LncRNA BCYRN1 as a Potential Therapeutic Target and Diagnostic Marker in Serum Exosomes in Bladder Cancer
Abstract
1. Introduction
2. Results
2.1. Knockdown of BCYRN1 Decreased Cell Viability in BC Cells
2.2. Cell Viability Was Decreased by BCYRN1 Knockdown in a Xenograft Mouse Model
2.3. BCYRN1 Downregulation Affected the Cell Cycle and Cell Apoptosis
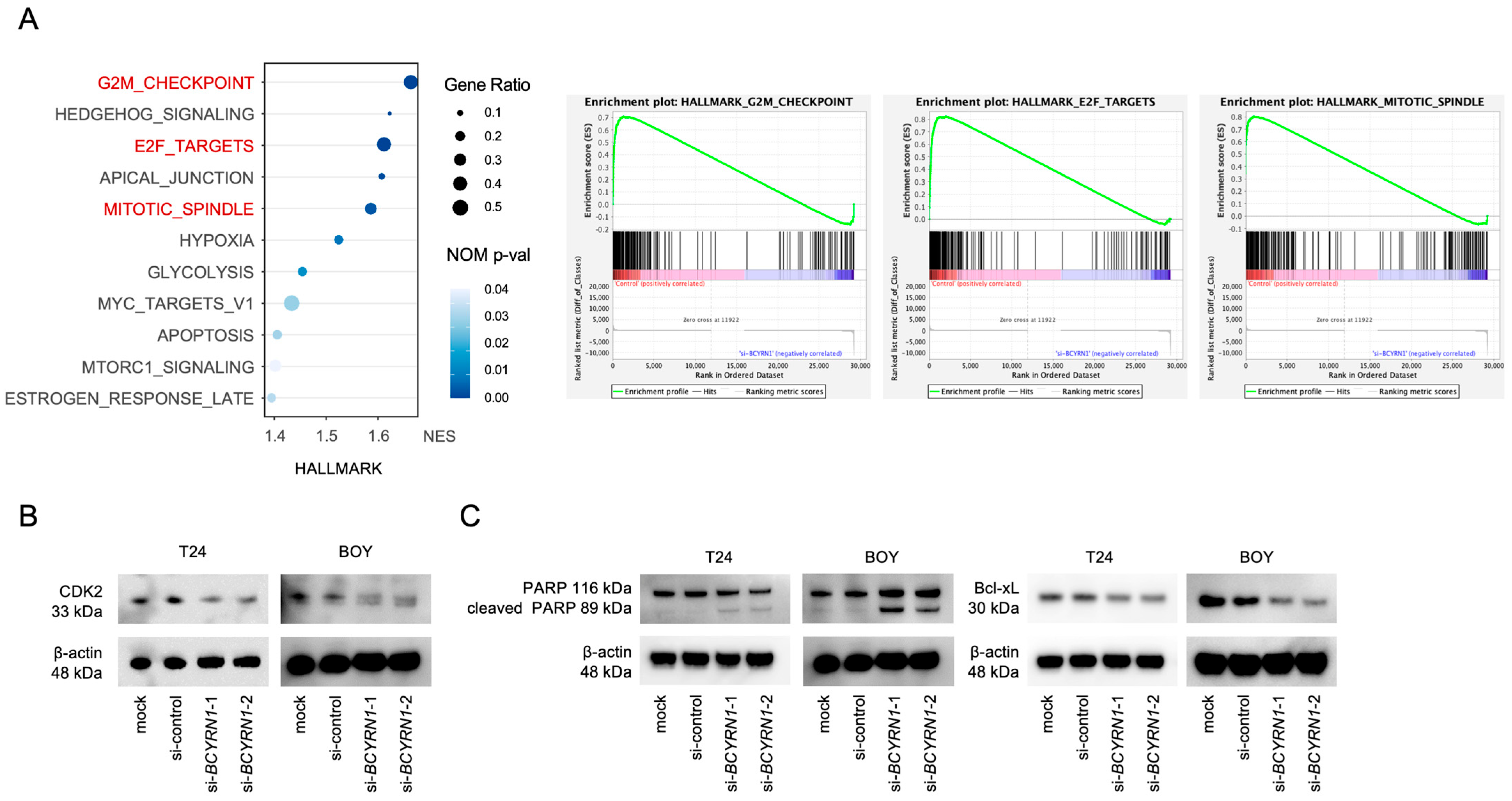
2.4. Knockdown of BCYRN1 Induced G2/M Arrest and Apoptosis in BC Cells
2.5. High Serum Exosomal BCYRN1 Expression Was Observed in Patients with BC Tumors
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transfection with siRNA
4.3. RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.4. RNA Sequencing and Gene Set Enrichment Analysis (GSEA)
4.5. Cell Proliferation, Three-Dimensional (3D) Spheroid Formation, Migration, and Invasion Assays
4.6. Apoptosis and Cell Cycle Analysis
4.7. Western Blotting and Immunohistochemistry
4.8. Xenograft Model
4.9. Immunohistochemistry
4.10. Isolation of Exosomes from the Sera of Patients with BC
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Kolahi, A.-A.; Naghavi, M. Global, Regional and National Burden of Bladder Cancer and Its Attributable Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. BMJ Glob. Health 2021, 6, e004128. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, Screening, and Prevention of Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, S.; Alfred Witjes, J. Long-Term Cancer-Specific Survival in Patients with High-Risk, Non–Muscle-Invasive Bladder Cancer and Tumour Progression: A Systematic Review. Eur. Urol. 2011, 60, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Necchi, A.; Park, S.H.; García-Donas, J.; Huddart, R.A.; Burgess, E.F.; Fleming, M.T.; Rezazadeh Kalebasty, A.; Mellado, B.; Varlamov, S.; et al. Efficacy and Safety of Erdafitinib in Patients with Locally Advanced or Metastatic Urothelial Carcinoma: Long-Term Follow-up of a Phase 2 Study. Lancet Oncol. 2022, 23, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.K.; Foerster, B.; Catto, J.W.F.; Kamat, A.M.; Kassouf, W.; Jubber, I.; Shariat, S.F.; Sylvester, R.J.; Gontero, P. Repeat Transurethral Resection in Non–Muscle-Invasive Bladder Cancer: A Systematic Review. Eur. Urol. 2018, 73, 925–933. [Google Scholar] [CrossRef]
- Divrik, R.T.; Şahin, A.F.; Yildirim, Ü.; Altok, M.; Zorlu, F. Impact of Routine Second Transurethral Resection on the Long-Term Outcome of Patients with Newly Diagnosed PT1 Urothelial Carcinoma with Respect to Recurrence, Progression Rate, and Disease-Specific Survival: A Prospective Randomised Clinical Trial. Eur. Urol. 2010, 58, 185–190. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Name V.X.202X. © National Comprehensive Cancer Network, Inc. 202X. All Rights Reserved. To View the Most Recent and Complete Version of the Guideline. Available online: www.NCCN.org.com.
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-Coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Enokida, H.; Yoshino, H.; Matsushita, R.; Nakagawa, M. The Role of MicroRNAs in Bladder Cancer. Investig. Clin. Urol. 2016, 57, S60. [Google Scholar] [CrossRef]
