A Comprehensive Investigation of Stimulatory Agents on MAIT and Vα7.2+/CD161− T Cell Response and Effects of Immunomodulatory Drugs
Abstract
:1. Introduction
2. Results
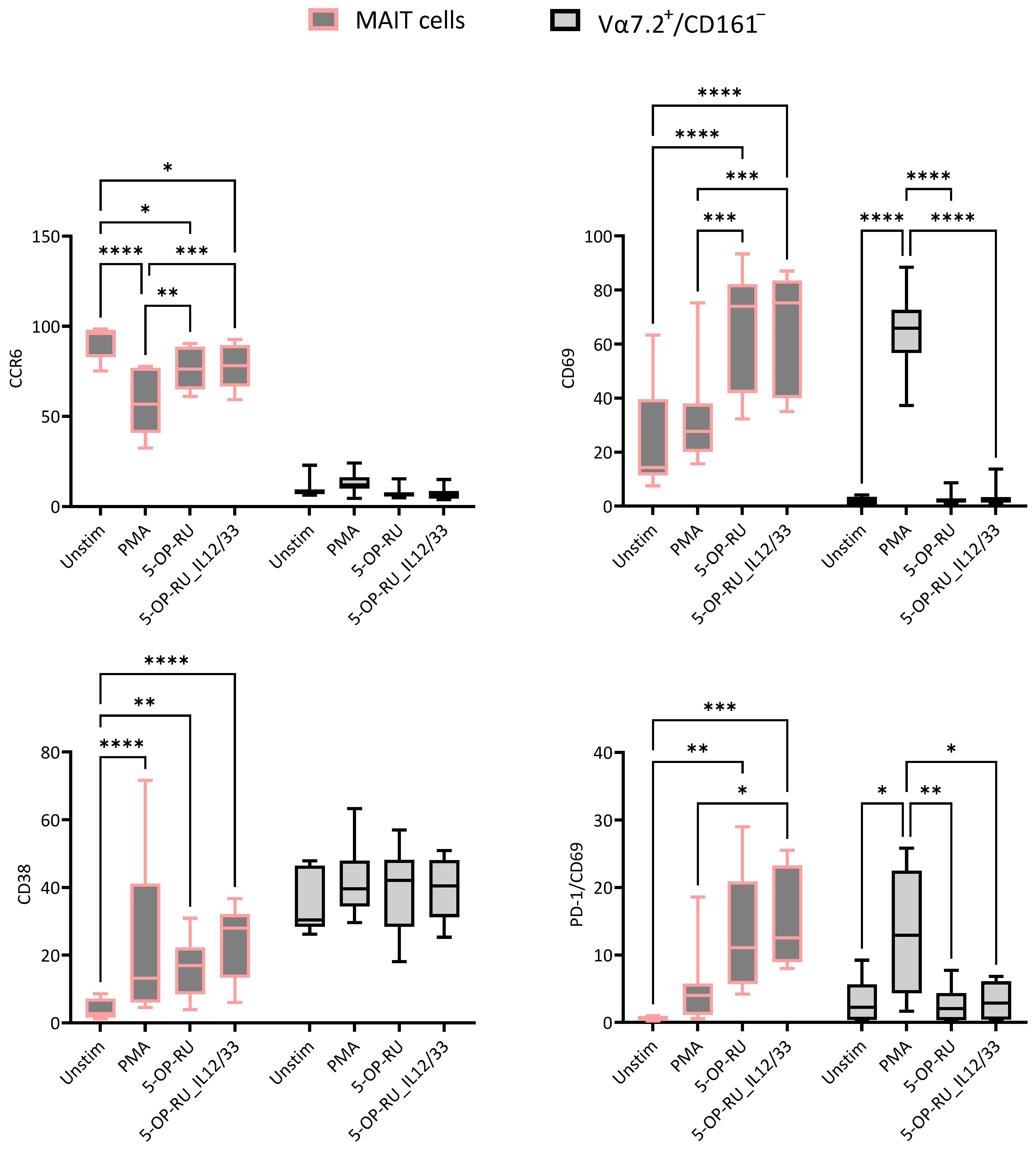
2.1. Effects of Different Stimulants on MAIT and Vα7.2+/CD161− T Cells
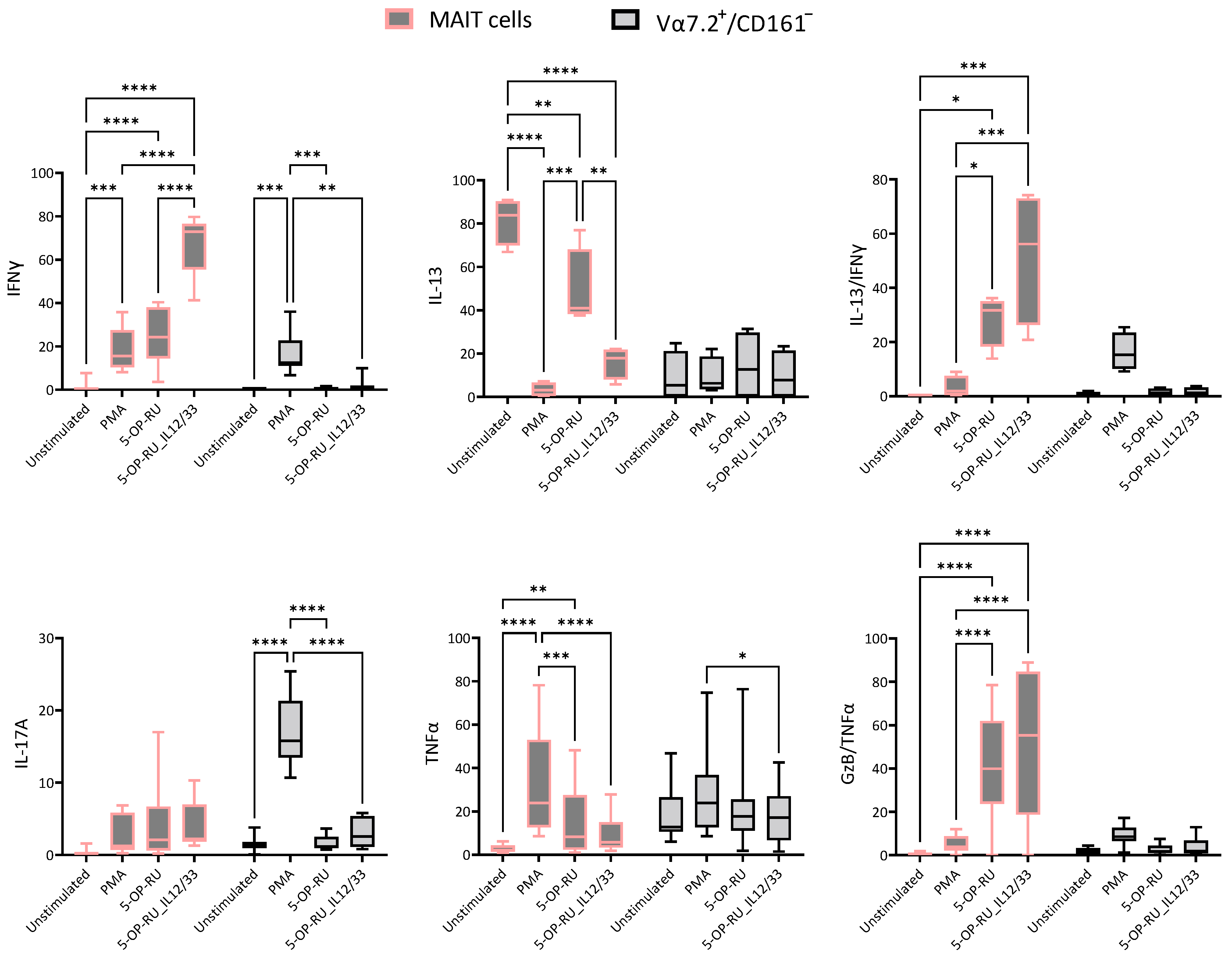
2.2. Cytokine Production Fluctuates with Varied Stimuli
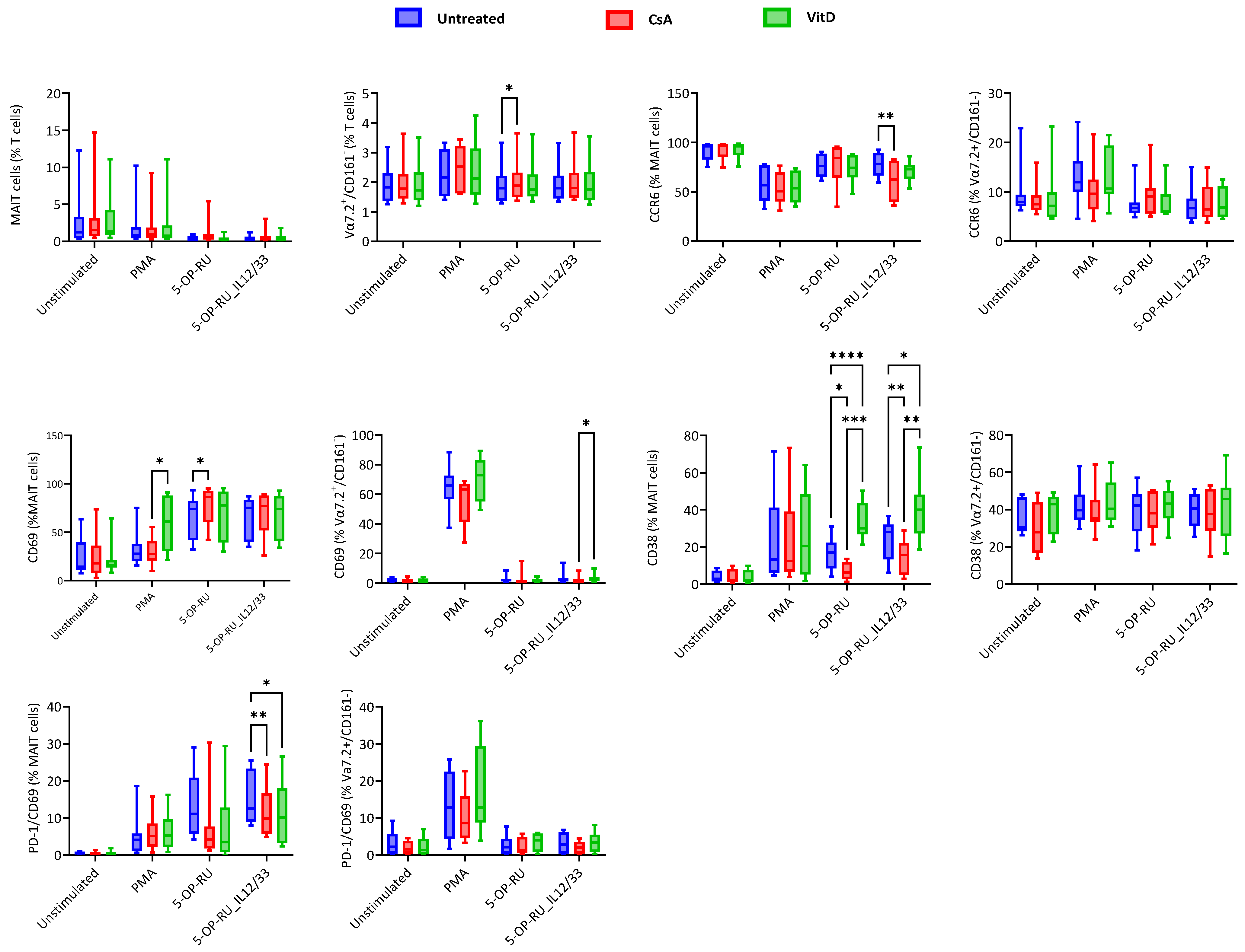
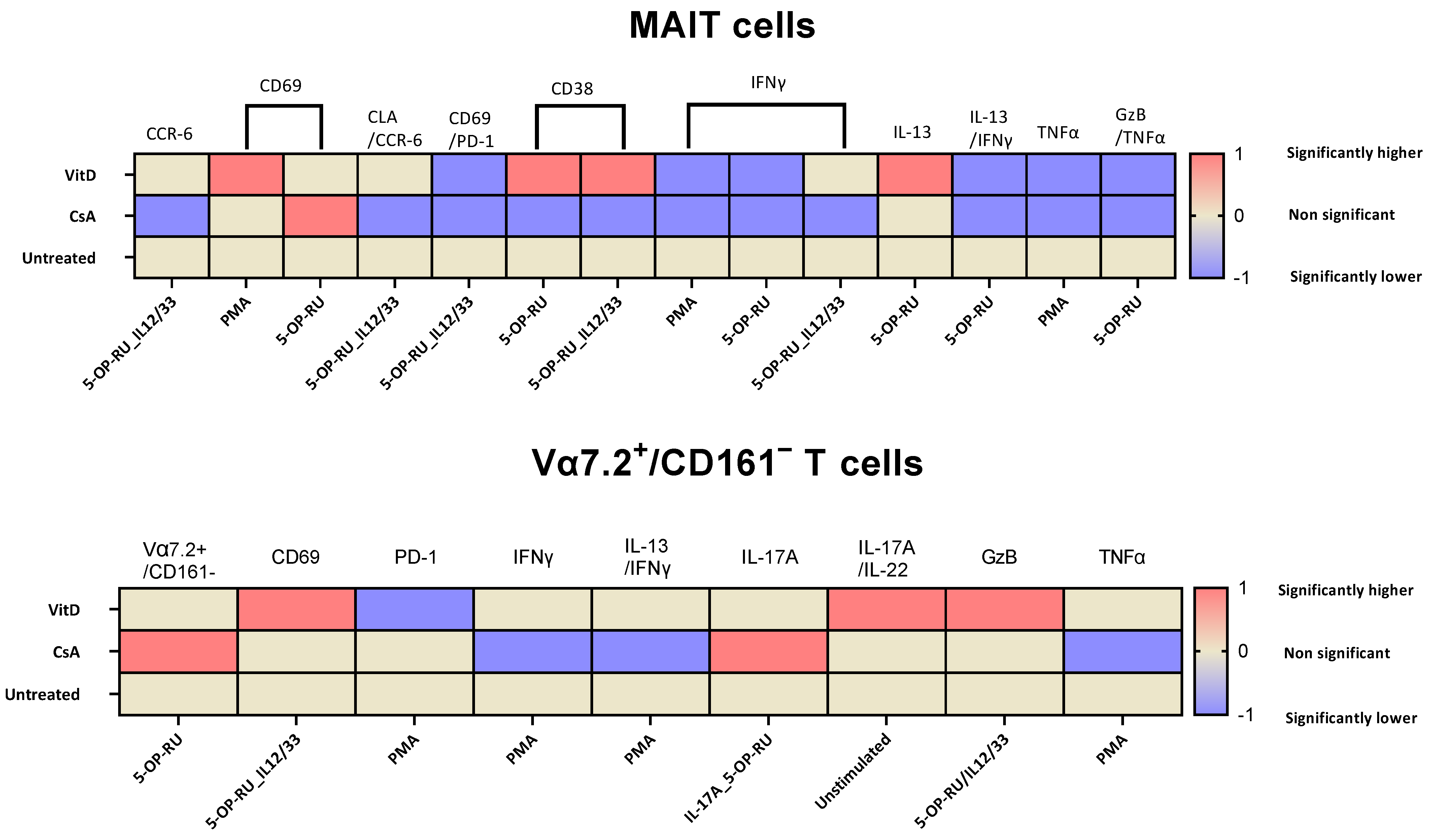
2.3. Cyclosporin A and Vitamin D3 Modulatory Changes in the Cellular Surface of MAIT and Vα7.2+/CD161− T Cells under Different Stimulation
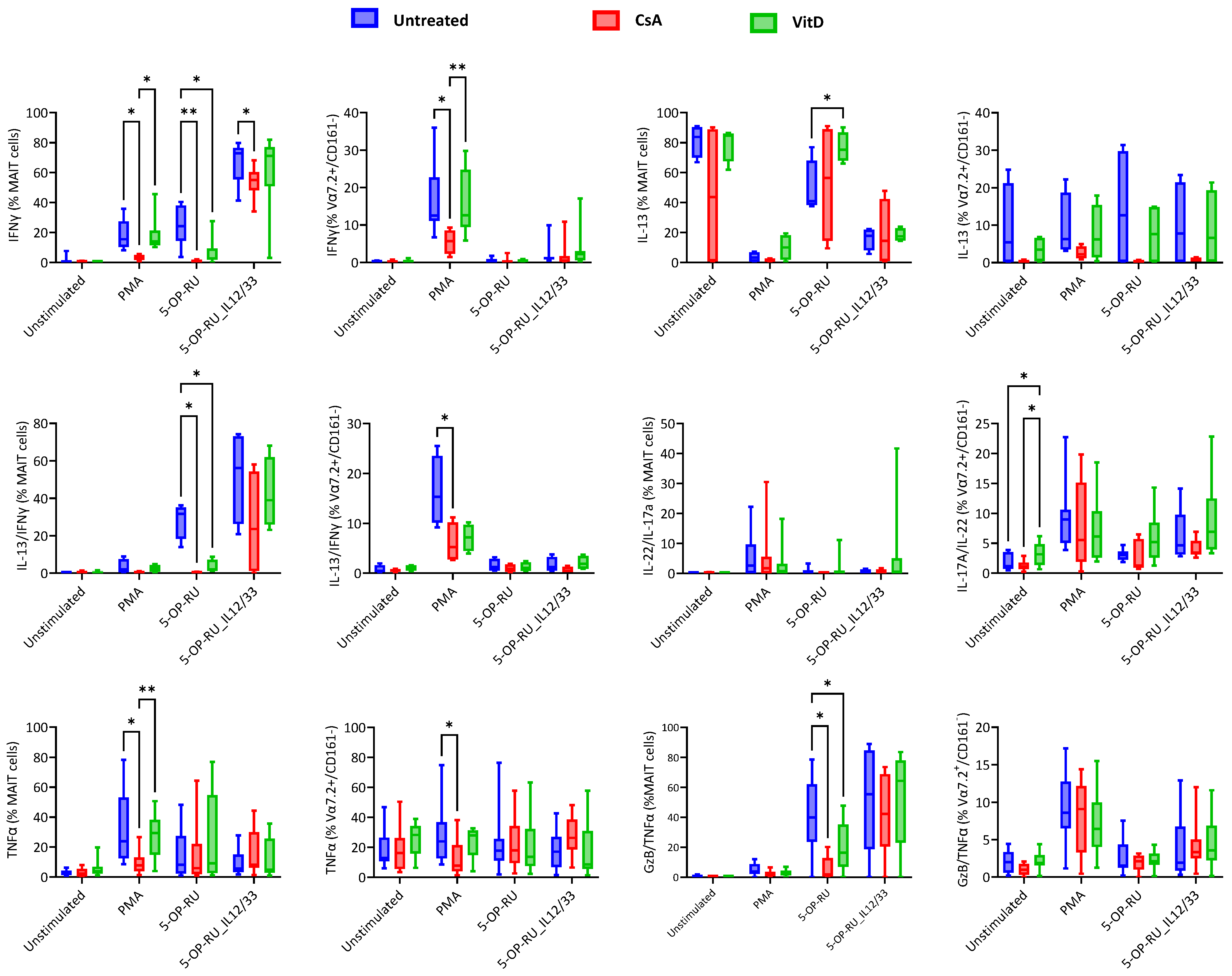
2.4. Cyclosporin A and Vitamin D3 Modulatory Changes in Cytokine Production of MAIT and Vα7.2+/CD161− T Cells under Varied Stimulation
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Peripheral Blood Mononuclear Cells (PBMCs) Isolation
4.2. PBMC Isolation
4.3. Reagent Preparation
4.4. Experiment Design
4.5. Flow Cytometry
4.6. Data Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Sagaseta, J.; Dulberger, C.L.; Crooks, J.E.; Parks, C.D.; Luoma, A.M.; McFedries, A.; Van Rhijn, I.; Saghatelian, A.; Adams, E.J. The molecular basis for Mucosal-Associated Invariant T cell recognition of MR1 proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E1771–E1778. [Google Scholar] [CrossRef] [PubMed]
- Dusseaux, M.; Martin, E.; Serriari, N.; Péguillet, I.; Premel, V.; Louis, D.; Milder, M.; Le Bourhis, L.; Soudais, C.; Treiner, E.; et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161 hi IL-17-secreting T cells. Blood 2011, 117, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Szaraz-Szeles, M.; Mezei, Z.; Barath, S.; Hevessy, Z. Age-dependent frequency of unconventional T cells in a healthy adult Caucasian population: A combinational study of invariant natural killer T cells, γδ T cells, and mucosa-associated invariant T cells. GeroScience 2022, 44, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Szaraz-Szeles, M.; Mezei, Z.; Barath, S.; Hevessy, Z. Gender-dependent frequency of unconventional T cells in a healthy adult Caucasian population: A combinational study of invariant NKT cells, γδ T cells, and mucosa-associated invariant T cells. J. Leukoc. Biol. 2022, 112, 1155–1165. [Google Scholar] [CrossRef]
