto, T.; Ichioka, H.; Honjo, K.-i.; Yamamoto, K.; Oseko, F.; Kita, M.; Mazda, O.; Kanamura, N. β-cryptoxanthin regulates bone resorption related-cytokine production in human periodontal ligament cells. Arch. Oral. Biol. 2013, 58, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, The bone anabolic carotenoids p-hydroxycinnamic acid and β-cryptoxanthin antagonize NF-κB activation in MC3T3 preosteoblasts. Mol. Med. Rep. 2009, 2, 641–644.
- Hirata, N.; Ichimaru, R.; Tominari, T.; Matsumoto, C.; Watanabe, K.; Taniguchi, K.; Hirata, M.; Ma, S.; Suzuki, K.; Grundler, F.; et al. Beta-Cryptoxanthin Inhibits Lipopolysaccharide-Induced Osteoclast Differentiation and Bone Resorption via the Suppression of Inhibitor of NF-κB Kinase Activity. Nutrients 2019, 11, 368. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. β-cryptoxanthin stimulates apoptotic cell death and suppresses cell function in osteoclastic cells: Change in their related gene expression. J. Cell. Biochem. 2006, 98, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.; Ashida, N.; Yokoyama, S.; Tominari, T.; Hirata, M.; Ogawa, K.; Sugiura, M.; Yano, M.; Inada, M.; Miyaura, C. The Protective Effects of β-Cryptoxanthin on Inflammatory Bone Resorption in a Mouse Experimental Model of Periodontitis. Biosci. Biotechnol. Biochem. 2013, 77, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Okamoto, M.; Fukasawa, K.; Iezaki, T.; Onishi, Y.; Yoneda, Y.; Sugiura, M.; Hinoi, E. Daily intake of β-cryptoxanthin prevents bone loss by preferential disturbance of osteoclastic activation in ovariectomized mice. J. Pharmacol. Sci. 2015, 129, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Uchiyama, S.; Ishiyama, K.; Hashimoto, K. Oral Administration in Combination with Zinc Enhances.BETA.-Cryptoxanthin-Induced Anabolic Effects on Bone Components in the Femoral Tissues of Rats in Vivo. Biol. Pharm. Bull. 2006, 29, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Yamaguchi, M. Oral administration of beta-cryptoxanthin prevents bone loss in ovariectomized rats. Int. J. Mol. Med. 2006, 17, 15–20. [Google Scholar] [PubMed]
- Uchiyama, S.; Yamaguchi, M. Oral Administration of.BETA.-Cryptoxanthin Prevents Bone Loss in Streptozotocin-Diabetic Rats in Vivo. Biol. Pharm. Bull. 2005, 28, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Igarashi, A.; Uchiyama, S.; Morita, S.; Sugawara, K.; Sumida, T. Prolonged Intake of Juice (Citrus Unshiu) Reinforced with.BETA.-Crypthoxanthin Has an Effect on Circulating Bone Biochemical Markers in Normal Individuals. J. Health Sci. 2004, 50, 619–624. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Igarashi, A.; Morita, S.; Sumida, T.; Sugawara, K. Relationship between Serum β-Cryptoxanthin and Circulating Bone Metabolic Markers in Healthy Individuals with the Intake of Juice (Citrus unshiu) Containing β-Cryptoxanthin. J. Health Sci. 2005, 51, 738–743. [Google Scholar] [CrossRef]
- Kim, S.J.; Anh, N.H.; Diem, N.C.; Park, S.; Cho, Y.H.; Long, N.P.; Hwang, I.G.; Lim, J.; Kwon, S.W. Effects of β-Cryptoxanthin on Improvement in Osteoporosis Risk: A Systematic Review and Meta-Analysis of Observational Studies. Foods 2021, 10, 296. [Google Scholar] [CrossRef]
- Mao, L.; Wang, F.; Li, Y.; Dai, Y.; Liu, Y.; Wang, J.; Xue, C. Oil from Antarctic krill (Euphausia superba) facilitates bone formation in dexamethasone-treated mice. Food Sci. Biotechnol. 2018, 28, 539–545. [Google Scholar] [CrossRef]
- Cugno, C.; Kizhakayil, D.; Calzone, R.; Rahman, S.M.; Halade, G.V.; Rahman, M.M. Omega-3 fatty acid-rich fish oil supplementation prevents rosiglitazone-induced osteopenia in aging C57BL/6 mice and in vitro studies. Sci. Rep. 2021, 11, 10364. [Google Scholar]
