A Pilot Study on Circulating, Cellular, and Tissue Biomarkers in Osteosarcopenic Patients
Abstract
1. Introduction
2. Results
2.1. Demographics Data and Baseline Clinical Characteristics
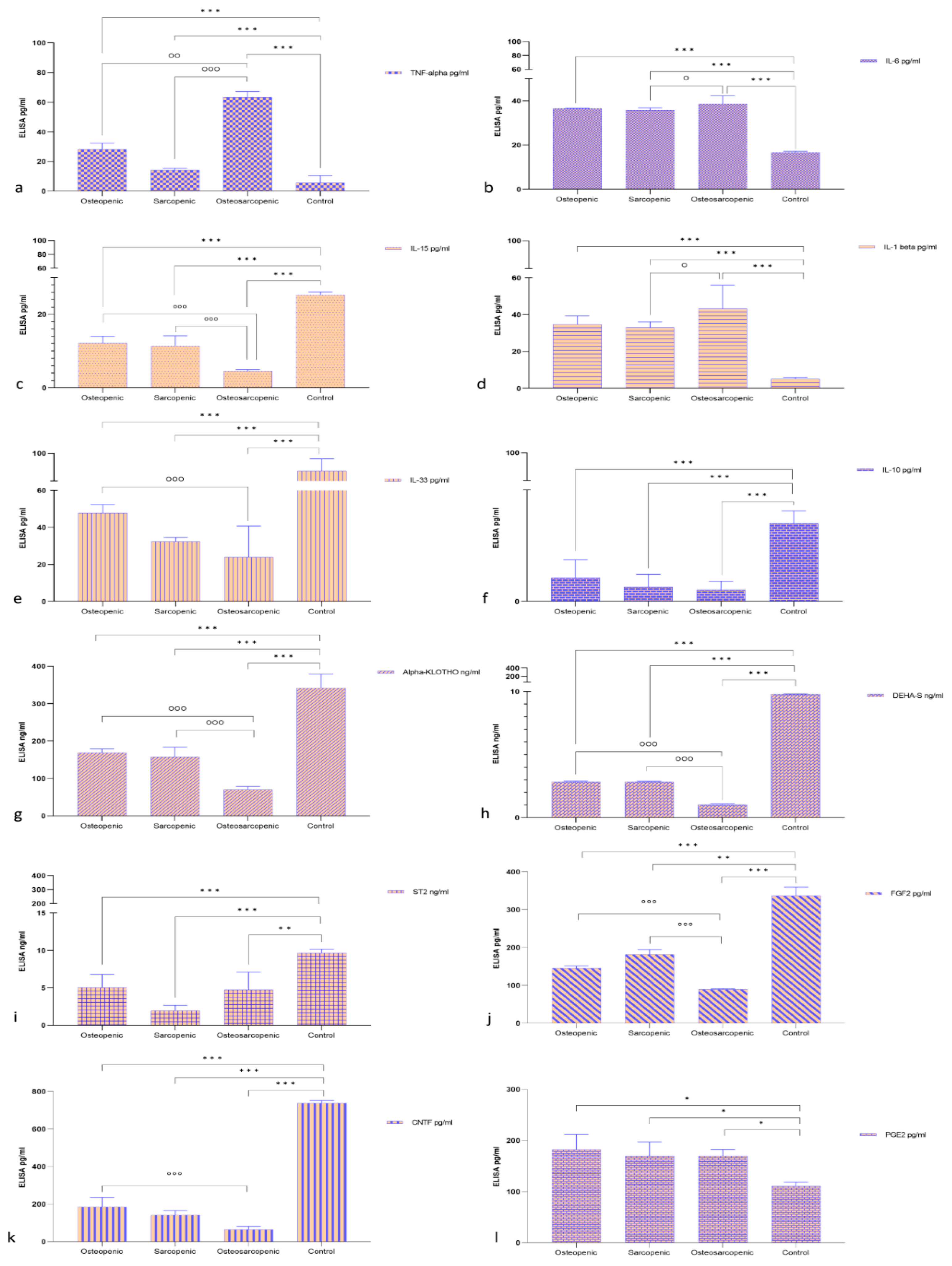
2.2. Serum Measurement