- Ruf, B.; Catania, V.V.; Wabitsch, S.; Ma, C.; Diggs, L.P.; Zhang, Q.; Heinrich, B.; Subramanyam, V.; Cui, L.L.; Pouzolles, M.; et al. Activating Mucosal-Associated Invariant T Cells Induces a Broad Antitumor Response. Cancer Immunol. Res. 2021, 9, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Hagel, J.P.; Garner, L.C.; Bilton, M.; Mehta, H.; Leng, T.; Hackstein, C.-P.; Phalora, P.; Amini, A.; Akther, H.D.; Provine, N.M.; et al. Human MAIT Cell Activation In Vitro. Methods Mol. Biol. 2020, 2098, 97–124. [Google Scholar] [CrossRef] [PubMed]
- Hinks, T.S.C.; Zhang, X.-W. MAIT Cell Activation and Functions. Front. Immunol. 2020, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Reantragoon, R.; Corbett, A.J.; Sakala, I.G.; Gherardin, N.A.; Furness, J.B.; Chen, Z.; Eckle, S.B.G.; Uldrich, A.P.; Birkinshaw, R.W.; Patel, O.; et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 2013, 210, 2305–2320. [Google Scholar] [CrossRef] [PubMed]
- Barathan, M.; Mohamed, R.; Vadivelu, J.; Chang, L.Y.; Saeidi, A.; Yong, Y.K.; Ravishankar Ram, M.; Gopal, K.; Velu, V.; Larsson, M.; et al. Peripheral loss of CD8(+) CD161(++) TCRVα7·2(+) mucosal-associated invariant T cells in chronic hepatitis C virus-infected patients. Eur. J. Clin. Investig. 2016, 46, 170–180. [Google Scholar] [CrossRef]
- Leeansyah, E.; Ganesh, A.; Quigley, M.F.; Sönnerborg, A.; Andersson, J.; Hunt, P.W.; Somsouk, M.; Deeks, S.G.; Martin, J.N.; Moll, M.; et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 2013, 121, 1124–1135. [Google Scholar] [CrossRef]