- Wan, Y.; Chong, L.-W.; Evans, R.M. PPAR-γ regulates osteoclastogenesis in mice. Nat. Med. 2007, 13, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, A.; Kruger, M.C.; Coetzee, M. Activation of PPARs Modulates Signalling Pathways and Expression of Regulatory Genes in Osteoclasts Derived from Human CD14+ Monocytes. Int. J. Mol. Sci. 2019, 20, 1798. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Tsukamoto, I. n-3 polyunsaturated fatty acids stimulate osteoclastogenesis through PPARγ-mediated enhancement of c-Fos expression, and suppress osteoclastogenesis through PPARγ-dependent inhibition of NFkB activation. J. Nutr. Biochem. 2015, 26, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Li, Y.; Allen, K.G.D.; Hoffmann, W.E.; Seifert, M.F. Dietary Ratio of (n-6)/(n-3) Polyunsaturated Fatty Acids Alters the Fatty Acid Composition of Bone Compartments and Biomarkers of Bone Formation in Rats. J. Nutr. 2000, 130, 2274–2284. [Google Scholar] [CrossRef]
- Watkins, B.A.; Hutchins, H.; Li, Y.; Seifert, M.F. The endocannabinoid signaling system: A marriage of PUFA and musculoskeletal health. J. Nutr. Biochem. 2010, 21, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Li, L.; Wang, K.; Wu, X.; Chen, H.; Shi, J.; Zhou, C.; Zhang, W.; Hang, K.; et al. Eicosapentaenoic acid supplementation modulates the osteoblast/osteoclast balance in inflammatory environments and protects against estrogen deficiency-induced bone loss in mice. Clin. Nutr. 2023, 42, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Farahnak, Z.; Freundorfer, M.T.; Lavery, P.; Weiler, H.A. Dietary docosahexaenoic acid contributes to increased bone mineral accretion and strength in young female Sprague-Dawley rats. Prostaglandins Leukot. Essent. Fat. Acids 2019, 144, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, H.; Sun, D.; Lin, C.; Wang, L.; Huang, M.; Jiang, H.; Zhang, Z.; Jin, D.; Zhang, B.; et al. Endogenous Production of n-3 Polyunsaturated Fatty Acids Promotes Fracture Healing in Mice. J. Healthc. Eng. 2017, 2017, 3571267. [Google Scholar] [CrossRef]
- Sakakibara, M.; Lavado-García, J.; Roncero-Martin, R.; Moran, J.M.; Pedrera-Canal, M.; Aliaga, I.; Leal-Hernandez, O.; Rico-Martin, S.; Canal-Macias, M.L. Long-chain omega-3 polyunsaturated fatty acid dietary intake is positively associated with bone mineral density in normal and osteopenic Spanish women. PLoS ONE 2018, 13, e0190539. [Google Scholar]
- Feehan, O.; Magee, P.J.; Pourshahidi, L.K.; Armstrong, D.J.; Slevin, M.M.; Allsopp, P.J.; Conway, M.C.; Strain, J.J.; McSorley, E.M. Associations of long chain polyunsaturated fatty acids with bone mineral density and bone turnover in postmenopausal women. Eur. J. Nutr. 2022, 62, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Mediterr. J. Nutr. Metab. 2009, 3, 15–23. [Google Scholar] [CrossRef]
- Kamaruzzaman, M.A.; Chin, K.-Y.; Mohd Ramli, E.S. A Review of Potential Beneficial Effects of Honey on Bone Health. Evid. -Based Complement. Altern. Med. 2019, 2019, 8543618. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.-K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef]
- Kim, T.-K.; Atigadda, V.; Brzeminski, P.; Fabisiak, A.; Tang, E.K.Y.; Tuckey, R.C.; Slominski, A.T. Detection of 7-Dehydrocholesterol and Vitamin D3 Derivatives in Honey. Molecules 2020, 25, 2583. [Google Scholar] [CrossRef]
- Ariefdjohan, M.W.; Martin, B.R.; Lachcik, P.J.; Weaver, C.M. Acute and Chronic Effects of Honey and Its Carbohydrate Constituents on Calcium Absorption in Rats. J. Agric. Food Chem. 2008, 56, 2649–2654. [Google Scholar] [CrossRef]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.-G.; Marcazzan, G.L.; Piro, R.; et al. Honey quality and international regulatory standards: Review by the International Honey Commission. Bee World 2015, 80, 61–69. [Google Scholar] [CrossRef]
- Chepulis, L.; Starkey, N. The Long-Term Effects of Feeding Honey Compared with Sucrose and a Sugar-Free Diet on Weight Gain, Lipid Profiles, and DEXA Measurements in Rats. J. Food Sci. 2007, 73, H1–H7. [Google Scholar] [CrossRef]
- Ramli, E.; Kamaruzzaman, M.; Thanu, A.; Yusof, M.; Soelaiman, I. Kelulut honey ameliorates glucocorticoid induced osteoporosis via its antioxidant activity in rats. Asian Pac. J. Trop. Biomed. 2019, 9, 493–500. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Chin, K.-Y.; Ramli, N.Z.; Zarkasi, K.A.; Ahmad, F. Effect of Kelulut honey supplementation on bone health in male rats on high-carbohydrate high-fat diet. Trop. J. Pharm. Res. 2022, 20, 1185–1192. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Wong, S.K.; Ekeuku, S.O.; Pang, K.-L. Relationship Between Metabolic Syndrome and Bone Health–An Evaluation of Epidemiological Studies and Mechanisms Involved. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3667–3690. [Google Scholar] [CrossRef]