- Yoshino, H.; Seki, N.; Itesako, T.; Chiyomaru, T.; Nakagawa, M.; Enokida, H. Aberrant Expression of MicroRNAs in Bladder Cancer. Nat. Rev. Urol. 2013, 10, 396–404. [Google Scholar] [CrossRef]
- Okamura, S.; Yoshino, H.; Kuroshima, K.; Tsuruda, M.; Osako, Y.; Sakaguchi, T.; Yonemori, M.; Yamada, Y.; Tatarano, S.; Nakagawa, M.; et al. EHHADH Contributes to Cisplatin Resistance through Regulation by Tumor-Suppressive MicroRNAs in Bladder Cancer. BMC Cancer 2021, 21, 48. [Google Scholar] [CrossRef]
- Yoshino, H.; Chiyomaru, T.; Enokida, H.; Kawakami, K.; Tatarano, S.; Nishiyama, K.; Nohata, N.; Seki, N.; Nakagawa, M. The Tumour-Suppressive Function of MiR-1 and MiR-133a Targeting TAGLN2 in Bladder Cancer. Br. J. Cancer 2011, 104, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein Inhibits Prostate Cancer Cell Growth by Targeting MiR-34a and Oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef] [PubMed]
- Itesako, T.; Seki, N.; Yoshino, H.; Chiyomaru, T.; Yamasaki, T.; Hidaka, H.; Yonezawa, T.; Nohata, N.; Kinoshita, T.; Nakagawa, M.; et al. The MicroRNA Expression Signature of Bladder Cancer by Deep Sequencing: The Functional Significance of the MiR-195/497 Cluster. PLoS ONE 2014, 9, e84311. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Yoshino, H.; Yonemori, M.; Miyamoto, K.; Sugita, S.; Matsushita, R.; Itesako, T.; Tatarano, S.; Nakagawa, M.; Enokida, H. Regulation of ITGA3 by the Dual-Stranded MicroRNA-199 Family as a Potential Prognostic Marker in Bladder Cancer. Br. J. Cancer 2017, 116, 1077–1087. [Google Scholar] [CrossRef]
- Yamada, Y.; Enokida, H.; Kojima, S.; Kawakami, K.; Chiyomaru, T.; Tatarano, S.; Yoshino, H.; Kawahara, K.; Nishiyama, K.; Seki, N.; et al. MiR-96 and MiR-183 Detection in Urine Serve as Potential Tumor Markers of Urothelial Carcinoma: Correlation with Stage and Grade, and Comparison with Urinary Cytology. Cancer Sci. 2011, 102, 522–529. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Khan, Y.S. Histology, Extracellular Vesicles. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary Extracellular Vesicles: A Position Paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-Based Liquid Biopsies in Cancer: Opportunities and Challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Wang, M.; Ji, S.; Shao, G.; Zhang, J.; Zhao, K.; Wang, Z.; Wu, A. Effect of Exosome Biomarkers for Diagnosis and Prognosis of Breast Cancer Patients. Clin. Transl. Oncol. 2018, 20, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Picon, M.A.; Wang, L.; Da Fonseca Ferreira, A.; Dong, C.; Marzouka, G.R. Extracellular Vesicles as Delivery Systems in Disease Therapy. Int. J. Mol. Sci. 2023, 24, 17134. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Liu, L.; Zhu, Z.; He, C. Exosomes in Lung Cancer Metastasis, Diagnosis, and Immunologically Relevant Advances. Front. Immunol. 2023, 14, 1326667. [Google Scholar] [CrossRef]
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomed. 2023, 18, 7923–7940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D. Exosomes in Cancer Development, Metastasis, and Immunity. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef]