- Singh, P.; Gaspar, K.; Szegedi, A.; Sajtos, L.; Barath, S.; Hevessy, Z. Investigating Vα7.2+/CD161− T Cell and MAIT Cell Profiles Using Flow Cytometry in Healthy Subjects and Subjects with Atopic Dermatitis. Int. J. Mol. Sci. 2024, 25, 3486. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, R.; Schneider, M.; de la Harpe, S.M.; Harrop, T.W.R.; Hannaway, R.F.; Dearden, P.K.; Kirman, J.R.; Tyndall, J.D.A.; Vernall, A.J.; Ussher, J.E. TCR- or Cytokine-Activated CD8+ Mucosal-Associated Invariant T Cells Are Rapid Polyfunctional Effectors That Can Coordinate Immune Responses. Cell Rep. 2019, 28, 3061–3076.e5. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Marchi, E.; Jabeen, M.; Olshansky, M.; Kurioka, A.; Pediongco, T.J.; Meehan, B.S.; Kostenko, L.; Turner, S.J.; Corbett, A.J.; et al. Activation and In Vivo Evolution of the MAIT Cell Transcriptome in Mice and Humans Reveals Tissue Repair Functionality. Cell Rep. 2019, 28, 3249–3262.e5. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Murray, J.; Simpson, C.; Okoye, R.; Tyson, K.; Griffiths, M.; Baeten, D.; Shaw, S.; Maroof, A. Interleukin (IL)-12 and IL-18 Synergize to Promote MAIT Cell IL-17A and IL-17F Production Independently of IL-23 Signaling. Front. Immunol. 2020, 11, 585134. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Trivedi, S.; Li, K.; Aubé, J.; Hale, J.S.; Ryan, E.T.; Leung, D.T. Use of a MAIT-Activating Ligand, 5-OP-RU, as a Mucosal Adjuvant in a Murine Model of Vibrio cholerae O1 Vaccination. Pathog. Immun. 2022, 7, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Azzout, M.; Dietrich, C.; Machavoine, F.; Gastineau, P.; Bottier, A.; Lezmi, G.; Leite-de-Moraes, M. IL-33 Enhances IFNγ and TNFα Production by Human MAIT Cells: A New Pro-Th1 Effect of IL-33. Int. J. Mol. Sci. 2021, 22, 10602. [Google Scholar] [CrossRef] [PubMed]
- Gherardin, N.A.; Loh, L.; Admojo, L.; Davenport, A.J.; Richardson, K.; Rogers, A.; Darcy, P.K.; Jenkins, M.R.; Prince, H.M.; Harrison, S.J.; et al. Enumeration, functional responses and cytotoxic capacity of MAIT cells in newly diagnosed and relapsed multiple myeloma. Sci. Rep. 2018, 8, 4159. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, E.-M.; Collings, S.; Authier-Hall, A.; Dasyam, N.; Luey, B.; Nacey, J.; Painter, G.F.; Delahunt, B.; Hermans, I.F.; Weinkove, R. Mucosal-Associated Invariant T (MAIT) Cell Dysfunction and PD-1 Expression in Prostate Cancer: Implications for Immunotherapy. Front. Immunol. 2021, 12, 748741. [Google Scholar] [CrossRef]