- Mohd Ramli, E.S.; Sukalingam, K.; Kamaruzzaman, M.A.; Soelaiman, I.N.; Pang, K.-L.; Chin, K.-Y. Direct and Indirect Effect of Honey as a Functional Food Against Metabolic Syndrome and Its Skeletal Complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Abu-Serie, M.M.; Habashy, N.H. The ameliorating effect of the combined extract from GreekThymus vulgarisand bee’s honey on the hydrocortisone-induced osteoporosis in rat bone cellsviamodulating the bone turnover, oxidative stress, and inflammation. RSC Adv. 2018, 8, 28341–28355. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Sulaiman, S.A.; Sirajudeen, K.N.M.; Othman, N.H. The effects of tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats-animal model for menopause. BMC Complement. Altern. Med. 2010, 10, 82. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Sulaiman, S.A.; Othman, N.H.; Soelaiman, I.-N.; Shuid, A.N.; Mohamad, N.; Muhamad, N. Protective effects of Tualang honey on bone structure in experimental postmenopausal rats. Clinics 2012, 67, 779–784. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldra, F. Handbook of Analysis of Active Compounds in Functional Foods; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Swift, S.; Chepulis, L.M.; Uy, B.; Radcliff, F.J. Enhanced Antibacterial Activity of MGOTM Manuka Honey complexed with a- cyclodextrin (Manuka Honey with CycloPowerTM). Funct. Foods Health Dis. 2014, 4, 172. [Google Scholar] [CrossRef]
- Katsumata, S.; Wolber, F.; Tadaishi, M.; Tousen, Y.; Ishimi, Y.; Kruger, M. Effects of manuka honey combined with α-cyclodextrin on bone metabolism and caecal bacterial contents in ovariectomized mice. Int. J. Food Nutr. Sci. 2015, 2, 86–91. [Google Scholar]
- Yudaniayanti, I.S.; Primarizky, H.; Nangoi, L. The effects of honey (Apis dorsata) supplements on increased bone strength in ovariectomized rat as animal model of osteoporosis. AIP Conf. Proc. 2018, 1945, 020004. [Google Scholar]
- Ibrahim, M.H.R.; Hasib, A.H.; Rohmah, S.N.; Abani, S.; Yordan, S.; Yudaniayanti, I.S. Utilization of Honey Apis dorsata as Antiosteoporosis on Requirements of Bone Calcium Ash Density on Ovariohysterectomized White Rat (Ratus norvegicus). KnE Life Sci. 2017, 3, 627–632. [Google Scholar] [CrossRef]
- Hasib, A.; Wahjuningrum, D.A.; Ibrahim, M.H.R.; Kurniawan, H.J.; Ernawati, R.; Hadinoto, M.E.K.; Mooduto, L. ALP (alkaline phosphatase) expression in simple fracture incident in rat (Rattus norvegicus) femur bone supplemented by apis mellifera honey. J. Int. Dent. Med. Res. 2020, 13, 887–891. [Google Scholar]
- Bigham-Sadegh, A.; Karimi, I.; Hoseini, F.; Oryan, A.; Sharifi, S.; Pakzad, A. Effects of honey and hydroxyapatite on bone healing in rats. Trauma. Mon. 2018, 23, e56119. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Derakhshan, B.; Peimani, A.; Abbasi, Z. Effect of Topical Honey on Mandibular Bone Defect Healing in Rats. J. Contemp. Dent. Pr. 2018, 19, 47–51. [Google Scholar]
- Ooi, F.K.; Tavafzadeh, S.S.; Hung, L.; Hung, W.; He, Y. Tibial Bone Mineral Density, Geometry, and Mechanical Properties in Response to High-Impact Exercise and Honey Supplementation in Rats. Asian J. Exerc. Sports Sci. 2014, 11, 12–24. [Google Scholar]
- Tavafzadeh, S.S.; Ooi, F.-K.; Oleksandr, K.; Sulaiman, S. Effect of a combination of jumping exercise and honey supplementation on the mass, strength and physical dimensions of bones in young female rats. J. ApiProduct ApiMedical Sci. 2011, 3, 26–32. [Google Scholar] [CrossRef]
- Tavafzadeh, S.S.; Ooi, F.K.; Chen, C.K.; Sulaiman, S.A.; Hung, L. Changes in bone metabolism and antioxidant status with combined exercise and honey supplementation in young female rats. J. Exerc. Sports Orthop. 2015, 2, 1–8. [Google Scholar]
- Tavafzadeh, S.S.; Ooi, F.K.; Chen, C.K.; Sulaiman, S.A.; Hung, L.K. Bone mechanical properties and mineral density in response to cessation of jumping exercise and honey supplementation in young female rats. BioMed Res. Int. 2015, 2015, 938782. [Google Scholar] [CrossRef]
