Identification and Functional Analysis of KH Family Genes Associated with Salt Stress in Rice
Abstract
1. Introduction
2. Results
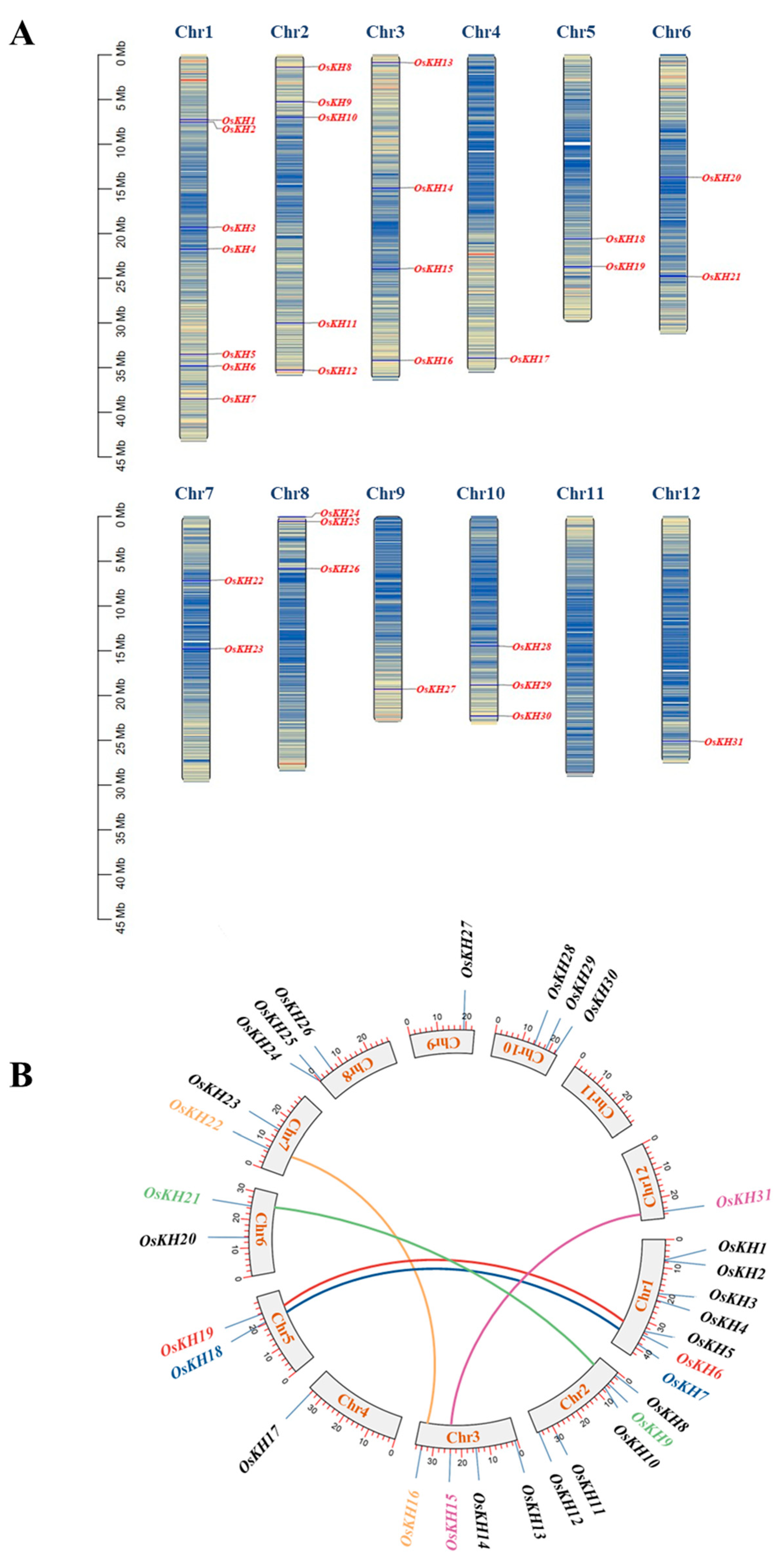
2.1. Identification and Chromosomal Location of KH Family Genes in Rice
2.2. Phylogenetic and Synteny Analysis of KH Genes in Arabidopsis thaliana and Oryza sativa
2.3. Conserved Motif and Domain Analysis of the KH Family Proteins
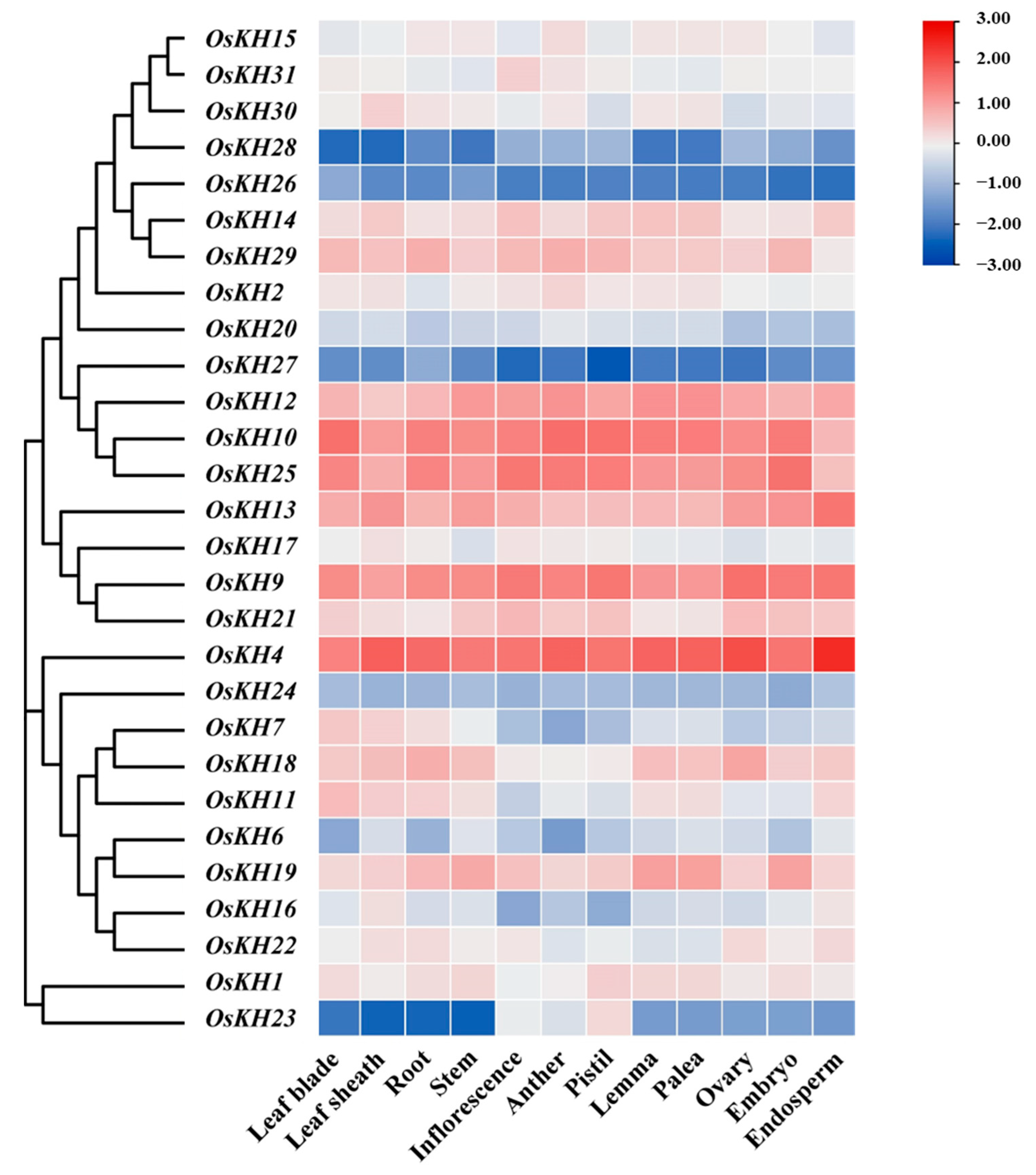
2.4. Tissues Expression Analysis of KH Members in Oryza sativa
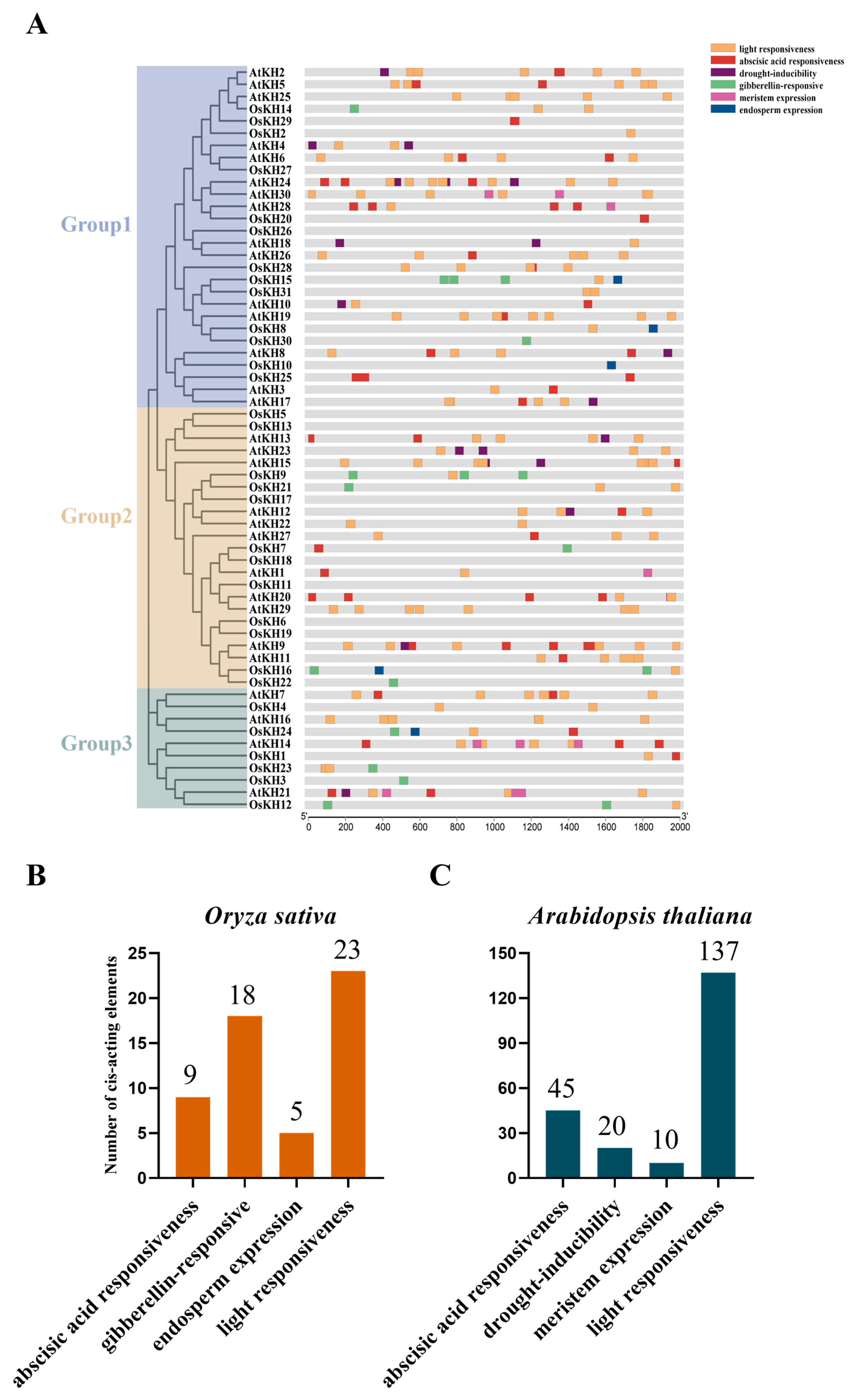
2.5. Cis-Acting Element Analysis of KH Gene Promoters
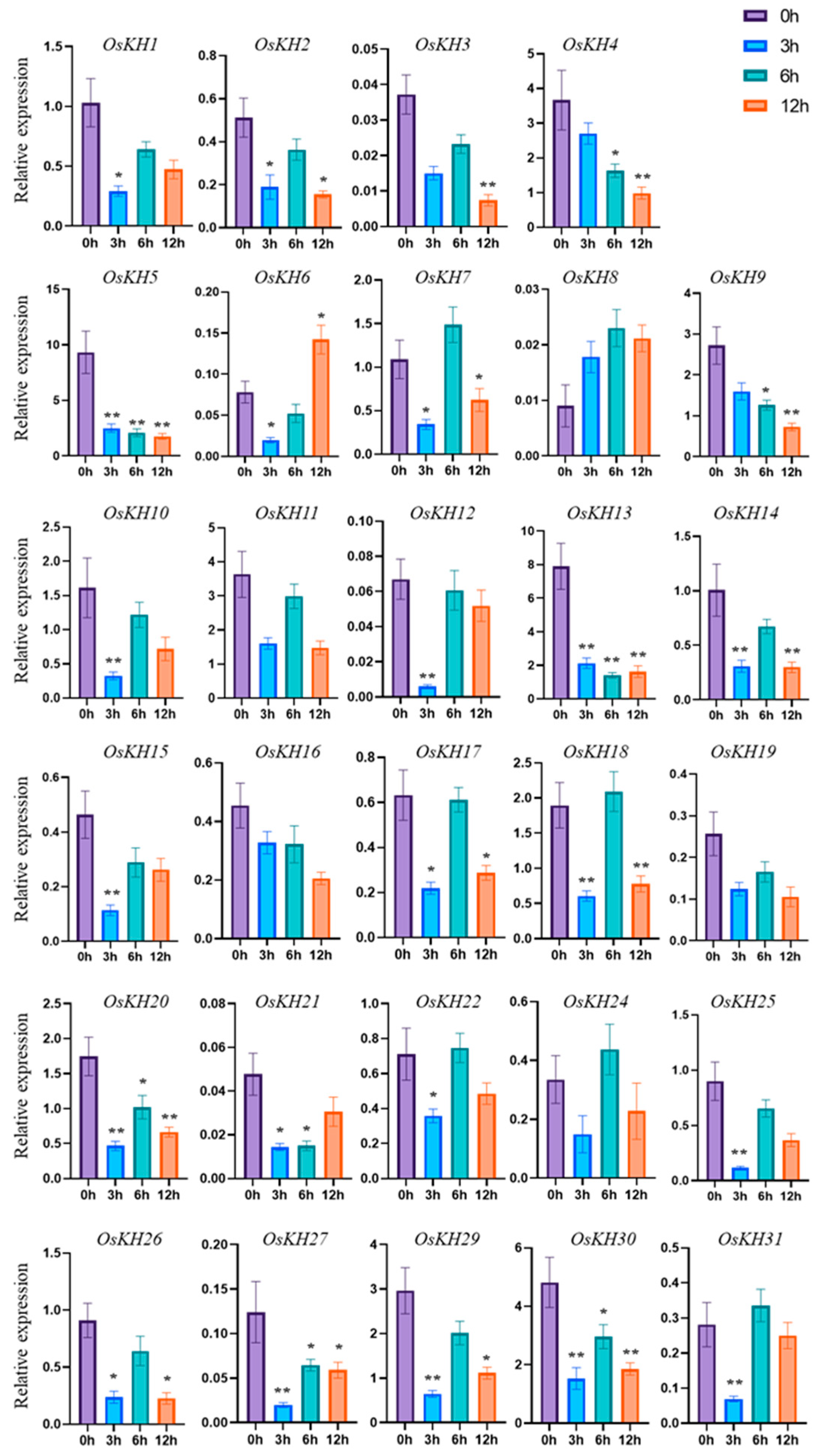
2.6. Expression Pattern Analysis of OsKHs under Salt Stress and ABA Treatment
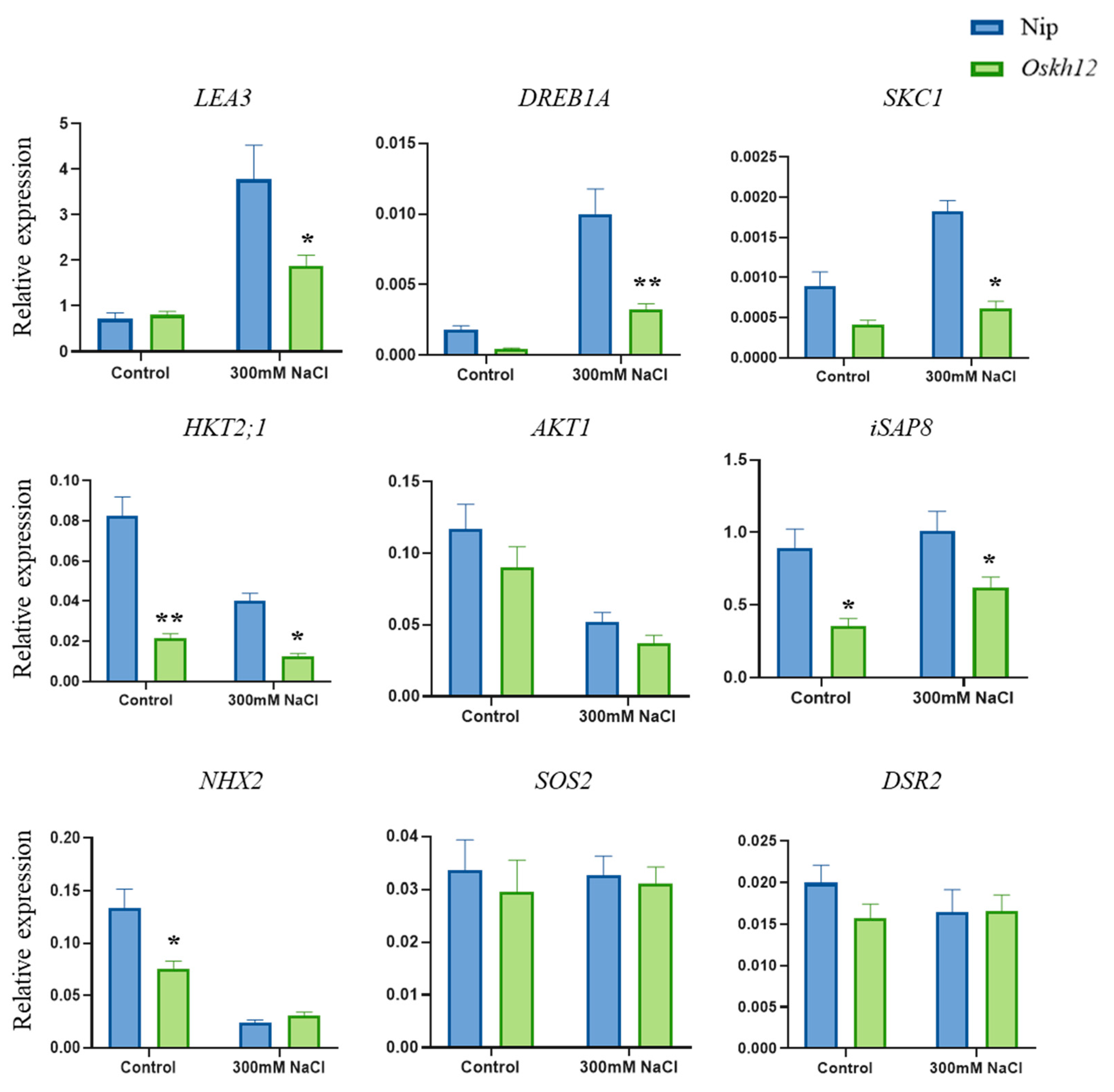
2.7. The Oskh12-Knockout Mutant Decreased Tolerance to Salt Stress in Rice
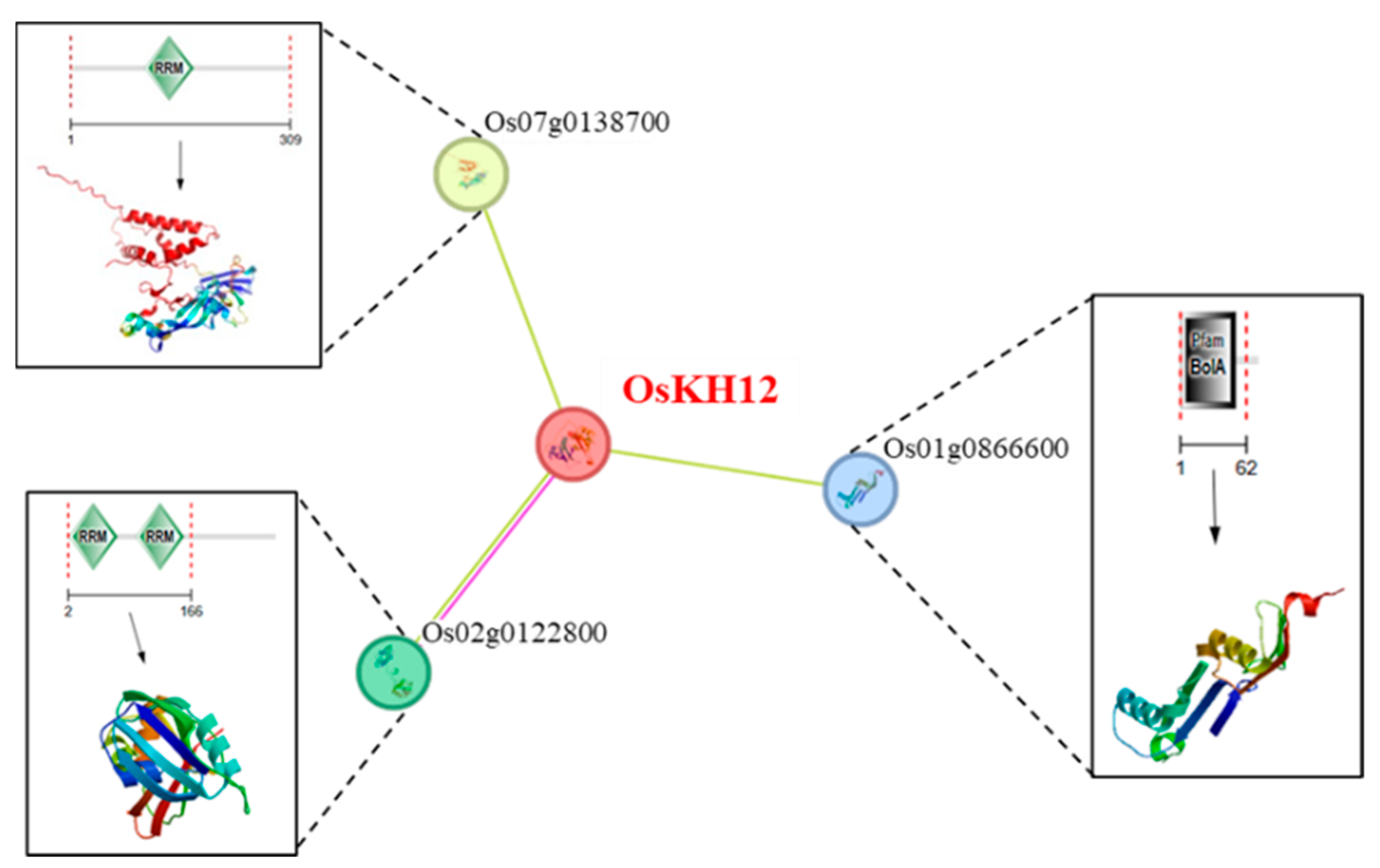
2.8. OsKH12 Is Related to the Proteins Containing the RRM Domain
3. Discussion
4. Materials and Methods
4.1. Identification of the KH Members in Oryza sativa
4.2. Chromosomal Location and Duplication Analysis of KH Genes in Oryza sativa
4.3. Phylogenetic Tree and Collinearity Analysis of KH Proteins
4.4. Conserved Motif and Domain Analysis of KH Family Proteins
4.5. Expression Pattern Analysis of KH Genes in Oryza sativa
4.6. Cis-Regulatory Elements Analysis of KH Gene Promoters
4.7. Plant Materials and Methods of Stress Treatment
4.8. RNA Extraction and qRT-PCR Analysis
4.9. ROS Staining
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siomi, H.; Matunis, M.J.; Michael, W.M.; Dreyfuss, G. The pre-mRNA binding K protein contains a novel evolutionary conserved motif. Nucleic Acids Res. 1993, 21, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Grishin, N.V. KH domain: One motif, two folds. Nucleic Acids Res. 2001, 29, 638–643. [Google Scholar] [CrossRef]
- Valverde, R.; Edwards, L.; Regan, L. Structure and function of KH domains. FEBS J. 2008, 275, 2712–2726. [Google Scholar] [CrossRef] [PubMed]
- Miska, E.A.; Li, L.; Veksler-Lublinsky, I.; Zinovyeva, A. HRPK-1, a conserved KH-domain protein, modulates microRNA activity during Caenorhabditis elegans development. PLoS Genet. 2019, 15, e1008067. [Google Scholar] [CrossRef] [PubMed]
- Briata, P.; Bordo, D.; Puppo, M.; Gorlero, F.; Rossi, M.; Perrone-Bizzozero, N.; Gherzi, R. Diverse roles of the nucleic acid-binding protein KHSRP in cell differentiation and disease. WIREs RNA 2015, 7, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, W.; Wang, J.; Qi, L.; Pan, H.; Feng, Z.; Tian, D. SAM68 promotes tumorigenesis in lung adenocarcinoma by regulating metabolic conversion via PKM alternative splicing. Theranostics 2021, 11, 3359–3375. [Google Scholar] [CrossRef] [PubMed]