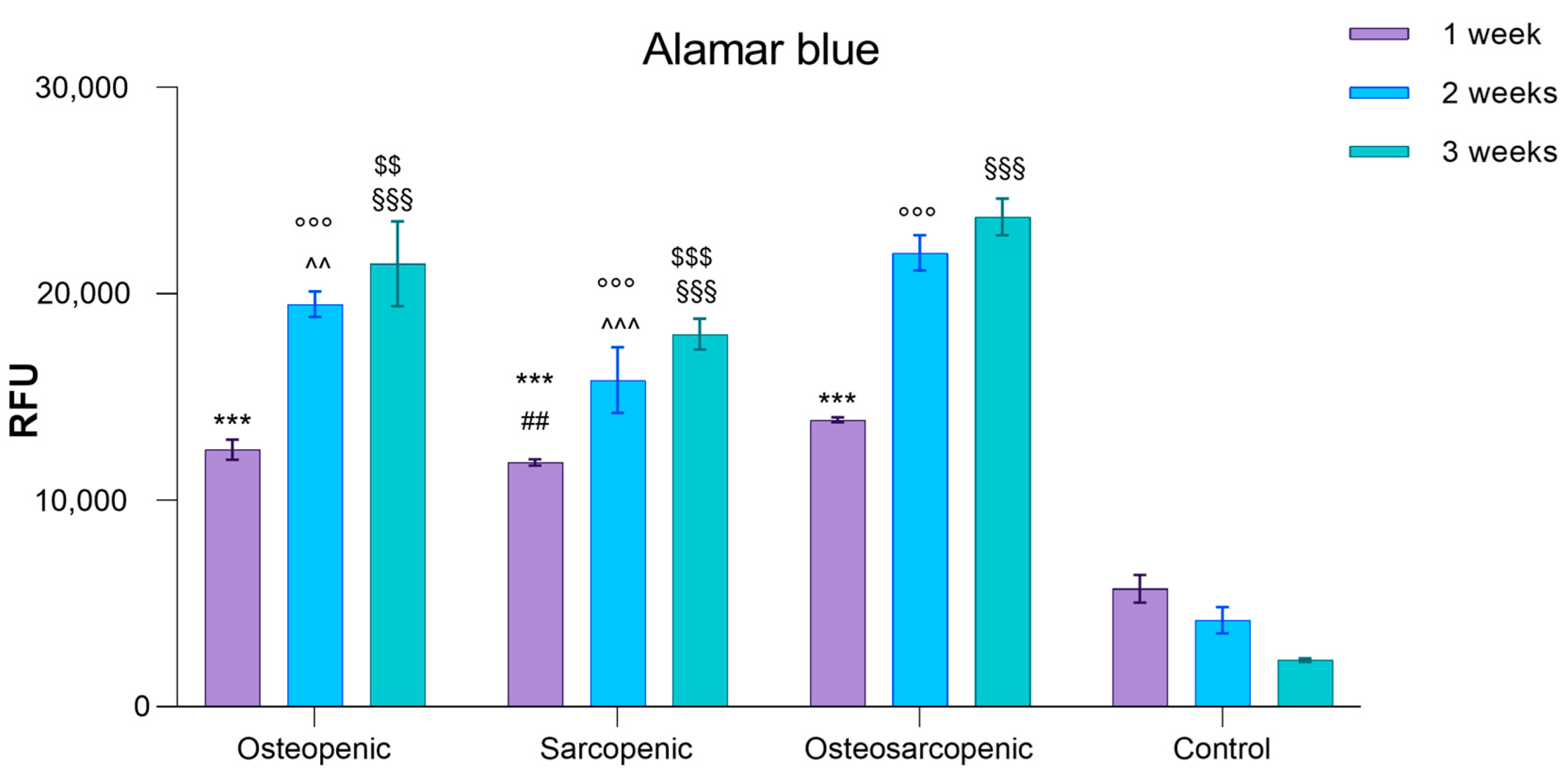
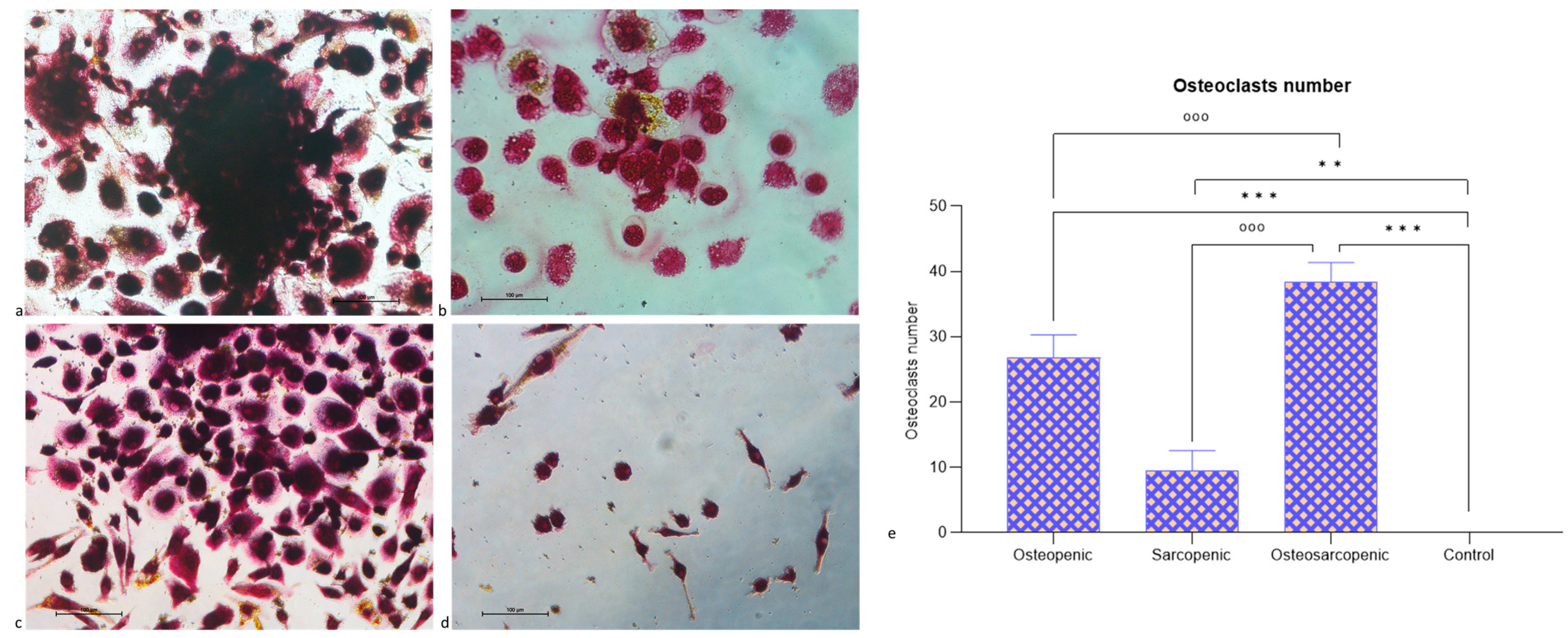
2.3. Spontaneous Osteoclastogenesis
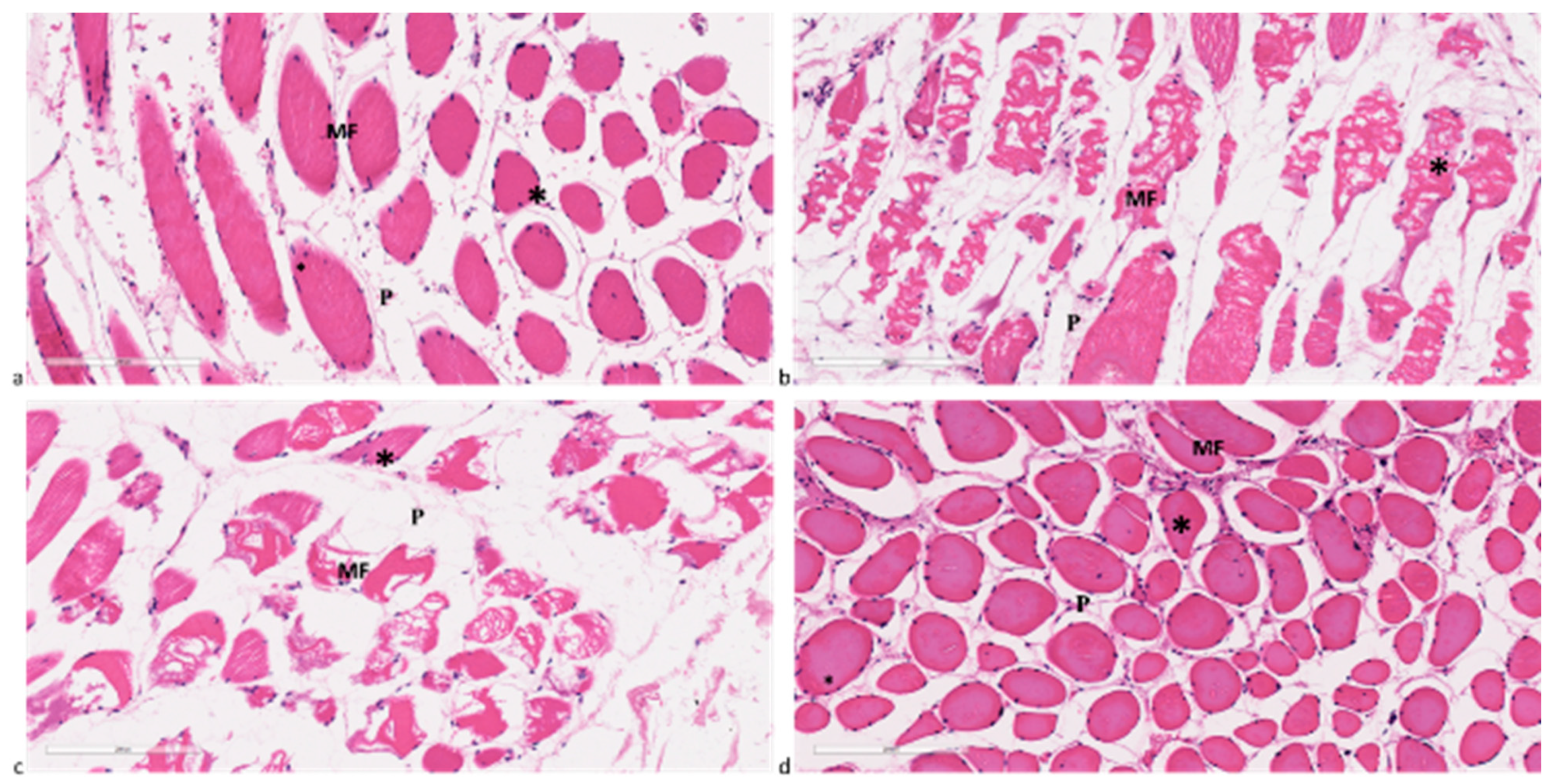
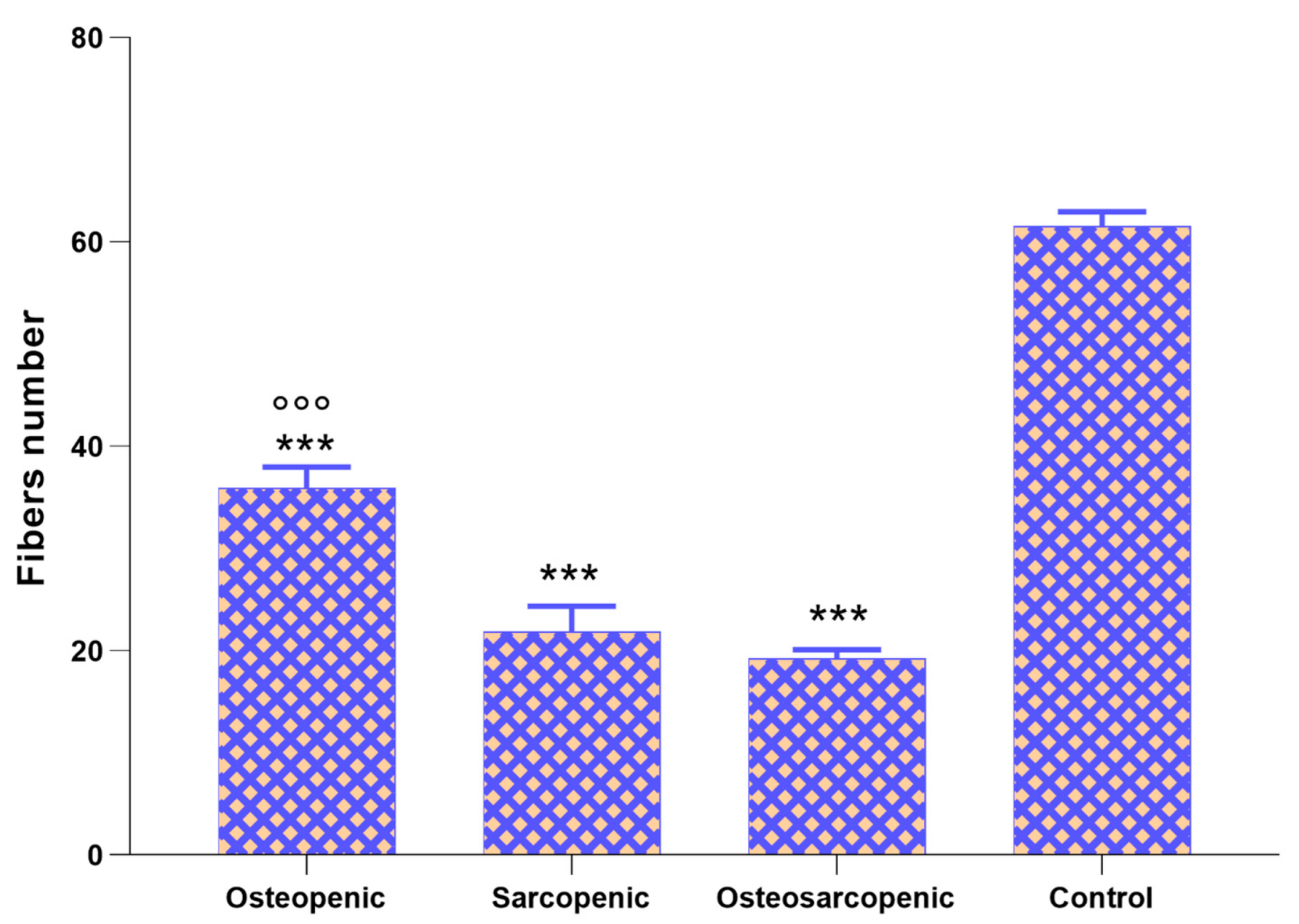
2.4. Histology and Histomorphometry