- Mosavat, M.; Ooi, F.K.; Mohamed, M. Effects of honey supplementation combined with different jumping exercise intensities on bone mass, serum bone metabolism markers and gonadotropins in female rats. BMC Complement. Altern. Med. 2014, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Nik Hussain, N.H.; Sulaiman, S.A.; Idiana Hassan, I.; Abdul Kadir, A.; Mohd Nor, N.; Bahari Ismail, S.; Yaacob, L.H.; Zakaria, R.; Shaniza Shafie, N.; Haron, J.; et al. Randomized Controlled Trial on the Effects of Tualang Honey and Hormonal Replacement Therapy (HRT) on Cardiovascular Risk Factors, Hormonal Profiles and Bone Density Among Postmenopausal Women: A Pilot Study. J. Food Res. 2012, 1, 171. [Google Scholar] [CrossRef]
- Ooi, F.K. Effects of Combined Aerobic Dance Exercise and Honey Supplementation on Bone Turnover Markers in Young Females. Asian J. Exerc. Sports Sci. 2011, 8, 1–11. [Google Scholar]
- Tavafzadeh, S.S.; Chee Keong, C.; Foong Kiew, O.; Hamzah, N.A.; Sulaiman, S.A.; Meor Osman, J. Effects of Aerobic Dance Exercise and Honey Supplementation Followed by Their Subsequent Cessation on Bone Metabolism Markers and Antioxidant Status in Young Collegiate Females. Malays. J. Med. Sci. 2023, 30, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.; Ooi, F.K.; Hamid, W.Z.W.A. Changes of Bone Metabolism Markers and Muscular Performance with Combined Aerobic Dance Exercise and Honey Supplementation in Adult Women. Sports Exerc. Med. Open J. 2016, 1, 186–197. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Katan, M.B. Health Effects and Bioavailability of Dietary Flavonols. Free Radic. Res. 2016, 31 (Suppl. S1), 75–80. [Google Scholar] [CrossRef] [PubMed]
- Das, A.S.; Mukherjee, M.; Mitra, C. Evidence for a prospective anti-osteoporosis effect of black tea (Camellia Sinensis) extract in a bilaterally ovariectomized rat model. Asia Pac. J. Clin. Nutr. 2004, 13, 210–216. [Google Scholar] [PubMed]
- Das, A.S.; Das, D.; Mukherjee, M.; Mukherjee, S.; Mitra, C. Phytoestrogenic effects of black tea extract (Camellia sinensis) in an oophorectomized rat (Rattus norvegicus) model of osteoporosis. Life Sci. 2005, 77, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Das, A.S.; Mukherjee, M.; Das, D.; Mitra, C. Protective action of aqueous black tea (Camellia sinensis) extract (BTE) against ovariectomy-induced oxidative stress of mononuclear cells and its associated progression of bone loss. Phytother. Res. 2009, 23, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Das, A.S.; Banerjee, M.; Das, D.; Mukherjee, S.; Mitra, C. Black tea may be a prospective adjunct for calcium supplementation to prevent early menopausal bone loss in a rat model of osteoporosis. J. Osteoporos. 2013, 2013, 760586. [Google Scholar] [CrossRef]
- Shen, C.-L.; Yeh, J.K.; Stoecker, B.J.; Chyu, M.-C.; Wang, J.-S. Green tea polyphenols mitigate deterioration of bone microarchitecture in middle-aged female rats. Bone 2009, 44, 684–690. [Google Scholar] [CrossRef]
- Shen, C.-L.; Wang, P.; Guerrieri, J.; Yeh, J.; Wang, J.-S. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos. Int. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Shao, C.; Chen, L.; Lu, C.; Shen, C.-L.; Gao, W. A gel-based proteomic analysis of the effects of green tea polyphenols on ovariectomized rats. Nutrition 2011, 27, 681–686. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, W.; Shen, C.-L.; Gao, W. Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PLoS ONE 2012, 7, e38332. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Majumdar, S.; Maiti, A.; Choudhury, M.; Ghosh, A.; Das, A.S.; Mitra, C. Protective role of black tea extract against nonalcoholic steatohepatitis-induced skeletal dysfunction. J. Osteoporos. 2011, 2011, 426863. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Cao, J.J.; Dagda, R.Y.; Tenner, T.E.; Chyu, M.-C.; Yeh, J.K. Supplementation with green tea polyphenols improves bone microstructure and quality in aged, orchidectomized rats. Calcif. Tissue Int. 2011, 88, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.