- Qiao, A.; Zhou, J.; Xu, S.; Ma, W.; Boriboun, C.; Kim, T.; Yan, B.; Deng, J.; Yang, L.; Zhang, E.; et al. Sam68 promotes hepatic gluconeogenesis via CRTC2. Nat. Commun. 2021, 12, 3340. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Yuan, M.; Kuang, Z.; Zou, Y.; Tang, T.; Zhang, W.; Hu, X.; Xia, T.; Cao, T.; et al. Sam68 Promotes the Progression of Human Breast Cancer through inducing Activation of EphA3. Curr. Cancer Drug Targets 2020, 20, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Mockler, T.C.; Yu, X.; Shalitin, D.; Parikh, D.; Michael, T.P.; Liou, J.; Huang, J.; Smith, Z.; Alonso, J.M.; Ecker, J.R.; et al. Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 12759–12764. [Google Scholar] [CrossRef]
- Ripoll, J.J.; Rodríguez-Cazorla, E.; González-Reig, S.; Andújar, A.; Alonso-Cantabrana, H.; Perez-Amador, M.A.; Carbonell, J.; Martínez-Laborda, A.; Vera, A. Antagonistic interactions between Arabidopsis K-homology domain genes uncover PEPPER as a positive regulator of the central floral repressor FLOWERING LOCUS C. Dev. Biol. 2009, 333, 251–262. [Google Scholar] [CrossRef]
- Steffen, A.; Elgner, M.; Staiger, D. Regulation of Flowering Time by the RNA-Binding Proteins AtGRP7 and AtGRP8. Plant Cell Physiol. 2019, 60, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Wellmer, F.; Han, Y.; Jing, M.; Liu, Y.; Shi, H.; Yan, Z. KHZ1 and KHZ2, novel members of the autonomous pathway, repress the splicing efficiency of FLC pre-mRNA in Arabidopsis. J. Exp. Bot. 2020, 71, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Jia, J.; Yan, X.; Shi, H.; Han, Y. Arabidopsis KHZ1 and KHZ2, two novel non-tandem CCCH zinc-finger and K-homolog domain proteins, have redundant roles in the regulation of flowering and senescence. Plant Mol. Biol. 2017, 95, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.; Gao, M.; Zhang, X.N.; Shi, J.; Vrydagh, L.; Kim, S.H.; Patel, P.; Hu, A.R.; Lu, H. The flowering time regulator FLK controls pathogen defense in Arabidopsis thaliana. Plant Physiol. 2023, 191, 2461–2474. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.Y.; Chen, D.K.; Sun, Y.P.; Liang, W.Y.; Liu, Y.; Huang, L.Q.; Li, Y.K.; He, J.F.; Yao, N. The Arabidopsis KH-domain protein FLOWERING LOCUS Y delays flowering by upregulating FLOWERING LOCUS C family members. Plant Cell Rep. 2020, 39, 1705–1717. [Google Scholar] [CrossRef]