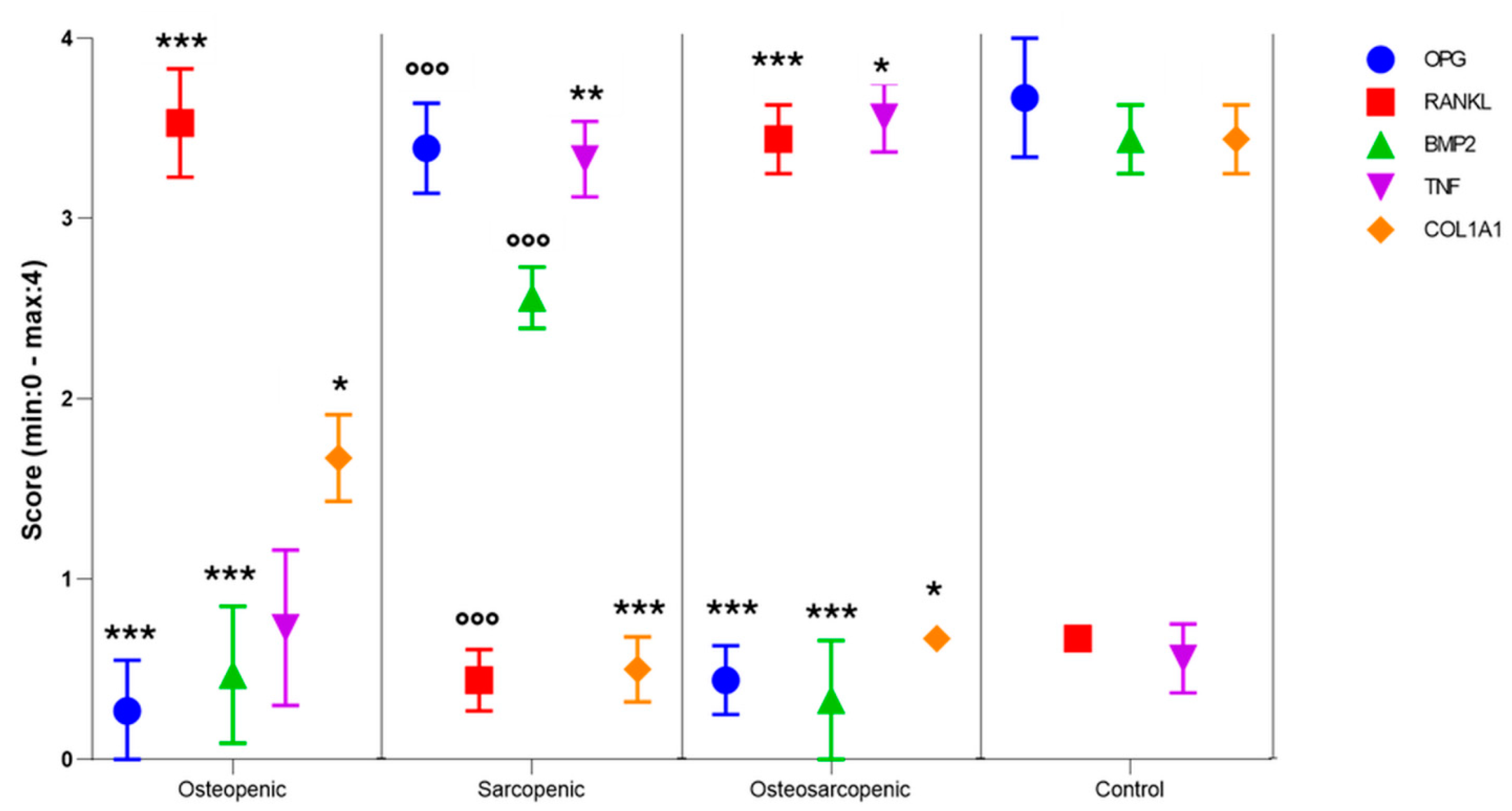
2.5. Immunohistochemistry
2.6. Linear Discriminant Analysis (LDA)
3. Discussion
4. Materials and Methods
4.1. Patients Population
4.2. Baseline Characteristics, Demographics, and OS Definition
- The M-score was calculated based on the T1W spin-echo sequence of the preoperative MRI. The M-score was derived using the mean and standard deviation of a reference population, as follows: M-Score = (Signal-to-Noise Ratio (SNR) L1-L4—SNRref)/SDref [54].