-S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J. Nutr. Biochem. 2010, 21, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Yeh, J.; Samathanam, C.; Cao, J.; Stoecker, B.; Dagda, R.; Chyu, M.-C.; Dunn, D.; Wang, J.-S. Green tea polyphenols attenuate deterioration of bone microarchitecture in female rats with systemic chronic inflammation. Osteoporos. Int. 2011, 22, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.-C.; Gao, W.; Wang, J.-S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet–induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Chyu, M.-C.; Cao, J.J.; Yeh, J.K. Green tea polyphenols improve bone microarchitecture in high-fat-diet–induced obese female rats through suppressing bone formation and erosion. J. Med. Food 2013, 16, 421–427. [Google Scholar] [CrossRef]
- Shen, C.; Syapin, P.; Graef, J.; Smith, B.; Brackee, G.; Fowler, A.; Segura-Ulate, I.; Wang, J.; Bergeson, S. Alcohol-induced bone loss and quality during adolescence is improved by green tea polyphenols. J Clin Toxicol. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Jin, S.; Park, J.-Y.; Hong, J.-M.; Kim, T.-H.; Shin, H.-I.; Park, E.K.; Kim, S.-Y. Inhibitory effect of (-)-epigallocatechin gallate on titanium particle-induced TNF-α release and in vivo osteolysis. Exp. Mol. Med. 2011, 43, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, G.; Claudino, M.; Cestari, T.M.; Ceolin, D.; Germino, P.; Garlet, G.P.; De Assis, G.F. Green tea modulates cytokine expression in the periodontium and attenuates alveolar bone resorption in type 1 diabetic rats. PLoS ONE 2015, 10, e0134784. [Google Scholar] [CrossRef]
- Nakamura, H.; Ukai, T.; Yoshimura, A.; Kozuka, Y.; Yoshioka, H.; Yoshinaga, Y.; Abe, Y.; Hara, Y. Green tea catechin inhibits lipopolysaccharide-induced bone resorption in vivo. J. Periodontal Res. 2010, 45, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Iwaniec, U.T.; Turner, R.T.; Koo, S.I.; Kaur, R.; Ho, E.; Wong, C.P.; Bruno, R.S. Consumption of green tea extract results in osteopenia in growing male mice. J. Nutr. 2009, 139, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Zhang, J.; Chen, J.; Liu, Z.; Guo, P.; Li, X.; Li, L.; Zhang, Q. Oolong tea drinking boosts calcaneus bone mineral density in postmenopausal women: A population-based study in southern China. Arch. Osteoporos. 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. Habitual Tea Consumption and Risk of Osteoporosis: A Prospective Study in the Women’s Health Initiative Observational Cohort. Am. J. Epidemiol. 2003, 158, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Kurzer, M.S. Long-Term Supplementation of Green Tea Extract Does Not Modify Adiposity or Bone Mineral Density in a Randomized Trial of Overweight and Obese Postmenopausal Women. J. Nutr. 2016, 146, 256–264. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Song, J. A Meta-Analysis on the Association between Tea Consumption and the Risk of Osteoporotic Fractures. Altern. Ther. Health Med. 2023, 29, 290–296. [Google Scholar]
- Zhou, F.; Wang, T.; Li, L.; Yu, J.; Liu, Z.; Zhang, J.; Wang, G.; Li, J.; Shao, C.; Wang, P.; et al. Tea consumption and risk of bone health: An updated systematic review and meta-analysis. J. Bone Min. Metab. 2024, 42, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kunze, G.; Qiu, R.; Cao, W.-t.; Tian, H.-y.; He, J.; Chen, G.-d.; Chen, Y.-m. Greater Intake of Fruit and Vegetables Is Associated with Greater Bone Mineral Density and Lower Osteoporosis Risk in Middle-Aged and Elderly Adults. PLoS ONE 2017, 12, e0168906. [Google Scholar]
- New, S.A.; Bolton-Smith, C.; Grubb, D.A.; Reid, D.M. Nutritional influences on bone mineral density: A cross-sectional study in premenopausal women. Am. J. Clin. Nutr. 1997, 65, 1831–1839. [Google Scholar] [CrossRef]
- Li, J.-J.; Huang, Z.-W.; Wang, R.-Q.; Ma, X.-M.; Zhang, Z.-Q.; Liu, Z.; Chen, Y.-M.; Su, Y.-X. Fruit and vegetable intake and bone mass in Chinese adolescents, young and postmenopausal women. Public Health Nutr. 2012, 16, 78–86. [Google Scholar] [CrossRef]