- Amara, U.; Hu, J.; Cai, J.; Kang, H. FLK is an mRNA m6A reader that regulates floral transition by modulating the stability and splicing of FLC in Arabidopsis. Mol. Plant 2023, 16, 919–929. [Google Scholar] [CrossRef]
- Vega-Sánchez, M.E.; Zeng, L.; Chen, S.; Leung, H.; Wang, G.L. SPIN1, a K homology domain protein negatively regulated and ubiquitinated by the E3 ubiquitin ligase SPL11, is involved in flowering time control in rice. Plant Cell 2008, 20, 1456–1469. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Liu, R.; Li, G. Genome-Wide Characterization and Expression Analysis of KH Family Genes Response to ABA and SA in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 511. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Wen, C.; Zeng, H.; Zhu, J. A KH Domain-Containing Putative RNA-Binding Protein Is Critical for Heat Stress-Responsive Gene Regulation and Thermotolerance in Arabidopsis. Mol. Plant 2013, 6, 386–395. [Google Scholar] [CrossRef]
- Chen, T.; Cui, P.; Xiong, L. The RNA-binding protein HOS5 and serine/arginine-rich proteins RS40 and RS41 participate in miRNA biogenesis in Arabidopsis. Nucleic Acids Res. 2015, 43, 8283–8298. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Foley, R.; Casarotto, H.J.; Gao, L.-L.; Kamphuis, L.G.; Melser, S.; Singh, K.B. The Arabidopsis RNA Polymerase II Carboxyl Terminal Domain (CTD) Phosphatase-Like1 (CPL1) is a biotic stress susceptibility gene. Sci. Rep. 2018, 8, 13454. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cui, P.; Chen, H.; Ali, S.; Zhang, S.; Xiong, L. A KH-domain RNA-binding protein interacts with FIERY2/CTD phosphatase-like 1 and splicing factors and is important for pre-mRNA splicing in Arabidopsis. PLoS Genet. 2013, 9, e1003875. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Q.; Xiang, L.; Lei, Z.; Huang, Q.; Zhang, J.; Cai, M.; Chen, T. AtSIEK, an EXD1-like protein with KH domain, involves in salt stress response by interacting with FRY2/CPL1. Int. J. Biol. Macromol. 2023, 233, 123369. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Molla, K.A.; Henry, R.J.; Bhat, K.V.; Mondal, T.K. Advances in understanding salt tolerance in rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Pascale, S.D.; Maggio, A.; Barbieri, G. Soil salinization affects growth, yield and mineral composition of cauliflower and broccoli. Eur. J. Agron. 2005, 23, 254–264. [Google Scholar] [CrossRef]