- Han, J.; Ma, S.; Zhao, Y.; Wang, B.; Ding, S.; Hu, Y. The Function, Underlying Mechanism and Clinical Potential of Exosomes in Colorectal Cancer. Front. Biosci.-Landmark 2023, 28, 302. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.C.; Chandrashekar, C.; Kulkarni, S.; Shetty, N.; Pandey, A. Exosomes: Mediators of Cellular Communication in Potentially Malignant Oral Lesions and Head and Neck Cancers. F1000Research 2023, 12, 58. [Google Scholar] [CrossRef]
- Yoshino, H.; Tatarano, S.; Tamai, M.; Tsuruda, M.; Iizasa, S.; Arima, J.; Kawakami, I.; Fukumoto, W.; Kawahara, I.; Li, G.; et al. Exosomal MicroRNA-1 and MYO15A as a Target for Therapy and Diagnosis in Renal Cell Carcinoma. Biochem. Biophys. Res. Commun. 2022, 630, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Bu, P. Non-Coding RNA in Cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, K.; Zhou, H.; Wu, Y.; Li, C.; Liu, Y.; Liu, Z.; Xu, Q.; Liu, S.; Xiao, D.; et al. Role of Non-Coding RNAs and RNA Modifiers in Cancer Therapy Resistance. Mol. Cancer 2020, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Assaraf, Y.G.; Gacche, R.N. Long Non-Coding RNA Mediated Drug Resistance in Breast Cancer. Drug Resist. Updates 2022, 63, 100851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, P.; Yuan, S.; Wang, Y.; Cao, P.; Wen, F.; Li, H.; Zhu, L.; Liang, L.; Wang, Z.; et al. LncRNA BC200/MiR-150-5p/MYB Positive Feedback Loop Promotes the Malignant Proliferation of Myelodysplastic Syndrome. Cell Death Dis. 2022, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Muslimov, I.A.; Tsokas, P.; Berardi, V.; Zhong, J.; Sacktor, T.C.; Tiedge, H. Neuronal BC RNAs Cooperate with EIF4B to Mediate Activity-Dependent Translational Control. J. Cell Biol. 2014, 207, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Berardi, V.; Zhong, J.; Risuleo, G.; Tiedge, H. Dual Nature of Translational Control by Regulatory BC RNAs. Mol. Cell Biol. 2011, 31, 4538–4549. [Google Scholar] [CrossRef] [PubMed]
- Muslimov, I.A.; Berardi, V.; Stephenson, S.; Ginzler, E.M.; Hanly, J.G.; Tiedge, H. Autoimmune RNA Dysregulation and Seizures: Therapeutic Prospects in Neuropsychiatric Lupus. Life Sci. Alliance 2022, 5, e202201496. [Google Scholar] [CrossRef] [PubMed]
- Khodayi, M.; Khalaj-Kondori, M.; Hoseinpour Feizi, M.A.; Jabarpour Bonyadi, M.; Talebi, M. Plasma LncRNA Profiling Identified BC200 and NEAT1 LncRNAs as Potential Blood-Based Biomarkers for Late-Onset Alzheimer’s Disease. EXCLI J. 2022, 21, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Swellam, M.; Shalaby, N.M.; Darwish, M.K.; M. El-Nahrery, E. Long Non-Coding RNAs BACE1-AS and BC200 in Multiple Sclerosis and Their Relation to Cognitive Function: A Gene Expression Analysis. Brain Res. 2023, 1814, 148424. [Google Scholar] [CrossRef]
- Lang, N.; Wang, C.; Zhao, J.; Shi, F.; Wu, T.; Cao, H. Long Non-coding RNA BCYRN1 Promotes Glycolysis and Tumor Progression by Regulating the MiR-149/PKM2 Axis in Non-small-cell Lung Cancer. Mol. Med. Rep. 2020, 21, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Bian, W.-G.; Qin, Z.; Zeng, D.; Xu, J.-J.; Tang, H.-C. LncRNA BCYRN1 Promotes Cell Migration and Invasion of Non-Small Cell Lung Cancer via the MiR-30b-3p/ROCK1 Axis. Neoplasma 2022, 69, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, W.; Wang, M.; Yu, T.; Zhang, W. Long Non-Coding RNA Brain Cytoplasmic RNA 1 Acts as an Oncogene and Regulates Cell Proliferation and Metastasis in Non-Small Cell Lung Cancer. J. Nanosci. Nanotechnol. 2019, 19, 1978–1985. [Google Scholar] [CrossRef]