- Favreau, M.; Venken, K.; Faict, S.; Maes, K.; De Veirman, K.; De Bruyne, E.; Leleu, X.; Boon, L.; Elewaut, D.; Vanderkerken, K.; et al. Both mucosal-associated invariant and natural killer T-cell deficiency in multiple myeloma can be countered by PD-1 inhibition. Haematologica 2017, 102, e266–e270. [Google Scholar] [CrossRef]
- De Biasi, S.; Gibellini, L.; Lo Tartaro, D.; Puccio, S.; Rabacchi, C.; Mazza, E.M.C.; Brummelman, J.; Williams, B.; Kaihara, K.; Forcato, M.; et al. Circulating mucosal-associated invariant T cells identify patients responding to anti-PD-1 therapy. Nat. Commun. 2021, 12, 1669. [Google Scholar] [CrossRef]
- Kobayashi, T.; Momoi, Y.; Iwasaki, T. Cyclosporine A inhibits the mRNA expressions of IL-2, IL-4 and IFN-gamma, but not TNF-alpha, in canine mononuclear cells. J. Vet. Med. Sci. 2007, 69, 887–892. [Google Scholar] [CrossRef]
- Tsuda, K.; Yamanaka, K.; Kitagawa, H.; Akeda, T.; Naka, M.; Niwa, K.; Nakanishi, T.; Kakeda, M.; Gabazza, E.C.; Mizutani, H. Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naïve T cells into cytokine-producing mature T cells. PLoS ONE 2012, 7, e31465. [Google Scholar] [CrossRef] [PubMed]
- Wißfeld, J.; Hering, M.; Ten Bosch, N.; Cui, G. The immunosuppressive drug cyclosporin A has an immunostimulatory function in CD8(+) T cells. Eur. J. Immunol. 2024, Apr, e2350825. [Google Scholar] [CrossRef] [PubMed]
- Konijeti, G.G.; Arora, P.; Boylan, M.R.; Song, Y.; Huang, S.; Harrell, F.; Newton-Cheh, C.; O’Neill, D.; Korzenik, J.; Wang, T.J.; et al. Vitamin D Supplementation Modulates T Cell-Mediated Immunity in Humans: Results from a Randomized Control Trial. J. Clin. Endocrinol. Metab. 2016, 101, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Singh, S.; Singh, B.; Goswami, B.; Gupta, S.K.; Jain, A. Role of Active Vitamin D3 in Immunity. Indian J. Med. Biochem. 2017, 21, 166–175. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Zhou, X.; Staples, K.J.; Dimitrov, B.D.; Manta, A.; Petrossian, T.; Lum, P.Y.; Smith, C.G.; Ward, J.A.; Howarth, P.H.; et al. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J. Allergy Clin. Immunol. 2015, 136, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Nel, I.; Bertrand, L.; Toubal, A.; Lehuen, A. MAIT cells, guardians of skin and mucosa? Mucosal Immunol. 2021, 14, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Konecny, A.J.; Huang, Y.; Setty, M.; Prlic, M. Signals that control MAIT cell function in healthy and inflamed human tissues. Immunol. Rev. 2024, 323, 13325. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, J.R.; Ussher, J.E.; Kurioka, A.; Klenerman, P.; Walker, L.J. High MDR-1 expression by MAIT cells confers resistance to cytotoxic but not immunosuppressive MDR-1 substrates. Clin. Exp. Immunol. 2018, 194, 180–191. [Google Scholar] [CrossRef]
- Kurioka, A.; Jahun, A.S.; Hannaway, R.F.; Walker, L.J.; Fergusson, J.R.; Sverremark-Ekström, E.; Corbett, A.J.; Ussher, J.E.; Willberg, C.B.; Klenerman, P. Shared and Distinct Phenotypes and Functions of Human CD161++ Vα7.2+ T Cell Subsets. Front. Immunol. 2017, 8, 1031. [Google Scholar] [CrossRef]
- Fergusson, J.R.; Smith, K.E.; Fleming, V.M.; Rajoriya, N.; Newell, E.W.; Simmons, R.; Marchi, E.; Björkander, S.; Kang, Y.-H.; Swadling, L.; et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep. 2014, 9, 1075–1088. [Google Scholar] [CrossRef]
- Martínez-Martín, N.; Fernández-Arenas, E.; Cemerski, S.; Delgado, P.; Turner, M.; Heuser, J.; Irvine, D.J.; Huang, B.; Bustelo, X.R.; Shaw, A.; et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity 2011, 35, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, A.; Liu, L.; Mak, J.Y.W.; Fairlie, D.P.; Cowley, S. CXCL16 Stimulates Antigen-Induced MAIT Cell Accumulation but Trafficking During Lung Infection Is CXCR6-Independent. Front. Immunol. 2020, 11, 1773. [Google Scholar] [CrossRef] [PubMed]