- Zhang, J.-T.; Mu, C.-S. Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius. Soil Sci. Plant Nutr. 2009, 55, 685–697. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Ismail, M.R.; Hossain, M.A.; Othman, R.; Rahim, A.A. Effect of Salinity Stress on Nutrient Uptake and Chlorophyll Content of Tropical Turfgrass Species. Aust. J. Crop Sci. 2011, 5, 620–629. [Google Scholar]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Xie, Q.; Zhou, Y.; Jiang, X. Structure, Function, and Regulation of the Plasma Membrane Na+/H+ Antiporter Salt Overly Sensitive 1 in Plants. Front. Plant Sci. 2022, 13, 866265. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tang, L.H.; Nie, J.W.; Zhang, C.R.; Han, X.; Li, Q.Y.; Qin, L.; Wang, M.H.; Huang, X.; Yu, F.; et al. Structure and activation mechanism of the rice Salt Overly Sensitive 1 (SOS1) Na+/H+ antiporter. Nat. Plants 2023, 9, 1924–1936. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Deng, Y.; Ding, Y.; Guo, J.; Qiu, J.; Wang, B.; Wang, C.; Xie, Y.; Zhang, Z.; Chen, J.; et al. Rice functional genomics: Decades’ efforts and roads ahead. Sci. China Life Sci. 2022, 65, 33–92. [Google Scholar] [CrossRef] [PubMed]
- de Zelicourt, A.; Colcombet, J.; Hirt, H. The Role of MAPK Modules and ABA during Abiotic Stress Signaling. Trends Plant Sci. 2016, 21, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Tang, N.; Du, H.; Ye, H.; Xiong, L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008, 148, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, N.; Ji, D.; Zhang, W.; Wang, Y.; Yu, Y.; Zhao, S.; Lyu, M.; You, J.; Zhang, Y.; et al. A GmSIN1/GmNCED3s/GmRbohBs Feed-Forward Loop Acts as a Signal Amplifier That Regulates Root Growth in Soybean Exposed to Salt Stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef] [PubMed]
- Moons, A.; De Keyser, A.; Van Montagu, M. A group 3 LEA cDNA of rice, responsive to abscisic acid, but not to jasmonic acid, shows variety-specific differences in salt stress response. Gene 1997, 191, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Moons, A.; Bauw, G.; Prinsen, E.; Van Montagu, M.; Van der Straeten, D. Molecular and physiological responses to abscisic acid and salts in roots of salt-sensitive and salt-tolerant Indica rice varieties. Plant Physiol. 1995, 107, 177–186. [Google Scholar] [CrossRef]
- Kanneganti, V.; Gupta, A.K. Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant Mol. Biol. 2008, 66, 445–462. [Google Scholar] [CrossRef]