- Zhai, H.; Li, Y. BCYRN1 Is Correlated with Progression and Prognosis in Gastric Cancer. Biosci. Rep. 2019, 39, BSR20190505. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Yang, X.; Yang, Y.; Zhang, X.; Zhao, R.; Wei, R.; Zhang, X.; Zhang, Y. Upregulation of LncRNA BCYRN1 Promotes Tumor Progression and Enhances EpCAM Expression in Gastric Carcinoma. Oncotarget 2018, 9, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yu, T.; Ou, X.; Cao, D.; Xie, T.; Chen, X. Potential LncRNA Diagnostic Biomarkers for Early Gastric Cancer. Mol. Med. Rep. 2017, 16, 9545–9552. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Chen, Y. Clinical Significance of LncRNA BCYRN1 in Colorectal Cancer and Its Role in Cell Metastasis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9371–9378. [Google Scholar] [CrossRef]
- Gu, L.; Lu, L.; Zhou, D.; Liu, Z. Long Noncoding RNA BCYRN1 Promotes the Proliferation of Colorectal Cancer Cells via Up-Regulating NPR3 Expression. Cell. Physiol. Biochem. 2018, 48, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Bao, J.; Feng, J.-F. Long Non-Coding RNA BCYRN1 Exerts an Oncogenic Role in Colorectal Cancer by Regulating the MiR-204-3p/KRAS Axis. Cancer Cell Int. 2020, 20, 453. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Wang, H.; Fu, R.; Liu, X.; Piao, Y.; Wei, L.; Wang, J.; Zhang, L. LncRNA BCYRN1-Induced Autophagy Enhances Asparaginase Resistance in Extranodal NK/T-Cell Lymphoma. Theranostics 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Su, Y.-K.; Lin, J.W.; Shih, J.-W.; Chuang, H.-Y.; Fong, I.-H.; Yeh, C.-T.; Lin, C.-M. Targeting BC200/MiR218-5p Signaling Axis for Overcoming Temozolomide Resistance and Suppressing Glioma Stemness. Cells 2020, 9, 1859. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Afzal, O.; Afzal, M.; Gupta, G.; Thapa, R.; Ali, H.; Hassan almalki, W.; Kazmi, I.; Alzarea, S.I.; Saleem, S.; et al. MALAT1: A Key Regulator in Lung Cancer Pathogenesis and Therapeutic Targeting. Pathol. Res. Pract. 2024, 253, 154991. [Google Scholar] [CrossRef]
- Booy, E.P.; McRae, E.K.; Koul, A.; Lin, F.; McKenna, S.A. The Long Non-Coding RNA BC200 (BCYRN1) Is Critical for Cancer Cell Survival and Proliferation. Mol. Cancer 2017, 16, 109. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C.; Luo, Y.; Yu, M.; He, W.; An, M.; Gao, B.; Kong, Y.; Ya, Y.; Lin, Y.; et al. Tumor-derived Exosomal BCYRN1 Activates WNT5A/VEGF-C/VEGFR3 Feedforward Loop to Drive Lymphatic Metastasis of Bladder Cancer. Clin. Transl. Med. 2021, 11, e497. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Jiao, M.; Jiang, L.; Fu, X.; Wang, W. Long Noncoding RNA BCYRN1 Recruits BATF to Promote TM4SF1 Upregulation and Enhance HCC Cell Proliferation and Invasion. Dis. Markers 2022, 2022, 1561607. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, J.; Gutiérrez-Kobeh, L. Apoptosis and Its Pathways as Targets for Intracellular Pathogens to Persist in Cells. Parasitol. Res. 2024, 123, 60. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Zavarykina, T.M.; Lomskova, P.K.; Pronina, I.V.; Khokhlova, S.V.; Stenina, M.B.; Sukhikh, G.T. Circulating Tumor DNA Is a Variant of Liquid Biopsy with Predictive and Prognostic Clinical Value in Breast Cancer Patients. Int. J. Mol. Sci. 2023, 24, 17073. [Google Scholar] [CrossRef]
- Marassi, V.; Giordani, S.; Placci, A.; Punzo, A.; Caliceti, C.; Zattoni, A.; Reschiglian, P.; Roda, B.; Roda, A. Emerging Microfluidic Tools for Simultaneous Exosomes and Cargo Biosensing in Liquid Biopsy: New Integrated Miniaturized FFF-Assisted Approach for Colon Cancer Diagnosis. Sensors 2023, 23, 9432. [Google Scholar] [CrossRef]
- Ouyang, D.; Ye, N.; Yang, K.; Wang, Y.; Hu, L.; Chao, S.; Toner, M.; Li, Y. Precision Isolation of Circulating Leukemia Cells in Chronic Myelogenous Leukemia Patients Using a Novel Microfluidic Device and Its Clinical Applications. Cancers 2023, 15, 5696. [Google Scholar] [CrossRef]
- Crocetto, F.; Russo, G.; Di Zazzo, E.; Pisapia, P.; Mirto, B.F.; Palmieri, A.; Pepe, F.; Bellevicine, C.; Russo, A.; La Civita, E.; et al. Liquid Biopsy in Prostate Cancer Management—Current Challenges and Future Perspectives. Cancers 2022, 14, 3272. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Janku, F.; Zhan, Q.; Fan, J.-B. Accessing Genetic Information with Liquid Biopsies. Trends Genet. 2015, 31, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Miao, L.; Lu, Y.; Sun, Y.; Wang, S. Exosome, the Glass Slipper for Cinderella of Cancer—Bladder Cancer? J. Nanobiotechnol. 2023, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qi, Y.; Yang, C.; Tai, Q.; Zhang, M.; Shen, X.-Z.; Deng, C.; Guo, J.; Jiang, S.; Sun, N. Heterogeneous MXene Hybrid-Oriented Exosome Isolation and Metabolic Profiling for Early Screening, Subtyping and Follow-up Evaluation of Bladder Cancer. ACS Nano 2023, 17, 23924–23935. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guo, K.; Jiang, J.; Lin, S. Mesenchymal Stem Cell-Derived Exosomes as Delivery Vehicles for Non-Coding RNAs in Lung Diseases. Biomed. Pharmacother. 2024, 170, 116008. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar]
- Yang, C.; Wu, S.; Mou, Z.; Zhou, Q.; Dai, X.; Ou, Y.; Chen, X.; Chen, Y.; Xu, C.; Hu, Y.; et al. Exosome-Derived CircTRPS1 Promotes Malignant Phenotype and CD8+ T Cell Exhaustion in Bladder Cancer Microenvironments. Mol. Ther. 2022, 30, 1054–1070. [Google Scholar] [CrossRef]
- Chen, C.; Luo, Y.; He, W.; Zhao, Y.; Kong, Y.; Liu, H.; Zhong, G.; Li, Y.; Li, J.; Huang, J.; et al. Exosomal Long Noncoding RNA LNMAT2 Promotes Lymphatic Metastasis in Bladder Cancer. J. Clin. Investig. 2019, 130, 404–421. [Google Scholar] [CrossRef]
- Zheng, R.; Du, M.; Wang, X.; Xu, W.; Liang, J.; Wang, W.; Lv, Q.; Qin, C.; Chu, H.; Wang, M.; et al. Exosome–Transmitted Long Non-Coding RNA PTENP1 Suppresses Bladder Cancer Progression. Mol. Cancer 2018, 17, 143. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-Based Immunotherapy: A Promising Approach for Cancer Treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2017, 7, 237856. [Google Scholar] [CrossRef] [PubMed]