- Paquin-Proulx, D.; Greenspun, B.C.; Costa, E.A.S.; Segurado, A.C.; Kallas, E.G.; Nixon, D.F.; Leal, F.E. MAIT cells are reduced in frequency and functionally impaired in human T lymphotropic virus type 1 infection: Potential clinical implications. PLoS ONE 2017, 12, e0175345. [Google Scholar] [CrossRef]
- Li, S.; Simoni, Y.; Becht, E.; Loh, C.Y.; Li, N.; Lachance, D.; Koo, S.-L.; Lim, T.P.; Tan, E.K.W.; Mathew, R.; et al. Human Tumor-Infiltrating MAIT Cells Display Hallmarks of Bacterial Antigen Recognition in Colorectal Cancer. Cell Rep. Med. 2020, 1, 100039. [Google Scholar] [CrossRef]
- Pincikova, T.; Paquin-Proulx, D.; Sandberg, J.K.; Flodström-Tullberg, M.; Hjelte, L. Vitamin D treatment modulates immune activation in cystic fibrosis. Clin. Exp. Immunol. 2017, 189, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Slichter, C.K.; McDavid, A.; Miller, H.W.; Finak, G.; Seymour, B.J.; McNevin, J.P.; Diaz, G.; Czartoski, J.L.; McElrath, M.J.; Gottardo, R.; et al. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight 2016, 1, 86292. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Akther, H.D.; Hackstein, C.-P.; Powell, K.; King, T.; Friedrich, M.; Christoforidou, Z.; McCuaig, S.; Neyazi, M.; Arancibia-Cárcamo, C.V.; et al. TCR and Inflammatory Signals Tune Human MAIT Cells to Exert Specific Tissue Repair and Effector Functions. Cell Rep. 2019, 28, 3077–3091.e5. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Zhang, S.; Niu, L.; Lewinsohn, D.M.; Zhang, X.; Huang, S. Mucosal-Associated Invariant T Cells Develop an Innate-Like Transcriptomic Program in Anti-mycobacterial Responses. Front. Immunol. 2020, 11, 1136. [Google Scholar] [CrossRef]
- Kelly, J.; Minoda, Y.; Meredith, T.; Cameron, G.; Philipp, M.-S.; Pellicci, D.G.; Corbett, A.J.; Kurts, C.; Gray, D.H.; Godfrey, D.I.; et al. Chronically stimulated human MAIT cells are unexpectedly potent IL-13 producers. Immunol. Cell Biol. 2019, 97, 689–699. [Google Scholar] [CrossRef]