- Luo, C.; Guo, C.; Wang, W.; Wang, L.; Chen, L. Overexpression of a new stress-repressive gene OsDSR2 encoding a protein with a DUF966 domain increases salt and simulated drought stress sensitivities and reduces ABA sensitivity in rice. Plant Cell Rep. 2014, 33, 323–336. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nan, N.; Li, N.; Liu, Y.; Wang, T.J.; Hwang, I.; Liu, B.; Xu, Z.Y. A DNA Methylation Reader-Chaperone Regulator-Transcription Factor Complex Activates OsHKT1;5 Expression during Salinity Stress. Plant Cell 2020, 32, 3535–3558. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Seidel, T.; Golldack, D.; Lindberg, S. Expressions of OsHKT1, OsHKT2, and OsVHA are differentially regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J. Exp. Bot. 2006, 57, 4257–4268. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Nakamura, A.; Hara, N.; Toki, S.; Tanaka, Y. Molecular and functional analyses of rice NHX-type Na+/H+ antiporter genes. Planta 2011, 233, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Hayashi, S.; Abe, T.; Igura, M.; Kuramata, M.; Tanikawa, H.; Iino, M.; Saito, T.; Ono, Y.; Ishikawa, T.; et al. Low-cesium rice: Mutation in OsSOS2 reduces radiocesium in rice grains. Sci. Rep. 2017, 7, 2432. [Google Scholar] [CrossRef]
- Mahalingam, R.; Thatcher, L.F.; Kamphuis, L.G.; Hane, J.K.; Oñate-Sánchez, L.; Singh, K.B. The Arabidopsis KH-Domain RNA-Binding Protein ESR1 Functions in Components of Jasmonate Signalling, Unlinking Growth Restraint and Resistance to Stress. PLoS ONE 2015, 10, e0126978. [Google Scholar] [CrossRef] [PubMed]
- Rout, A.K.; Singh, H.; Patel, S.; Raghvan, V.; Gautam, S.; Minda, R.; Rao, B.J.; Chary, K.V.R. Structural characterization of a novel KH-domain containing plant chloroplast endonuclease. Sci. Rep. 2018, 8, 13750. [Google Scholar] [CrossRef] [PubMed]
- Koiwa, H.; Hausmann, S.; Bang, W.Y.; Ueda, A.; Kondo, N.; Hiraguri, A.; Fukuhara, T.; Bahk, J.D.; Yun, D.J.; Bressan, R.A.; et al. Arabidopsis C-terminal domain phosphatase-like 1 and 2 are essential Ser-5-specific C-terminal domain phosphatases. Proc. Natl. Acad. Sci. USA 2004, 101, 14539–14544. [Google Scholar] [CrossRef]
- Ortuño-Miquel, S.; Rodríguez-Cazorla, E.; Zavala-Gonzalez, E.A.; Martínez-Laborda, A.; Vera, A. Arabidopsis HUA ENHANCER 4 delays flowering by upregulating the MADS-box repressor genes FLC and MAF4. Sci. Rep. 2019, 9, 1478. [Google Scholar] [CrossRef]
- Butt, H.; Bazin, J.; Prasad, K.V.S.K.; Awad, N.; Crespi, M.; Reddy, A.S.N.; Mahfouz, M.M. The Rice Serine/Arginine Splicing Factor RS33 Regulates Pre-mRNA Splicing during Abiotic Stress Responses. Cells 2022, 11, 1796. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.D.; Allen, J.; Amode, R.M.; Azov, A.G.; Barba, M.; Becerra, A.; Bhai, J.; Campbell, L.I.; Carbajo Martinez, M.; Chakiachvili, M.; et al. Ensembl Genomes 2022: An expanding genome resource for non-vertebrates. Nucleic Acids Res. 2022, 50, D996–D1003. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cai, M.; Zhang, S.; Chai, J.; Sun, M.; Wang, Y.; Xie, Q.; Chen, Y.; Wang, H.; Chen, T. Genome-Wide Identification of AP2/ERF Transcription Factor Family and Functional Analysis of DcAP2/ERF#96 Associated with Abiotic Stress in Dendrobium catenatum. Int. J. Mol. Sci. 2022, 23, 13603. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Cai, M.; Zeng, Q.; Han, Y.; Zhang, S.; Wang, Y.; Xie, Q.; Chen, Y.; Zeng, Y.; Chen, T. Genome-Wide Identification and Expression Analysis of UBiA Family Genes Associated with Abiotic Stress in Sunflowers (Helianthus annuus L.). Int. J. Mol. Sci. 2023, 24, 1883. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Takehisa, H.; Kamatsuki, K.; Minami, H.; Namiki, N.; Ikawa, H.; Ohyanagi, H.; Sugimoto, K.; Antonio, B.A.; Nagamura, Y. RiceXPro Version 3.0: Expanding the informatics resource for rice transcriptome. Nucleic Acids Res. 2013, 41, D1206–D1213. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]




| ID | Rename | Number of Amino Acids | MW (Da) | PI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Location |
|---|---|---|---|---|---|---|---|---|
| At1g09660 | AtKH1 | 298 | 33,687.72 | 8.42 | 61.41 | 75.87 | −0.541 | Nucleus |
| At1g14170 | AtKH2 | 479 | 52,540.33 | 5.85 | 49.27 | 87.93 | −0.361 | Chloroplast |
| At1g33680 | AtKH3 | 763 | 80,438.61 | 4.71 | 53.96 | 47.23 | −0.96 | Nucleus |
| At1g51580 | AtKH4 | 621 | 67,115.01 | 5.74 | 50.65 | 75.17 | −0.363 | Chloroplast |
| At2g03110 | AtKH5 | 153 | 17,376.29 | 4.16 | 34.48 | 90.39 | −0.373 | Peroxisome |
| At2g22600 | AtKH6 | 632 | 67,754.64 | 5.94 | 37.66 | 96.33 | −0.173 | Chloroplast |
| At2g25910 | AtKH7 | 342 | 38,474.69 | 5.53 | 49.65 | 94.09 | −0.125 | Mitochondria |
| At2g25970 | AtKH8 | 632 | 64,606.8 | 5.32 | 57.97 | 40.32 | −0.853 | Nucleus |
| At2g38610 | AtKH9 | 286 | 31,720.93 | 8.99 | 64.05 | 76.36 | −0.56 | Chloroplast |
| At3g04610 | AtKH10 | 577 | 63,403.92 | 4.57 | 51.8 | 66.2 | −0.753 | Cytoplasm |
| At3g08620 | AtKH11 | 283 | 31,500.94 | 9.3 | 59.94 | 79.93 | −0.57 | Nucleus |
| At3g12130 | AtKH12 | 248 | 25,952.1 | 9.51 | 27.54 | 49.96 | −0.623 | Nucleus |
| At3g13230 | AtKH13 | 215 | 24,009.02 | 9.71 | 27.82 | 88.93 | −0.284 | Cytoplasm |
| At3g16230 | AtKH14 | 449 | 50,078.63 | 9.17 | 39.03 | 88.11 | −0.275 | Chloroplast |
| At3g29390 | AtKH15 | 578 | 62,535.11 | 8.77 | 61.83 | 76.61 | −0.51 | Nucleus |
| At3g32940 | AtKH16 | 607 | 66,479.76 | 9.01 | 47.82 | 69.11 | −0.546 | Nucleus |