- Chen, Y.M.; Ho, S.C.; Woo, J.L. Greater fruit and vegetable intake is associated with increased bone mass among postmenopausal Chinese women. Br. J. Nutr. 2006, 96, 745–751. [Google Scholar] [PubMed]
- Prynne, C.J.; Mishra, G.D.; O’Connell, M.A.; Muniz, G.; Laskey, M.A.; Yan, L.; Prentice, A.; Ginty, F. Fruit and vegetable intakes and bone mineral status: A cross-sectional study in 5 age and sex cohorts. Am. J. Clin. Nutr. 2006, 83, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Tylavsky, F.A.; Holliday, K.; Danish, R.; Womack, C.; Norwood, J.; Carbone, L. Fruit and vegetable intakes are an independent predictor of bone size in early pubertal children. Am. J. Clin. Nutr. 2004, 79, 311–317. [Google Scholar] [CrossRef]
- McGartland, C.P.; Robson, P.J.; Murray, L.J.; Cran, G.W.; Savage, M.J.; Watkins, D.C.; Rooney, M.M.; Boreham, C.A. Fruit and vegetable consumption and bone mineral density: The Northern Ireland Young Hearts Project. Am. J. Clin. Nutr. 2004, 80, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, H.; Baxter-Jones, A.; Faulkner, R.A.; Bailey, D.A.; Whiting, S.J. Positive effects of vegetable and fruit consumption and calcium intake on bone mineral accrual in boys during growth from childhood to adolescence: The University of Saskatchewan Pediatric Bone Mineral Accrual Study. Am. J. Clin. Nutr. 2005, 82, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Damani, J.J.; De Souza, M.J.; VanEvery, H.L.; Strock, N.C.A.; Rogers, C.J. The Role of Prunes in Modulating Inflammatory Pathways to Improve Bone Health in Postmenopausal Women. Adv. Nutr. 2022, 13, 1476–1492. [Google Scholar] [CrossRef]
- Stacewicz-Sapuntzakis, M. Dried Plums and Their Products: Composition and Health Effects—An Updated Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1277–1302. [Google Scholar] [CrossRef]
- Treutter, D.; Wang, D.; Farag, M.A.; Baires, G.D.A.; Rühmann, S.; Neumüller, M. Diversity of Phenolic Profiles in the Fruit Skin of Prunus domestica Plums and Related Species. J. Agric. Food Chem. 2012, 60, 12011–12019. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Dried Plums, Prunes and Bone Health: A Comprehensive Review. Nutrients. 2017, 9, 401. [Google Scholar] [CrossRef]
- Bu, S.Y.; Hunt, T.S.; Smith, B.J. Dried plum polyphenols attenuate the detrimental effects of TNF-α on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-I. J. Nutr. Biochem. 2009, 20, 35–44. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kumar, A.; Zhang, J.Y.; Johnson, S.A.; Chai, S.C.; Arjmandi, B.H. Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food Funct. 2015, 6, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Lerner, M.; Stoecker, B.J.; Boldrin, E.; Brackett, D.J.; Lucas, E.A.; Smith, B.J. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif. Tissue Int. 2008, 82, 475–488. [Google Scholar] [CrossRef]
- Schreurs, A.S.; Shirazi-Fard, Y.; Shahnazari, M.; Alwood, J.S.; Truong, T.A.; Tahimic, C.G.T.; Limoli, C.L.; Turner, N.D.; Halloran, B.; Globus, R.K. Dried plum diet protects from bone loss caused by ionizing radiation. Sci. Rep. 2016, 6, 21343. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.; Lucas, E.; Juma, S.; Soliman, A.; Stoecker, B.; Khalil, D.; Smith, B.; Wang, C. Dried plums prevent ovariectomy-induced bone loss in rats. JANA 2001, 4, 50–56. [Google Scholar]
- Deyhim, F.; Stoecker, B.J.; Brusewitz, G.H.; Devareddy, L.; Arjmandi, B.H. Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause 2005, 12, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Muhlbauer, R.C.; Lozano, A.; Reinli, A.; Wetli, H. Various selected vegetables, fruits, mushrooms and red wine residue inhibit bone resorption in rats. J. Nutr. 2003, 133, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.; Bu, S.Y.; Lerner, M.R.; Lancaster, E.A.; Bellmer, D.; Marlow, D.; Lightfoot, S.A.; Arjmandi, B.H.; Brackett, D.J.; Lucas, E.A.; et al. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone 2006, 39, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.H.; Khalil, D.A.; Lucas, E.A.; Georgis, A.; Stoecker, B.J.; Hardin, C.; Payton, M.E.; Wild, R.A. Dried Plums Improve Indices of Bone Formation in Postmenopausal Women. J. Women’s Health Gend. Based Med. 2002, 11, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Chai, S.C.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br. J. Nutr. 2011, 106, 923–930. [Google Scholar] [CrossRef]