4.3. Serum Measurement
4.4. Spontaneous Osteoclastogenesis
4.5. Histology and Histomorphometry
4.6. Immunohistochemistry
4.7. Linear Discriminant Analysis (LDA)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanis, J.A.; Oden, A.; Johansson, H.; McCloskey, E. Pitfalls in the external validation of FRAX: Response to Bolland et al. Osteoporos. Int. 2013, 24, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Tseng, W.C.; Yang, Y.H.; Chen, C.L.; Lin, L.L.; Chen, F.P.; Wong, A.M.K. Calf Circumference as an Optimal Choice of Four Screening Tools for Sarcopenia Among Ethnic Chinese Older Adults in Assisted Living. Clin. Interv. Aging 2020, 15, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, Y.; Zhan, J.K.; Tang, Z.Y.; He, J.Y.; Tan, P.; Deng, H.Q.; Huang, W.; Liu, Y.S. Sarco-Osteoporosis: Prevalence and Association with Frailty in Chinese Community-Dwelling Older Adults. Int. J. Endocrinol. 2015, 2015, 482940. [Google Scholar] [CrossRef]
- Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Hotta, K.; Morishita, S.; Tsubaki, A. Related Factors and Clinical Outcomes of Osteosarcopenia: A Narrative Review. Nutrients 2021, 13, 291. [Google Scholar] [CrossRef]
- Hamad, B.; Basaran, S.; Coskun Benlidayi, I. Osteosarcopenia among postmenopausal women and handgrip strength as a practical method for predicting the risk. Aging Clin. Exp. Res. 2020, 32, 1923–1930. [Google Scholar] [CrossRef]
- Inoue, T.; Shinjo, T.; Matsuoka, M.; Tamashiro, M.; Oba, K.; Arasaki, O.; Moromizato, T.; Arima, H. The association between frailty and chronic kidney disease; cross-sectional analysis of the Nambu Cohort Study. Clin. Exp. Nephrol. 2021, 25, 1311–1318. [Google Scholar] [CrossRef]
- Saeki, C.; Takano, K.; Oikawa, T.; Aoki, Y.; Kanai, T.; Takakura, K.; Nakano, M.; Torisu, Y.; Sasaki, N.; Abo, M.; et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet. Disord. 2019, 20, 615. [Google Scholar] [CrossRef]
- Kirk, B.; Miller, S.; Zanker, J.; Duque, G. A clinical guide to the pathophysiology, diagnosis and treatment of osteosarcopenia. Maturitas 2020, 140, 27–33. [Google Scholar] [CrossRef]
- Rittweger, J.; Frost, H.M.; Schiessl, H.; Ohshima, H.; Alkner, B.; Tesch, P.; Felsenberg, D. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: Results from the LTBR study. Bone 2005, 36, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Pierre, N.; Appriou, Z.; Gratas-Delamarche, A.; Derbré, F. From physical inactivity to immobilization: Dissecting the role of oxidative stress in skeletal muscle insulin resistance and atrophy. Free Radic. Biol. Med. 2016, 98, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- From the American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI); Society of Interventional Radiology (SIR); et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, L.P.; Parreira Batista, P.; Sirineu Pereira, D.; Pereira, L.S.M.; Scianni, A.; Amorim Ribeiro-Samora, G. Comparison between parameters of muscle performance and inflammatory biomarkers of non-sarcopenic and sarcopenic elderly women. Clin. Interv. Aging 2017, 12, 1183–1191. [Google Scholar] [CrossRef]
- Thoma, A.; Lightfoot, A.P. NF-kB and inflammatory cytokine signalling: Role in skeletal muscle atrophy. Adv. Exp. Med. Biol. 2018, 1088, 267–279. [Google Scholar] [PubMed]
- Pijet, B.; Pijet, M.; Litwiniuk, A.; Gajewska, M.; Pająk, B.; Orzechowski, A. TNF-α and IFN-s-dependent muscle decay is linked to NF-κB- and STAT-1α-stimulated Atrogin1 and MuRF1 genes in C2C12 myotubes. Mediat. Inflamm. 2013, 2013, 171437. [Google Scholar] [CrossRef]
- Brown, J.P.; Roux, C.; Ho, P.R.; Bolognese, M.A.; Hall, J.; Bone, H.G.; Bonnick, S.; van den Bergh, J.P.; Ferreira, I.; Dakin, P.; et al. Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos. Int. 2014, 25, 1953–1961. [Google Scholar]
- Kawao, N.; Kaji, H. Interactions between muscle tissues and bone metabolism. J. Cell Biochem. 2015, 116, 687–695. [Google Scholar] [CrossRef]
- Lutz, C.T.; Quinn, L.S. Sarcopenia, obesity, and natural killer cell immune senescence in aging: Altered cytokine levels as a common mechanism. Aging 2012, 4, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Carter, C.S.; Wohlgemuth, S.E.; Lees, H.A.; Giovannini, S.; Anderson, B.; Quinn, L.S.; Leeuwenburgh, C. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech. Ageing Dev. 2009, 130, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, A.; Silay, K.; Balik, A.R.; Avcioğlu, G.; Aydin, A.S. The relationship between plasma interleukin-15 levels and sarcopenia in outpatient older people. Aging Clin. Exp. Res. 2018, 30, 783–790. [Google Scholar] [CrossRef]