| At4g10070 | AtKH17 | 725 | 77,201.51 | 4.64 | 56.61 | 48.36 | −0.939 | Nucleus |
| At4g18375 | AtKH18 | 606 | 65,760.82 | 8.04 | 50.66 | 87.94 | −0.234 | Chloroplast |
| At4g26000 | AtKH19 | 495 | 54,024.67 | 4.92 | 51.46 | 86.28 | −0.291 | Cytoplasm |
| At4g26480 | AtKH20 | 308 | 33,588.38 | 9.05 | 61.03 | 81.66 | −0.391 | Nucleus |
| At5g04430 | AtKH21 | 334 | 36,018.23 | 5.77 | 50.4 | 76.86 | −0.422 | Nucleus |
| At5g06770 | AtKH22 | 240 | 25,367.57 | 9.66 | 35.72 | 56.54 | −0.628 | Cytoplasm |
| At5g08420 | AtKH23 | 391 | 45,323.08 | 9.53 | 57.5 | 64.83 | −1.053 | Nucleus |
| At5g09560 | AtKH24 | 612 | 67,954 | 6.62 | 46.76 | 85.16 | −0.35 | Nucleus |
| At5g15270 | AtKH25 | 548 | 59,712.05 | 8.33 | 58.14 | 80.24 | −0.51 | Nucleus |
| At5g46190 | AtKH26 | 644 | 69,676.84 | 8.35 | 45.72 | 86.99 | −0.283 | Nucleus |
| At5g51300 | AtKH27 | 804 | 87,050.31 | 5.88 | 55.54 | 55.11 | −0.804 | Nucleus |
| At5g53060 | AtKH28 | 652 | 71,345 | 5.75 | 62.6 | 82.96 | −0.478 | Cytoplasm |
| At5g56140 | AtKH29 | 315 | 33,984.66 | 7.87 | 62.06 | 80.48 | −0.378 | Nucleus |
| At5g64390 | AtKH30 | 866 | 94,703.74 | 7.2 | 47.21 | 81.29 | −0.395 | Nucleus |
| Os01g0231900 | OsKH1 | 487 | 54,345.92 | 8.42 | 48.93 | 80.1 | −0.48 | Chloroplast |
| Os01g0235800 | OsKH2 | 705 | 76,040.62 | 5.96 | 36.44 | 83.21 | −0.391 | Mitochondria |
| Os01g0533450 | OsKH3 | 133 | 14,786.94 | 10.07 | 31.48 | 95.26 | −0.236 | Chloroplast |
| Os01g0566900 | OsKH4 | 307 | 34,744.41 | 5.25 | 43.35 | 95.21 | −0.096 | Cytoplasm |
| Os01g0788950 | OsKH5 | 220 | 24,409.36 | 9.74 | 36.72 | 89.64 | −0.198 | Cytoskeleton |
| Os01g0818300 | OsKH6 | 283 | 31,302.83 | 7.73 | 43.94 | 83.07 | −0.451 | Cytoplasm |
| Os01g0886300 | OsKH7 | 290 | 32,556.19 | 8.77 | 54.67 | 84.31 | −0.562 | Nucleus |
| Os02g0125500 | OsKH8 | 458 | 49,993.26 | 5.04 | 42.18 | 91.11 | −0.352 | Cytoplasm |
| Os02g0194200 | OsKH9 | 300 | 31,153.44 | 9.58 | 35.7 | 48.23 | −0.532 | Nucleus |
| Os02g0224300 | OsKH10 | 699 | 71,068.1 | 5.56 | 61.59 | 39.31 | −0.887 | Nucleus |
| Os02g0722700 | OsKH11 | 286 | 31,368.76 | 9.27 | 46.16 | 83.25 | −0.43 | Chloroplast |
| Os02g0822300 | OsKH12 | 343 | 36,718.26 | 6.19 | 53.38 | 76.82 | −0.394 | Cytoskeleton |
| Os03g0115200 | OsKH13 | 227 | 24,965.89 | 9.74 | 40.16 | 86.43 | −0.236 | Cytoplasm |
| Os03g0376800 | OsKH14 | 542 | 57,843.61 | 6.41 | 44.49 | 80.22 | −0.395 | Nucleus |
| Os03g0627500 | OsKH15 | 510 | 54,819.89 | 4.81 | 53.11 | 65.43 | −0.624 | Nucleus |
| Os03g0815700 | OsKH16 | 281 | 31,285.46 | 9.48 | 63.61 | 72.49 | −0.617 | Nucleus |