- Kinjo, Y.; Tupin, E.; Wu, D.; Fujio, M.; Garcia-Navarro, R.; Benhnia, M.R.E.I.; Zajonc, D.M.; Ben-Menachem, G.; Ainge, G.D.; Painter, G.F.; et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 2006, 7, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.J.; Eckle, S.B.G.; Birkinshaw, R.W.; Liu, L.; Patel, O.; Mahony, J.; Chen, Z.; Reantragoon, R.; Meehan, B.; Cao, H.; et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014, 509, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.J.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Koay, H.-F.; McCluskey, J.; Gherardin, N.A. The biology and functional importance of MAIT cells. Nat. Immunol. 2019, 20, 1110–1128. [Google Scholar] [CrossRef]
- Gibbs, A.; Leeansyah, E.; Introini, A.; Paquin-Proulx, D.; Hasselrot, K.; Andersson, E.; Broliden, K.; Sandberg, J.K.; Tjernlund, A. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017, 10, 35–45. [Google Scholar] [CrossRef]
- Vazquez, J.; Chavarria, M.; Chasman, D.A.; Schwartz, R.W.; Tyler, C.T.; Lopez, G.; Fisher, R.C.; Ong, I.M.; Stanic, A.K. Multiomic analysis reveals decidual-specific transcriptional programing of MAIT cells. Am. J. Reprod. Immunol. 2021, 86, e13495. [Google Scholar] [CrossRef]
- Sobkowiak, M.J.; Davanian, H.; Heymann, R.; Gibbs, A.; Emgård, J.; Dias, J.; Aleman, S.; Krüger-Weiner, C.; Moll, M.; Tjernlund, A.; et al. Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur. J. Immunol. 2018, 49, 133–143. [Google Scholar] [CrossRef]
- Gherardin, N.A.; Souter, M.N.T.; Koay, H.F.; Mangas, K.M.; Seemann, T.; Stinear, T.P.; Eckle, S.B.G.; Berzins, S.P.; d’Udekem, Y.; Konstantinov, I.E.; et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 2018, 96, 507–525. [Google Scholar] [CrossRef]
- Ito, E.; Inuki, S.; Izumi, Y.; Takahashi, M.; Dambayashi, Y.; Ciacchi, L.; Awad, W.; Takeyama, A.; Shibata, K.; Mori, S.; et al. Sulfated bile acid is a host-derived ligand for MAIT cells. Sci. Immunol. 2024, 9, eade6924. [Google Scholar] [CrossRef]
- Park, D.; Kim, H.G.; Kim, M.; Park, T.; Ha, H.-H.; Lee, D.H.; Park, K.-S.; Park, S.J.; Lim, H.J.; Lee, C.H. Differences in the molecular signatures of mucosal-associated invariant T cells and conventional T cells. Sci. Rep. 2019, 9, 7094. [Google Scholar] [CrossRef]