- Hooshmand, S.; Brisco, J.R.Y.; Arjmandi, B.H. The effect of dried plum on serum levels of receptor activator of NF-κB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: A randomised controlled trial. Br. J. Nutr. 2014, 112, 55–60. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: A randomized, controlled trial. Osteoporos. Int. 2016, 27, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Strock, N.C.A.; Williams, N.I.; Lee, H.; Koltun, K.J.; Rogers, C.; Ferruzzi, M.G.; Nakatsu, C.H.; Weaver, C. Prunes preserve hip bone mineral density in a 12-month randomized controlled trial in postmenopausal women: The Prune Study. Am. J. Clin. Nutr. 2022, 116, 897–910. [Google Scholar] [CrossRef] [PubMed]
- George, K.S.; Munoz, J.; Ormsbee, L.T.; Akhavan, N.S.; Foley, E.M.; Siebert, S.C.; Kim, J.-S.; Hickner, R.C.; Arjmandi, B.H. The Short-Term Effect of Prunes in Improving Bone in Men. Nutrients 2022, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lazarenko, O.P.; Zhang, J.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M. Diet-Derived Phenolic Acids Regulate Osteoblast and Adipocyte Lineage Commitment and Differentiation in Young Mice. J. Bone Miner. Res. 2014, 29, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Wankhade, U.D.; Alund, A.W.; Blackburn, M.L.; Shankar, K.; Lazarenko, O.P. 3-(3-Hydroxyphenyl)-Propionic Acid (PPA) Suppresses Osteoblastic Cell Senescence to Promote Bone Accretion in Mice. JBMR Plus 2019, 3, e10201. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-M.; Zhang, J.; Lazarenko, O.P.; Kang, J.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M.; Chen, J.-R. Feeding Blueberry Diets to Young Rats Dose-Dependently Inhibits Bone Resorption through Suppression of RANKL in Stromal Cells. PLoS ONE 2013, 8, e70438. [Google Scholar]
- Domazetovic, V.; Marcucci, G.; Pierucci, F.; Bruno, G.; Di Cesare Mannelli, L.; Ghelardini, C.; Brandi, M.L.; Iantomasi, T.; Meacci, E.; Vincenzini, M.T. Blueberry juice protects osteocytes and bone precursor cells against oxidative stress partly through SIRT1. FEBS Open Bio. 2019, 9, 1082–1096. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Falsetti, I.; Bilia, A.R.; Vincenzini, M.T.; Brandi, M.L.; Iantomasi, T. Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1. Antioxidants 2020, 9, 125. [Google Scholar] [CrossRef]
- Cladis, D.P.; Swallow, E.A.; Allen, M.R.; Hill Gallant, K.M.; Weaver, C.M. Blueberry Polyphenols do not Improve Bone Mineral Density or Mechanical Properties in Ovariectomized Rats. Calcif. Tissue Int. 2021, 110, 260–265. [Google Scholar] [CrossRef]
- Hodges, J.K.; Maiz, M.; Cao, S.; Lachcik, P.J.; Peacock, M.; McCabe, G.P.; McCabe, L.D.; Cladis, D.P.; Jackson, G.S.; Ferruzzi, M.G.; et al. Moderate consumption of freeze-dried blueberry powder increased net bone calcium retention compared with no treatment in healthy postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2023, 118, 382–390. [Google Scholar] [CrossRef]
- Savanelli, M.C.; Barrea, L.; Macchia, P.E.; Savastano, S.; Falco, A.; Renzullo, A.; Scarano, E.; Nettore, I.C.; Colao, A.; Di Somma, C. Preliminary results demonstrating the impact of Mediterranean diet on bone health. J. Transl. Med. 2017, 15, 81. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Crupi, P.; Desantis, A.; Rondanelli, M.; Corbo, F.; Clodoveo, M.L. Olive Oil Polyphenols Improve HDL Cholesterol and Promote Maintenance of Lipid Metabolism: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Metabolites 2023, 13, 1187. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Muraglia, M.; Crupi, P.; Hbaieb, R.H.; De Santis, S.; Desantis, A.; Corbo, F. The Tower of Babel of Pharma-Food Study on Extra Virgin Olive Oil Polyphenols. Foods 2022, 11, 1915. [Google Scholar] [CrossRef]