- Loro, E.; Ramaswamy, G.; Chandra, A.; Tseng, W.J.; Mishra, M.K.; Shore, E.M.; Khurana, T.S. IL15RA is required for osteoblast function and bone mineralization. Bone 2017, 103, 20–30. [Google Scholar] [CrossRef]
- Ogata, Y.; Kukita, A.; Kukita, T.; Komine, M.; Miyahara, A.; Miyazaki, S.; Kohashi, O. A novel role of IL-15 in the development of osteoclasts: Inability to replace its activity with IL-2. J. Immunol. 1999, 162, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Xiao, L.; Doetschman, T.; Coffin, D.J.; Hurley, M.M. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J. Biol. Chem. 2011, 286, 40575–40583. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ueno, D.; Catros, S.; Homer-Bouthiette, C.; Charles, L.; Kuhn, L.; Hurley, M.H. Fibroblast growth factor-2 isoform (low molecular weight/18 kDa) overexpression in preosteoblast cells promotes bone regeneration in critical size calvarial defects in male mice. Endocrinology 2014, 155, 965–974. [Google Scholar] [CrossRef]
- Du, Y.; Xu, C.; Shi, H.; Jiang, X.; Tang, W.; Wu, X.; Chen, M.; Li, H.; Zhang, X.; Cheng, Q. Serum concentrations of oxytocin, DHEA and follistatin are associated with osteoporosis or sarcopenia in community-dwelling postmenopausal women. BMC Geriatr. 2021, 21, 542. [Google Scholar] [CrossRef] [PubMed]
- Ghebre, M.A.; Hart, D.J.; Hakim, A.J.; Kato, B.S.; Thompson, V.; Arden, N.K.; Spector, T.D.; Zhai, G. Association between DHEAS and bone loss in postmenopausal women: A 15-year longitudinal population-based study. Calcif. Tissue Int. 2011, 89, 295–302. [Google Scholar] [CrossRef]
- Labrie, F.; Bélanger, A.; Luu-The, V.; Labrie, C.; Simard, J.; Cusan, L.; Gomez, J.L.; Candas, B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: Its role during aging. Steroids 1998, 63, 322–328. [Google Scholar] [CrossRef]
- García-Alfaro, P.; García, S.; Rodriguez, I.; Pascual, M.A.; Pérez-López, F.R. Association of Endogenous Hormones and Bone Mineral Density in Postmenopausal Women. J. Midlife Health 2023, 14, 196–204. [Google Scholar] [CrossRef]
- Johnson, R.W.; White, J.D.; Walker, E.C.; Martin, T.J.; Sims, N.A. Myokines (muscle-derived cytokines and chemokines) including ciliary neurotrophic factor (CNTF) inhibit osteoblast differentiation. Bone 2014, 64, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Borsari, V.; Cherubini, A.; Fini, M. Association of Klotho with physical performance and frailty in middle-aged and older adults: A systematic review. Exp. Gerontol. 2021, 154, 111518. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, K.H.; Park, K.S.; Kong, I.D.; Cha, S.K. Biological role of antiageing protein klotho. J. Lifestyle Med. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in ageing and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [PubMed]
- Zhou, H.; Pu, S.; Zhou, H.; Guo, Y. Klotho as Potential Autophagy Regulator and Therapeutic Target. Front. Pharmacol. 2021, 12, 755366. [Google Scholar] [CrossRef]
- Kanbay, M.; Copur, S.; Ozbek, L.; Mutlu, A.; Cejka, D.; Ciceri, P.; Cozzolino, M.; Haarhaus, M.L. Klotho: A potential therapeutic target in aging and neurodegeneration beyond chronic kidney disease-a comprehensive review from the ERA CKD-MBD working group. Clin. Kidney J. 2023, 17, sfad276. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Giardino, R.; Fini, M. Spontaneous osteoclastogenesis: Hypothesis for gender-unrelated osteoporosis screening and diagnosis. Med. Hypotheses 2017, 109, 70–72. [Google Scholar] [CrossRef]
- Roato, I.; Grano, M.; Brunetti, G.; Colucci, S.; Mussa, A.; Bertetto, O.; Ferracini, R. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. 2005, 19, 228–230. [Google Scholar] [CrossRef]
- Roato, I.; Porta, F.; Mussa, A.; D’Amico, L.; Fiore, L.; Garelli, D.; Spada, M.; Ferracini, R. Bone impairment in phenylketonuria is characterized by circulating osteoclast precursors and activated T cell increase. PLoS ONE 2010, 5, e14167. [Google Scholar] [CrossRef]