| Os04g0665700 | OsKH17 | 309 | 31,787.8 | 9.48 | 41.21 | 47.9 | −0.544 | Cytoplasm |
| Os05g0419500 | OsKH18 | 291 | 32,510.16 | 8.8 | 55.79 | 80 | −0.53 | Cytoplasm |
| Os05g0481500 | OsKH19 | 282 | 31,240.65 | 8.65 | 63.45 | 74.04 | −0.565 | Nucleus |
| Os06g0342500 | OsKH20 | 662 | 72,249.2 | 5.91 | 55.74 | 76.18 | −0.577 | Nucleus |
| Os06g0618100 | OsKH21 | 295 | 30,451.57 | 9.53 | 38.8 | 49.46 | −0.479 | Nucleus |
| Os07g0227400 | OsKH22 | 286 | 32,135.56 | 9.16 | 56.99 | 70.91 | −0.685 | Nucleus |
| Os07g0439100 | OsKH23 | 233 | 24,955.9 | 4.61 | 28.88 | 79.53 | −0.344 | Nucleus |
| Os08g0100700 | OsKH24 | 753 | 79,140.08 | 9.43 | 61.72 | 66.02 | −0.504 | Nucleus |
| Os08g0110800 | OsKH25 | 700 | 72,186.11 | 4.88 | 60.17 | 41.61 | −0.95 | Nucleus |
| Os08g0200400 | OsKH26 | 454 | 48,932.73 | 6.39 | 42.88 | 94.91 | −0.207 | Cytoplasm |
| Os09g0498600 | OsKH27 | 616 | 65,484.48 | 7.31 | 49.72 | 72.44 | −0.325 | Mitochondria |
| Os10g0414700 | OsKH28 | 586 | 65,147.67 | 5.68 | 59.13 | 80.24 | −0.336 | Cytoplasm |
| Os10g0495000 | OsKH29 | 762 | 83,489.63 | 8.26 | 50.23 | 79.76 | −0.594 | Nucleus |
| Os10g0564000 | OsKH30 | 458 | 48,410.67 | 5.21 | 43.83 | 90.37 | −0.139 | Cytoplasm |
| Os12g0597600 | OsKH31 | 517 | 54,962.92 | 4.89 | 56.58 | 64.35 | −0.66 | Cytoplasm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Zhang, Y.; Wu, M.; Chen, Y.; Wang, Y.; Zeng, Q.; Han, Y.; Zhang, S.; Zhang, J.; Chen, T.; et al. Identification and Functional Analysis of KH Family Genes Associated with Salt Stress in Rice. Int. J. Mol. Sci. 2024, 25, 5950. https://doi.org/10.3390/ijms25115950
Xie Q, Zhang Y, Wu M, Chen Y, Wang Y, Zeng Q, Han Y, Zhang S, Zhang J, Chen T, et al. Identification and Functional Analysis of KH Family Genes Associated with Salt Stress in Rice. International Journal of Molecular Sciences. 2024; 25(11):5950. https://doi.org/10.3390/ijms25115950
Chicago/Turabian StyleXie, Qinyu, Yutong Zhang, Mingming Wu, Youheng Chen, Yingwei Wang, Qinzong Zeng, Yuliang Han, Siqi Zhang, Juncheng Zhang, Tao Chen, and et al. 2024. "Identification and Functional Analysis of KH Family Genes Associated with Salt Stress in Rice" International Journal of Molecular Sciences 25, no. 11: 5950. https://doi.org/10.3390/ijms25115950
APA StyleXie, Q., Zhang, Y., Wu, M., Chen, Y., Wang, Y., Zeng, Q., Han, Y., Zhang, S., Zhang, J., Chen, T., & Cai, M. (2024). Identification and Functional Analysis of KH Family Genes Associated with Salt Stress in Rice. International Journal of Molecular Sciences, 25(11), 5950. https://doi.org/10.3390/ijms25115950