- Held, K.; Bhonsle-Deeng, L.; Siewert, K.; Sato, W.; Beltrán, E.; Schmidt, S.; Rühl, G.; Ng, J.K.M.; Engerer, P.; Moser, M.; et al. αβ T-cell receptors from multiple sclerosis brain lesions show MAIT cell–related features. Neurol.-Neuroimmunol. Neuroinflammation 2015, 2, e107. [Google Scholar] [CrossRef] [PubMed]
| Tube No | Stimulation | Cyclosporin A | Vitamin D3 |
|---|---|---|---|
| 1 | Unstimulated | − | − |
| 2 | PMA/Ionomycin | − | − |
| 3 | 5-OP-RU | − | − |
| 4 | 5-OP-RU+IL-12 and IL-33 | − | − |
| 5 | Unstimulated | + | − |
| 6 | PMA/Ionomycin | + | − |
| 7 | 5-OP-RU | + | − |
| 8 | 5-OP-RU+IL-12 and IL-33 | + | − |
| 9 | Unstimulated | − | + |
| 10 | PMA/Ionomycin | − | + |
| 11 | 5-OP-RU | − | + |
| 12 | 5-OP-RU+IL-12 and IL-33 | − | + |
| Tube | FITC | PE | PerCP/Cy5.5 | PC7 | APC | APC-H7 | PB | PO |
|---|---|---|---|---|---|---|---|---|
| Tube 1 | CLA | CCR6 | Vα7.2 | CD161 | PD-1 | CD3 | CD69 | CD45 |
| Tube 2 | cyIFN-g | cyIL-4/IL-13 | Vα7.2 | CD161 | cyIL-22 | CD3 | cyIL-17A | CD45 |
| Tube 3 | Syto | cyTNF-α | Vα7.2 | CD161 | CD38 | CD3 | cyGzB | CD45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.; Száraz-Széles, M.; Baráth, S.; Hevessy, Z. A Comprehensive Investigation of Stimulatory Agents on MAIT and Vα7.2+/CD161− T Cell Response and Effects of Immunomodulatory Drugs. Int. J. Mol. Sci. 2024, 25, 5895. https://doi.org/10.3390/ijms25115895
Singh P, Száraz-Széles M, Baráth S, Hevessy Z. A Comprehensive Investigation of Stimulatory Agents on MAIT and Vα7.2+/CD161− T Cell Response and Effects of Immunomodulatory Drugs. International Journal of Molecular Sciences. 2024; 25(11):5895. https://doi.org/10.3390/ijms25115895
Chicago/Turabian StyleSingh, Parvind, Marianna Száraz-Széles, Sándor Baráth, and Zsuzsanna Hevessy. 2024. "A Comprehensive Investigation of Stimulatory Agents on MAIT and Vα7.2+/CD161− T Cell Response and Effects of Immunomodulatory Drugs" International Journal of Molecular Sciences 25, no. 11: 5895. https://doi.org/10.3390/ijms25115895