- Salamanna, F.; Maglio, M.; Borsari, V.; Giavaresi, G.; Aldini, N.N.; Fini, M. Peripheral Blood Mononuclear Cells Spontaneous Osteoclastogenesis: Mechanisms Driving the Process and Clinical Relevance in Skeletal Disease. J. Cell Physiol. 2016, 231, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Grimes, S.N.; Li, S.; Hu, X.; Ivashkiv, L.B. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J. Exp. Med. 2012, 209, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Blouin, S.; Gallois, Y.; Moreau, M.F.; Basle, M.F.; Chappard, D. Disuse and orchidectomy have additional effects on bone loss in the aged male rat. Osteoporos. Int. 2007, 18, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.G.; Park, H.S.; Lee, S.H.; Park, I.S.; An, C.H. Influence of unilateral masseter muscle atrophy on craniofacial morphology in growing rabbits. J. Oral. Maxillofac. Surg. 2007, 65, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Libouban, H.; Blouin, S.; Moreau, M.F.; Baslé, M.F.; Audran, M.; Chappard, D. Effects of risedronate in a rat model of osteopenia due to orchidectomy and disuse: Densitometric, histomorphometric and microtomographic studies. Micron 2008, 39, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Manske, S.L.; Boyd, S.K.; Zernicke, R.F. Muscle and bone follow similar temporal patterns of recovery from muscle-induced disuse due to botulinum toxin injection. Bone 2010, 46, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Ren, H.; Jiang, J.; Shao, C.; Shi, Y.; Li, P. Dying to Defend: Neutrophil Death Pathways and their Implications in Immunity. Adv. Sci. 2023, 11, e2306457. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Kirschenbaum, A.; Yao, S.; Levine, A.C. Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann. N. Y. Acad. Sci. 2006, 1068, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P. Sarcopenia, a neurogenic syndrome? J. Aging Res. 2013, 2013, 791679. [Google Scholar] [CrossRef]
- McGregor, N.E.; Poulton, I.J.; Walker, E.C.; Pompolo, S.; Quinn, J.M.W.; Martin, T.J.; Sims, N.A. Ciliary neurotrophic factor inhibits bone formation and plays a sex-specific role in bone growth and remodeling. Calcif. Tissue Int. 2010, 86, 261–270. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. IL-33/IL-31 Axis in Osteoporosis. Int. J. Mol. Sci. 2020, 21, 1239. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior. Research. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Ebbeling, L.; Grabo, D.J.; Shashaty, M.; Dua, R.; Sonnad, S.S.; Sims, C.A.; Pascual, J.L.; Schwab, C.W.; Holena, D.N. Psoas:lumbar vertebra index: Central sarcopenia independently predicts morbidity in elderly trauma patients. Eur. J. Trauma. Emerg. Surg. 2014, 40, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.M.; Ahmed, A.T.; Mohamed, K.E.; Habba, M.R. Role of lumbar spine signal intensity measurement by MRI in the diagnosis of osteoporosis in post-menopausal women. Egypt. J. Radiol. Nucl. Med. 2019, 50, 35. [Google Scholar] [CrossRef]
- Ruffilli, A.; Manzetti, M.; Barile, F.; Ialuna, M.; Cerasoli, T.; Viroli, G.; Salamanna, F.; Contartese, D.; Giavaresi, G.; Faldini, C. Complications after Posterior Lumbar Fusion for Degenerative Disc Disease: Sarcopenia and Osteopenia as Independent Risk Factors for Infection and Proximal Junctional Disease. J. Clin. Med. 2023, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Ruffilli, A.; Manzetti, M.; Cerasoli, T.; Barile, F.; Viroli, G.; Traversari, M.; Salamanna, F.; Fini, M.; Faldini, C. Osteopenia and Sarcopenia as Potential Risk Factors for Surgical Site Infection after Posterior Lumbar Fusion: A Retrospective Study. Microorganisms 2022, 10, 1905. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone istomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2013, 28, 2–17. [Google Scholar] [CrossRef]
- de la Torre, N.G.; Buley, I.; Wass, J.A.H.; Turner, H.E. Angiogenesis and lymphangiogenesis in thyroid proliferative lesions: Relationship to type and tumour behaviour. Endocr. Relat. Cancer 2006, 13, 931–944. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 3 April 2024).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]


| Characteristics | Total (n = 18) | OP (n = 5) | SP (n = 6) | OS (n = 4) | Control (n = 3) |
|---|---|---|---|---|---|
| Sex (female %) | 67% | 11% | 28% | 22% | 6% |
| Age at surgery (years) | 63.9 [59.8, 69.1] | 64.4 [56.3, 72.5] | 68.0 [60.4, 75.6] | 65.8 [61.2, 70.3] | 52.7 [42.9, 62.4] |
| Age Class | n (%) | n | |||