- Kayajima, T.; Shirahama, T.; Yanase, I.; Ohi, Y. Characterization of a New Cell Line Established from a Human Urinary-Bladder Cancer, with Specialreference to Metastatic Ability to the Lung. Jpn. J. Urol. Surg. 1989, 2, 577–580. [Google Scholar]
- Ichimi, T.; Enokida, H.; Okuno, Y.; Kunimoto, R.; Chiyomaru, T.; Kawamoto, K.; Kawahara, K.; Toki, K.; Kawakami, K.; Nishiyama, K.; et al. Identification of Novel MicroRNA Targets Based on MicroRNA Signatures in Bladder Cancer. Int. J. Cancer 2009, 125, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hidaka, H.; Seki, N.; Yoshino, H.; Yamasaki, T.; Itesako, T.; Nakagawa, M.; Enokida, H. Tumor-suppressive Micro RNA-135a Inhibits Cancer Cell Proliferation by Targeting the C-MYC Oncogene in Renal Cell Carcinoma. Cancer Sci. 2013, 104, 304–312. [Google Scholar] [CrossRef]
- Hidaka, H.; Seki, N.; Yoshino, H.; Yamasaki, T.; Yamada, Y.; Nohata, N.; Fuse, M.; Nakagawa, M.; Enokida, H. Tumor Suppressive MicroRNA-1285 Regulates Novel Molecular Targets: Aberrant Expression and Functional Significance in Renal Cell Carcinoma. Oncotarget 2012, 3, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the Welfare and Use of Animals in Cancer Research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]
- Tamai, M.; Tatarano, S.; Okamura, S.; Fukumoto, W.; Kawakami, I.; Osako, Y.; Sakaguchi, T.; Sugita, S.; Yonemori, M.; Yamada, Y.; et al. MicroRNA-99a-5p Induces Cellular Senescence in Gemcitabine-Resistant Bladder Cancer by Targeting SMARCD1. Mol. Oncol. 2022, 16, 1329–1346. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arima, J.; Yoshino, H.; Fukumoto, W.; Kawahara, I.; Saito, S.; Li, G.; Fukuda, I.; Iizasa, S.; Mitsuke, A.; Sakaguchi, T.; et al. LncRNA BCYRN1 as a Potential Therapeutic Target and Diagnostic Marker in Serum Exosomes in Bladder Cancer. Int. J. Mol. Sci. 2024, 25, 5955. https://doi.org/10.3390/ijms25115955
Arima J, Yoshino H, Fukumoto W, Kawahara I, Saito S, Li G, Fukuda I, Iizasa S, Mitsuke A, Sakaguchi T, et al. LncRNA BCYRN1 as a Potential Therapeutic Target and Diagnostic Marker in Serum Exosomes in Bladder Cancer. International Journal of Molecular Sciences. 2024; 25(11):5955. https://doi.org/10.3390/ijms25115955
Chicago/Turabian StyleArima, Junya, Hirofumi Yoshino, Wataru Fukumoto, Ichiro Kawahara, Saeki Saito, Gang Li, Ikumi Fukuda, Sayaka Iizasa, Akihiko Mitsuke, Takashi Sakaguchi, and et al. 2024. "LncRNA BCYRN1 as a Potential Therapeutic Target and Diagnostic Marker in Serum Exosomes in Bladder Cancer" International Journal of Molecular Sciences 25, no. 11: 5955. https://doi.org/10.3390/ijms25115955
APA StyleArima, J., Yoshino, H., Fukumoto, W., Kawahara, I., Saito, S., Li, G., Fukuda, I., Iizasa, S., Mitsuke, A., Sakaguchi, T., Inoguchi, S., Matsushita, R., Nakagawa, M., Tatarano, S., Yamada, Y., & Enokida, H. (2024). LncRNA BCYRN1 as a Potential Therapeutic Target and Diagnostic Marker in Serum Exosomes in Bladder Cancer. International Journal of Molecular Sciences, 25(11), 5955. https://doi.org/10.3390/ijms25115955