- Saleh, N.K.; Saleh, H.A. Olive oil effectively mitigates ovariectomy-induced osteoporosis in rats. BMC Complement. Altern. Med. 2011, 11, 10. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Li, B.; Wu, D.; Wang, F.; Zheng, X.H.; Chen, Q.; Wu, B.; Fan, X. Olive oil in the prevention and treatment of osteoporosis after artificial menopause. Clin. Interv. Aging 2014, 9, 2087–2095. [Google Scholar] [CrossRef]
- Keiler, A.M.; Zierau, O.; Bernhardt, R.; Scharnweber, D.; Lemonakis, N.; Termetzi, A.; Skaltsounis, L.; Vollmer, G.; Halabalaki, M. Impact of a functionalized olive oil extract on the uterus and the bone in a model of postmenopausal osteoporosis. Eur. J. Nutr. 2014, 53, 1073–1081. [Google Scholar] [CrossRef]
- Puel, C.; Mardon, J.; Agalias, A.; Davicco, M.-J.; Lebecque, P.; Mazur, A.; Horcajada, M.-N.; Skaltsounis, A.-L.; Coxam, V. Major phenolic compounds in olive oil modulate bone loss in an ovariectomy/inflammation experimental model. J. Agric. Food Chem. 2008, 56, 9417–9422. [Google Scholar] [CrossRef]
- Wichers, H.J.; Soler-rivas, C.; Espı, J.C. Review Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar]
- Hagiwara, K.; Goto, T.; Araki, M.; Miyazaki, H.; Hagiwara, H. Olive polyphenol hydroxytyrosol prevents bone loss. Eur. J. Pharmacol. 2011, 662, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; García-Martínez, O.; Mazzaglia, G.; Sánchez-Ortiz, A.; Ocaña-Peinado, F.M. Phenolic content of Sicilian virgin olive oils and their effect on MG-63 human osteoblastic cell proliferation. Grasas Aceites 2014, 65, e032. [Google Scholar] [CrossRef]
- Caruso, C.; García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar]
- Santiago-Mora, R.; Casado-Díaz, A.; De Castro, M.D.; Quesada-Gómez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2010, 22, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Puel, C.; Mathey, J.; Agalias, A.; Kati-coulibaly, S.; Mardon, J.; Obled, C.; Davicco, M.-J.; Lebecque, P.; Horcajada, M.-N.; Skaltsounis, A.L.; et al. Dose–response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clin. Nutr. 2006, 25, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Puel, C.; Quintin, A.; Agalias, A.; Mathey, J.; Obled, C.; Mazur, A.; Davicco, M.J.; Lebecque, P.; Skaltsounis, A.L.; Coxam, V. Olive oil and its main phenolic micronutrient (oleuropein) prevent inflammation-induced bone loss in the ovariectomised rat. Br. J. Nutr. 2007, 92, 119–127. [Google Scholar] [CrossRef]
- Roncero-Martín, R.; Aliaga Vera, I.; Moreno-Corral, L.; Moran, J.; Lavado-Garcia, J.; Pedrera-Zamorano, J.; Pedrera-Canal, M. Olive Oil Consumption and Bone Microarchitecture in Spanish Women. Nutrients 2018, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; Bulló, M.; Moreno-Navarrete, J.M.; Ricart, W.; Ros, E.; Estruch, R.; Salas-Salvadó, J. A Mediterranean Diet Enriched with Olive Oil Is Associated with Higher Serum Total Osteocalcin Levels in Elderly Men at High Cardiovascular Risk. J. Clin. Endocrinol. Metab. 2012, 97, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- García-Gavilán, J.F.; Bulló, M.; Canudas, S.; Martínez-González, M.A.; Estruch, R.; Giardina, S.; Fitó, M.; Corella, D.; Ros, E.; Salas-Salvadó, J. Extra virgin olive oil consumption reduces the risk of osteoporotic fractures in the PREDIMED trial. Clin. Nutr. 2018, 37, 329–335. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faienza, M.F.; Giardinelli, S.; Annicchiarico, A.; Chiarito, M.; Barile, B.; Corbo, F.; Brunetti, G. Nutraceuticals and Functional Foods: A Comprehensive Review of Their Role in Bone Health. Int. J. Mol. Sci. 2024, 25, 5873. https://doi.org/10.3390/ijms25115873
Faienza MF, Giardinelli S, Annicchiarico A, Chiarito M, Barile B, Corbo F, Brunetti G. Nutraceuticals and Functional Foods: A Comprehensive Review of Their Role in Bone Health. International Journal of Molecular Sciences. 2024; 25(11):5873. https://doi.org/10.3390/ijms25115873
Chicago/Turabian StyleFaienza, Maria Felicia, Silvia Giardinelli, Alessia Annicchiarico, Mariangela Chiarito, Barbara Barile, Filomena Corbo, and Giacomina Brunetti. 2024. "Nutraceuticals and Functional Foods: A Comprehensive Review of Their Role in Bone Health" International Journal of Molecular Sciences 25, no. 11: 5873. https://doi.org/10.3390/ijms25115873