| 40–60 | 6 (33.3) | 2 | 1 | 1 | 2 |
| >60 | 12 (66.7) | 3 | 5 | 3 | 1 |
| BMI | 25.19 [23.8, 26.6] | 28.65 [26.73, 30.57] | 23.88 [21.57, 26.20] | 23.38 [21.30, 25.45] | 24.43 [20.21, 28.66] |
| BMI Class | n (%) | n | |||
| Healthy weight 18.5–24.9 | 10 (55.6) | 0 | 5 | 3 | 2 |
| Overweight 25.0–29.9 | 6 (33.3) | 3 | 1 | 1 | 1 |
| Class 1 Obesity 30.0–34.9 | 2 (11.1) | 2 | 0 | 0 | 0 |
| PLVI | 0.83 [0.69, 0.98] | 1.23 [0.86, 1.60] | 0.69 [0.64, 0.74] | 0.50 [0.49 0.51] | 0.89 [0.71, 1.09] |
| M-score | 2.62 [2.14, 3.09] | 1.54 [1.07, 2.01] | 3.71 [3.22, 4.20] | 1.65 [1.08, 2.21] | 3.51 [2.85, 4.17] |
| Inpatient diagnosis | n (%) | n | |||
| Lumbar disc herniation | 4 (22.2) | 2 | 1 | 0 | 1 |
| Lumbar spondylolisthesis | 2 (11.1) | 0 | 0 | 1 | 1 |
| Lumbar/lumbosacral stenosis | 10 (55.7) | 3 | 4 | 2 | 1 |
| Wound dehiscence in lumbosacral arthrodesis | 1 (5.5) | 0 | 0 | 1 | 0 |
| Thoraco-lumbosacral idiopathic kyphosis | 1 (5.5) | 0 | 1 | 0 | 0 |
| Length of stay (day) | 12.75 [9.35, 16.15] | 16.50 [9.09, 23.91] | 9.50 [7.59, 11.41] | 19.33 [1.81, 36.85] | 7.66 [1.95, 13.37] |
| Blood Parameters | Reference Values | Total (n = 18) | OP (n = 5) | SP (n = 6) | OS (n = 4) | Control (n = 3) | F, p |
|---|---|---|---|---|---|---|---|
| Neutrophils | 42–77% | 53.8 [46.5, 61.0] | 56.9 * [48.1, 65.8] | 43.8 ** [39.1, 48.5] | 42.5 ** [28.3, 56.6] | 83.6 [69.8, 97.3] | 8.36, 0.002 |
| Basophils | 0–1.8% | 0.44 [0.32, 0.56] | 0.44 ° [0.27, 0.59] | 0.30 °° [0.11, 0.49] | 0.85 * [0.68, 1.02] | 0.20 [0.21, 0.22] | 6.30, 0.006 |
| INR | <1.2 | 1.03 [1.01, 1.06] | 1.04 ° [1.01, 1.07] | 1.05 ° [1.02, 1.08] | 0.95 ** [0.91, 0.99] | 1.10 [1.04, 1.16] | 6.34, 0.006 |
| aPPT | 0.82–1.25 | 0.95 [0.90, 1.01] | 0.99 ° [0.90, 1.08] | 1.00 ° [0.93, 1.07] | 0.80 * [0.68, 0.91] | 1.02 [0.95, 1.08] | 3.61, 0.040 |
| Total (n = 18) | OP (n = 5) | SP (n = 6) | OS (n = 4) | Control (n = 3) | |
|---|---|---|---|---|---|
| ECI | |||||
| Score | −1.3 [−3.6, 0.9] | −3.6 [−6.7, −0.5] | −1.5 [−6.5, 3.6] | 3.0 [−2.1, 8.1] | −3.0 [−4.6, −1.4] |
| n | |||||
| <0 | 12 | 3 | 5 | 1 | 3 |
| 0 | 3 | 2 | 0 | 1 | 0 |
| 1 to 4 | 0 | 0 | 0 | 0 | 0 |
| ≥5 | 3 | 0 | 1 | 2 | 0 |
| Polypharmacy | |||||
| Drugs taken | 4.6 [3.2, 6.0] | 5.0 [2.3, 7.7] | 4.1 [1.4, 6.9] | 5.3 [2.2, 8.3] | 4.0 [0.2, 7.8] |
| n | |||||
| ‘no polypharmacy’ 0–4 drugs | 10 | 2 | 5 | 1 | 2 |
| ‘polypharmacy’ > 5 drugs | 6 | 3 | 0 | 2 | 1 |
| ‘excessive polypharmacy’ >10 drugs | 2 | 0 | 1 | 1 | 0 |
| Parameters | OP (n = 5) | SP (n = 6) | OS (n = 4) | Control (n = 3) | F, p |
|---|---|---|---|---|---|
| BV/TV (%) | 25.2 ± 3.7 *** | 55.2 ± 6.7 ***,°°° | 33.5 ± 8.6 *** | 81.0 ± 7.6 | 52.75, <0.0005 |
| Tb.Th, µm | 86 ± 9 *** | 147 ± 33 *,°° | 83 ± 11 *** | 202 ± 27 | 21.59, <0.0005 |
| Tb.N, 1/mm | 0.29 ± 0.06 ° | 0.39 ± 0.08 | 0.42 ± 0.04 | 0.40 ± 0.02 | 4.51, 0.021 |
| Tb.Sp, µm | 262 ± 55 ***,°° | 119 ± 30 | 166 ± 38 ** | 46 ± 17 | 22.21, <0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanna, F.; Faldini, C.; Veronesi, F.; Borsari, V.; Ruffilli, A.; Manzetti, M.; Viroli, G.; Traversari, M.; Marchese, L.; Fini, M.; et al. A Pilot Study on Circulating, Cellular, and Tissue Biomarkers in Osteosarcopenic Patients. Int. J. Mol. Sci. 2024, 25, 5879. https://doi.org/10.3390/ijms25115879
Salamanna F, Faldini C, Veronesi F, Borsari V, Ruffilli A, Manzetti M, Viroli G, Traversari M, Marchese L, Fini M, et al. A Pilot Study on Circulating, Cellular, and Tissue Biomarkers in Osteosarcopenic Patients. International Journal of Molecular Sciences. 2024; 25(11):5879. https://doi.org/10.3390/ijms25115879
Chicago/Turabian StyleSalamanna, Francesca, Cesare Faldini, Francesca Veronesi, Veronica Borsari, Alberto Ruffilli, Marco Manzetti, Giovanni Viroli, Matteo Traversari, Laura Marchese, Milena Fini, and et al. 2024. "A Pilot Study on Circulating, Cellular, and Tissue Biomarkers in Osteosarcopenic Patients" International Journal of Molecular Sciences 25, no. 11: 5879. https://doi.org/10.3390/ijms25115879
APA StyleSalamanna, F., Faldini, C., Veronesi, F., Borsari, V., Ruffilli, A., Manzetti, M., Viroli, G., Traversari, M., Marchese, L., Fini, M., & Giavaresi, G. (2024). A Pilot Study on Circulating, Cellular, and Tissue Biomarkers in Osteosarcopenic Patients. International Journal of Molecular Sciences, 25(11), 5879. https://doi.org/10.3390/ijms25115879









